Abstract
Attention Deficit/Hyperactivity Disorder (ADHD) is associated with different impairment profiles in the symptom domains of hyperactivity/impulsivity and/or inattention. An additional symptom domain of sluggish cognitive tempo (SCT) has also been proposed. Although there is a degree of correlation between the SCT symptom domain and inattention, it has been proposed as a distinct disorder independent of ADHD. The objective of this study was to examine the neural substrates of cue-related preparatory processes associated with SCT symptoms versus inattentive symptoms in a group of adolescents with ADHD. We also compared cue-related effects in the entire ADHD group compared with a group of typically developing (TD) peers. A modified cued flanker paradigm and fMRI examined brain activity associated with attention preparation and motor response preparation. Between group contrasts between the ADHD and TD group revealed significant hypoactivity in the ADHD group during general attention preparation in the supplementary motor area (SMA) and in the right superior parietal lobe (SPL) during response preparation. In the ADHD group, greater numbers of SCT symptoms were associated with hypoactivity in the left SPL to cues in general whereas greater numbers of inattentive symptoms were associated with greater activity in the SMA to cues that provided no information and less activity in the thalamus during response preparation. Hypoactivity in the SPL with increasing SCT symptoms may be associated with impaired reorienting or shifting of attention. Altered activity in the SMA and thalamus with increasing inattention may be associated with a general problem with response preparation, which may also reflect inefficient processing of the response preparation cue. Our results support a degree of differentiation between SCT and inattentive symptom profiles within adolescents with ADHD.
Keywords: fMRI, Cognitive control, ADHD, Adolescent, Sluggish cognitive tempo
Highlights
-
•
We examine cognitive control activity in ADHD SCT versus inattentive symptomatology.
-
•
An increase in SCT symptoms was associated with impaired attention orienting.
-
•
More inattentive symptoms were associated with impaired response preparation.
-
•
SCT activity differed from inattentive activity in adolescents with ADHD.
1. Introduction
Attention Deficit/Hyperactivity Disorder (ADHD) is a heterogeneous disorder with impairments in the symptom domains of inattention and/or hyperactivity/impulsivity. Although these domains are distinct, there is also a high degree of correlation between them (Willcutt et al., 2012). More recently, an additional symptom domain has been associated with ADHD, namely sluggish cognitive tempo (SCT). SCT is associated with symptoms including daydreaming, drowsiness, sluggishness/slowness to respond and hypoactivity (Barkley, 2014; Penny et al., 2009). It is also highly correlated with the symptom domain of inattention across studies (see Willcutt et al., 2012) and may be significantly negatively correlated with hyperactivity/impulsivity (Lee et al., 2014; Penny et al., 2009). It has been estimated that 30–63% of individuals with ADHD inattentive subtype have high levels of SCT (Carlson and Mann, 2002; Garner et al., 2010; McBurnett et al., 2001). However, some evidence suggests that SCT may be a distinct disorder, as factor analysis studies demonstrate a clear separation of these symptom types (Lee et al., 2014; McBurnett et al., 2014; Willcutt et al., 2014) and approximately half of individuals with ADHD may not qualify for SCT and vice versa (Barkley, 2013; Garner et al., 2010). One very recent study examined whether SCT fits better with the construct of a symptom domain within ADHD or a distinct factor separate from ADHD (Garner et al., 2014). Although SCT correlated strongly positively with inattention and negatively with hyperactivity/impulsivity, the best fitting model was one that represented SCT as structurally distinct not only from ADHD symptoms but an ADHD diagnosis itself.
The underlying dysfunction in SCT is as yet unknown. It is possible that the symptoms represent a problem with vigilance or arousal (Barkley, 2014; Penny et al., 2009), or that they constitute a pathological form of mind-wandering, which differs from other types of attentional lapses in that it is task-unrelated and stimulus-independent (Barkley, 2014). Other impairments linked to SCT include deficits in early information processing or selective attention (Huang-Pollock et al., 2005), spatial memory (Skirbekk et al., 2011), organization and problem solving (Barkley, 2012, 2013) and motor speed problems (Garner et al., 2010). Unlike ADHD, SCT has not been associated with significant executive functioning problems (Barkley, 2012, 2013; Bauermeister et al., 2012; Wahlstedt and Bohlin, 2010). SCT has been linked to impairments in working memory (McBurnett et al., 2014), although this study was conducted in a primarily ADHD sample, which may bias the results somewhat (Barkley, 2014). SCT appears to be somewhat less heritable than ADHD and, while sharing some genetic liability with ADHD, also has unique genetic contributions as well as greater unique environmental influences on the trait(s) (Moruzzi et al., 2014). The three symptom dimensions (ADHD-inattentive, ADHD-hyperactive/impulsive, and SCT) are observed to be distinct yet partly correlated at the genetic level of analysis (Moruzzi et al., 2014).
