Abstract
Background
Outcomes for patients with metastatic Ewing sarcoma (ES) remain poor. We investigated whether the intensification of ifosfamide improved survival for patients with metastatic ES.
Procedure
We conducted a retrospective chart review of 30 patients with metastatic ES treated with the MSKCC “EFT regimen”. The regimen included an intensification of ifosfamide dosing from 1,800 mg/m2/day × 5 days per cycle to 2,800 mg/m2/day × 5 days per cycle.
Results
Twenty six of the 30 patients completed planned chemotherapy. Two patients experienced disease progression during therapy. There were no toxic deaths. One patient developed secondary leukemia. The 4-year event free survival (EFS) was 27% and the overall survival (OS) was 39%.
Conclusions
Intensification of ifosfamide was tolerated and did not increase toxicity in patients with metastatic ES. The intensification did not improve outcomes for these patients with metastatic disease.
Keywords: Ewing sarcoma, chemotherapy, outcomes
INTRODUCTION
Ewing sarcoma (ES) is the second most common primary bone tumor affecting children and young adults with an incidence of 3 cases per 1,000,000 per year in the United States. Approximately twenty five percent of new patients will present with metastatic disease [1]. The presence of metastatic disease at diagnosis confers a poor prognosis with an estimated event free survival of 22–30% at five years [2–4]. Other prognostic factors associated with a poor prognosis include central/pelvic tumors[5, 6], large tumors[2, 6], age > 18 years [2], and an elevated serum lactate dehydrogenase (LDH) at diagnosis[7]. In patients with metastatic disease, those with metastases limited to the lungs have a better prognosis than those with metastases to the bone, bone marrow or a combination of bone and lung[5, 6].
In 2003 we reported the results of the MSKCC P6 trial for treatment of newly diagnosed patients with Ewing sarcoma. The trial included patients with both localized and metastatic disease. With this dose intense regimen the 4 year event free survival (EFS) and overall survival (OS) for patients with localized disease were excellent at 82% and 89% respectively. The results for patients with metastatic disease were disappointing with EFS and OS of 12% and 17.8% [8]. The MSKCC EFT regimen was developed as a further dose intensification of the P6 regimen. The cumulative dose of ifosfamide was increased from 27 grams/m2 to 42 grams/m2 to investigate whether further intensification could improve prognosis for patients with metastatic ES.
METHODS
Patients
From 2004 to 2012, 30 patients with newly diagnosed, previously untreated, metastatic Ewing sarcoma were treated according to a modified version of the P6 protocol. This treatment was renamed the “EFT regimen”. All patients were confirmed pathologically to have Ewing sarcoma. Pretreatment extent of disease evaluation included computed tomography (CT) and/or magnetic resonance imaging of the primary site and all sites of metastatic disease; CT of the chest, a technetium-99m bone scan; and bone marrow analysis by aspiration and biopsy. A retrospective chart review was conducted to analyze prognostic factors and outcomes of patients treated according to this regimen. Age, gender, site of primary disease, extent and site(s) of metastatic disease, primary tumor size, pre-treatment LDH, local control modality employed and events during or following therapy including recurrence, death and secondary malignancy were reviewed for all patients. The institutional review board at MSKCC approved the review of medical records for this analysis.
Therapy
The EFT regimen is a modified version of the P6 regimen [9]. The major modification was an increase in the dosing of ifosfamide from 1,800 mg/m2/day × 5 days per cycle to 2,800 mg/m2/day × 5 days per cycle. Other minor changes included a change in the ordering of cycles 6 and 7 and a change from continuous infusion doxorubicin 75 mg/m2 with vincristine 2 mg/m2 over 72 hours to the same dosing in two divided doses of doxorubicin on days 1 and 2 and one bolus dose of vincristine on day 1 of each cycle.
