Abstract
Objective
To investigate the relationship between tissue-specific alterations in brain volume and neurobehavioral status in newborns with complex congenital heart defects preoperatively.
Study design
Three-dimensional volumetric magnetic resonance imaging was used to calculate tissue-specific brain volumes and a standardized neurobehavioral assessment was performed to assess neurobehavioral status in 35 full-term newborns admitted to the hospital before cardiopulmonary bypass surgery. Multiple linear regression models were performed to evaluate relationships between neurobehavioral status and brain volumes.
Results
Reduced subcortical gray matter (SCGM) volume and increased cerebrospinal fluid (CSF) volume were associated with poor behavioral state regulation (SCGM, P = .04; CSF, P = .007) and poor visual orienting (CSF, P = .003). In cyanotic newborns, reduced SCGM was associated with higher overall abnormal scores on the assessment (P = .001) and poor behavioral state regulation (P = .04), and increased CSF volume was associated with poor behavioral state regulation (P = .02), and poor visual orienting (P = .02). Conversely, acyanotic newborns showed associations between reduced cerebellar volume and poor behavioral state regulation (P = .03).
Conclusion
Abnormal neurobehavior is associated with impaired volumetric brain growth before open heart surgery in infants with complex congenital heart defects. This study highlights a need for routine preoperative screening and early intervention to improve neurodevelopmental outcomes.
Brain injury and neurodevelopmental impairments have emerged as salient comorbidities of congenital heart defects (CHDs), especially in newborns with complex cardiac lesions.1–4 Moreover, there is increasing awareness of neurologic compromise presenting even before corrective or palliative surgery, with up to 60% of newborns demonstrating neuroimaging abnormalities1–3,5,6 and up to 70% showing signs of neurobehavioral impairment.7–10 Studies using advanced magnetic resonance imaging (MRI) techniques are adding to this growing body of literature by providing quantitative in vivo measurements of brain structure.11–15 For instance, recent studies using 3-dimensional (3D) volumetric MRI have shown that fetuses with CHD have progressively lower total and tissue-specific brain volumes as well as delayed gyrification in the third trimester compared with healthy controls.14,15
The high prevalence of neurologic and neurobehavioral abnormalities, in addition to more recent quantitative indicators of impairment, points to a need for routine monitoring of neurologic status in these high-risk infants. Moreover, a better understanding of the association between early brain structure and function may help to determine the most appropriate methods for evaluating this high-risk population. Very few studies have investigated the relationship between neurologic status and brain injury in newborns with complex CHD, and available data are equivocal. 8,9 Furthermore, no study to date has examined the association between clinical neurobehavioral status and volumetric brain growth in newborns with CHD before open heart surgery. Therefore, the objective of this study was to examine the association between preoperative neurobehavioral status, as measured with a standardized neurobehavioral assessment, and global and tissue-specific brain volumes, as measured using 3D volumetric MRI.
Methods
The study cohort included full-term newborns (>36 weeks’ gestational age) diagnosed with complex CHD requiring corrective or palliative cardiopulmonary bypass surgery. Subjects were enrolled prospectively, either antenatally or postnatally. Mothers of fetuses with confirmed complex CHD following a fetal echocardiogram were enrolled as part of a larger, longitudinal study of brain development in fetuses and infants with CHD. Postnatally, newborns were enrolled following admission to the cardiac intensive care unit at our center with complex CHD confirmed by echocardiography.
Newborns were excluded when there was evidence of central nervous system dysfunction that could be plausibly attributed to causes that were unrelated to complications of CHD. This included subjects with central nervous system infections; congenital malformations, known chromosomal anomalies, or syndromes; or documented perinatal insults. Informed, parental consent was obtained before acquiring any patient information. This study was approved by the institutional review board at our center as part of the longitudinal study protocol.
Preoperative brain MRI studies were conducted for enrolled newborns as per the standard clinical protocol at our center for newborns undergoing cardiopulmonary bypass surgery. In certain cases, surgery was scheduled beyond the neonatal period and studies were performed strictly for research purposes. Studies took place as soon as the clinical care team determined that the newborn was stable enough to be transported to the MRI scanner or at the parents’ earliest convenience when newborns were scanned as outpatients.