SCT and ADHD symptoms differ in their association with various external factors, including demographics, comorbidities and impairments. ADHD symptoms demonstrate age and sex differences, whereas SCT symptoms do not, but may be more associated with low socioeconomic status (Barkley, 2013; Garner et al., 2010). Unlike ADHD, SCT is not comorbid with oppositional defiant disorder (Lee et al., 2014), but is more associated with internalizing symptoms such as anxiety and depression (Becker et al., 2014). SCT is also notably associated with unique social impairments, especially negative social preference and peer impairment, even when controlling for other psychopathologies, baseline peer functioning and demographics (Becker et al., 2014).
The current study investigated whether the differences between ADHD and SCT can be extended to observable differences in brain function in a group of adolescents with ADHD. To our knowledge this is the first study examining the neural signature associated with SCT symptoms in a group of individuals with ADHD. We tested the relationship between cognitive control-related fMRI activation during a cued flanker task and both SCT and ADHD symptoms in youth with ADHD compared with typically developing (TD) youth (12–17 years old). Specifically, as a first step we investigated differences in behavior as well as brain activity related to cognitive control on the flanker task between the overall ADHD and TD groups. To probe the contribution of SCT symptoms to neural differences we examined the correlation between activation and parent ratings of (1) SCT symptoms and (2) ADHD inattentive symptoms. We hypothesized that these symptom types would be associated with different patterns of activation.
2. Materials and methods
2.1. Participants
Thirteen TD adolescents and sixteen adolescents with either combined type ADHD (involving both inattention and hyperactivity/impulsivity, n = 7) or primarily inattentive type ADHD (n = 9) were included in analyses (an additional ten participants were excluded from analysis due to excessive movement: 2 TD, 8 ADHD) after both informed, written parental consent and written assent by all participants. The study was approved by the Institutional Review Board of the University of California Davis. Participants were 12–17 years old (see Table 1 for participant information).
Table 1.
Demographic information.
| TD | ADHD | |
|---|---|---|
| n | 13 | 16 |
| Age | 15.2 (1.7) | 15.1 (2.1) |
| Female | 54% | 38% |
| IQ | 116 (10) | 114 (13) |
| Left-handed | 0% | 6% |
| Conners' inattentive score* | 42 (2) | 73 (11) |
| Conners' hyperactive–impulsive score* | 44 (2) | 65 (17) |
| Conners' DSM inattentive score* | 42 (2) | 77 (10) |
| Conners' DSM hyperactive–impulsive score* | 45 (4) | 64 (15) |
| SCT score* | 0.8 (1.0) | 11.2 (5.1) |
| History of ADHD medication | N/A | 81% |
Significant group difference at p < .001
Participants were excluded if they met any of the following criteria: (a) having an IQ score ≤80; (b) evidence of a DSM-IV-TR math/reading disorder or academic learning disability; (c) history of head trauma, neurological disorder or major medical problem; (d) prescribed psychoactive medication besides stimulant medication in the ADHD group; and (e) meeting DSM-IV-TR criteria for any other Axis I diagnosis besides ADHD (in the ADHD group) or oppositional defiant disorder. Typically developing children were excluded if they had a t-score of 65 or higher on Conners' parent reports. They were also excluded if they had a biological sibling with ADHD and all participants were excluded if they had a first degree family member with any Axis I disorder (except ADHD in the ADHD group). Participants with ADHD were included if they had a Conners' Total ADHD score of 65 or greater on the parent report forms in conjunction with a significant indication of ADHD on the structured clinical interview. Of the 16 ADHD participants, 13 had a history of taking stimulant medication, all of whom were also currently taking medication (none had a history of taking non-stimulant medication).