The EFT regimen consists of seven cycles of chemotherapy planned every 21 days. Patients must have recovered from the toxicities of the prior cycle and have an absolute neutrophil count of 500/µL or higher and a platelet count of 75,000/µL or higher before proceeding with each cycle. Cycles 1, 2, 3, and 7 consist of cyclophosphamide 2,100 mg/m2/day × 2 days, doxorubicin 37.5 mg/m2/day × 2 days and vincristine 2 mg/m2/dose to maximum of 2 mg on day 1. Cycles 4, 5, and 6 consist of ifosfamide 2,800 mg/m2/day × 5 days and etoposide 100 mg/m2/day × 5 days. Cyclophosphamide and ifosfamide are given with vigorous intravenous hydration and an equivalent daily dose of mesna continuously over 24 hours. Dexrazoxane 375 mg/m2/dose is given 15 minutes prior to each dose of doxorubicin. Filgrastim or peg-filgrastim is given for neutrophil support with each cycle.
For those patients in whom gross total resection of the primary tumor was feasible, surgical local control was performed following recovery from cycle 3 of chemotherapy. If radiation therapy was used for primary local control it was administered concurrent with cycles 4–6 of chemotherapy. Local control of metastatic sites of disease, including whole lung irradiation for pulmonary disease, was performed following cycle 7 of chemotherapy.
Statistics
Event free survival (EFS) and overall survival (OS) were calculated for all patients from the time of treatment initiation. For EFS, events included recurrence, second malignancy or death. Prognostic factors for EFS and OS were evaluated using the log-rank test. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Toxicity
Outpatient clinic notes, inpatient admission and discharge summaries, and laboratory reports were reviewed for assessment of toxicity. Acute toxicities were documented from the start of the first cycle of the EFT regimen through recovery from the final administered cycle of this regimen. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
RESULTS
Patient Characteristics
Thirty patients with newly diagnosed, previously untreated, metastatic Ewing sarcoma were treated according to the EFT regimen. The characteristics of all patients are described in Table I. Five (17%) of these patients had a primary tumor in an extremity, 13 (43%) in the pelvis and the remainder in other locations in the axial skeleton. Twelve patients had metastatic disease in the lungs only (40%); the remainder had metastases to other sites or a combination of lung and other sites. Three patients had disease in the bone marrow.
Table I.
Patient Characteristics (n = 30)
| Gender | |
| Male | 22 |
| Female | 8 |
| Age, years | |
| Median | 19 |
| Range | 10–38 |
| Primary Tumor Location | |
| Pelvis | 13 |
| Extremity | 5 |
| Other axial skeleton | 12 |
| Site of Metastasis | |
| Lung Only | 12 |
| Other | 18 |
| LDH at diagnosis, Units/L | |
| Median (range) | 305 (234–436) |
| Less than/equal to 200 | 7 |
| Greater than 200 | 23 |
| Size of primary tumor | |
| Less than/equal to 8 cm | 11 |
| Greater than 8 cm | 19 |
| Local Control of primary site | |
| Surgery | 3 |
| Irradiation | 16 |
| Both | 10 |
Outcomes
Twenty six of the 30 patients completed planned chemotherapy. One patient progressed after 4 cycles of chemotherapy. This patient had a 5 week delay in starting cycle 4 as a surgical plan was made and cancelled. A second patient progressed after cycle 5 of chemotherapy. He had not received chemotherapy in almost 8 weeks due to thrombocytopenia associated with a large radiation field. A third patient was changed to a regimen with a lesser risk of cytopenias to allow for continued administration of therapy while healing from Grade 3 hemorrhagic cystitis. The fourth patient to not complete planned chemotherapy elected to return to his home country after 4 cycles and was lost to follow-up.
For local control of the primary site, 3 patients were treated with surgery, 16 with radiation therapy and 10 with a combination of surgery and radiation therapy. The patient who progressed after cycle 4 did not receive local control to the primary site until after progression. Twenty four (80%) patients received local control (radiation therapy in all cases) to all sites of metastatic disease. Two patients with pulmonary disease did not receive whole lung irradiation (WLI) because the pulmonary disease recurred or progressed prior to initiation of WLI. In 3 patients the boney lesions were too numerous to deliver RT to all sites of disease. One patient elected to discontinue RT.