No sedation was used during the MRI scan unless it was necessary for clinical reasons. Newborns who were not clinically sedated were quieted by feeding and swaddling and were immobilized through using an Infant Vacuum Immobilizer (Newmatic Medical, Caledonia, Michigan). All infants were provided with 2 layers of hearing protection: ear plugs (Mighty Plugs; Beneficial Products Inc, Ashland, Oregon) and Neonatal Noise Guards (Natus Medical Inc, Seattle, Washington). Oxygen saturation, heart rate, and temperature were monitored and recorded throughout the scanning process by a nurse using a Veris MR vital signs monitoring system (MEDRAD, Inc, Indianola, Pennsylvania).
MRI pulse sequences were acquired on a 3-T magnetic resonance scanner (Discovery MR750, GE Healthcare, Milwaukee, Wisconsin) with a 32-channel receive-only head coil (MR Instruments, Inc, Minneapolis, Minnesota). Two sequences were required to compute brain volumes: a T1-weighted fluid attenuated inversion recovery sequence with periodically rotated overlapping parallel lines with enhanced reconstruction, 2-mm contiguous axial slices, 38.6-ms echo time, 1525-ms repetition time, 565.42-ms inversion time, 256 × 256 matrix, 1 excitation, and 25.6-cm field of view, as well as a 2-mm axial T2-weighted periodically rotated overlapping parallel lines with enhanced reconstruction sequence with 83.72-ms echo time, 9640-ms repetition time, 256 × 256 matrix, 2 excitations, and 25.6-cm field of view. A pediatric neuroradiologist blinded to clinical diagnosis and neurobehavioral findings reviewed each MRI study.
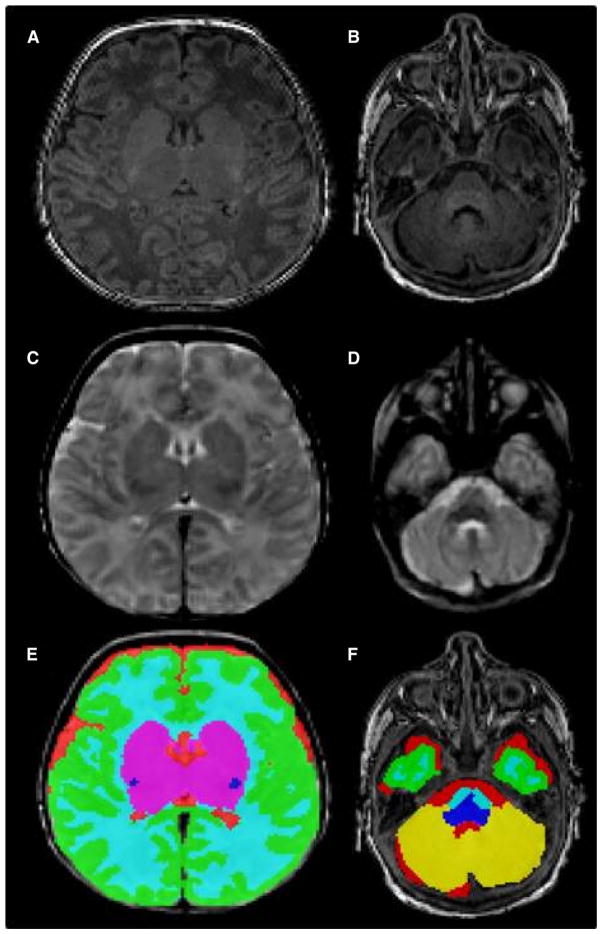
Image processing was performed on Linux workstations. A previously validated automatic segmentation software program16,17 was used to segment each brain volume into 5 major tissue classes and the cerebellum (Figure 1). The resulting images were inspected and corrected manually using MINC software (www.bic.mni.mcgill.ca/software/minc). Manual corrections were performed by a single investigator, and intrarater reliability was assessed by performing the manual corrections a second time for 5 cases. Using the intraclass correlation coefficient, intrarater reliability was calculated as 0.998 overall: total brain volume, 0.96; cortical gray matter (CGM), 0.89; myelinated white matter (mWM), 0.77; unmyelinated white matter, 0.97; subcortical gray matter (SCGM), 0.91; and cerebellar volume, 0.91. Following automatic segmentation and manual corrections, each tissue volume was determined by multiplying the number of voxels in the appropriate tissue’s segmentation mask (eg, CGM) by the volume of each voxel (www.bic.mni.mcgill.ca/software/minc).
