Abstract
Introduction
Approximately 10-15% of women reportedly take an antihistamine during pregnancy for the relief of nausea and vomiting, allergy and asthma symptoms, or indigestion. Antihistamines include histamine H1-receptor and H2-receptor antagonists.
Areas covered
This is a systematic evaluation of the peer-reviewed epidemiologic literature published through February 2014 on the association between prenatal exposure to antihistamines and birth defects. Papers addressing histamine H1- or H2-receptor antagonists are included. Papers addressing pyridoxine plus doxylamine (Bendectin in the United States, Debendox in the United Kingdom, Diclectin in Canada, Lenotan and Merbental in other countries) prior to the year 2001 were excluded post-hoc because of several previously published meta-analyses and commentaries on this medication.
Expert opinion
The literature on the safety of antihistamine use during pregnancy with respect to birth defects is generally reassuring though the positive findings from a few large studies warrant corroboration in other populations. The findings in the literature are considered in light of three critical methodological issues: (1) selection of appropriate study population; (2) ascertainment of antihistamine exposures; and (3) ascertainment of birth defects outcomes. Selected antihistamines have been very well-studied (e.g. loratadine); others, especially H2- receptor antagonists, require additional study before an assessment of safety with respect to birth defects risk could be made.
Keywords: antihistamines, birth defects, pregnancy, prenatal exposure
Introduction
Antihistamines, available in both prescription and over-the-counter formulations, are commonly used during early pregnancy for the treatment of nausea and vomiting, symptoms of asthma and allergies, and relief of indigestion1, 2. Collectively, antihistamine use is reported by 10-15% of pregnant women 2, 3; a recent analysis of pooled data from two large national studies showed that several individual antihistamine components (i.e. promethazine, diphenhydramine, loratadine, cetirizine, doxylamine, chlorpheniramine, and fexofenadine) are each used by 1-4% of pregnant women during the first trimester 1. First generation H1-receptor antagonists (e.g. diphenhydramine [Benadryl®], dimenhydrinate [Dramamine], doxylamine plus pyridoxine (vitamin B6) [Bendectin in the United States, Debendox in the United Kingdom, Diclectin in Canada, Lenotan and Merbental in other countries] can cross the blood-brain barrier with resulting sedative and anticholinergic side effects and are frequently used to treat allergic reactions and nausea and vomiting of pregnancy (NVP). Second generation H1-receptor antagonists (e.g. loratadine [Claritin], cetirizine [Zyrtec], fexofenadine [Allegra]) lack those side effects and are primarily used to treat symptoms of asthma and allergies. H2-receptor antagonists (e.g. ranitidine [Zantac], cimetidine [Tagamet], famotidine [Pepcid], nizatidine [Axid]) used to treat indigestion, are less commonly used during pregnancy, though recent data indicate that approximately 1 in 100 pregnant women take ranitidine during the first trimester 1.
The most controversial antihistamine ever in widespread use in the United States, the combination of doxylamine plus pyridoxine (originally a three-component drug which also included dicyclomine [an antispasmodic]), was reformulated to the more commonly-used two-component version in 1976 after a double-blind evaluation demonstrated that dicyclomine did not contribute to the drug's effectiveness (reported in Brent, 2003) 4. Sold in the United States from 1957-1983, an estimated 30-35% of pregnant women used doxylamine plus pyridoxine for the treatment of NVP during the height of its popularity the 1970s 4. However, because of concerns about fetal safety, doxylamine plus pyridoxine has been the topic of at least 27 original research peer-reviewed publications 5, three published meta-analyses 5-7, and at least 30 commentaries, editorials, and letters to the editor (4, 8-11 ). The meta-analysis by McKeigue and colleagues based on 16 cohort and 11 case-control studies reported a pooled relative risk showing no association between any birth defect and first trimester exposure to doxylamine plus pyridoxine (0.95; 95% confidence interval (CI) 0.88-1.04). Separate analyses were conducted on types of birth defects including neural tube defects, oral clefts, congenital heart defects (CHD), and limb reductions, with relative risks ranging from 0.81 to 1.12, all of which had confidence intervals that contained the null value of 1.0 5. However in the face of numerous lawsuits stating that the drug caused birth defects, including a class action case with over 1,000 plaintiffs, 4 its manufacturer stopped marketing doxylamine plus pyridoxine in the United States in 1983. As of April 2013, however, the US Food and Drug Administration has granted approval for a marketing a formulation of doxylamine plus pyridoxine for the treatment of NVP in women who do not respond to conservative management.
This review evaluates the peer-reviewed epidemiologic literature on the association between prenatal exposure to antihistamine medications and birth defects. It is limited in scope to only structural birth defects; other potential adverse pregnancy outcomes such as fetal loss, preterm delivery, or low birth weight, or longer-term neurodevelopmental outcomes, are not included in this review. Papers addressing either histamine H1- or H2-receptor antagonists (or both antihistamine subtypes) are included. The findings in the literature are considered in light of three critical methodological issues: (1) selection of appropriate study population; (2) ascertainment of antihistamine exposures; and (3) ascertainment of birth defects outcomes.
Methods
2.1 Data sources and search terms
The authors conducted a search of the peer-reviewed literature using PubMed (National Center for Biotechnology Information, United States National Library of Medicine) with a filter for human studies in the English language published through February 4, 2014. Two distinct search strings were used, the results of which were combined and de-duplicated in a single EndNote X7 (Thomson Reuters, 1998-2013) library:
-
(1)
antihistamines AND (pregnancy OR birth defects OR congenital defects OR congenital malformations)
-
(2)
(nausea OR vomiting) AND (pregnancy OR birth defects OR congenital defects OR congenital malformations)
Search string (2) was used to ensure that papers which had a primary focus on NVP, but reported analyses for antihistamine use were captured. Papers that only reported on the associations with NVP did not meet inclusion criteria and would be excluded. Two co-authors each initially screened one-half of the article titles and abstracts for relevance; two additional coauthors then independently re-screened the article titles and abstracts. If both co-authors who reviewed a title and/or abstract agreed that the article could be excluded, then it was excluded without further review. If one of the two co-authors determined that the screened title and/or abstract needed a review of the full text, either because it appeared to meet inclusion criteria or it could not be determined whether it met inclusion criteria, the full text was retrieved and reviewed. The reference lists of the selected articles were also searched for additional papers that were not ascertained through the PubMed search (see flowchart, Figure 1). Through personal communication with the author, one additional article that was electronically published in September, 2013 but was not indexed in PubMed until March 2014 was also included in the review (in flowchart box entitled “Articles identified through other sources”).
Figure 1.
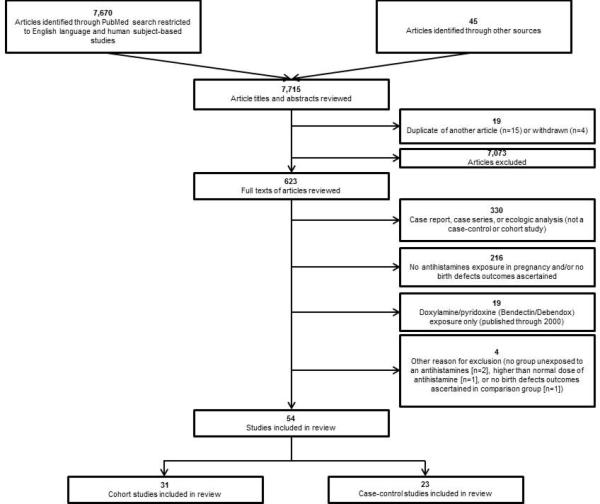
Flowchart for inclusion of articles in systematic review
2.2 Inclusion criteria
A study was included in this review if it:
-
(1)
was written in English,
-
(2)
was based on human data,
-
(3)
reported the results of original research (excluded case reports, case series, editorials [without original data], commentaries [without original data], review papers, clinical guideline documents, and duplicate reports),
-
(4)
reported on exposure during pregnancy to histamine H1-receptor (first or second generation) and/or H2-receptor antagonists at a clinically appropriate dose (i.e. excluded one study that investigated fetal effects of maternal suicide attempts through promethazine overdose) and an etiologically relevant time period for birth defects (i.e. studies of antihistamine exposure administered during labor and delivery to prevent nausea were excluded),
-
(5)
reported structural birth defects (collectively or as individual subtypes) as an outcome, and
-
(6)
included a comparison population (e.g. comparison of the prevalence of birth defects among antihistamine exposed and unexposed pregnancies in a cohort design or comparison of prevalence of antihistamine exposure among mothers of infants born with birth defects and mothers of infants born without birth defects [or with a different birth defect other than the birth defect of interest] in a case-control design).
Studies that focused exclusively on doxylamine plus pyridoxine use and were published during the 1980s and 1990s (through the year 2000) were excluded post-hoc, as it was determined that re-reviewing this literature was unnecessary in light of the several previously published meta-analyses and commentaries on this medication. In addition, if other studies included doxylamine plus pyridoxine as one of several antihistamines under investigation, the results of the other antihistamines are included in this review and the doxylamine plus pyridoxine results are not discussed, though the table notes for Tables 1 and 2 indicate the papers with published results for doxylamine plus pyridoxine use. Recent papers (since January 2001) that included doxylamine plus pyridoxine exposures are abstracted and included in this review because these are subsequent to the last published meta-analysis.
Table 1.
Cohort studies investigating association between antihistamines and birth defects included in systematic review (n=31)
| Author, Year | Setting | Antihistamines studied | Comparison group |
Exposure ascertainment |
Birth defects outcomes |
Total # pregnancies; # exposed; # unexposed |
# Birth defects among exposed and unexposed |
Measures of association |
|---|---|---|---|---|---|---|---|---|
| Aselton et al. 198525* | United States (Washington, Group Health Cooperative of Puget Sound), 1980-1982 | Diphenhydramine, triprolidine + pseudoephedrine, chlorpheniramine, brompheniramine, promethazine; use during first trimester | Baseline risk in total cohort | Pharmacy claims data from Group Health Cooperative | Any major birth defect ascertained through hospital discharge data with medical record reviews for suspected cases; clinical geneticist review of selected records | Total pregnancies: 6,509; diphenhydramine: 270; triprolidine + pseudoephedrine: 244; chlorpheniramine: 175; brompheniramine + phenylephrine + phenylpropanolamine: 172; promethazine: 63; phenylpropanolamine + chlorpheniramine: 82 | Diphenhydramine: 4/270 (1.5%); triprolidine + pseudoephedrine: 3/244 (1.3%); chlorpheniramine: 2/175 (1.1%); brompheniramine + phenylephrine + phenylpropanolamine: 5/172 (2.9%); promethazine: 0/63 (0%); phenylpropanolamine + chlorpheniramine: 2/82 (2.4%); baseline risk of birth defects: 16 per 1,000 (1.6%) | Not calculated |
| Ashkenazi-Hoffnung et al. 201320 | Israel Teratogen Information Service, 2008-2010 | Doxylamine + pyridoxine; use typically began during first trimester | Women taking metoclopramide | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about exposure | Any major birth defect ascertained through follow-up interview with mothers up to two years after initial inquiry | Total pregnancies: 58 (59 infants); doxylamine + pyridoxine: 29; metoclopramide: 29 | Doxylamine + pyridoxine: 0/29; metoclopramide: 1/29 (3.4%) | Not calculated |
| Asker et al. 200513 | Sweden, 1995-2002 | Meclizine; cyclizine; promethazine; diphenhydramine; use during first, second, and/or third trimester | Baseline risk in total cohort | Interview at first prenatal care visit (~10-12 weeks gestation) captured first trimester exposures; drugs prescribed during prenatal care were documented prospectively in medical record; analysis focused on first trimester use | Any major birth defect ascertained through record linkage with other national databases | Total pregnant women: 665,572 (676,198 infants); first trimester exposure: meclizine: 18,008; cyclizine: 1,221; promethazine:1,961; diphenhydramine: 147; | Any antiemetic: 3.1%; meclizine: 3.1%; cyclizine: 3.6%; promethazine: 3.2% diphenhydramine: 1.1% Baseline risk of birth defects: 3.5%; | Adjusted OR (95% CI) for exposure anytime during pregnancy and any birth defect: meclizine: 0.89 (0.82-0.96); cyclizine: 1.08 (0.86-1.35); promethazine: 0.91 (0.75-1.11); diphenhydramine: not calculated |
| Boskovic et al. 200421 | Canada Teratogen Information Service (Motherisk), 2001-2003 | Doxylamine + pyridoxine: standard dose and “supradose”; use typically began late in the first trimester (mean: 9 ± 2 weeks gestation) Note: Primary exposure of interest was lack of morning sickness |
Pregnancies without NVP | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about first trimester exposure | Any major birth defect ascertained through postnatal follow-up interview with mother; maternal reporting of birth defects verified by written report requested from pediatrician | NVP + standard dose of doxylamine + pyridoxine: 122; NVP + “supradose” of doxylamine + pyridoxine: 124 No NVP: 130 | NVP + standard dose of doxylamine + pyridoxine: 2/122 (1.6%); NVP + “supradose” of doxylamine + pyridoxine: 0/124; No NVP in pregnancy: 0/130 | Not calculated |
| Bsat et al. 200328 | United States (Clinical outpatient setting), 1994-1996 | Promethazine; use typically began late in the first trimester (mean: 8.6 ± 2 weeks gestation) | Women taking B6-metoclopramide or prochlorperazine | Administered through randomized clinical trial | Any major birth defect; method of ascertainment not stated | Singleton pregnanices: 156; promethazine: 52 B6-metoclopramide: 54 prochlorperazine: 50 | Promethazine: 0/52 B6-metoclopramide: 0/54;prochlorperazine: 1/50 | Not calculated |
| Colin Jones et al. 198559 | Scotland, 1978-1979 | Cimetidine; use anytime during pregnancy | Unexposed to cimetidine | Prescriptions reported by general practitioners | Any “abnormal children” reported by general practitioners | Total pregnancies:42; cimetidine: 20; unexposed: 22 | Cimetidine: 1/20 (5%); unexposed: 0/22 | Not calculated |
| Diav-Citrin et al. 200329 | Israel, Teratogen Information Service, 1995-2001 (loratadine exposure); 1990-2001 (comparison populations) | Loratadine; other antihistamines (astemizole, chlorpheniramine, terfenadine, hydroxyzine, promethazine);use during first trimester and anytime during pregnancy | Exposed to NTS | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about exposure | Any major birth defect ascertained through postnatal follow-up interview with mother, typically within one year after delivery | Total pregnancies:1,406; loratadine: 210; OAH: 267; NTS: 929 [Note: these numbers differ from the number of exposed with pregnancy outcome information due to loss to follow-up] |