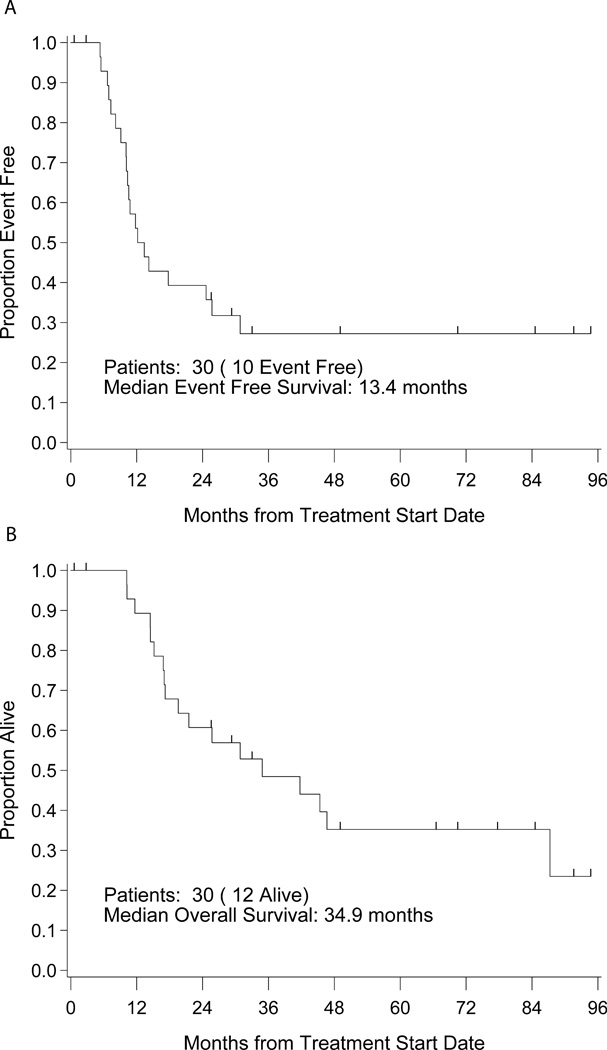
The four year EFS and OS are 27% (95% CI: 12–45%) and 39% (95% CI: 22–57%) respectively (Figure 1). Ten patients remain in a continuous first remission. Twelve patients were alive at the time of last follow-up; two having received several lines of additional therapy for recurrent disease. The median follow-up for survivors is 58 months (range 0.6–95). None of the patients who did not receive local control to all sites of metastatic disease survived event free though two were alive (one in remission) at the time of last follow-up. All patients with bone marrow disease (n=3) died. There were no toxic deaths. All deaths were attributed to progressive Ewing sarcoma.
Figure 1.
A. Event free survival of all patients. B. Overall survival of all patients.
Toxicity
Two hundred and one cycles of chemotherapy, 85 of them ifosfamide/etoposide cycles, were assessed for toxicity. Common toxicities are summarized in Table III. The “other” infections included a peri-rectal abscess that required incision and drainage, 2 urinary tract infections (UTIs)- one associated with a Foley catheter, the other with ureteral stents, and a herpes zoster infection. The perirectal abscess and one UTI were associated with ifosfamide/etoposide cycles.
Table III.