Figure 1.
Input and output for the automatic segmentation of brain tissue volumes. A, Axial T1 slice at the level of the thalami and B, cerebellum; C, axial T2 slice at the level of the thalami and D, cerebellum; E, automatic segmentation of brain tissue volumes at the level of the thalami and F, cerebellum. The tissue classes depicted are CGM (green), unmyelinated white matter (light blue), mWM (dark blue), SCGM (magenta), CSF (red), and cerebellum (yellow).
The Einstein Neonatal Neurobehavioral Assessment Scale (ENNAS)18 was administered by 1 of 2 evaluators trained in assessing neonatal neurobehavioral status and blinded to clinical neurologic findings and MRI results. The ENNAS was selected as a comprehensive yet efficient assessment of neonatal neurologic and neurobehavioral status, which has been previously used to evaluate newborns with CHD.4,7,19,20 It takes approximately 20 minutes to perform at the newborn’s bedside and consists of 20 test items, which evaluate muscle tone, passive and active movements, primitive reflexes, as well as visual and auditory orienting. In addition, 4 summary items assess overall impression of “cuddliness,” spontaneous movements, tonus, and tremor (both incidence and quality). Of the 24 scored items, 19 contribute to an overall score reflecting the number of failed items, with the remaining items informing the summary scores. The infant’s behavioral state is also observed throughout the assessment, with predominant state documented independently of the scored items. Assessments with up to 2 failed items were classified as normal, 3–6 failed items constituted a suspect assessment, and >6 failed items was considered abnormal.
The ENNAS has excellent concurrent validity with the standard neonatal neurologic examination.20 The negative predictive value of this instrument is very good (83%–92%) for intellectual function, communication, and socialization and good (64%–83%) for neuromotor status and daily living skills at school age.21 The sensitivity of the ENNAS is also good, ranging from 64% to 78%, although positive predictive value and specificity remain limited due to a susceptibility for false-positive results.21 Interrater reliability was not calculated in the present study as the critical condition of the newborns in this cohort was not compatible with an increase in handling to accommodate 2 successive assessments. Conducting temporally separate assessments was ruled out due to the possibility of intervening clinical events affecting reliability. The interrater reliability has been previously calculated as 0.97.19
Medical records for each newborn were reviewed for clinical information (eg, gestational age at birth, birth weight, cardiac diagnosis, cardiac catheterization procedures). In addition, the Score for Neonatal Acute Physiology-II (SNAP-II)22 was calculated for each newborn to assess illness severity within the first 12 hours of life.
Statistical Analyses
Descriptive statistics were defined as means and SDs for continuous values, medians and ranges for ordinal values, and proportions for categorical values. Multiple linear regression models, both unstratified and stratified, were performed to evaluate the relationships between overall ENNAS scores as well as individual items or clusters (active motility, muscle tone, visual orienting, auditory orienting, and behavioral state) and brain volumes. The stratification factor was cardiac physiology (cyanotic vs acyanotic defects) as determined by anatomic classification and oxygen saturation. All analyses controlled for differences in postconceptional age at time of MRI, birth weight, sex, and SNAP-II score to rule out the effects of maturation and general illness severity. This study was intended primarily to generate hypotheses; as such, all probability values with a significance of ≤.05 are reported.
Results
At the antenatal entry point, 39 women were pregnant with fetuses meeting the study criteria. Of these, 20 were enrolled (12 declined, 2 pregnancies were terminated, 1 pregnancy ended in fetal death, and 4 were not approached out of sensitivity to patients’ needs [ie, schedule, new diagnosis]). Once delivered, 2 of these subjects were excluded (1 due to prematurity, 1 due to insufficient time before surgery to perform MRI). At the postnatal entry point, 35 newborns were eligible, and 20 were enrolled (7 declined, 5 had insufficient time for study evaluation before surgery, 1 had insufficient time before discharge, 1 began extracorporeal membrane oxygenation before surgery, 1 was not approached as study staff were not available). Of those enrolled in the study, 3 were excluded (2 due to insufficient time before surgery to perform MRI, 1 no longer required surgery). Newborns who were not included in the study did not differ in their CHD diagnoses from those who were included. In total, 35 newborns (18 enrolled antenatally, 17 enrolled postnatally) underwent preoperative brain MRI and neurobehavioral assessment. Table I presents characteristics of the cohort. Postnatally enrolled newborns did not differ from those enrolled prenatally with respect to ENNAS score, conventional MRI findings, or measures of brain volume. Table II (available at www.jpeds.com) provides a breakdown of the cardiac diagnoses of enrolled newborns.