Anytime during pregnancy: Loratadineloratadine: 4/175 (2.3%), OAH: 10/247 (4.0%), NTS: 25/844 (3.0%); During first trimester: loratadine: 1/126 (0.8%), OAH: 7/146 (4.8%), NTS:25/844 (3.0%) |
Unadjusted RR (95% CI) for exposure anytime during pregnancy: loratadine vs. OAH: 0.56 (0.18-1.77); loratadine vs. NTS: 0.77 (0.27-2.19); for first trimester exposure: loratadine vs. OAH: 0.17 (0.02-1.33); loratadine vs. NTS: 0.27 (0.04-1.94) |
| Einarson et al. 199722 | Canada Teratogen Information Service (Motherisk), 1989-1994; | Hydroxyzine, cetirizine; use during first trimester and entire pregnancy | Unexposed to antihistamines; unexposed matched to exposed on several potential confounders | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about exposure | Any major birth defect ascertained through maternal self-report during postnatal follow-up telephone interview; outcomes of interest confirmed whenever possible by the child's pediatrician. | Hydroxyzine: 81 (43 with first trimester exposure and live births); cetirizine: 39 (33 with first trimester exposure and live births); unexposed: 120 | Hydroxyzine: 2/43 (4.6%); cetirizine: 0/33; unexposed: 0/120 | Not calculated |
| Erez et al. 197123 | Turkey, 1967-1969 | Hydroxyzine; administered twice daily for 3 weeks during first two months of pregnancy | Placebo | Administered through double-blind placebo controlled trial | Any major birth defect ascertained by study pediatrician and obstetrician examining each delivered infant within 40 hours after birth (115 out of 150 were followed up until delivery) | Total pregnancies: 150; hydroxyzine: 100; placebo: 50 | Hydroxyzine: 1/100 (1%); placebo: 0/50 | Not calculated |
| Garbis et al. 200560 | European Network of Teratology Information Services (ENTIS), 1990-1997 for exposed cohort; 1990-1999 for unexposed cohort | H2-receptor antagonists: ranitidine (n=335), cimetidine (n=113); famotidine (n=75); nizatidine (n=15); roxatidine (n=15); use anytime during pregnancy though most were exposed during first trimester | Exposed to NTS | Self-reported through structured questionnaire; women (or their healthcare providers) called one of the 18 participating TIS because of question about exposure | Any major birth defect ascertained by each center through follow-up interview with the mother within a year of estimated date of delivery. | H2-receptor antagonists: 635; (lost to follow-up: 82; followed for pregnancy outcome: 553); NTS: 1,390 | H2-receptor antagonists: 13/553 (2.7%); NTS: 44/1,390 (3.5%) | Unadjusted RR (95% CI) for H2-receptor antagonist use at any time during pregnancy and any major birth defect: 0.78 (0.42 - 1.44) |
| Jick et al. 198126* | United States (Washington, Group Health Cooperative of Puget Sound), 1977-1979 | Triprolidine hydrochloride + pseudoephedrine hydrochloride; diphenhydramine; use during first trimester | Baseline risk in total cohort | Pharmacy records | Any major birth defect identified from hospital discharge codes; all potential cases had medical records review to confirm diagnosis | Total pregnancies: 6,837; triprolidine + pseudoephedrine: 384; diphenhydramine: 361 | Triprolidine + pseudoephedrine: 6/384 (16 per 1,000); diphenhydramine: 1/361 (3 per 1,000); baseline risk of birth defects: 11.7 per 1,000 (1.2%) | Not calculated |
| Källén and Mottet, 200319 | Sweden, 1995-2001 | Meclizine; use during early pregnancy (~before 10-12 weeks gestation) | Unexposed to meclizine | Exposure during “early pregnancy” ascertained prospectively during prenatal care by midwives, typically by 10-12 weeks gestation | Spectrum of major birth defects ascertained through linkage with other national databases | Total pregnancies: 540,660 (549,569 infants); meclizine: 16,536 | Meclizine: 524/16,536 (3.16%); prevalence among unexposed not stated | Unadjusted OR (95% CI) for any birth defect identified in the Medical Birth Registry: 0.91 (0.83-0.99); table 6 shows observed/expected analyses (RRs and 95% CIs) for several selected defects; none are significantly elevated or decreased; potential signals for anal atresia (2.29; 0.99-4.50) and body wall defect (2.33; 0.94-4.81) |
| Källén and Olausson, 200130‡ | Sweden, 1995-2001 | Loratadine; use during early pregnancy (~before 10-12 weeks gestation) | Baseline risk in total cohort | Exposure during “early pregnancy” ascertained prospectively during prenatal care by midwives; for cases, medical records were gathered to validate exposure though timing of loratadine use was not well-reported and unreliable. | Hypospadias as documented by ICD codes in the Swedish ; Registry of Congenital Malformations | Total pregnancies: 540,660 (549,569 infants); loratadine: 2,780 | Loratadine: 15/2,780 (0.5%) baseline hypospadias prevalence: 1/500; | Adjusted OR (95% CI) for association between loratadine exposure and hypospadias: 2.39 (1.43-3.38) if the twins were counted separately and 2.27 (1.33-3.87) if the twins were counted as a single occurrence. |
| Källén and Olausson, 200631‡ | Sweden, 1995-2004 | Loratadine; use during early pregnancy (~before 10-12 weeks gestation) | Expected number of hypospadias cases in birth population exposed to loratadine | Exposure during “early pregnancy” ascertained prospectively during prenatal care typically by 10-12 weeks gestation by midwives | Hypospadias as documented by ICD codes in the Swedish Registry of Congenital Malformations and Hospital Discharge Register | Updating of 2001 analysis: loratadine (1995-2001): 2,780; loratadine (2002-2004): 1,911 | Prevalence of hypospadias among women exposed to loratadine: 1995-2001: 25/2,780 (0.9 %), 2002-2004: 2/1,911(0.1%), 1995-2004 combined: 27/4,691 (0.6%); expected number of hypospadias cases in birth population exposed to loratadine: 1995-2001:12.5, 2002-2004:4.3; 1995-2004 combined: 16.8. | Calculated ratio of observed to expected (95% CI) using an exact Poisson distribution; for re-analysis of 1995-2001 data: 2.0 (1.29-2.95); for 2002-2004: 0.47 (0.06-1.68); for 1995-2004: 1.61 (1.04-2.34) |
| Källén, 199861 | Sweden, 1995-1996 | H2-receptor antagonists (included cimetidine, ranitidine, famotidine, nizatidine, cimetidine + famotidine, ranitidine + famotidine); use during early pregnancy (~before 10-12 weeks gestation) | Baseline risk in total cohort | Maternal self-report of medication used before first prenatal visit at 10-12 weeks gestation | Any major birth defect ascertained through linkages with several national registries | Acid-suppressing drugs: 547 pregnancies (553 infants); H2-antagonists only or H2-antagonists + proton pump inhibitors: 275 cimetidine (n=35), ranitidine (n=156), famotidine (n=58), nizatidine (n=3), cimetidine + famotidine (n=1), ranitidine + famotidine (n=2) | H2-receptor antagonists only: 6/255 (2.4%) Baseline birth defects prevalence: 3.9%; | Adjusted OR (95% CI) for H2-receptor antagonists use in first trimester and any major birth defect: 0.46 (0.17-1.20) |
| Källén, 200212 | Sweden, 1995-1999 | Wide array of first and second generation H1 receptor antagonists (see paper table 1 for complete list); use during early pregnancy (~ before 10-12 weeks gestation) Note: all analyses conducted by indication (NVP or allergy), not by medication |
Baseline risk in total cohort | Exposure during “early pregnancy” ascertained prospectively during prenatal care by midwives (typically at 10-12 weeks gestation) | Spectrum of specific defects ascertained through linkage with other national databases | Total exposed pregnancies: 17,776 (18,197 infants) | Total birth defects among exposed: 579; NVP indication: 402; Allergy indication: 177 Baseline birth defects prevalence: 3.16%; | Unadjusted OR (95% CI); among those with NVP indication: association with all birth defects: 0.98 (0.89-1.09); “selected” major birth defects: 0.94 (0.83-1.07); congenital heart defects: 0.89 (0.71-1.11); specific congenital heart defects: 0.78 (0.60-1.01); among those with allergy indication: all birth defect: 1.03 (0.89-1.20); “selected” major birth defects: 1.06 (0.88-1.28); congenital heart defects: 0.89 (0.63-1.24); specific congenital heart defects: 0.81 (0.55-1.20) [Reported in paper table 5]; only association reported for a specific defect was for congenital hip subluxation (previously associated with promethazine [Kullander and Källén14]): 1.05 (0.83-1.32) for any antihistamine used for NVP and 0.77 (0.51-1.18) for any antihistamine used for allergy |
| Kullander and Källén, 197614 | Sweden, 1963-1965 | Promethazine, diphenhydramine, and meclizine; any “anti-emetic”; use during first trimester | No antiemetic use | Self-report | Any major birth defect as ascertained by pediatrician and documented in medical records up to one year after birth | Total pregnancies: 6,376; pregnancies with infant follow-up: 5,753: antiemetics: 778; promethazine: 617; prochlorperazine: 91; diphenhydramine: 46; meclizine: 19; no antiemetics: 4,975 | Any antiemetic: 34/778 (4.4%); promethazine: 27/617 (4.4%); diphenhydramine: 3/46 (6.5%); meclizine: 0/19; no antiemetics: 166/4,975 (3.3%) (See table V for more detailed results) | Not calculated; authors noted increased risk of congenital diaphragmatic hernia associated with promethazine exposure |
| Lalkin et al. 199865 | Canada, Italy (2) and France, Teratogen Information Services | “Histamine blockers”; any use during pregnancy [89% used in first trimester] Note: exposure of interest was omeprazole; exposure to “histamine blockers” was comparison exposure |
Exposed to NTS; unexposed matched to exposed on several potential confounders | Self-reported through structured questionnaire; women (or their healthcare providers) TIS because of question about exposure | Any major birth defect ascertained through maternal self-report during postnatal follow-up telephone interview | Exposure anytime during pregnancy: “Histamine blockers”: 113; NTS: 113 Exposure during first trimester and had live birth: “Histamine blockers”: 98; NTS: 66 | “Histamine blockers”: 3/98 (3.1%); NTS: 2/66 (3.0%) | Not calculated; there was no significant difference in prevalence of birth defects among those exposed and unexposed to medications during pregnancy |
| Loebstein et al. 199932 | Canada (Motherisk) and Italy, Teratogen Information Services | Terfenadine; use during first trimester and entire pregnancy | Exposed to NTS; unexposed matched to exposed on several potential confounders | Self-reported through structured questionnaire; women (or their healthcare providers) called Canada or Italy TIS because of question about exposure | Any major birth defect ascertained through maternal self-report during postnatal follow-up telephone interview; outcomes of interest confirmed in writing by the child's pediatrician. | Terfenadine: 118 from both Canada (102) and Italy (16); first trimester exposure: 65; unexposed: 118 | Terfenadine: 0/65; unexposed: 2/111 (1.8%) | Matched RR (95% CI); association between first trimester terfenadine exposure and any major birth defect: 0.57 (0.06-5.39) |
| Magee et al. 199662 | Canada Teratogen Information Service (Motherisk), 1985-1993 | H2-receptor antagonists (ranitidine, cimetidine, famotidine, nizatidine); use during first trimester | Exposed to NTS; unexposed matched to exposed on several potential confounders | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about exposure. | Any major birth defect ascertained in interview with mother within one year after delivery | H2-receptor antagonists: 142 live births; NTS: 143 | H2-receptor antagonists: 3/142 (2.1%); NTS: 5/143 (3.5%) | Risk difference (95% CI) comparing frequency of birth defects among users of H2-receptor antagonists during the first trimester and NTS: −1.4% (−5.2%, 2.4%) |
| Matok et al. 201063 | Israel (Southern District), 1998-2007 | H2-receptor antagonists (famotidine, ranitidine, cimetidine), alone and in combination; use during first trimester | Unexposed to H2-receptor antagonists (or to famotidine, ranitidine, or cimetidine) | Prescription records | Any major birth defect, excluding those with chromosomal malformations | Total pregnancies: 84,823; H2-receptor antagonists: 1,148; famotidine: 878; ranitidine: 276 | H2-receptor antagonist: 65/1,148 (5.7%) vs.unexposed to H2-receptor antagonist: 4,400/83,675 (5.3%); famotidine: 58/878 (6.6%) vs.unexposed to famotidine: 4,407/83,945 (5.2%); ranitidine: 6/276 (2.2%) vs. unexposed to ranitidine: 4,459/84,547 (5.3%) | Adjusted OR (95% CI) for association between H2-receptor antagonist use in the first trimester and major birth defects: any use: 1.03 (0.80-1.32); famotidine: 1.21 (0.92-1.58); ranitidine: not calculated (fewer than 7 birth defects noted) |
| McBride, 196915 | Austraila (Sydney), 1956-1961 | Cyclizine; use during first trimester | Unexposed to cyclizine | Not stated | CPO | Total pregnancies: 25,333; first trimester use of cyclizine: 1,125; unexposed: 24,208; women with NVP and unexposed: 1,100 | CPO among first trimester users of cyclizine: 5/1125 (4.4/1,000); CPO among unexposed: 19/24,208 (0.78/1,000); CPO among women with NVP (and unexposed): 4/1,100 (3.64/1,000) | Not calculated; among individuals with NVP, there was no significant difference in prevalence of CPO among those exposed and unexposed to cyclizine in the first trimester |
| Michaelis et al. 198324* | Germany, 1964-1972 | Miscellaneous antiemetics (meclizine, triflupromazine, dimenhydrinate, chlorpheniramine); use during first trimester | Unexposed to antiemetics; unexposed matched to exposed on several potential confounders | Self-report and report by physician | Major, minor, other abnormalities ascertained from pediatric records; suspected cases underwent clinical review by panel of physicians | Total pregnancies: 13,643; miscellaneous antiemetics: 628; unexposed: 628 | Miscellaneous antiemetics: 11/628; Unexposed: 12/628 | Matched OR (90% CI) for miscellaneous antiemetic use in the first trimester and any birth defect: 0.92 (90% CI: 0.42, 2.00) |
| Milkovich and van den Berg, 197616† | United States (Northern California, Kaiser Permanente), 1959-1966 | Meclizine, cyclizine; use during first trimester use and throughout pregnancy | Women with NVP but no prescription for treatment of condition | Medical record reviewed to identify first trimester prescriptions | Any major birth defect | Total pregnancies: 11,481; use of meclizine in the first trimester: 613; use of cyclizine in the first trimester: 111; NVP but no prescription for NVP medications: 4,353 | Prevalence of birth defects identified by 1 month, 1 year and 5 years of age: Meclizine use in first trimester: 2.0%, 2.9%, 4.1%; cyclizine use in first trimester: 1.8%, 1.8%, 3.7%; among those with NVP but no medication use:1.5%, 2.2%, 3.2% | Not calculated; there were no significant differences in prevalence of birth defects comparing those with meclizine or cyclizine use in the first trimester with the unmedicated group |
| Moretti et al. 200333 | Canada, Italy, Israel, Brazil Teratogen Information Services (dates not stated) | Loratadine; use during first trimester | NTS; unexposed matched to exposed on several potential confounders | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about exposure | Any major birth defect ascertained through maternal self-report during postnatal follow-up telephone interview; outcomes of interest confirmed in writing by the child's pediatrician. | Total pregnancies: 322; loratadine: 161; NTS: 161 | Loratadine: 5/143 (3.5%); NTS: 6/150 (4.0%) | Not calculated by authors, p-value of 0.95 provided; RR (95% CI) for comparison of proportion of exposed and unexposed with major birth defect: 0.87 (0.26-2.91) (calculated via EpiSheet) |