Toxicity
| Ifosfamide/Etoposide Cycles |
Entire Regimen |
|||
|---|---|---|---|---|
| Toxicity | No. of cycles | % | No. of cycles | % |
| Infection | ||||
| Febrile Neutropenia | 31 | 36 | 115 | 57 |
| Sepsis-ICU admission | 0 | 0 | 1 | 0.5 |
| Central line infection | 5 | 6 | 16 | 8 |
| Other Infection | 2 | 2.4 | 4 | 2 |
| Neutropenia-Grade 4 | 85 | 100 | 201 | 100 |
| Thrombocytopenia | ||||
| Grade 3 | 14 | 16 | 49 | 24 |
| Grade 4 | 50 | 59 | 117 | 58 |
| Encephalopathy | 1 | 1.2 | 1 | 0.5 |
| Fanconi Syndrome | 1 | 1.2 | 1 | 0.5 |
| Hemorrhagic Cystitis | 2 | 2.4 | 2 | 1 |
| Acute kidney injury | ||||
| Grade 2 | 1 | 1.2 | 1 | 0.5 |
| Grade 3 | 0 | 0 | 0 | 0 |
Eighty eight percent of ifosfamide/etoposide cycles were associated with Grade 3 or 4 thrombocytopenia. Only 8 of these cycles were given without concurrent radiation therapy.
The two patients who experienced hemorrhagic cystitis following administration of ifosfamide were both concurrently receiving radiation therapy to large pelvic masses. One of these patients later received an additional cycle of ifosfamide without incident supported by double dosing of mesna and continuous bladder irrigation during the week of chemotherapy. The second patient was changed to an alternate chemotherapy regimen. One patient experienced significant Fanconi syndrome following his final cycle of ifosfamide. This patient had presented with acute renal failure at the time of diagnosis due to ureteral obstruction by a large pelvic mass. The renal imaging findings at diagnosis were thought consistent with longstanding obstruction and resultant nephron loss.
One patient developed secondary AML (monosomy 7) 2 years and 10 months from completion of the EFT regimen. Prior to development of tAML this patient was also treated with 26 cycles of irinotecan/temozolomide and 3 cycles of cyclophosphamide/topotecan for recurrent Ewing sarcoma.
Prognostic Features
Of the various prognostic features examined (patient age, disease site, tumor size, pre-treatment LDH and site of metastatic disease) the only variable found to be significant in predicting poorer EFS was tumor size. Those patients with a tumor size >8 cm (n = 19 patients) had an EFS of 9% (95% CI: 1–33%) compared with 39% (95% CI: 4–61%) for patients with tumors less than or equal to 8 cm (p= 0.03). The OS for these patients was 18% (95% CI: 3–44%) versus 49% (95% CI: 23%–71%) with a p-value of 0.07. As demonstrated in Table II, patients under age 18, patients with an LDH less than or equal to 200 and patients with metastatic disease limited to the lungs had improved OS; however, none of these factors reached statistical significance.
Table II.
Prognostic Factors
| Overall Survival |
Event Free Survival |
||||||
|---|---|---|---|---|---|---|---|
| Events | 4 year estimate (95% CI) |
p- value |
Events | 4 year estimate (95% CI) |
p- value |
||
| Age | <18 years | 4 | 54 % (13–82) |
0.27 | 4 | 43% (10–73) |
0.54 |
| ≥18 years | 14 | 29 % (11–50) |
16 | 23% (8–43) |
|||
| Site | Central | 15 | 37% (17–56) |
0.67 | 17 | 26% (11–45) |
0.47 |
| Extremity | 3 | 27% (1–69) |
3 | 30% (1–72) |
|||
| Size | ≤8 cm | 9 | 49% (23–71) |
0.07 | 10 | 39% (16–61) |
0.03 |
| >8 cm | 9 | 18% (3–44) |
10 | 9% (1–33) |
|||
| LDH | ≤200 | 3 | 57% (17–85) |
0.17 | 5 | 29% (4–61) |
0.88 |
| >200 | 15 | 26% (9–48) |
15 | 26% (9–47) |
|||
| Metastatic Site | Lung | 6 | 53% (21–77) |
0.28 | 6 | 45% (17–71) |
0.16 |
| Other | 12 | 23% (6–47) |
14 | 16% (3–37) |
|||
DISCUSSION
Several studies have demonstrated that increasing dose intensity of chemotherapy improves outcomes for patients with localized ES [8, 10]. An increase in dose intensity can be achieved by interval compression as in the most recently completed Children’s Oncology Group (COG) trial for patients with localized ES (AEWS0031) [10] or by an escalation of dose as in the P6 protocol [8]. Both approaches increase the planned dose per unit time- mg/m2/week [11]. For more than 20 years we have treated patients at MSKCC with a short-duration (21 week), dose-intensified chemotherapy regimen. The mg/m2/week dose of cyclophosphamide is more than double the dosing used in COG studies and the doxorubicin and ifosfamide dosages/m2/week are comparable to those used in AEWS0031, Regimen B (interval compressed arm). Excellent outcomes were described for patients with localized ES treated on the P6 protocol while results for patients with metastatic disease remained poor [8]. While the current study was not prospective, further intensification of ifosfamide does not appear to have improved outcomes. It is notable though that our cohort of patients included a disproportionate number of older patients (median age 19 years) and few patients with primary tumors located in the extremities (17%).