Table I.
Perinatal characteristics
| Characteristics | All newborns (N = 35) |
|---|---|
| Gestational age at birth, wk, mean ± SD | 38.8 ± 0.9 |
| Birth weight, g, mean ± SD | 3195.9 ± 528.1 |
| Male sex, n (%) | 19 (54) |
| Cyanotic, n (%) | 26 (74) |
| Normal spontaneous vaginal delivery, n (%) | 7 (20) |
| Induced vaginal delivery, n (%) | 15 (43) |
| Elective cesarean delivery, n (%) | 10 (29) |
| Emergent cesarean delivery, n (%) | 3 (9) |
| Apgar score at 5 min, median (range) | 8 (7–9) |
| SNAP-II score, median (range) | 5 (0–28) |
| Respiratory resuscitation at birth, n (%) | 13 (37) |
| Postconceptional age at MRI, mean ± SD, wk | 39.5 ± 1.3 |
| Age at MRI, d, median (range) | 3 (1–21) |
| Postconceptional age at ENNAS, mean ± SD, wk | 39.6 ± 1.3 |
| Age at ENNAS, d, median (range) | 4 (1–21) |
| Time between MRI and ENNAS, d, median (range)* | 1 (0–5) |
| Pressor-inotrope support before MRI, n (%) | 19 (54) |
| Prostaglandin E1 before MRI, n (%) | 24 (69) |
| Balloon atrial septostomy or cardiac catheterization before MRI, n (%) | 9 (26) |
| Time to surgery, d, median (range) | 10.5 (4–248) |
The ENNAS was performed within 36 hours of the MRI in the majority of patients (91%). The span between MRI and neurobehavioral assessment for the remaining 3 newborns was 2 days (2) and 5 days (1).
Table II.
Heart defect classifications
| Cyanotic heart defects | |
| TGA | 8 TGA |
| HLHS | 5 HLHS |
| 1 HLHS + DORV + mitral atresia | |
| Single ventricle | 1 Tricuspid atresia + VSD |
| Single ventricle with coarctation of the aorta | 1 DORV + atrioventricular canal defect + coarctation |
| 1 DORV + VSD + coarctation | |
| 1 DILV + L-TGA + aortic stenosis | |
| Single ventricle with pulmonary stenosis/atresia | 1 DORV + hypoplastic right heart + pulmonary stenosis |
| 1 DORV + ventricular inversion + pulmonary stenosis | |
| 1 Pulmonary atresia + hypoplastic right heart | |
| 1 DILV + L-TGA + pulmonary stenosis | |
| 1 Single ventricle + pulmonary atresia with heterotaxy | |
| 1 Tricuspid atresia + VSD + pulmonary stenosis | |
| 2 ventricles | 2 Tetralogy of Fallot (SPO2 <80) |
| Acyanotic heart defects | |
| 2 Ventricles | 2 Tetralogy of Fallot (SPO2 >80) |
| 1 VSD | |
| 1 Truncus arteriosus | |
| 2 Ventricles with coarctation of the aorta | 2 Coarctation + mitral stenosis |
| 1 VSD + coarctation | |
| 1 VSD + interrupted aortic arch | |
| 1 Ebstein anomaly + VSD + coarctation + left ventricle dysfunction | |
DILV, double inlet left ventricle; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; L-TGA, levo-transposition of the great arteries; SPO2, blood oxygen saturation; TGA, transposition of the great arteries; VSD, ventricular septal defect.
Twenty-five (71%) newborns had a suspect or abnormal neurobehavioral assessment; almost one-half fell into the suspect category (16), and 9 were classified as abnormal. Muscle tone was abnormal in 18—10 were hypotonic, 5 were hypertonic, and 3 had mixed tone. Active movements and primitive reflexes were each abnormal in 21 newborns, and passive movements were abnormal in 23. Visual orienting and auditory orienting were abnormal in 26 and 21, respectively. Twenty-three newborns had difficulty maintaining an alert behavioral state, and were instead drowsy or irritable; in 10 of these cases, newborns fluctuated between drowsiness and irritability. One newborn was microcephalic (head circumference <3rd percentile) and 1 was macrocephalic (head circumference >97th percentile). Of note, only 1 newborn was receiving morphine during the neurobehavioral assessment, and this infant received a suspect score on limited examination.