| Pastuszak et al. 199634 | Canada and United States (Philadelphia) Teratogen Information Services, 1989-1994 | Astemizole; use during first trimester | NTS; unexposed matched to exposed on several potential confounders | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about exposure | Any major birth defect ascertained through maternal self-report during postnatal follow-up telephone interview | Astemizole: 114; NTS: 114 | Astemizole: 2/114 (1.8%) (one case of hypospadias and one case of spina bifida occulta); unexposed: 2/114 (1.8%) (one case of ventricular septal defect and one case of ocular myopathy) | Not calculated; there was no significant differences in prevalence of birth defects among those exposed and unexposed in the first trimester |
| Ruigómez et al. 199964 | United Kingdom and Italy, 1991-1996 | Cimetidine, ranitidine; use from 30 days before LMP date to 100 days after the LMP date | Unexposed to cimetidine, ranitidine, or omeprazole | Ascertained from medical records (United Kingdom) or prescriptions (Italy); women receiving more than one acid-suppressing drug during the first trimester were excluded from analysis | Any major or minor birth defect identified through general practitioners' records (United Kingdom) or hospital discharge data (Italy) | In both cohorts combined: liveborn infants: 2,261; stillbirths: 20; cimetidine: 237; ranitidine: 330; unexposed: 1,575 | United Kingdom cohort: cimetidine: 9/227 (4%); ranitidine: 17/229 (7.4%), unexposed: 37/651 (5.7%); Italy cohort: cimetidine: 2/10 (20%); ranitidine: 3/101 (3%); unexposed: 27/924 (2.9%) | Adjusted RR (95% CI) for medication use in the first trimester and any birth defect (in both cohorts combined): cimetidine: 1.3 (0.7-2.6), ranitidine: 1.5 (0.9-2.6) |
| Schatz et al. 199727 | United States (California, Kaiser-Permanente Prospective Study of Asthma During Pregnancy), 1978-1989 | Chlorpheniramine, tripelenamine or other antihistamine (not otherwise specified); use during first trimester and throughout pregnancy | Unexposed to chlorpheniramine tripelennamine, or other antihistamines; unexposed matched to exposed on several potential confounders | Maternal self-report at initial visit (< 28 weeks gestation) then daily diary for all medications through delivery | Any major birth defect ascertained from medical records | 824 women with and 678 women without asthma (total n = 1,502) who delivered a singleton birth later than 20 weeks' gestation and for whom complete information regarding medications taken during pregnancy was available; chlorpheniramine, tripelennamine, or other antihistamines used in first trimester: 321; unexposed: 1,175 | Chlorpheniramine, tripelennamine, or other antihistamines used in first trimester: 3.7%; unexposed: 5.5%; | Not calculated |
| Shapiro et al. 197817 | United States (Collaborative Perinatal Project), 1959-1966 | Meclizine; use during first four lunar months | Not exposed to meclizine in the first four lunar months | Self-report | Any major birth defect | “Mother-child pairs”: 50,282; meclizine: 1,014; unexposed: 49,268 | Meclizine: 36/1,014 (3.6%); unexposed: 1,357/49,268 (2.8%) | Standardized RR (95% CI) for meclizine use in the first four lunar months of pregnancy and major malformations: 1.20 (0.90-1.61) [See paper table for specific organ systems -- significant association with eye and ear defects (2.79 (1.12-5.73)] |
| Weber-Schoendorfer and Schaefer 200835 | Germany (Berlin Teratogen Information Service), 1992-2006 | Cetirizine; use during the first trimester | Exposed to NTS; unexposed matched to exposed on several potential confounders | Self-reported through structured questionnaire; women (or their healthcare providers) called TIS because of question about exposure | Any major birth defect, excluding genetic syndromes ascertained during postnatal questionnaire to the mother and/or physician 8 weeks after delivery | Cetirizine: 196; NTS: 1,686 | Cetirizine: 3/177 (1.7%); NTS: 24/1,521 (1.6%) | Unadjusted OR (95% CI) of the association of cetirizine use in the first trimester and any major birth defect: 1.07 (0.21-3.59) |
| Yerushalmy and Milkovich, 196518† | United States (Northern California, Kaiser Permanente), 1960-1964 | Meclizines, cyclizines, OAN; medications prescribed during the first 12 weeks of pregnancy | No antinauseant drugs prescribed | Maternal self-report at time of first prenatal visit (2/3 report in the first trimester); record of prescribed medications in medical record | Any “severe” birth defect, and by organ systems -- see Table 3 in paper | Total pregnancies: 4,277; meclizines/ cyclizines: 315; OAN: 449; unexposed: 3,902 | Meclizines/cyclizines: 9/315 (3.2%); OAN: 11/449 (3.3%); unexposed: 101/3,902 (3.8%) | Not calculated; there were no significant differences in prevalence of birth defects among those exposed and unexposed to medications |
CI, confidence interval; CL/P, cleft lip with or without cleft palate; CPO, cleft palate only; LMP, last menstrual period; NTD, neural tube defects; NTS, nonteratogenic substances; NVP, nausea and vomiting of pregnancy; OAN, other antinauseant; OAH, other antihistamines; OR, odds ratio; RR, risk ratio; TIS, Teratogen Information Service
Manuscript reported results for doxylamine+pyridoxine which are not included in this systematic review
Overlapping data from Kaiser Permanente
Overlapping data from Swedish surveillance systems
Table 2.
Case-control studies investigating association between antihistamines and birth defects included in systematic review (n=23)
| Author Year | Setting | Antihistamines studied | Exposure ascertainment | Birth defects outcomes | Total # cases and controls* | # Exposed cases and controls | Measures of association |
|---|---|---|---|---|---|---|---|
| Acs et al. 200966 | Hungary, (HCCSCA), 1980-1996 | H2-receptor antagonists (i.e. cimetidine, ranitidine); use during pregnancy | Documented in prenatal care records, self-reported in maternal questionnaire, or both | Spectrum of major birth defects ascertained through national birth defects surveillance system | Cases: 22,843; matched controls: 38,151 | H2-receptor antagonist (secondary exposure of interest) among those with severe chronic dyspepsia: 14/148 (9.5%) cases; 27/214 (12.6%) controls | Among those with severe chronic dyspepsia, the unadjusted OR (95% CI) for association between any birth defect and treatment with H2-receptor antagonists during pregnancy: 0.7 (0.4-1.4) |
| Anderka et al. 201253# | United States, (NBDPS), 1997-2004 | Antihistamine antiemetics (including promethazine, doxylamine), other antihistamines (including diphenhydramine, cetirizine [“OAH”]), antihistamine antiemetics + vitamin B6 (doxylamine + pyridoxine), H2-receptor antagonists (including ranitidine); use from one month before pregnancy through end of first trimester, | Self-reported during computer assisted telephone interview conducted between 6 weeks and 24 months after estimated date of delivery | CL/P, CPO, NTD, 2nd or 3rd degree hypospadias ascertained through population-based birth defects surveillance systems | CL/P cases: 1,546; CPO cases: 821; NTD cases: 1,038; hypospadias cases: 1,144; controls: 5,859 | Among those with NVP: antihistamine antiemetic: CL/P cases: 45/933 (4.8%), controls: 212/4,009 (5.3%); promethazine: CL/P cases: 38/933 (4.1%); controls: 162/4,009 (4.0%); OAH: CL/P cases: 22/933 (2.4%), controls: 97/4,009 (2.4%); diphenhydramine: CL/P cases: 17/933 (1.8%), controls: 68/4,009 (1.7%) cetirizine: CL/P cases: 6/933 (0.64%), controls: 30/4,009 (0.75%); H2-receptor antagonists: CL/P cases: 4/933 (0.43%), controls: 29/4,009 (0.72%) [See paper tables 4, 5, and 6 for exposure prevalences for other birth defects] | Adjusted OR (95% CI) for medication use and CLP: antihistamine antiemetic: 1.02 (0.72-1.44); promethazine: 1.11 (0.76-1.63); OAH 0.95 (0.59-1.55); diphenhydramine OR 1.03 (0.59-1.79); cetirizine OR 0.90 (0.36-2.22); H2-receptor antagonists: 0.57 (0.19-1.67) [See paper tables 4, 5, and 6 for results for other birth defects] |
| Aselton and Jick, 198542 | United States (Washington, Group Health Cooperative of Puget Sound), 1977-1982 | Non-doxylamine-pyridoxine antihistamines (i.e. pseudoephedrine + triprolidine and diphenhydramine); use during first trimester | Pharmacy claims data | Pyloric stenosis ascertained through hospital discharge data | Pyloric stenosis cases: 12; matched controls: 32 | Non-doxylamine-pyridoxine antihistamines: 3/12 (25%) cases;2/32 (6.3%) controls | Matched OR (95% CI) for association between pyloric stenosis and non-doxylamine-pyridoxine antihistamine use: 4.1 (0.8-21.7) |
| Bánhidy et al. 201167 | Hungary (HCCSCA), 1980-1996 | Cimetidine Note: primary exposure of interest was PUD |
Documented in prenatal care records, self-reported in maternal questionnaire, or both; dose, duration and route of administration were available | Spectrum of specific defects ascertained through national surveillance system | Cases: 22,843; matched controls: 38,151 | Cimetidine exposure (secondary exposure of interest) among those with definitive peptic ulcer disease (PUD): 6/20 (30%) cases; 7/58 (12.1%) controls | Among those with definitive peptic ulcer disease, unadjusted OR (95% CI) for association of any birth defect and treatment with cimetidine: 3.1 (0.9-10.8) |
| Bartfai et al. 200850 | Hungary (HCCSCA), 1980-1996 | Promethazine; use during 2nd/3rd gestational months and entire pregnancy | Documented in prenatal care records, self-reported in maternal questionnaire, or both; dose, duration and route of administration were available | Spectrum of specific defects ascertained through national surveillance system | Cases: 22,843; matched controls: 38,151; Down syndrome controls: 834 (selected from HCAR) | Promethazine exposure during entire pregnancy [during 2nd/3rd gestational months]: 3,648 (16.0%)[1179 (5.2%)] cases; 6,025 (15.8%) [1825 (4.8%)] controls without birth defects; 142 (17.0%) [43 (5.2%)] Down syndrome controls | Adjusted OR (95% CI) for any exposure to promethazine and any birth defect in cases compared to matched controls: during entire pregnancy: 1.06 (1.01-1.11); any 2nd/3rd gestational month exposure: 1.14 (1.05-1.23); “medically recorded” promethazine exposure during 2nd/3rd gestational month: 0.8 (0.7-0.9); also elevated OR for CL/P and polydactyly/ syndactyly [see paper table 6];in cases compared to Down syndrome controls: 0.9 (0.7-1.3); no statistically significant associations reported [see paper table 7] |
| Boneva et al. 199939† | United States (Metropolitan Atlanta), 1982-1983 | Any antinausea medication (not otherwise defined); use within the first 8 weeks of pregnancy | Self-reported in maternal telephone interview | Congenital heart defects as a group and specific subtypes ascertained through population-based active birth defects surveillance system | CHD cases: 998; controls: 3,029 | Among individuals with early, severe NVP: any antinausea medication (not otherwise specified): 145/624(23.2%) cases; 543/2,084 (26.1%) controls | Unadjusted OR (95% CI) for any antinausea medication: among those with the most NVP (level 1), compared with no NVP: 0.74 (0.56-0.97); among those with NVP levels 1-4, compared to no NVP: 0.77 (0.61-0.97) [see paper Table 3]; specific CHD effect estimates were provided for “NVP+med” vs “No NVP” and “NVP+med” vs “NVP+no med” |
| CDC, 200455§ | United States (NBDPS), 1997-2001 | Loratadine, non-sedating antihistamines (not further defined but included loratadine); sedating antihistamines (not further defined); use from one month before pregnancy through the first trimester | Use from one month before pregnancy through end of first trimester, self-reported during computer assisted telephone interview conducted between 6 weeks and 24 months after estimated date of delivery | Second or third degree hypospadias ascertained through population-based birth defects surveillance systems | Hypospadias cases: 563; male controls: 1,444 | Loratadine: 11/563 (1.9%) cases; 22/1,444 (1.5%) controls; non-sedating antihistamines (including loratadine): 17/563 (3.0%) cases; 33/1,444 (2.3%) controls; sedating antihistamines: 43/563 (7.6%) cases;104/1,444 (7.2%) controls | Adjusted OR (95% CI) for associations with hypospadias: loratadine: 0.96 (0.41-2.22); non-sedating: 0.95 (0.48-1.89); sedating: 1.02 (0.68-1.53) |
| Czeizel and Vargha, 200549 | Hungary (HCCSCA), 1980-1996 | Dimenhydrinate; use during first trimester | Documented in prenatal care records, self-reported in maternal questionnaire, or both; dose, duration and route of administration were available | Spectrum of specific defects ascertained through national surveillance system | Cases: 22,843 cases; matched controls: 38,151 | Dimenhydrinate: 914/22,843 (4.0%) cases; 1,726/38,151 (4.5%) controls | No statistically significantly elevated associations; one statistically significantly inverse association (with urinary tract obstructive defects, PR 0.2 [95% CI: 0.1-0.7]) [see paper table 3 for results of matched case-control analysis] |
| Czeizel et al. 200351 | Hungary (HCCSCA), 1980-1996 | Dimenhydrinate; use during pregnancy Note: exposure of interest was hyperemesis gravidarum; treatments included: pyridoxine, dimenhydrinate, thiethylperazine, magnesium |
Documented in prenatal care records, self-reported in maternal questionnaire, or both; dose, duration and route of administration were available | CL/P, CPO | 1368 cases with CL/P; 582 cases with CPO; 1374 controls matched to CL/P cases; 581 controls matched to CPO cases | Dimenhydrinate exposure among those with hyperemesis gravidarum 27/89 (30.3%) CL/P cases; 34/128 (26.5%) CL/P matched controls; 21/42 (50%) CPO cases;19/66 (28.7%) CPO matched controls | Among cases and controls with hyperemesis gravidarum unadjusted OR (95% CI) for CL/P 1.20 (0.66-2.19), CPO: 2.47 (1.10-5.54) [Calculated based on data in paper table 3 using: http://www.medcalc.org/calc/odds_ratio.php] |
| Eskenazi and Bracken, 198543 | United States (Connecticut, 5 urban hospitals), 1974-1976 | Diphenhydramine, chlorpheniramine, promethazine; chlorpheniramine+phenylpropanolamine Note: excluded exposures to doxylamine-pyridoxine; only included exposures to other antihistamines |
Self-reported in maternal interview | Pyloric stenosis ascertained through medical records of live births and stillbirths occurring at five main urban hospitals in Connecticut; some cases also arose from “community” | Pyloric stenosis cases: 71; “other malformed controls”: 1,356; controls: 3,002 | Diphenhydramine: 3/71 (4.2%) cases; 26/3,002 (0.87%) controls; chlorpheniramine: 1/71 (1.4%) cases; 2/3,002 (0.06%) controls; promethazine: 1/71 (1.4%) cases; 8/3,002 (0.26%) controls; chlorpheniramine+phenylpropanola mine: 1/71 (1.4%) cases; 4/3,002 (0.13%) controls | Unadjusted OR (95% CI) for association between any non-doxylamine-pyridoxine antihistamine exposure and pyloric stenosis: 5.61 (2.14-14.67) (control group without birth defects; 3.73 (1.39-9.99) (control group with other birth defects) |