As the number of patients evaluated was 30 our ability to determine prognostic factors was limited. The data did confirm tumor size greater than 8 cm as predictive of poorer EFS with a trend to the same for OS. Similar to observations made by other groups [5, 6, 12], patients with metastases limited to the lungs seemed to fare better than those with metastases to other sites.
The intensification of ifosfamide did not increase apparent toxicity as compared with the P6 regimen; there were no toxic deaths and one case of secondary cancer. The majority of patients were able to receive local control as planned without delays related to excess toxicity. New therapies are desperately needed to treat patients with metastatic ES. Intensification of therapy alone has not proven to be the answer but as we consider the addition of new active drug combinations to the current standard 5 chemotherapy agents, this EFT regimen could serve as a backbone that delivers dose density and intensity without toxicity greater than expected.
Acknowledgments
Grant Number: R25 CA020449
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30(6):425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 2.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 3.Cangir A, Vietti TJ, Gehan EA, et al. Ewing's sarcoma metastatic at diagnosis. Results and comparisons of two intergroup Ewing's sarcoma studies. Cancer. 1990;66(5):887–893. doi: 10.1002/1097-0142(19900901)66:5<887::aid-cncr2820660513>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Miser JS, Goldsby RE, Chen Z, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: evaluation of increasing the dose intensity of chemotherapy--a report from the Children's Oncology Group. Pediatr Blood Cancer. 2007;49(7):894–900. doi: 10.1002/pbc.21233. [DOI] [PubMed] [Google Scholar]
- 5.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18(17):3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Galindo C, Liu T, Krasin MJ, et al. Analysis of prognostic factors in ewing sarcoma family of tumors: review of St. Jude Children's Research Hospital studies. Cancer. 2007;110(2):375–384. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- 7.Bacci G, Longhi A, Ferrari S, et al. Prognostic factors in non-metastatic Ewing's sarcoma tumor of bone: an analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol. 2006;45(4):469–475. doi: 10.1080/02841860500519760. [DOI] [PubMed] [Google Scholar]
- 8.Kolb EA, Kushner BH, Gorlick R, et al. Long-term event-free survival after intensive chemotherapy for Ewing's family of tumors in children and young adults. J Clin Oncol. 2003;21(18):3423–3430. doi: 10.1200/JCO.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Kushner BH, Meyers PA, Gerald WL, et al. Very-high-dose short-term chemotherapy for poor-risk peripheral primitive neuroectodermal tumors, including Ewing's sarcoma, in children and young adults. J Clin Oncol. 1995;13(11):2796–2804. doi: 10.1200/JCO.1995.13.11.2796. [DOI] [PubMed] [Google Scholar]
- 10.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(33):4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton L. Evolving concepts in the systemic drug therapy of breast cancer. Seminars in oncology. 1997;24(4 Suppl 10):S10-S13-S10-10. [PubMed] [Google Scholar]
- 12.Paulussen M, Ahrens S, Burdach S, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol. 1998;9(3):275–281. doi: 10.1023/a:1008208511815. [DOI] [PubMed] [Google Scholar]