Sixteen (46%) newborns had evidence of injury or immaturity on conventional MRI. The most prevalent findings were hemorrhages (9), including 4 extra-axial, 2 cerebellar, 2 intra-ventricular, and 1 choroid plexus. Other abnormalities included prominent subarachnoid spaces (5), infarction (3), T2 hyperintensity (bilateral anterior limbs of the internal capsule [1], bilateral basal ganglia [1]), white matter injury (deep cerebral white matter [1], periventricular white matter [1]), asymmetric cerebral hemispheres and lateral ventricles (1), delayed opercular development (1), and diffuse edema (1).
There was no association between ENNAS scores and total brain or tissue-specific volumes after controlling for postconceptional age at time of MRI, sex, birth weight, and illness severity (SNAP-II score). Independent associations were observed, however, between individual items on the ENNAS and tissue-specific brain volumes after controlling for the same factors. Specifically, newborns that had difficulty maintaining an alert behavioral state had reduced SCGM volume (P = .04) as well as increased cerebrospinal fluid (CSF) volume (P = .007) (Figure 2; available at www.jpeds.com). Additionally, decreased visual orienting was associated with increased CSF volume (P = .003).
Figure 2.
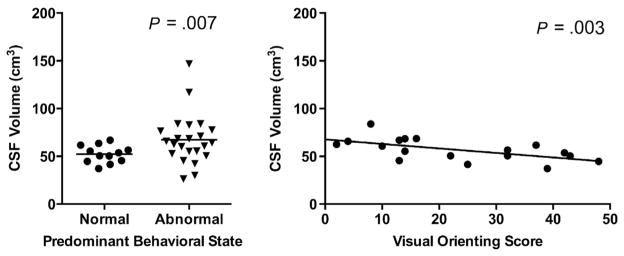
Relationships between CSF volume and predominant behavioral state (left) and between CSF volume and visual orienting score (right) for all newborns. Higher visual orienting score indicates better performance.
Stratifying for cyanotic vs acyanotic lesions revealed a significant relationship between reduced SCGM and higher (more abnormal) ENNAS scores (P = .001) in newborns with cyanotic defects (Figure 3). In addition, although decreased visual orienting was associated with increased CSF volume among the entire cohort, this association was primarily attributed to cyanotic newborns (P = .02). Newborns with cyanotic defects also demonstrated an association between poor behavioral state regulation and decreased SCGM volume (P = .04) as well as increased CSF (P = .02).
Figure 3.
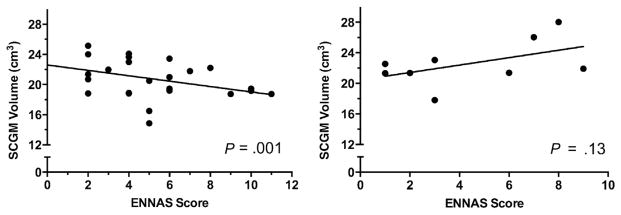
Relationship between SCGM volume and ENNAS scores in newborns with cyanotic (left) and acyanotic CHD (right). Higher ENNAS score indicates greater number of abnormal assessment items.
Conversely, in acyanotic newborns, there was no independent association between either SCGM or CSF volumes and behavioral state. There was, however, an association between decreased cerebellar volume and poor behavioral state regulation (P = .03; Figure 4).
Figure 4.
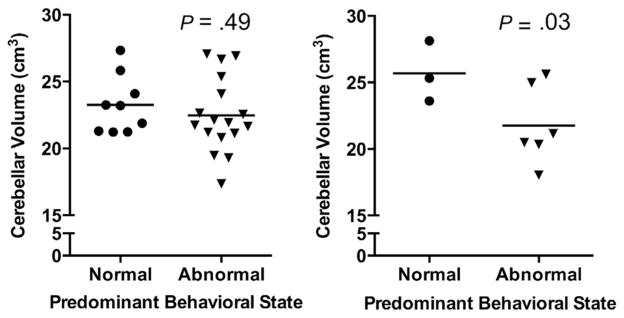
Relationship between cerebellar volume and predominant behavioral state in newborns with cyanotic (left) and acyanotic CHD (right).