| Gilboa et al. 20093§# | United States (NBDPS), 1997-2003 | Any antihistamine and cetirizine, clemastine, dimenhydrinate, diphenhydramine, doxylamine, hydroxyzine, fexofenadine, loratadine, meclizine, pheniramine, promethazine, triprolidine, and not otherwise specified antihistamine products; use from one month before pregnancy through the end of the first trimester | Self-reported during computer assisted telephone interview conducted between 6 weeks and 24 months after estimated date of delivery | Spectrum of selected major birth defects ascertained through population-based birth defects surveillance systems | CHD cases: 3,587; non-CHD cases: 5,491; controls: 4,982 | Any antihistamine: 421/3,587 (11.7%) CHD cases; 643/5,491 (11.7%); non-CHD cases; 518/4,982 (10.4%) controls [see paper tables for exposure prevalences for specific antihistamines] | 23 statistically significant associations; 24 when used Bayesian analysis [See results tables in manuscript] |
| Golding et al. 198347† | United Kingdom, 1965-1974 | Any antihistamine in 69 days since date of last menstrual period; sufficient number of exposures to analyze doxylamine and promethazine | Medical record review (as reported by general practitioners) | CL/P, CPO ascertained through birth and subsequent hospital records, vital records, birth defects registry, and genetics research unit | Oral cleft cases: 196; matched controls: 407 | Promethazine: 5/196 (2.5%) cases; 18/407 (4.4%) controls | Not calculated; frequency of promethazine use between case and control mothers was not statistically significantly different |
| Idanpaan-Heikkila and Saxen, 197345 | Finland, 1964-1972 | Fixed combination drug (imipramine 10 mg [a tricyclic antidepressant] + chloropyramine 10 mg [an antihistamine]); use during first trimester | Documented prospectively in prenatal record | Any major birth defect ascertained through Finish Register of Congenital Malformations | Cases: 2,784; matched controls: 2,784 | Fixed combination drug during the first trimester of pregnancy: 3/2,784 (0.1%) cases; 0/2,784 controls | Not calculated |
| Källén and Robert-Gnansia, 200536 | Sweden, 1995-2002 | Any antihistamine (not otherwise specified); use during first trimeste use and anytime during pregnancy | Documentation in medical record of maternal report of antihistamine use prior to first prenatal visit at 10-12 weeks gestation | Craniostenosis (excluding cases with chromosomal defects) ascertained through several national health registries | Cases: 398; comparison population: 728,822 pregnancies | Any antihistamine use in the first trimester: 22/398 (5.5%) cases | RR (95% CI) (based on comparison of observed and expected frequency of exposure) for association between any antihistamine use in the first trimester and craniostenosis: 1.4 (0.9-2.1)†† |
| Li et al. 201354‡ | United States and Canada (Slone Epidemiology Center Birth Defects Study), 1998-2010 | Any H1 antihistamine; 16 a priori analyses (based on previous findings in literature) and exploratory analyses limited to diphenhydramine, loratadine, chlorpheniramine, and doxylamine; use during first trimester | Mothers sent a letter describing the study and a medication identification booklet with pictures of medications and products; nurse-interviewer administered questionnaire by telephone within 6 months of delivery | Major birth defects identified within 5 months of delivery at 29 large, pediatric, tertiary-care centers across the US and Canada. | Cases:13,213; matched controls: 6,982 | Any antihistamine (first trimester): 14.9% cases; 13.7% controls [see paper for prevalences for specific antihistamines] | [See paper table 2 (a priori hypotheses) and table 3 (exploratory analyses) for specific estimates] |
| Mellin and Katzenstein, 196344 | United States (New York City), 1953-1957 | Meclizine, dimehydrinate, cyclizine; use during first trimester | Prospectively documented in medical record | Any major birth defect | Cases: 266; controls (group 1): 266; controls (group 2): 266 | Any of the included antihistamines: 30/266 (11.3%) cases; 31/266 (11.7%) controls (group 1); 32/266 (12.0%) controls (group 2) | Not calculated; frequency of antihistamine use between case and control mothers was not statistically significantly different |
| Nelson and Forfar, 197148 | Scotland, 2 years (unspecified dates) | Promethazine hydrochloride, diphenhydramine, triprolidine hydrochloride, mepyramine maleate, diphenylpyraline, chlorpheniramine, chlorcyclizine, and trimeprazine; use during first trimester | Self-report at time of delivery; use of prescription medications verified by physician report, hospital record, or pharmacy record | Major and minor defects | Cases (major defects): 175; cases (minor defects): 283; matched controls: 911 | Any antihistamine: 2/175 (1.1%) cases; 26/911 (2.9%) controls | Not calculated; frequency of antihistamine use between case and control mothers was not statistically significantly different |
| Pedersen et al. 200658* | Denmark (4 counties), 1989-2002 | Loratadine; OAH; use from 30 days before conception through end of first trimester (other time periods of pregnancy also analyzed) | Linkage of case data with prescription databases | Hypospadias ascertained from national hospital discharge registry | Hypospadias cases: 227; matched controls: 2270 | Loratadine: 1/227 (0.4%) cases and 8/2270 (0.35%) controls; OAH: 4/227 (1.8%) cases and 23/2270 (1.0%) controls | Adjusted OR (95% CI) for exposure during the first trimester or 30 days before conception: loratadine: 1.4 (0.0-10.5); OAH: 1.9 (0.5-5.8); for exposure in first or second trimester: loratadine: 0.8 (0.0-4.9); OAH: 1.6 (0.3-5.5); for exposure anytime during pregnancy: loratadine: 0.5 (0.0-3.3); OAH: 1.0 (0.2-3.4) |
| Pedersen et al. 200657* | Denmark, 1998-2002 | Loratadine; OAH; use from 30 days before conception through pregnancy | At first prenatal visit (around 12 weeks gestation), women completed questionnaire asking about medication use in three months before pregnancy through the present; subsequent interviews (around 30 weeks), 6 months and 18 months post-delivery captured additional information on exposures and outcomes | Hypospadias ascertained through linkage with other national databases | Hypospadias cases: 203; matched controls: 2,030 | Exposure during pregnancy or 30 days before conception: 1/203 (0.5%) loratadine exposed case; 25/2030 (1.2%) loratadine exposed controls; 4/203 (2.0%) OAH exposed cases, 48/2030 (2.4%) OAH exposed controls.; exposure 30 days before conception and in first trimester only: 1/203 (0.5%) loratadine exposed case, 12/2030 (0.5%) loratadine exposed controls; 2/203 (1%) OAH exposed cases, 37/2030 (1.8%) OAH exposed controls. | Adjusted OR (95% CI) for exposure during the first trimester or 30 days before conception: loratadine: 0.9 (0.1-6.9); OAH: 0.5 (0.1-1.9); for exposure anytime during pregnancy or 30 days before conception: loratadine: 0.4 (0.1-2.8); OAH: 0.7 (0.3-2.1) |
| Pedersen et al. 200856* | Denmark, 1996-2004 | Loratadine; OAH; use from 30 days before conception through first trimester (other time periods of pregnancy also analyzed) | Linkage of case data with prescription databases | Hypospadias ascertained from national hospital discharge registry; up to 10 controls (live male births) randomly selected from national birth registry without a diagnosis of hypospadias, matched by birth year and mother's residence | Cases: 1,575; matched controls: 14,660 | Loratadine: 7/1,575 (0.44%) cases;88/14,660 (0.6%) controls; OAH: 28/1,575 (1.8%) cases;217/14,660 (1.5%) controls | Adjusted PR (95% CI) for loratadine use 30 days before conception and during first trimester: 0.6 (0.3-1.4); for OAH: 1.3 (0.9-1.9) |
| Saxen, 197446 | Finland, 1967-1971 | Any antihistamine; cyclizines, diphenhydramine, and other antihistamines; use during first trimeste | Prospectively ascertained through maternal self-report | Oral clefts ascertained from national birth defects registry | All oral clefts: 599; CPO: 232; CL/P: 232; clefts with other defects: 134; matched controls: 590 | Any antihistamine: 5.6% CPO, 2.6% CL/P, 5.4% all oral clefts, 2.3% controls; diphenhydramine: 8/232 CPO (3.4%), 4/232 (1.7%) CL/P, 20/599 (3.3%) all oral clefts, 6/590 (1%) controls | Not calculated; for both any antihstamine use and diphenhydramine use, there were significant differences between controls and all clefts combined and CPO; for diphenhydramine use, there were significant differences between controls and all clefts combined and CPO; no association with cyclizine use. |
| Werler et al. 199237 | United States and Canada, (Slone Epidemiology Center Birth Defects Study), 1976-1990 | Any antihistamine (excluding antiemetics which contained antihistamines); use during first trimester | Mothers sent a letter describing the study and a medication identification booklet with pictures of medications and products; nurse-interviewer administered questionnaire by telephone within 6 months of delivery | Gastroschisis identified within five months of delivery at 29 large, pediatric, tertiary-care centers across the US and Canada | Gastroschisis cases: 76; malformed controls (birth defects other than gastroschisis and several other gastrointestinal, vascular and other defects): 2,142 | Any antihistamine: 10/76 (13.2%) cases; 173/2,142 (8.1%) controls | Adjusted OR (95% CI) for association between any antihistamine use in the first trimester and gastroschisis: 1.3 (0.5-3.1) |
| Werler et al. 200238‡ | United States and Canada, (Slone Epidemiology Center Birth Defects Study), 1995-1999 | Any antihistamine; use during first three lunar months | Mothers sent a letter describing the study and a medication identification booklet with pictures of medications and products; nurse-interviewer administered questionnaire by telephone within six months of delivery | Gastroschisis and small intestinal atresia identified within five months of delivery at 29 large, pediatric, tertiary-care centers across the US and Canada | Gastroschisis cases: 206; small intestinal atresia cases: 126; age-matched controls: 382 malformed (with birth defects other than gastroschisis, small intestinal atresia or other gastrointestinal defects); 416 nonmalformed (infants with medical conditions requiring hospitalization such as infections, preterm delivery, hyperbilirubinemia) | Any antihistamine: 26/206 (12.6%) gastroschisis cases; 18/126 (14.3%) small intestinal atresia cases; 48/382 (12.5%) malformed controls; 56/416 (13.5%) nonmalformed controls | Adjusted OR (95% CI) for gastroschisis: 0.6 (0.3-1.2); small intestinal atresia: 0.9 (0.4-1.8) |
CHD, congenital heart defect; CI, confidence interval; CL/P, cleft lip with or without cleft palate; CPO, cleft palate only; GP, general practitioner; HCCSCA, Hungarian Case-Control Surveillance of Congenital Abnormalities; HCAR, Hungarian Congenital Abnormalities Registry; NBDPS, National Birth Defects Prevention Study; NTD, neural tube defects; NVP, nausea and vomiting of pregnancy; OAH, other antihistamines; OR, odds ratio; PR, prevalence ratio; PUD, peptic ulcer disease; RR, risk ratio
Controls are infants born without major birth defects unless otherwise specified
Manuscript reported results for doxylamine+pyridoxine which are not included in this systematic review
Overlapping data from Slone Epidemiology Center Birth Defects Study
Overlapping data from NBDPS
Overlapping data from NBDPS
Potential overlapping data from Denmark
Note: Re-calculated confidence interval for this review because confidence limit reported in paper was incorrect. Used Rothman's EpiSheet (http://www.epidemiolog.net/studymat/).
Included studies were not required to report a measure of association (i.e. odds ratio, prevalence ratio, risk ratio) or the results of statistical testing. For selected studies in which adequate data were available but a measure of association was not reported in the original publication, we calculated a measure of association. When distinct publications included overlapping data, it was noted in the text and notes for Tables 1 and 2.
3. Results
Out of the 7,670 articles identified through PubMed and 45 identified through other sources, 54 papers met the inclusion criteria for this review (Figure 1). Thirty-one are cohort studies (Table 1); 23 are case-control studies (Table 2).
3.1 Histamine H1-receptor antagonists: Findings from cohort studies
A total of 24 cohort studies reported findings with respect to histamine H1-receptor antagonists (Table 1). Eight of these 24 cohort studies were based on data from a Teratogen Information Service (TIS). Briefly, TIS locations in the United States and in several countries provide evidence-based information to mothers, health care professionals, and the general public about medications and other exposures during pregnancy and while breastfeeding. This typically occurs when physicians or women call the service concerned about a chemical or medication exposure. During the call, information on pregnancy exposures is collected. For TIS conducting studies of pregnancy outcomes, within a year of delivery, a follow-up survey is sent to the women to gather information on the outcome of the pregnancy. In some services, the outcome of the pregnancy is verified with a health care provider. Additional data sources are sometimes linked to the information gathered during the initial telephone call or in the follow-up interview. Many of the studies using TIS data derive an “unexposed” group from women calling about exposures deemed to be “non-teratogenic”, such as dental examinations, x-ray exposures, and use of medications such as acetaminophen.
One cohort study analyzed antihistamines in the aggregate only 12 though reported frequencies of selected birth defects by specific medication exposures. Källén explored a wide array of first and second generation H1-receptor antagonists in relation to several pregnancy outcomes, but reported estimates of association by indication only (not specific antihistamines), and analyzed medications used to treat NVP separately from those used to treat allergies. Among the reported analyses of aggregated outcomes such as all birth defects, selected birth defects, all CHDs, specified CHDs, and all birth defects except CHDs, there were no significantly elevated associations with either antihistamines used to treat NVP or those used to treat allergies 12.
3.1.1 First generation H1-receptor antagonists
3.1.1.1 Cyclizine or meclizine
Seven cohort studies investigated the association between cyclizine or meclizine exposure and birth defects 13-19. A Northern California Kaiser Permanente cohort study of 4,277 pregnancies between 1960-1964 included detailed maternal interviews to ascertain medication use in addition to validation from the prenatal record 18. There were 315 pregnancies exposed to meclizine or cyclizine in the first trimester compared with 3,902 unexposed to any antinauseant medication. The prevalence of birth defects was comparable in the two groups (3.2% in the meclizine/cyclizine group and 3.8% in the unexposed comparison group) 18. In a follow-up study of the same population including several additional years of data, Milkovich and van den Berg found no difference in the prevalence of birth defects among women exposed to meclizine or cyclizine compared with a group of women with unmedicated NVP 16.
Based on data from over 25,000 pregnancies in Sydney, Australia from 1956-1961, McBride reported a prevalence of cleft palate of 4.4 per 1,000 among 1,125 women who took cyclizine during pregnancy, compared with a baseline prevalence of 0.78 per 1,000 among 24,208 unexposed pregnancies. However, in a sub-analysis comparing the cyclizine-exposed group to a group with untreated NVP (i.e. controlling for indication), the difference in the two groups was no longer noteworthy (4.4 per 1,000 compared with 3.6 per 1,000) 15.
Using data from the United States Collaborative Perinatal Project (CPP), Shapiro and colleagues 17 analyzed data for 1,014 pregnant women who took meclizine in the first four lunar months of pregnancy compared with 49,268 unexposed pregnancies. A wide array of birth defects were investigated; eye and ear defects were the only significant associations identified (standardized relative risk: 2.79; 95% CI: 1.12-5.73). However, further investigation of subtypes of eye and ear defects did not reveal a relationship between meclizine and any specific birth defect 17.
Three analyses of Swedish data explored meclizine in relation to birth defects 13, 14, 19. The earliest included 5,753 women, 778 of whom used an antiemetic (i.e. promethazine, prochlorperazine [an antiemetic with no antihistaminic properties], diphenhydramine, or meclizine) during pregnancy. Meclizine exposure was not associated with an increased risk of any of the birth defects under study 14. In a more recent analysis of Swedish surveillance data that focused only on meclizine, the frequency of birth defects was 3.2% among 16,536 meclizine exposed pregnancies; the association was slightly protective (odds ratio (OR): 0.91; 95% CI: 0.83-0.99) when compared with an unexposed group 19. Lastly, in a 2005 study by Asker and colleagues, there was no reported association between birth defects and cyclizine, and again a protective effect was noted for meclizine (cyclizine OR: 1.08; 95% CI: 0.86-1.35; meclizine OR: 0.89; 95% CI 0.82-0.96). In this Swedish population, meclizine was the most commonly used antihistamine (there were over 18,000 meclizine-exposed pregnancies compared with less than 2,000 pregnancies exposed to any other antihistamine) 13.
3.1.1.2 Doxylamine plus pyridoxine
Two relatively recent papers have investigated doxylamine plus pyridoxine20, 21. Using data obtained from the Canadian TIS (the “Motherisk Program”) from 2001-2003, Boskovic and colleagues compared women with no NVP with two groups of women with NVP – the first group of women took a standard dose of doxylamine plus pyridoxine and the second group took a “supradose”. Among the group without NVP, there were no birth defects reported; the frequency of birth defects was 2/122 (1.6%) among the women on the standard dose and 0/124 among women on the supradose 21. Askenazi-Hoffnung and colleagues compared doxylamine plus pyridoxine to metoclopramide for the treatment of NVP using data from an Israel TIS. There were no birth defects among the 29 women exposed to doxylamine plus pyridoxine and one among the 29 women exposed to metoclopramide 20.
3.1.1.3 Hydroxyzine
There were two cohort studies that examined the association between hydroxyzine exposure and birth defects 22, 23. In a 1971 study conducted in Turkey, hydroxyzine (n=100) or placebo (n=50) was administered during the first two months of pregnancy 23. Among the 150 pregnancies enrolled, 115 had information on fetal outcomes. There was one birth defect in the hydroxyzine group; and none in the placebo group. Hydroxyzine was also investigated by Einarson and colleagues using data from Motherisk Program 22. Among 43 women with first trimester hydroxyzine exposure, 2 infants were born with major birth defects; in the comparison population of 120 women exposed to non-teratogens, there were none.
3.1.1.4 Other first generation H1-receptor antagonists – brompheniramine, chlorpheniramine, dimenhydrinate, diphenhydramine, promethazine, triprolidine
In a matched analysis using data from a German population based cohort study from 1964-1976, the association between first trimester use of “miscellaneous antiemetics” (including meclizine, dimenhydrinate, and chlopheniramine) and birth defects was 0.92 (90% CI: 0.42-2.00) based on 11 birth defects among 628 exposed pregnancies and 12 birth defects among 628 unexposed pregnancies 24.
Two analyses of data from the Seattle-based Group Health Cooperative of Puget Sound analyzed a wide range of first trimester antihistamine use in relation to risk for birth defects 25, 26. Based on data from 1977-1979, Jick and colleagues compared the prevalence of birth defects among women exposed to selected antihistamines in the first trimester of pregnancy to a baseline risk of birth defects of 11.7 per 1,000. The prevalence among those exposed to triprolidine (6/384; 15.6 per 1,000) and diphenhydramine (1/361; 2.8 per 1,000) were not statistically significantly different from the baseline 26. In an update of this analysis using data from 1980-1982, Aselton and colleagues reported a baseline birth defects prevalence of 16.1 per 1,000. Among women with first trimester exposures to diphenhydramine, triprolidine, chlorpheniramine, brompheniramine or promethazine, the prevalence of birth defects was not statistically significantly different from the baseline 25. Schatz and colleagues, using data from the Kaiser-Permanente Prospective Study of Asthma During Pregnancy, reported a prevalence of birth defects of 5.5% among unexposed pregnancies and 3.7% among pregnancies exposed to chlorpheniramine, tripelennamine, or other antihistamines (not otherwise specified) in the first trimester 27.
Bsat and colleagues published a prospective evaluation of three outpatient regimens for NVP: promethazine, prochlorperazine (an antiemetic with no antihistaminic properties), and pyridoxine plus metoclopramide. There were no birth defects noted among the 52 women exposed to promethazine; one birth defect was reported among the women exposed to prochlorperazine 28.
Kullander and Kallen's analysis of a Swedish prospective cohort found no association between first trimester exposure to diphenhydramine and birth defects. The one association reported in that paper was between promethazine exposure and congenital dysplasia of the hip (based on 11 observed cases; 4 were expected) 14. Asker and colleagues’ later analysis of Swedish surveillance data found no association with promethazine (OR: 0.91; 95% CI: 0.75-1.11)13.
3.1.2 Second generation H1-receptor antagonists
Eight cohort studies in this review investigated the association between one or more second generation H1-receptor antagonists and birth defects 22, 29-35
3.1.2.1 Cetirizine
Two cohort studies have investigated the association between cetirizine use during pregnancy and birth defects 22, 35. Einarson and colleagues, using data from the Motherisk Program, observed no pregnancies affected by a major birth defect among the 33 exposed to cetirizine in the first trimester 22; no birth defects were reported in the non-teratogen comparison group as well. An analysis of the Berlin TIS data documented three pregnancies affected by birth defects among the 177 exposed to cetirizine in the first trimester (1.7%) compared with 24/1,521 (1.6%) in the non-teratogen comparison group (OR: 1.07; 95% CI: 0.21-3.59) 35.
3.1.2.2 Loratadine
Four cohort studies have investigated the association between loratadine use during pregnancy and birth defects 29-31, 33. The first investigation to report an association between loratadine and hypospadias was by Källén and Olausson using data from the Swedish Medical Birth Registry 30. In a cohort of over 540,000 women with deliveries from 1995-2001, 2,780 infants were exposed to loratadine in early pregnancy. The observed prevalence of hypospadias was 5.4 per 1,000 among the loratadine-exposed pregnancies, significantly higher than the expected prevalence of 1 in 500 (OR: 2.27; 95% CI: 1.33-3.87) 30. Five years later, after linkage with additional national registries, the same authors published a follow-up study and proposed that the 2001 “signal” had been a chance finding. In the revised analysis, which included three additional years of data (2002-2004), the prevalence of hypospadias among the loratadine-exposed pregnancies was 1.04 per 1,000 during 2002-2004, dramatically lower than during 1995-2001. When analyzing the full time period, the association was attenuated from what had been previously published (RR: 1.61; 95% CI: 1.04-2.34) 31. Two papers published in 2003 based on TIS data also investigated the fetal safety of loratadine 29, 33. Using data form the Israel TIS, Diav-Citrin and colleagues reported one birth defect among 126 first trimester loratadine-exposed pregnancies (0.8%), compared with 7/146 (4.8%) pregnancies exposed to “other antihistamines”, and 25/844 (3.0%) pregnancies exposed to non-teratogens. Moretti and colleagues pooled data across four TIS (Canada [Motherisk], Israel, Italy, Brazil) in their analysis of loratadine use and birth defects. Among those exposed to loratadine, 5/143 (3.5%) had a major birth defect, compared with 6/150 (4.0%) in the comparison group of women exposed to non-teratogens; these prevalence estimates were not significantly different 33.
3.1.2.3 Terfenadine and astemizole
Three cohort studies, all based on TIS data, investigated terfenadine and/or astemizole use during pregnancy in relation to birth defects 29, 32, 34. Using data from the Motherisk Program and the Italy TIS, Loebstein and colleagues reported no cases of birth defects among 65 women with first trimester exposure to terfenadine compared with 2 birth defects among 111 exposed to a non-teratogen (RR: 0.57; 95% CI: 0.06-5.39) 32. A second analysis from the Motherisk Program (in collaboration with the Pregnancy Healthline in Philadelphia) focused on astemizole. 34. Among 114 women exposed to astemizole there were 2 reported cases of major birth defects (1.8%); there were 2 reported among the 114 women in the non-teratogen comparison group as well 34. Although not the primary exposure of interest in a study by Diav-Citrin and colleagues using Israel TIS data (exposure of interest was loratadine, see above), the authors reported the prevalence of major birth defects for specific antihistamines included in their “other antihistamines” analytic group. The frequencies of birth defects among women exposed to astemizole (3/50; 6%) or terfenadine (2/27; 7.4%) during pregnancy were not significantly different from the comparison group 29.
3.2 Histamine H1-receptor antagonists: Findings from case-control studies
A total of 21 case-control studies that met inclusion criteria for this review reported findings with respect to histamine H1-receptor antagonists (Table 2). Four studies analyzed antihistamines in the aggregate only 36-39, the remainder reported aggregated analyses in addition to analyses of specific antihistamines.
In a paper focused on risk factors for infant craniostenosis (more commonly referred to as craniosynostosis), Källén and Robert-Gnansia 36 compared prenatal use of medications among the mothers of 398 infants born with craniostenosis between 1995-2002 with the nearly 730,000 Swedish births during the same time period. Twenty-two case mothers had documentation in their prenatal record of first trimester exposure to an antihistamine compared with an expected frequency of 15.6 based on the larger population data (observed/expected ratio: 1.4; 95% CI: 0.9-2.1).
Two manuscripts based on data from the Slone Epidemiology Center Birth Defects Study (Slone BDS) also known as the Pregnancy Health Interview Study 37, 38 reported on the associations between any antihistamine use and gastroschisis; the more recent paper also reported on the association with small intestinal atresias 38. In data from 1976-1990, there were 76 gastroschisis cases, among whom 10 (13.2%) were exposed to antihistamines (excluding antiemetics) and 2,142 controls affected by other, non-related birth defects, among whom 173 (8.1%) were exposed to antihistamines leading to an adjusted OR of 1.3 (95% CI: 0.5-3.1) 37; in more recent Slone BDS data (1995-1999) based on 206 gastroschisis cases, the association was closer to the null (OR: 0.6; 95% CI: 0.3-1.2). There was also no association with small intestinal atresia (OR: 0.9; 95% CI: 0.4-1.8) (based on 126 small intestinal atresia cases) 38.
Boneva and colleagues, using data from metropolitan Atlanta from 1982-1983, analyzed antihistamine use for the treatment of severe first trimester NVP among 998 mothers of infants with a CHD and 3,029 mothers of infants born without any major birth defect. The analysis focused on Bendectin use (not reported in this review) and antihistamines in general. Comparing women with the most severe level of NVP to those with no NVP, the association between CHD and using any antinausea medication was protective with a relative risk estimate of 0.74 (95% CI: 0.56-0.97). Considering specific CHD subtypes, all associations among women with NVP, comparing those who took medications with those who did not, were below the null (but not statistically significant), with the exception of tetralogy of Fallot, which was greater than 1.0 (OR: 2.19; 95% CI: 0.39-22.32), based on 8 cases, 6 of whom took medication to treat NVP, compared with 2 who did not take medication 39.
3.2.1. First generation H1-receptor antagonists
3.2.1.1 Antihistamines other than doxylamine plus pyridoxine
The authors of 1982 and 1984 reports on the association between doxylamine plus pyridoxine exposure and pyloric stenosis 40, 41 re-analyzed their data to explore the role of antihistamines excluding doxylamine plus pyridoxine antihistamines, and reported findings in letters to the editor in 1985 42, 43. Based on data from the mothers of 71 cases with pyloric stenosis (5 exposed) and 3,002 control mothers (40 exposed), Eskenazi and Bracken reported a 5-fold increase in risk (OR: 5.61; 95% CI: 2.14-14.67) for use of antihistamines excluding doxylamine plus pyridoxine 41. Aselton and Jick, using the Puget Sound Group Health Cooperative data from the mothers of 12 cases of pyloric stenosis and 32 matched control mothers, reported an elevated but not statistically significant association (matched OR: 4.1; 95% CI: 0.8-21.7).
3.2.2.2 Other first generation H1-receptor antagonists
The oldest case-control study included in this review was a letter to the editor published in The Lancet in 1961 reporting on the frequency of first trimester use of meclizine, dimenhydrinate, and cyclizine among mothers of 266 infants with birth defects, and mothers of two groups of control infants (n=266 in each control group) 44. Considering the three antihistamines combined, there were no differences across the three groups in the prevalence of medication use (11.3% of cases; 11.7% of control group 1; 12.0% of control group 2). A 1973 report using data from 1964-1972 from the Finnish Register of Congenital Malformations 45 investigated whether exposure to a combination drug, imipramine (a tricyclic antidepressant) plus chloropyramine (an antihistamine) was more common among mothers of 2,784 birth defect cases than among mothers of 2,784 matched controls. Three case mothers were exposed to imipramine/chloropyramine; no control mothers were exposed. The following year Saxén, in a letter to the editor of The Lancet, reported on the association between antihistamine intake and oral clefts (cleft palate only [CPO], cleft lip with or without cleft palate [CL/P]), using data from the Finnish Register. There was no association between cyclizine use and oral clefts, yet there was a significant difference between the frequency of diphenhydramine use among mothers of CPO cases (8/232; 3.4%) and mothers of controls (6/590; 1.0%) 46. Concern about oral clefts continued; in 1983, Golding and colleagues reported on data from the United Kingdom based on 196 oral cleft cases and 407 matched controls. Exposure to several antihistamines during the first 69 days of pregnancy was considered; only promethazine (and doxylamine plus pyridoxine) had a sufficient prevalence of exposure for analysis. The frequency of promethazine use was not significantly different among mothers of oral clefts cases and controls 47.
In 1971, Nelson and Forfar reported on a Scottish case-control study with 175 cases with major defects, 283 cases with minor defects, and 911 controls in which mothers were interviewed before hospital discharge about medication use during pregnancy. An attempt was made to validate maternal report of medication use by following up with the pharmacy that filled the prescription. Two of the 175 (1.1%) case mothers were exposed to an antihistamine (i.e. promethazine, diphenhydramine, triprolidine, mepyramine, diphenylpyraline, chlorpheniramine, chlorcyclizine, and trimeprazine) during the first trimester; 26 of the 911 (2.9%) control mothers were exposed and no individual antihistamine was more commonly used in the first trimester among case mothers than control mothers 48.