Discussion
The results of the present study support mounting evidence demonstrating an increased risk of brain injury and concomitant neurobehavioral impairment in newborns with complex CHD before surgery.1–3,5–10 Almost one-half of the newborns in our cohort showed signs of brain injury or immaturity on conventional MRI, and more than two-thirds had suspect or abnormal neurobehavioral assessments. Moreover, this study describes an association between abnormal neurobehavioral performance and reduced tissue-specific brain volumes.
As the relationship between neurobehavior and brain volumes has not been previously examined in this population, a direct comparison of these results is not possible. Watanabe et al, 23 however, described an association between reduced frontal gray matter volume and lower scores on the Bayley Psychomotor Developmental Index in older infants following corrective cardiac surgery. Similarly, there is a growing body of evidence suggesting an association between abnormal neurobehavior and reductions in specific brain tissue volumes in premature infants.24–26 In preterm-born infants evaluated at term equivalent age, lower behavior scores on the Hammersmith Neonatal Neurological Examination have been associated with white matter volume loss in this population,24 and premature infants with abnormal general movements at 1 and 3 months of age have smaller cerebellar diameters.26 Taken together, these data suggest that abnormal neurobehavioral status is associated with tissue-specific brain growth disturbances in these high-risk infant populations.
The aim of the present study was to determine if quantification of brain growth using advanced 3D volumetric MRI is related to newborn function as measured by a standardized neurobehavioral assessment. Although there was no relationship between total brain volume and overall score on the EN-NAS, there were several observable independent associations between tissue-specific volumes and behavioral items. In newborns with cyanotic heart defects, those who could not maintain an alert behavioral state throughout the majority of the assessment had increased CSF volumes. A similar relationship was observed for newborns who did not attend to a moving stimulus in the visual field. Increased CSF volume has been correlated with prematurity27,28; thus, we propose that abnormal behavioral state regulation and poor performance on visual orienting items suggest impaired brain development in cyanotic newborns. A potential antenatal origin for this increased CSF volume is supported by a recent study reporting progressively higher volumes of CSF in third trimester fetuses with CHD relative to controls.14
Our data also demonstrate that newborns with cyanotic heart defects show an association between reduced SCGM volume and higher (more abnormal) ENNAS scores as well as poor behavioral state organization. Although SCGM was composed collectively of the thalamus and the basal ganglia in this study, the role of the thalamus as a relay center between the environment and other brain regions, as well as its strong connections to the “emotion” centers of the limbic system (eg, periaqueductal gray, hypothalamus), may drive this particular relationship.29 Because the emotional (and thus behavioral) state is regulated on the basis of external cues, it is conceivable that if the thalamus is underdeveloped in a newborn, such an infant may become easily overwhelmed and consequently irritable or drowsy. Furthermore, studies of local cerebral metabolic rates for glucose and regional cerebral blood flow have established the thalamus as a region with one of the highest metabolic rates in the newborn.30–32 Such a metabolically demanding region may be more susceptible to decreased oxygenation resulting from cyanotic CHD, and consequently to impairments in regional brain growth and neurobehavior. Future studies are needed to address this intriguing question.
Newborns with acyanotic defects demonstrated associations between reduced cerebellar volumes and abnormal behavioral state regulation. This observation is consistent with the evolving view of the cerebellum, which is increasingly recognized as contributing to high-order cognitive and behavioral abilities.33–37 The cerebellum, and particularly the central vermis, is another region with an active metabolism and one of the highest regional cerebral blood flow requirements during late gestation and the early postnatal period.30–32,38,39 Therefore, it can be inferred that the increased perfusion requirements within the cerebellum during this transitional period likely render it susceptible to growth disturbances in acyanotic newborns with poor cerebral perfusion.
The different patterns of association that emerged among newborns with cyanotic defects and those with acyanotic defects are of particular interest. Our findings suggest cardiac physiology may have a role in mediating the relationship between brain development and neurobehavior; structural maturation may indeed differ depending on relative perfusion and oxygenation experienced in utero and in the early postnatal period.5,40,41 Animal models have shown that chronic cerebral hypoperfusion is associated with white matter injury in the corpus callosum and internal capsule, which in turn correlates with deficits in object recognition and spatial memory.42,43 Moreover, exposure to chronic hypoxic conditions has been linked to reduced cerebellar and hippocampal volumes and impaired learning and memory in mice.44 Clinical studies explicitly focusing on this line of inquiry are in progress.