The Hungarian Case Control Surveillance of Congenital Anomalies (HCCSCA) has been used to examine the risks associated with several antihistamines. The HCCSCA uses national birth defects surveillance data, the Hungarian Congenital Abnormality Registry (HCAR). Notification of cases with birth defects to HCAR is mandatory for physicians in Hungary; cases among live births, fetal deaths or terminations of pregnancy are reported. Medication data for cases and controls were gathered in one of two ways: (1) prenatal care “logbooks” and other medical records (gathered prospectively); or (2) responses to maternal questionnaires (self-reported, gathered retrospectively). In an investigation of 23 subtypes of major birth defects, dimenhydrinate was found to be inversely associated with risk for obstructive defects of the urinary tract (prevalence ratio (PR): 0.2; 95% CI: 0.1-0.7)49. In a similarly structured analysis, medically recorded promethazine use (excluding promethazine use that was only self-reported by mothers and not validated with documentation of use in the medial record) in the first trimester of pregnancy was also inversely associated with obstructive urinary tract defects, as well as hypospadias, undescended testes, clubfoot, and the aggregation of all defects in the analysis (OR: 0.8; 95% CI: 0.7-0.9) 50. In a 2003 paper, Czeizel and colleagues 51 limited their analysis of the association between oral clefts and antihistamine exposure to women with hyperemesis gravidarum, a severe form of NVP often resulting in hospitalization 52. They found no association between dimenhydrinate use and CL/P (OR: 1.20; 95% CI: 0.66-2.19) but a positive association between the medication and CPO (OR: 2.47; 95% CI: 1.10-5.54). The prevalence of dimenhydrinate use among mothers of two oral cleft subtypes was substantially different, with 25-30% of CL/P case and control mothers reporting use of the medication, but nearly 50% of the mothers of CPO cases reporting use 51.
Using data from the National Birth Defects Prevention Study (NBDPS), Gilboa and colleagues3 and Anderka and colleagues53 conducted analyses of the association between first trimester antihistamine use and selected birth defects. Gilboa and colleagues, using data from 1997-2003, conducted frequentist and Bayesian analyses of the association between self-reported first trimester use of 10 specific first generation antihistamines (i.e. clemastine, dimenhydrinate, diphenhydramine, doxylamine, hydroxyzine, meclizine, pheniramines [chlorpheniramine and brompheniramine], promethazine, triprolidine, and antihistamine “not otherwise specified”) or any antihistamine, and 26 isolated major birth defects 3. Three second generation antihistamines were also investigated (i.e. cetirizine, fexofenadine, and loratadine) (see below in Second generation H1-receptor antagonists). Only one association had a magnitude greater than 3.0 – the association between prenatal meclizine exposure and cleft palate (Bayesian posterior OR: 6.2; 95% Posterior Interval: 1.8-21.3) – based on 5 exposed cases and 4 exposed controls. The authors identified several modest, but elevated associations with diphenhydramine, and doxylamine. Many of these were not previously identified in the literature and could represent chance findings. The authors conducted several sensitivity analyses to explore the robustness of these findings to residual confounding by indication, but the results were largely unchanged.
Anderka and colleagues’ analysis focused on the subpopulation of mothers of cases and controls with NVP, and four birth defect subtypes - CL/P, CPO, neural tube defects (NTD), and hypospadias using data from 1997-2004. Among NBDPS controls, the prevalence of NVP was nearly 70%; among those with NVP, approximately 15% reported treatment. The associations between NVP itself and the four birth defect subtypes included in the analyses were either protective or null, with OR estimates ranging from 0.84 to 1.00. The reported associations with antihistamine antiemetics (including promethazine as an itemized subgroup) and the other antihistamines (including diphenhydramine, cetirizine, and doxylamine as itemized subgroups) ranged from 0.9 to 1.4, all with confidence intervals including the null value of 1.0. The only exception was an elevated, but not statistically significant OR of the association between NTD and doxylamine plus pyridoxine of 1.84 (95% CI: 0.71-4.78).
In the most recent paper included in this review, Li and colleagues, using data from the Slone Birth Defects Study (BDS) (gastroschisis cases overlapped somewhat with those analyzed by Werler and colleagues38), divided their study into a priori (hypothesis testing) and exploratory (hypothesis generating) analyses 54. The 16 a priori analyses, selected based on previous reports in the literature, were: loratadine and hypospadias (see below in Second generation H1-receptor antagonists); diphenhydramine and CPO, CL/P, NTD, spina bifida, limb reduction defects, and gastroschisis; chlorpheniramine and eye defects, ear defects, spina bifida, and CL/P; and doxylamine and oral clefts, pyloric stenosis, hypoplastic left heart syndrome, spina bifida, and NTD. None of the a priori analyses demonstrated a significantly elevated association. In their exploratory analyses, there were a few elevated associations: diphenhydramine and transposition of the great arteries (OR: 2.3; 95% CI: 1.1-5.0), right ventricular outflow tract obstruction defects (OR: 1.6; 95% CI: 1.0-2.7), renal collecting system anomalies (OR: 1.5; 95% CI: 1.0-2.2); chlorpheniramine and NTD (OR: 2.6; 95% CI: 1.1-6.1), tetralogy of Fallot (OR: 3.1; 95% CI: 1.2-8.4), hypoplastic left heart syndrome (OR: 4.9; 95% CI: 1.6-14.9) and anomalies of the great veins (OR: 3.3; 95% CI: 1.1-10.0); and doxylamine and renal collecting system anomalies (OR: 2.7; 95% CI: 1.3-5.6) 54. These were all novel associations, and like the novel associations reported by Gilboa and colleagues, could represent chance findings and are in need of replication in other datasets.
3.2.3 Second generation H1-receptor antagonists
Gilboa and colleagues published the only case-control study investigating exposure to cetirizine and fexofenadine; there were no elevated associations observed for either antihistamine 3. Loratadine, however has been much more thoroughly studied, and has been of particular interest in the literature, in part due to the 2002 Swedish study (discussed above) that suggested an association with hypospadias 12. Several case-control studies have since explored this association – one using data from the Slone BDS 54, two using data from the NBDPS 3, 55, and three using data from Denmark 56-58. Li and colleagues considered the hypospadias – loratadine association as one of their a priori hypotheses (based on previous suggestions in the literature). Based on self-reported medication use data from the mothers of 632 cases with hypospadias and 3,448 mothers of controls, there was no association found between first trimester loratadine use and hypospadias (OR: 0.8; 95% CI: 0.4-1.7) 54. Li and colleagues investigated the association between loratadine and 20 other major birth defects in their “exploratory” analyses; all of the adjusted OR were between 0.5 and 1.7 with 95% confidence intervals all including the null value of 1.0 54. A 2004 Morbidity and Mortality Weekly Report reported the results of an NBDPS analysis of maternal loratadine use from one month before pregnancy through the end of the first trimester among 563 male infants with 2nd or 3rd degree hypospadias (cases with first degree hypospadias are excluded from the NBDPS) and 1,444 male control infants 55. The analysis also included a larger group of nonsedating antihistamines (which included loratadine) and sedating antihistamines (not otherwise specified). All associations with hypospadias were null; loratadine OR: 0.96; 95% CI: 0.41-2.22, nonsedating antihistamines OR: 0.95; 95% CI: 0.48-1.89, sedating antihistamines OR: 1.02; 95% CI: 0.68-1.53 55. Gilboa and colleagues, using two additional years of data from NBDPS and exploring a wide array of birth defects, did not observe an elevated risk with loratadine for hypospadias or any other major birth defect, with the exception of transverse limb deficiencies (OR: 2.16; 95% CI: 1.08-4.30) 3. Two reports from Denmark published in 2006 reported on analyses of the association between first trimester loratadine use and hypospadias using case-control data57, 58. One was based on data from four Danish counties where 227 cases of hypospadias and 10 matched controls per case (n=2,270) were identified using linked national hospital discharge and birth registry datasets from 1989-2002. Medication use during pregnancy was identified through further linkage with pharmacy records. One case and eight control mothers were exposed to loratadine in the period from 30 days before conception through the end of the first trimester; there was no elevated risk of hypospadias associated with this exposure (OR: 1.4; 95% CI: 0.0-10.5) 58. The second Danish report was based on a case-control study nested within the Danish National Birth Cohort, in which 203 cases of hypospadias and 2,030 matched controls were identified. During maternal interview, one case and 25 control mothers self-reported use of loratadine from one month before pregnancy through the end of the first trimester. Similar to the results from the record-linkage based analysis, there was no association with hypospadias (OR: 0.9; 95% CI: 0.1-6.9) 57. Lastly, in 2008, in a nationwide record-linkage analysis using data from 1996-2004, 1,575 cases of hypospadias were identified, 7 (0.4%) of whom had prenatal loratadine exposure. Among 14,660 matched controls, 88 (0.6%) were exposed to loratadine. No association was observed (prevalence ratio [PR]: 0.6; 95% CI: 0.3-1.4) 56.
3.3 Histamine H2-receptor antagonists: Findings from cohort and case-control studies
The body of literature investigating the association between histamine H2-receptor antagonists and birth defects is substantially smaller than that for H1-receptor antagonists, with seven cohort studies 59-65 and three case-control studies 53, 66, 67 meeting inclusion criteria for this review. Colin Jones and colleagues reported on 12-month post-marketing surveillance in Scotland of 9,809 users of cimetidine and a comparison population of 9,140 non-users. Over the surveillance, there were 20 pregnancies exposed to cimetidine and 22 unexposed pregnancies; among cimetidine users there was one birth defect reported – a case of Down syndrome. There were no birth defects among the non-users 59.
Two studies used data from a TIS – the first based on data from the Motherisk Program 62 and the second based on pooled data from 18 members of the European Network of Teratogen Information Service (ENTIS) 60. In the analysis of Motherisk data, there were 185 pregnancies exposed to histamine H2-receptor antagonists (i.e. ranitidine [the most common], cimetidine, famotidine, nizatidine), 142 of them were first trimester exposed and resulted in a live birth. Three birth defects occurred among these pregnancies (2.1%) compared with 5 birth defects among 143 pregnancies in the comparison group (3.5%). The difference between the prevalence of birth defects among these two groups was not statistically significant (p=0.55) 62. The analysis of pooled ENTIS data (n=553 exposed to H2-receptor antagonists; n=1,390 in comparison group exposed to “non-teratogenic” substances) had similar findings with a prevalence of birth defects of 2.7% among the exposed pregnancies and 3.5% among those unexposed to H2-receptor antagonists 60. In another analysis of the Motherisk data (combined with data from Italy and France TIS), first trimester exposure to H2-receptor antagonists (not otherwise specified; n=113; n=98 with live births) was compared with exposure to non-teratogens (n=113; n=66 with live births) 65. The prevalence of birth defects among those with live births was similar in the exposed and unexposed groups: H2-receptor antagonists: 3.1%; non-teratogens: 3.0%.
In a 1998 publication of data from the Swedish Medical Birth Registry, Källén reported on the association between acid-suppressing drugs (e.g. proton pump inhibitors, H2-receptor antagonists) and birth defects. The baseline birth defects prevalence was 3.9%; among users of H -receptor antagonists, the prevalence was 2.4% (6/255) (OR: 0.46; 95% CI: 0.17-1.20)61 Ruigómez and colleagues reported on two cohorts of cimetidine- and ranitidine-exposed pregnancies – one from Italy and one from the United Kingdom 64. In the United Kingdom cohort, prevalence of birth defects among cimetidine-exposed pregnancies was 4.0% (9/227) and among ranitidine-exposed pregnancies was 7.4% (17/229), compared with the baseline prevalence of 5.7% among the unexposed population. In the Italy cohort, 2/10 (20%) cimetidine-exposed pregnancies and 3/101 (3.0%) rantidine-exposed pregnancies were compared with the unexposed baseline of 2.9%. Measures of association for each medication were calculated from pooled data: cimetidine (RR (95% CI)): 1.3 (0.7-2.6); ranitidine: 1.5 (0.9-2.6) 64. In the most recently published cohort analysis, Matok and colleagues used data from the largest health maintenance organization in Israel to examine the safety of H2-receptor antagonists during pregnancy 63. The associations between any major birth defect and first trimester exposure to any H2-receptor antagonist (n=1,148 exposed pregnancies) or famotidine (n=878 exposed pregnancies) were both null (OR: 1.03; 95% CI: 0.80-1.32 for any H2-receptor antagonist and OR: 1.21; 95% CI: 0.92-1.58 for famotidine). No measure of association was calculated for ranitidine because there were fewer than seven birth defects noted (n=276 exposed pregnancies) 63.
Anderka and colleagues, using NBDPS data, reported on H2-receptor antagonists as a group, and where sample size permitted, ranitidine. There was no association found between H2-receptor antagonists and CL/P (OR: 0.57; 95% CI: 0.19-1.67), CPO (OR: 1.04; 95% CI: 0.39-2.75), or hypospadias (OR: 1.07; 95% CI: 0.41-2.83). There was an elevated, though not statistically significant association with NTD (OR: 1.80; 95% CI: 0.80-4.05), though when restricted to ranitidine exposure only, the association did not persist (OR: 1.28; 95% CI: 0.43-3.82) 53.
Data from the HCCSCA have been used in two recent case control analyses exploring H2-receptor antagonists and birth defects. In the first study, Ács and colleagues explored the association among mothers with severe chronic dyspepsia. Severe chronic dyspepsia affected 148 case mothers and 214 control mothers; 9.5% (14/148) case mothers and 12.6% (27/214) control mothers took an H2-receptor antagonist (i.e. cimetidine or ranitidine, not specified in the paper) (OR: 0.7; 95% CI: 0.4-1.4) 66. In the second study, Bánhidy and colleagues, using data from HCCSCA restricted their analyses of the association between cimetidine exposure and major birth defects to mothers with definitive peptic ulcer disease (PUD). There were 20 mothers of cases (6 exposed) and 58 mothers of matched controls (7 exposed); the association was elevated (OR: 3.1; 95% CI: 0.9-10.8) 67.
Conclusion
In summary, the majority of findings reported in this body of literature demonstrate a lack of association between prenatal antihistamine exposure and birth defects. Among the 31 cohort studies included in this review, two identified any statistically significant positive associations between antihistamines and birth defects (Table 3). Among the 23 case-control studies included in this review, seven identified any statistically significant positive associations (Table 3). Supplemental online tables are provided that detail the frequencies of antihistamine exposure – birth defect outcome combinations. Although this lack of reported associations is reassuring, and suggests that antihistamines are unlikely to be strong risk factors for the major birth defects considered in the literature, a few large studies did identify associations that might warrant follow-up and corroboration in other study populations 3, 54. Considering the populations included in this review, the vast majority of the 54 studies are from the U.S., Canada, or Scandinavia. Given this, there is likely a lack of heterogeneity in the reviewed literature with respect to race-ethnicity and population diversity. The inclusion criterion of an English-language publication likely contributed to this.
Table 3.
Summary of significant positive associations among 54 studies included in systematic review of antihistamines and birth defects
| Cohort | Case-Control | |||
|---|---|---|---|---|
| N [References] | Number of studies showing any significantly increased risks [References] | References | Number of studies showing any significantly increased risks [References] | |
| H1-receptor antagonists | ||||
| Brompheniramine | 1 [25] | 0 | 0 | |
| Chlorpheniramine | 2 [25, 27] | 0 | 2 [43, 54] | 2 [43, 54] |
| Chlorocyclizine | 0 | 1 [48] | 0 | |
| Chloropyramine | 0 | 1 [45] | 0 | |
| Clemastine | 1 [12] | 0 | 1 [3] | 1 [3] |
| Cyclizine | 4 [12, 13, 15, 16] | 0 | 1 [46] | 0 |
| Cyclizine/meclizine | 1 [18] | 0 | 0 | |
| Dimenhydrinate | 0 | 3 [3, 49, 51] | 1 [51] | |
| Diphenhydramine | 4 [13, 14, 25, 26] | 0 | 5 [3, 43, 46, 53, 54] | 4 [3, 42, 46, 54] |
| Doxylamine | 2 [20, 21] | 0 | 3 [3, 53, 54] | 2 [3, 54] |
| Hydroxyzine | 2 [22, 23] | 0 | 1 [3] | 1 [3] |
| Meclizine | 6 [12, 13, 14, 16, 17, 19] | 0 | 1 [3] | 1 [3] |
| Pheniramine* | 0 | 1 [3] | 1 [3] | |
| Promethazine | 5 [12, 13, 14, 25, 28] | 1 [14] | 5 [3, 43, 47, 50, 53] | 2 [3, 50] |
| Triprolidine | 2 [25, 26] | 0 | 1 [3] | 0 |
| Astemizole | 1 [34] | 0 | 0 | |
| Cetirizine | 3 [12, 22, 35] | 0 | 2 [3, 53] | 0 |
| Fexofenadine | 0 | 1 [3] | 0 | |
| Loratadine | 5 [12, 29-31, 33] | 1 [30] | 6 [3, 54-58] | 1 [3] |
| Terfenadine | 2 [12, 32] | 0 | 0 | |
| H2-receptor antagonists | ||||
| Cimetidine | 2 [59,64] | 0 | 1[67] | 0† |
| Famotidine | 1 [63] | 0 | 0 | |
| Ranitidine | 2 [63,64] | 0 | 1 [66] | 0 |
| Unspecified antihistamines | 1 [65] | 0 | 4 [36-39] | 0 |
Pheniramine = brompheniramine or chlorpheniramine
Borderline significant association (Odds Ratio 3.1; 95% Confidence Interval 0.9-10.8) in Bánhidy et al. 201167
It is important to note, however, that these might be chance findings only, given the large number of comparisons conducted. The most thoroughly studied “signal” in the recent literature, that of the association between loratadine and hypospadias, first identified in data from the Swedish Medical Birth Registry 30, has not been confirmed in a subsequent case-control nor cohort analysis 3, 31, 54-58. This, too, is reassuring and confirms the need for continued research using different designs and study populations. However, other antihistamines, especially H2-receptor antagonists (e.g. ranitidine, cimetidine, famotidine, nizatidine) have much less literature on which an assessment of safety can be based and further study to understand the potential fetal effects of H2-receptor antagonists is needed.
Expert Opinion
The findings in this literature should be considered in light of three critical methodological issues: (1) selection of appropriate study population; (2) ascertainment of antihistamine exposures; and (3) ascertainment of birth defects outcomes.
5.1 Selection of appropriate study population
The distinction between population-based studies and studies based on a convenience sample is important. The population-based case-control studies included in this review 3, 39, 49, 53, 55, 66, 67 have the advantage of deriving cases from surveillance systems that, in general, attempt to capture all major birth defects cases occurring in a population. Most of the significant findings were reported in case-control studies which are typically better able to assess individual types of birth defects rather than being limited to an assessment of all birth defects as an aggregate group. Given the rarity of major birth defects in the population (approximately 3% overall 68; specific birth defects are less common) population-based cohort studies for birth defects are less frequent in the literature; however, cohort studies in both Sweden 14, 19, 30, 31 and the United States 17, 69 have been conducted making important contributions to the literature. The Swedish cohorts were built from extensive linkages of national registries; while the Collaborative Perinatal Project gathered data through maternal interviews of pregnant women enrolled at medical centers around the United States 70, 71. Cohorts derived from health maintenance organizations are designed to simulate population-based cohorts 18, 25-27, 63 and in some situations do capture the vast majority of the population in a given geographic area. These cohorts have access to rich clinical information as well as prescription claims. Cohorts based on a convenience sample, however, such as those derived from the TIS around the United States and internationally, while likely having acceptable internal validity, have questionable external generalizability. The TIS serve a critically important role – providing counsel to concerned women and providers about chemical and medication exposures during pregnancy and might provide important initial signals of teratogenicity. However, TIS study cohorts are comprised exclusively of individuals who choose to inquire about an exposure with a TIS 20-22, 29, 32, 33, 35, 60,65. Those who inquire about an exposure are likely to be qualitatively different from those who do not; those who do not inquire cannot, by definition, be in a TIS cohort. In addition, the TIS studies, by necessity, must select a comparison group of women exposed to “something” – since this is the entirety of their cohort. The studies tend to choose a comparison group of women exposed to “nonteratogenic agents” that is consisting of women exposed to dental x-rays or acetaminophen (see Table 2).
5.2 Ascertainment and analysis of antihistamine exposures
A second important issue to consider is the ascertainment and analysis of antihistamine exposures. Medication exposure during pregnancy is typically ascertained in one of two ways: (1) self-report by the mother, either prospectively or retrospectively and reported either in prenatal care records or during a maternal interview for the purposes of the study; or (2) linkage with pharmacy records or pharmaceutical claims. Both approaches are represented in the 54 papers included in this review though self-report by the mother was the most common; over 30 studies used the approach either alone or in combination with one of the other approaches. Given that many of the antihistamines of interest are currently sold over-the-counter, self-reported exposure assessment is a critical tool. In addition, given concerns about the safety of medication use (including antihistamine use) during pregnancy 72, it is not uncommon for women to fill a prescription, but decide not to take it during pregnancy 73. However, retrospective self-reporting has limitations – the most concerning is the potential for biased or inaccurate recall 74. The Slone BDS and NBDPS attempt to limit inaccurate recall by asking about specific indications of medications, categories of medications, and specific medications, including both generic and brand names. Study participants in the BDS are also provided with picture booklets of medications as well as a calendar to highlighting key dates and events to aid recall 2, 37, 38, 54. Earlier BDS analyses also used a “malformed control” group 2, 37, 38; this design was chosen under the assumption that mothers of control infants born with birth defects other than the birth defect of interest might have less biased recall than mothers of control infants born without any major birth defects. In addition, NBDPS has approximately a 70% response rate among cases and controls; analyses have suggested that NBDPS participants are representative of the populations from which they were selected 75. However if mothers of cases and controls have differential participation with respect to pregnancy exposures, study findings could be subject to selection bias.
In the analytic phase of a study, steps can be taken to enhance the accuracy of the exposure data. One of the most common approaches in studies of birth defects is to limit the medication exposure to the first trimester (sometimes also including the 30 days prior to conception to account for the fact that women who are prescribed a medication prior to conception may take it during after conception as well). Since the primary period of susceptibility to human teratogens is the first eight weeks of pregnancy (approximately 10 weeks when counting from the date of last menstrual period) 76, a focus on medication exposure during the first trimester (since medication exposure data are often too imprecise to accurately focus on specific weeks) is appropriate. While most studies limited the period of exposure to the first trimester, a handful explored the association with exposure any time during pregnancy 13, 60, potentially leading to an underestimate of the association with birth defects. In another analytic approach, the Slone BDS has developed and utilized an exposure classification algorithm based on the certainty of recall to help categorize individuals as “likely” or “possibly” exposed to a medication 77. Furthermore, the investigators of several case-control studies 39, 51, 53, 66, 67 have restricted analyses to individuals with a particular indication for medication use (e.g. NVP, peptic ulcer disease and severe dyspepsia in the papers noted above) as a method of minimizing confounding by indication 78. This is an advantage in this body of literature, especially since a lack of NVP is considered a risk factor for a poor pregnancy outcome. In addition, because women often take multiple medications during pregnancy79 analyses can exclude women who took medications during pregnancy known to be teratogenic or adjust for other medication use.
5.3 Ascertainment of birth defects outcomes
Among the studies included in this review, the data sources for the ascertainment of birth defects varied widely with respect to data quality and specificity. In studies using birth defects data derived from state or national surveillance programs, the quality and accuracy of the data were likely to be high 3, 12, 19, 30, 31, 39, 46, 49, 51, 53, 55-58, 61. In datasets developed exclusively through linkages between national birth registry data and hospital discharge data with cases of birth defects identified by International Classification of Diseases (ICD) coding, the quality might be somewhat lower than data that are actively abstracted from medical records, however, the statistical power that comes with larger sample sizes as well as the ability to create small, relatively homogeneous categories of birth defects is beneficial. Additionally, in some studies 39, 54, 55 rigorous clinical review of every case 80-82 was undertaken to verify reported diagnoses and determine whether the case met the study's inclusion criteria. Similarly, a few studies in the review are based on data from health maintenance organizations 18, 25-27, 42, 63 with medical records forming the foundation of the birth defects ascertainment and in some of these studies, additional clinical review of potential cases was also undertaken.
In contrast, in other studies, such as those based on the TIS included in this review 20-22, 29,32, 33, 35, 60, 65, the pregnancy outcome data were self-reported by mother who initially called the TIS, up to a year or more after their initial inquiry. This delay could lead to a substantial loss to follow-up, if mothers could not be located in order to report on the pregnancy outcome. While the reported pregnancy outcome was verified with the child's pediatrician in some of the included studies, this was not universally done across all TIS-based analyses.
A final methodological issue pertaining to birth defects outcome ascertainment is follow-up – the time period after birth during which birth defects could be identified and reported. In most state and national surveillance systems, this period of time is at least one year, accounting for the fact that not all birth defects are identified prenatally or immediately after birth. Some studies ascertained birth defects over a shorter period of time, for example, at delivery 23 or before age 5 months 37, 38, 54. In the BDS, the 5-month cut-off was intentional, to help ensure the conduct of maternal interviews no later than 6 months after delivery.
Despite these methodological issues, the body of literature on the risk of birth defects from antihistamine use in early pregnancy remains reassuring, particularly for the first generation H1-receptor antagonists. There is still a need for larger studies with clinical verification of birth defect subtypes, validation of maternal recall of medication exposures, and appropriate selection of study populations to follow-up on some of the signals found in previous studies, as well as a need to enrich the body of literature on H2-receptor antagonists, which are currently relatively understudied.
Finally, as stated earlier, this review was limited in scope to only structural birth defects; other potential adverse pregnancy outcomes such as fetal loss, preterm delivery, or low birth weight, or longer-term neurodevelopmental outcomes were not included. To have a complete understanding of the safety of medication for use during pregnancy, an understanding of the full spectrum of adverse outcomes associated with that medication is required. At this time the body of literature investigating other adverse pregnancy or developmental outcomes associated with prenatal use of antihistamines is much smaller than that focused on birth defects.
Supplementary Material
Article Highlights.
A systematic evaluation was undertaken of the peer-reviewed epidemiologic literature published through February 2014 on the association between prenatal exposure to antihistamines and birth defects.
A total of 54 studies met inclusion criteria for the review.
Among the 31 cohort studies in the review, two identified significant positive associations; among the 23 case-control studies, seven identified significant positive associations.
Histamine H1-receptor antagonists have been more thoroughly studied than H2-receptor antagonists.
The literature on the safety of antihistamine use during pregnancy with respect to birth defects is generally reassuring though the positive findings from a few large studies warrant corroboration in other populations.