This study has several limitations. Enrolling newborns prospectively with a wide range of complex CHD may have masked potential effects present within certain diagnostic categories. Although the relatively small sample size precluded analysis of effects within each lesion type, we stratified the cohort into cyanotic versus acyanotic CHD to account for variations in physiology. Studies with larger, equivalent samples of newborns with cyanotic and acyanotic CHD are needed to further elucidate these initial observations. A further limitation was the restriction of the neurobehavioral assessment for some infants (eg, intubated newborns could not be assessed in the prone position). In these instances, fewer items were scored, possibly resulting in an underestimation of abnormal findings in these subjects. In addition, due to the small volume of mWM in the neonatal brain and the lower intrarater reliability coefficient we obtained for mWM volumes, important observations may have been missed. Finally, as we conducted multiple tests, we consider the results of these exploratory analyses hypothesis generating for future research. Long-term developmental follow-up of our cohort is currently under way to determine the predictive value of these initial findings.
Acknowledgments
Support by the Canadian Institutes for Health Research (CIHR; MOP-81116). M.O. was supported by a Canada Graduate Scholarships Master’s Award, CIHR, and a Montreal Children’s Hospital Research Institute Studentship.
We would like to thank Jianping He, PhD, for his assistance with data analysis. In addition, we extend our gratitude to the families of the infants enrolled in this study, whose contribution made this work possible.
Glossary
- 3D
Three-dimensional
- CGM
Cortical gray matter
- CHD
Congenital heart defect
- CSF
Cerebrospinal fluid, ENNAS, Einstein Neonatal Neurobehavioral Assessment Scale
- MRI
Magnetic resonance imaging
- mWM
Myelinated white matter
- SCGM
Subcortical gray matter
- SNAP-II
Score for Neonatal Acute Physiology-II
Footnotes
The authors declare no conflicts of interest.
References
- 1.Van Houten J, Rothman A, Bejar R. High incidence of cranial ultrasound abnormalities in full-term infants with congenital heart disease. Am J Perinatol. 1996;13:47–53. doi: 10.1055/s-2007-994202. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–14. [PubMed] [Google Scholar]
- 3.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 4.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr. 2000;137:638–45. doi: 10.1067/mpd.2000.109152. [DOI] [PubMed] [Google Scholar]
- 5.Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841–9. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Beca J, Gunn J, Coleman L, Hope A, Whelan L-C, Gentles T, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53:1807–11. doi: 10.1016/j.jacc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 7.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics. 1999;103:402–8. doi: 10.1542/peds.103.2.402. [DOI] [PubMed] [Google Scholar]
- 8.Miller SP, McQuillen PS, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM, et al. Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg. 2004;77:1698–706. doi: 10.1016/j.athoracsur.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 9.Tavani F, Zimmerman R, Clancy R, Licht D, Mahle W. Incidental intracranial hemorrhage after uncomplicated birth: MRI before and after neonatal heart surgery. Neuroradiology. 2003;45:253–8. doi: 10.1007/s00234-003-0946-8. [DOI] [PubMed] [Google Scholar]
- 10.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–64. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 11.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 12.Partridge SC, Vigneron DB, Charlton NN, Berman JI, Henry RG, Mukherjee P, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640–51. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 13.Abdel Raheem MM, Mohamed WA. Impact of congenital heart disease on brain development in newborn infants. Ann Pediatr Cardiol. 2012;5:21–6. doi: 10.4103/0974-2069.93705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clouchoux C, du Plessis A, Bouyssi-Kobar M, Tworetzky W, McElhinney D, Newburger J, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2012:1–12. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- 16.Weisenfeld NI, Warfield SK. Automatic segmentation of newborn brain MRI. NeuroImage. 2009;47:564–72. doi: 10.1016/j.neuroimage.2009.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Als H, Duffy FH, McAnulty G, Butler SC, Lightbody L, Kosta S, et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J Perinatol. 2012 doi: 10.1038/jp.2011.201. Epub:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daum C, Grellong B, Kurtzberg D, Vaughan H. The Albert Einstein Neonatal Neurobehavioral Scale (Manual) 1977 [Google Scholar]