Acknowledgement
The authors would like to acknowledge the contribution of Emory Rollins School of Public Health graduate student, Valerie Godoshian, for her invaluable assistance with searching reference lists and editing the manuscript tables.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Thorpe PG, Gilboa SM, Hernandez-Diaz S, et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22:1013–1018. doi: 10.1002/pds.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstetr Gynecol. 2005;193:771–777. doi: 10.1016/j.ajog.2005.02.100. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa SM, Strickland MJ, Olshan AF, et al. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Molec Teratol. 2009;85:137–150. doi: 10.1002/bdra.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brent R. Bendectin and birth defects: hopefully, the final chapter. Birth Defects Res A Clin Molec Teratol. 2003;67:79–87. doi: 10.1002/bdra.10021. [DOI] [PubMed] [Google Scholar]
- 5*.McKeigue PM, Lamm SH, Linn S, Kutcher JS. Bendectin and birth defects: I. A meta-analysis of the epidemiologic studies. Teratology. 1994;50:27–37. doi: 10.1002/tera.1420500105. [Comprehensive review and meta-analysis of the literature on Bendectin and birth defects] [DOI] [PubMed] [Google Scholar]
- 6.Einarson TR, Leeder JS, Koren G. A method for meta-analysis of epidemiological studies. Drug Intell Clin Pharmacy. 1988;22:813–824. doi: 10.1177/106002808802201021. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon B. More on Bendectin. JAMA. 1981;246:371–372. [PubMed] [Google Scholar]
- 8.Beardsley T. Bendectin/debendox. Drug not guilty, says court. Nature. 1985;314:209. doi: 10.1038/314209b0. [DOI] [PubMed] [Google Scholar]
- 9.Brent RL. Bendectin: review of the medical literature of a comprehensively studied human nonteratogen and the most prevalent tortogen-litigen. Reprod Toxicol. 1995;9:337–349. doi: 10.1016/0890-6238(95)00020-b. [DOI] [PubMed] [Google Scholar]
- 10.Danks DM. Blame, compensation and birth defects. Med J Austr. 1985;143:135–136. doi: 10.5694/j.1326-5377.1985.tb122865.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis JM. Good-bye, Bendectin, old friend. Postgrad Med. 1984;75:20–24. doi: 10.1080/00325481.1984.11698609. [DOI] [PubMed] [Google Scholar]
- 12.Källén B. Use of antihistamine drugs in early pregnancy and delivery outcome. J Mat Fet Neonatal Med. 2002;11:146–152. doi: 10.1080/jmf.11.3.146.152. [DOI] [PubMed] [Google Scholar]
- 13.Asker C, Norstedt Wikner B, Kallen B. Use of antiemetic drugs during pregnancy in Sweden. Eur J Clin Pharmacol. 2005;61:899–906. doi: 10.1007/s00228-005-0055-1. [DOI] [PubMed] [Google Scholar]
- 14.Kullander S, Källén B. A prospective study of drugs and pregnancy. II. Anti-emetic drugs. Acta Obstetr Gynecol Scand. 1976;55:105–111. doi: 10.3109/00016347609156795. [DOI] [PubMed] [Google Scholar]
- 15.McBride WG. An aetiological study of drug ingestion by women who gave birth to babies with cleft palate. Austr N Zeal J Obstet Gynecol. 1969;9:103–104. doi: 10.1111/j.1479-828x.1969.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 16.Milkovich L, van den Berg BJ. An evaluation of the teratogenicity of certain antinauseant drugs. Am J Obstetr Gynecol. 1976;125:244–248. doi: 10.1016/0002-9378(76)90601-3. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro S, Kaufman DW, Rosenberg L, et al. Meclizine in pregnancy in relation to congenital malformations. BMJ. 1978;1:483. doi: 10.1136/bmj.1.6111.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yerushalmy J, Milkovich L. Evaluation of the teratogenic effect of meclizine in man. Am J Obstetr Gynecol. 1965;93:553–562. doi: 10.1016/0002-9378(65)90515-6. [DOI] [PubMed] [Google Scholar]
- 19.Källén B, Mottet I. Delivery outcome after the use of meclozine in early pregnancy. Eur J Epidemiol. 2003;18:665–669. doi: 10.1023/a:1024891618953. [DOI] [PubMed] [Google Scholar]
- 20.Ashkenazi-Hoffnung L, Merlob P, Stahl B, Klinger G. Evaluation of the efficacy and safety of bi-daily combination therapy with pyridoxine and doxylamine for nausea and vomiting of pregnancy. Israel Med Assoc J. 2013;15:23–26. [PubMed] [Google Scholar]
- 21.Boskovic R, Rudic N, Danieliewska-Nikiel B, et al. Is lack of morning sickness teratogenic? A prospective controlled study. Birth Defects Res A Clin Molec Teratol. 2004;70:528–530. doi: 10.1002/bdra.20040. [DOI] [PubMed] [Google Scholar]
- 22.Einarson A, Bailey B, Jung G, et al. Prospective controlled study of hydroxyzine and cetirizine in pregnancy. Ann Allergy Asthma Immunol. 1997;78:183–186. doi: 10.1016/S1081-1206(10)63385-6. [DOI] [PubMed] [Google Scholar]
- 23.Erez S, Schifrin BS, Dirim O. Double-blind evaluation of hydroxyzine as an antiemetic in pregancy. J Reprod Med. 1971;7:35–27. [PubMed] [Google Scholar]
- 24.Michaelis J, Michaelis H, Gluck E, Koller S. Prospective study of suspected associations between certain drugs administered during early pregnancy and congenital malformations. Teratology. 1983;27:57–64. doi: 10.1002/tera.1420270109. [DOI] [PubMed] [Google Scholar]
- 25.Aselton P, Jick H, Milunsky A, et al. First-trimester drug use and congenital disorders. Obstetr Gynecol. 1985;65:451–455. [PubMed] [Google Scholar]
- 26.Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343–346. [PubMed] [Google Scholar]
- 27.Schatz M, Zeiger RS, Harden K, et al. The safety of asthma and allergy medications during pregnancy. J Allergy Clin Immunol. 1997;100:301–306. doi: 10.1016/s0091-6749(97)70241-0. [DOI] [PubMed] [Google Scholar]
- 28.Bsat FA, Hoffman DE, Seubert DE. Comparison of three outpatient regimens in the management of nausea and vomiting in pregnancy. J Perinatol. 2003;23:531–535. doi: 10.1038/sj.jp.7210986. [DOI] [PubMed] [Google Scholar]
- 29.Diav-Citrin O, Shechtman S, Aharonovich A, et al. Pregnancy outcome after gestational exposure to loratadine or antihistamines: a prospective controlled cohort study. J Allergy Clin Immunol. 2003;111:1239–1243. doi: 10.1067/mai.2003.1499. [DOI] [PubMed] [Google Scholar]
- 30.Källén B, Olausson PO. Monitoring of maternal drug use and infant congenital malformations. Does loratadine cause hypospadias? Int J Risk Safety Med. 2001;14:115–119. [Google Scholar]
- 31.Källén B, Olausson PO. No increased risk of infant hypospadias after maternal use of loratadine in early pregnancy. Int J Med Sci. 2006;3:106–107. doi: 10.7150/ijms.3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loebstein R, Lalkin A, Addis A, et al. Pregnancy outcome after gestational exposure to terfenadine: A multicenter, prospective controlled study. J Allergy Clin Immunol. 1999;104:953–956. doi: 10.1016/s0091-6749(99)70074-6. [DOI] [PubMed] [Google Scholar]
- 33.Moretti ME, Caprara D, Coutinho CJ, et al. Fetal safety of loratadine use in the first trimester of pregnancy: a multicenter study. J Allergy Clin Immunol. 2003;111:479–483. doi: 10.1067/mai.2003.130. [DOI] [PubMed] [Google Scholar]
- 34.Pastuszak A, Schick B, D'Alimonte D, et al. The safety of astemizole in pregnancy. J Allergy Clin Immunol. 1996;98:748–750. doi: 10.1016/s0091-6749(96)70122-7. [DOI] [PubMed] [Google Scholar]
- 35.Weber-Schoendorfer C, Schaefer C. The safety of cetirizine during pregnancy. A prospective observational cohort study. Reprod Toxicol. 2008;26:19–23. doi: 10.1016/j.reprotox.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 36.Källén B, Robert-Gnansia E. Maternal drug use, fertility problems, and infant craniostenosis. Cleft Palate Craniofac J. 2005;42:589–593. doi: 10.1597/04-031.1. [DOI] [PubMed] [Google Scholar]
- 37.Werler MM, Mitchell AA, Shapiro S. First trimester maternal medication use in relation to gastroschisis. Teratology. 1992;45:361–367. doi: 10.1002/tera.1420450407. [DOI] [PubMed] [Google Scholar]
- 38.Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26–31. doi: 10.1093/aje/155.1.26. [DOI] [PubMed] [Google Scholar]
- 39.Boneva RS, Moore CA, Botto L, et al. Nausea during pregnancy and congenital heart defects: a population-based case-control study. Am J Epidemiol. 1999;149:717–725. doi: 10.1093/oxfordjournals.aje.a009880. [DOI] [PubMed] [Google Scholar]
- 40.Aselton P, Jick H, Chentow SJ, et al. Pyloric stenosis and maternal Bendectin exposure. Am J Epidemiol. 1984;120:251–256. doi: 10.1093/oxfordjournals.aje.a113887. [DOI] [PubMed] [Google Scholar]
- 41.Eskenazi B, Bracken MB. Bendectin (Debendox) as a risk factor for pyloric stenosis. Am J Obstetr Gynecol. 1982;144:919–924. doi: 10.1016/0002-9378(82)90185-5. [DOI] [PubMed] [Google Scholar]
- 42.Aselton P, Jick H. Re: Pyloric stenosis and maternal antihistamine exposure at group health cooperative. Am J Epidemiol. 1985;122:197. doi: 10.1093/oxfordjournals.aje.a114082. [DOI] [PubMed] [Google Scholar]
- 43.Eskenazi B, Bracken MB. Pyloric stenosis and antihistamines. Am J Epidemiol. 1985;122:196–197. doi: 10.1093/oxfordjournals.aje.a114081. [DOI] [PubMed] [Google Scholar]
- 44.Mellin GW, Katzenstein M. Meclozine and foetal abnormalities. Lancet. 1963;1:222–223. doi: 10.1016/s0140-6736(63)91243-1. [DOI] [PubMed] [Google Scholar]
- 45.Idanpaan-Heikkila J, Saxen L. Possible teratogenicity of imipramine-chloropyramine. Lancet. 1973;2:282–284. doi: 10.1016/s0140-6736(73)90790-3. [DOI] [PubMed] [Google Scholar]
- 46.Saxen I. Letter: Cleft palate and maternal diphenhydramine intake. Lancet. 1974;1:407–408. doi: 10.1016/s0140-6736(74)93172-9. [DOI] [PubMed] [Google Scholar]
- 47.Golding J, Vivian S, Baldwin JA. Maternal anti-nauseants and clefts of lip and palate. Human Toxicol. 1983;2:63–73. doi: 10.1177/096032718300200105. [DOI] [PubMed] [Google Scholar]
- 48.Nelson MM, Forfar JO. Associations between drugs administered during pregnancy and congenital abnormalities of the fetus. BMJ. 1971;1:523–527. doi: 10.1136/bmj.1.5748.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czeizel AE, Vargha P. A case-control study of congenital abnormality and dimenhydrinate usage during pregnancy. Arch Gynecol Obstetr. 2005;271:113–118. doi: 10.1007/s00404-004-0638-6. [DOI] [PubMed] [Google Scholar]
- 50.Bartfai Z, Kocsis J, Puho EH, Czeizel AE. A population-based case-control teratologic study of promethazine use during pregnancy. Reprod Toxicol. 2008;25:276–285. doi: 10.1016/j.reprotox.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Czeizel AE, Sarkozi A, Wyszynski DF. Protective effect of hyperemesis gravidarum for nonsyndromic oral clefts. Obstetr Gynecol. 2003;101:737–744. doi: 10.1016/s0029-7844(02)03125-3. [DOI] [PubMed] [Google Scholar]
- 52.Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update. 2005;11:527–539. doi: 10.1093/humupd/dmi021. [DOI] [PubMed] [Google Scholar]
- 53.Anderka M, Mitchell AA, Louik C, et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Molec Teratol. 2012;94:22–30. doi: 10.1002/bdra.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Li Q, Mitchell AA, Werler MM, et al. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol: In Practice. 2013;1:666–674. doi: 10.1016/j.jaip.2013.07.008. [A well-conducted analysis to test 16 previously reported associations between specific antihistamines as well as identify possible new associations using the Slone Epidemiology Center's Birth Defects Study] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention Evaluation of an association between loratadine and hypospadias--United States, 1997-2001. MMWR Morbidity and Mortality Weekly Report. 2004;53:219–221. [PubMed] [Google Scholar]
- 56.Pedersen L, Norgaard M, Rothman KJ, Sorensen HT. Loratadine during pregnancy and hypospadias. Epidemiology. 2008;19:359–360. doi: 10.1097/EDE.0b013e318162a934. [DOI] [PubMed] [Google Scholar]
- 57.Pedersen L, Norgaard M, Skriver MV, et al. Prenatal exposure to loratadine in children with hypospadias: a nested case-control study within the Danish National Birth Cohort. Am J Therapeutics. 2006;13:320–324. doi: 10.1097/00045391-200607000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Pedersen L, Skriver MV, Norgaard M, Sorensen HT. Maternal use of loratadine during pregnancy and risk of hypospadias in offspring. Int J Med Sci. 2006;3:21–25. doi: 10.7150/ijms.3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colin Jones DG, Langman MJ, Lawson DH, Vessey MP. Post-marketing surveillance of the safety of cimetidine: twelve-month morbidity report. Quarterly J Med. 1985;54:253–268. [PubMed] [Google Scholar]
- 60.Garbis H, Elefant E, Diav-Citrin O, et al. Pregnancy outcome after exposure to ranitidine and other H2-blockers. A collaborative study of the European Network of Teratology Information Services. Reprod Toxicol. 2005;19:453–458. doi: 10.1016/j.reprotox.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Källén B. Delivery outcome after the use of acid-suppressing drugs in early pregnancy with special reference to omeprazole. Br J Obstetr Gynecol. 1998;105:877–881. [PubMed] [Google Scholar]
- 62.Magee LA, Inocencion G, Kamboj L, et al. Safety of first trimester exposure to histamine H2 blockers. A prospective cohort study. Dig Dis Sci. 1996;41:1145–1149. doi: 10.1007/BF02088230. [DOI] [PubMed] [Google Scholar]
- 63.Matok I, Gorodischer R, Koren G, et al. The safety of H(2)-blockers use during pregnancy. J Clin Pharmacol. 2010;50:81–87. doi: 10.1177/0091270009350483. [DOI] [PubMed] [Google Scholar]
- 64.Ruigomez A, Garcia Rodriguez LA, Cattaruzzi C, et al. Use of cimetidine, omeprazole, and ranitidine in pregnant women and pregnancy outcomes. Am J Epidemiol. 1999;150:476–481. doi: 10.1093/oxfordjournals.aje.a010036. [DOI] [PubMed] [Google Scholar]
- 65.Lalkin A, Loebstein R, Addis A, et al. The safety of omeprazole during pregnancy: a multicenter prospective controlled study. Am J Obstetr Gynecol. 1998;179:727–730. doi: 10.1016/s0002-9378(98)70072-9. [DOI] [PubMed] [Google Scholar]
- 66.Ács N, Bánhidy F, Puho EH, Czeizel AE. A possible association between maternal dyspepsia and congenital rectal/anal atresia/stenosis in their children: a population-based case-control study. Acta Obstetr Gynecol Scand. 2009;88:1017–1023. doi: 10.1080/00016340903128447. [DOI] [PubMed] [Google Scholar]
- 67.Bánhidy F, Dakhlaoui A, Puho EH, Czeizel AE. Peptic ulcer disease with related drug treatment in pregnant women and congenital abnormalities in their offspring. Congen Anomal. 2011;51:26–33. doi: 10.1111/j.1741-4520.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978-2005. MMWR Morbidity and Mortality Weekly Report. 2008;57:1–5. [PubMed] [Google Scholar]
- 69.Shapiro S, Heinonen OP, Siskind V, et al. Antenatal exposure to doxylamine succinate and dicyclomine hydrochloride (Benedectin) in relation to congenital malformations, perinatal mortality rate, birth weight, and intelligence quotient score. Am J Obstetr Gynecol. 1977;128:480–485. doi: 10.1016/0002-9378(77)90028-x. [DOI] [PubMed] [Google Scholar]
- 70.Hardy JB. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol. 2003;13:303–311. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 71.Klebanoff MA. The Collaborative Perinatal Project: a 50-year retrospective. Paediatr Perinatal Epidemiol. 2009;23:2–8. doi: 10.1111/j.1365-3016.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Honein MA, Moore CA. The safety or risk of antihistamine use in pregnancy: reassuring data are helpful but not sufficient. J Allergy Clin Immunol: In Practice. 2013;1:675–676. doi: 10.1016/j.jaip.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olesen C, Sondergaard C, Thrane N, et al. Do pregnant women report use of dispensed medications? Epidemiology. 2001;12:497–501. doi: 10.1097/00001648-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Werler MM, Pober BR, Nelson K, Holmes LB. Reporting accuracy among mothers of malformed and nonmalformed infants. Am J Epidemiol. 1989;129:415–421. doi: 10.1093/oxfordjournals.aje.a115145. [DOI] [PubMed] [Google Scholar]
- 75.Cogswell ME, Bitsko RH, Anderka M, Caton AR, Feldkamp ML, Hockett Sherlock SM, Meyer RE, Ramadhani T, Robbins JM, Shaw GM, Mathews TJ, Royle M, Reefhuis J, National Birth Defects Prevention Study Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am J Epidemiol. 2009;170:975–985. doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]
- 76.Sadler TW. Langman's Medical Embryology. 8th Edition Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- 77.Yau WP, Lin KJ, Werler MM, et al. Drug certainty-response in interview-based studies. Pharmacoepidemiol Drug Saf. 2011;20:1210–1216. doi: 10.1002/pds.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatrics Soc. 1999;47:749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S, National Birth Defects Prevention Study Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 205:51.e1–8. doi: 10.1016/j.ajog.2011.02.029. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Botto LD, Lin AE, Riehle-Colarusso T, et al. Seeking causes: Classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Molec Teratol. 2007;79:714–727. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 81.Rasmussen SA, Olney RS, Holmes LB, et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Molec Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 82.Riehle-Colarusso T, Strickland MJ, Reller MD, et al. Improving the quality of surveillance data on congenital heart defects in the metropolitan Atlanta congenital defects program. Birth Defects Res A Clin Molec Teratol. 2007;79:743–753. doi: 10.1002/bdra.20412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