- 19.Kurtzberg D, Vaughan HG, Daum C, Grellong BA, Albin S, Rotkin L. Neurobehavioral performance of low-birthweight infants at 40 weeks conceptional age: comparison with normal full term infants. Dev Med Child Neurol. 1979;21:590–607. doi: 10.1111/j.1469-8749.1979.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 20.Limperopoulos C, Majnemer A, Rosenblatt B, Shevell MI, Rohlicek C, Tchervenkov C. Agreement between the neonatal neurological examination and a standardized assessment of neurobehavioural performance in a group of high-risk newborns. Pediatr Rehab. 1997;1:9–14. doi: 10.3109/17518429709060936. [DOI] [PubMed] [Google Scholar]
- 21.Majnemer A, Rosenblatt B. Prediction of outcome at school age in neonatal intensive care unit graduates using neonatal neurologic tools. J Child Neurol. 2000;15:645–51. doi: 10.1177/088307380001501002. [DOI] [PubMed] [Google Scholar]
- 22.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe K, Matsui M, Matsuzawa J, Tanaka C, Noguchi K, Yoshimura N, et al. Impaired neuroanatomic development in infants with congenital heart disease. J Thorac Cardiovasc Surg. 2009;137:146–53. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Brown NC, Inder TE, Bear MJ, Hunt RW, Anderson PJ, Doyle LW. Neurobehavior at term and white and gray matter abnormalities in very pre-term infants. J Pediatr. 2009;155:32–8. doi: 10.1016/j.jpeds.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Spittle AJ, Brown NC, Doyle LW, Boyd RN, Hunt RW, Bear M, et al. Quality of general movements is related to white matter pathology in very preterm infants. Pediatrics. 2008;121:e1184–9. doi: 10.1542/peds.2007-1924. [DOI] [PubMed] [Google Scholar]
- 26.Spittle AJ, Doyle LW, Anderson PJ, Inder TE, Lee KJ, Boyd RN, et al. Reduced cerebellar diameter in very preterm infants with abnormal general movements. Early Hum Dev. 2010;86:1–5. doi: 10.1016/j.earlhumdev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Zacharia A, Zimine S, Lovblad KO, Thoeny H, Ozdoba C, Bossi E, et al. Early assessment of brain maturation by MR imaging segmentation in neonates and premature infants. Am J Neuroradiol. 2006;27:972–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–3. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- 31.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–97. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 32.Tokumaru AM, Barkovich AJ, O’uchi T, Matsuo T, Kusano S. The evolution of cerebral blood flow in the developing brain: Evaluation with iodine-123 iodoamphetamine SPECT and correlation with MR imaging. Am J Neuroradiol. 1999;20:845–52. [PMC free article] [PubMed] [Google Scholar]
- 33.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 34.Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MH, Stewart AL, et al. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124:60–6. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- 35.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–93. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 36.Bolduc M-E, du Plessis AJ, Sullivan N, Khwaja OS, Zhang X, Barnes K, et al. Spectrum of neurodevelopmental disabilities in children with cerebellar malformations. Dev Med Child Neurol. 2011;53:409–16. doi: 10.1111/j.1469-8749.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 37.Bolduc M-E, du Plessis AJ, Sullivan N, Guizard N, Zhang X, Robertson RL, et al. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 2011;11:531–42. doi: 10.1007/s12311-011-0312-z. [DOI] [PubMed] [Google Scholar]
- 38.Volpe JJ. Cerebellum of the premature infant: Rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24:1085–104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield K, Bassan H, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115:688–95. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 40.Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: The brain sparing effect. Pediatr Cardiol. 2003;24:436–43. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 41.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32–6. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 42.Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, et al. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210:585–91. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Miki K, Ishibashi S, Sun L, Xu H, Ohashi W, Kuroiwa T, et al. Intensity of chronic cerebral hypoperfusion determines white/gray matter injury and cognitive/motor dysfunction in mice. J Neurosci Res. 2009;87:1270–81. doi: 10.1002/jnr.21925. [DOI] [PubMed] [Google Scholar]
- 44.Lan W-CJ, Priestley M, Mayoral SR, Tian L, Shamloo M, Penn AA. Sex-specific cognitive deficits and regional brain volume loss in mice exposed to chronic, sublethal hypoxia. Pediatr Res. 2011;70:15–20. doi: 10.1203/PDR.0b013e31821b98a3. [DOI] [PMC free article] [PubMed] [Google Scholar]