2.2. Diagnosis and assessments
Prior to enrollment in the study, demographic information and initial eligibility information were collected using a telephone screening interview. A parent completed Conners' Parent Rating Scale-Revised: Long Version (CPRS-R:L) (Conners, 1997) regarding behavior problems over the past month. Attempts were made to collect teacher ratings to substantiate diagnoses with all participants; 23 of the participants had at least one of their teachers return the rating scales. Many of the ADHD children prescribed medication were taking it during school hours; thus teacher ratings may reflect medicated behavior and were therefore only used as a screening tool.
The parent version of the computerized Diagnostic Interview for Children and Adolescents (DICA-IV) (Reich, 2000) provided additional diagnostic information to confirm the ADHD diagnosis and screen for other Axis I psychiatric disorders. To ensure that participants with sub-threshold combined type ADHD were not included in the inattentive subtype, participants were required to display three or fewer hyperactive and/or impulsive symptoms to be included in the inattentive subtype group. An experienced licensed psychologist conducted follow-up interviews with parents when clarification was needed to confirm diagnosis or its absence. The Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) assessed participants' intellectual abilities, except for two TD participants who received the Wechsler Intelligence Scale for Children (WISC-IV) (Wechsler, 2003). The Letter–Word Identification and Calculation scales of the Woodcock–Johnson Tests of Achievement — Third Edition NU (WJ-III NU) (Woodcock et al., 2001) assessed reading and mathematical performance to detect learning disabilities. Evidence of a math/reading disorder was considered present if a child performed below 80 on an achievement test and if there was a 1.5 SD or greater difference between IQ and WJ-III scores.
Two licensed psychologists with extensive experience in ADHD (JBS & J. Faye Dixon) evaluated all participants to determine eligibility and diagnosis. Information from the CPRS-R:L, the parent's DICA-IV responses, observations and any supplemental interview or information (i.e., teacher ratings, previous report cards or psychological evaluations) were used to determine whether each participant met Diagnostic and Statistical Manual of Mental Disorders, text revision (DSM-IV-TR) (Conners, 1997) criteria for ADHD combined or inattentive subtype. The presence of other psychiatric diagnoses was based on the DICA-IV and follow-up clinical interviews.
2.3. fMRI acquisition
2.3.1. Scan parameters
A Siemens 3 T TIM Trio MRI scanner (Siemens Medical, Erlangen, Germany) was used to acquire both anatomical and functional images. Stimulant medication was withheld for 24 h before fMRI measurements. The cued flanker task was completed in 8 separate fMRI runs, each of which contained between 145 and 163 functional T2*-weighted echoplanar images. Each run was collected with the following parameters: slice thickness voxel size = 3.4 mm isotropic, 36 slices, TR = 2 s, TE = 25 ms, flip angle = 90°, matrix 64 × 64, and FOV = 220 mm. In addition, an MPRAGE anatomical scan was collected with the following parameters: TR = 2.2 s, TE = 4.77 ms, FOV = 256 mm, matrix = 256 × 256, flip angle = 7°, slice thickness = 1 mm, and 192 slices.
2.3.2. Cued flanker task
Participants performed a cued variant of the Eriksen flanker paradigm (Fig. 1) (Eriksen and Eriksen, 1974) with stimuli presented using E-Prime (Psychology Software Tools, Inc., Sharpsburg, PA) and a mirror system with which participants viewed visual stimuli on a projection screen. Each trial began with a fixation (500 ms) followed by a cue (1000 ms), of which there were three types: null cue, response preparation cue, and warning cue. Each cue consisted of a pair of blue and/or yellow cartoon hands with the color providing cue information. For each participant, either a blue hand or a yellow hand represented a cue, while the other color represented a non-cue (the color meaning was counterbalanced across participants). In the null cue, there was no information about the following flanker (both hands were the non-cue color). In the response preparation cue one of the two hands was the cue color, signaling which hand was likely to be the correct response for the upcoming target. Invalid response preparation cues (16%) were included to encourage participants to attend to and process the central target arrow stimulus. To further encourage participants to attend to cues, 27.7% of response preparation cues were followed by a stimulus array made up only of plus signs (+++++). On these trials, participants were instructed to respond as the cue had indicated. In the warning cue, both hands were the cue color, and this cue informed participants with 100% accuracy that the following trial would be incongruent.
Fig. 1.
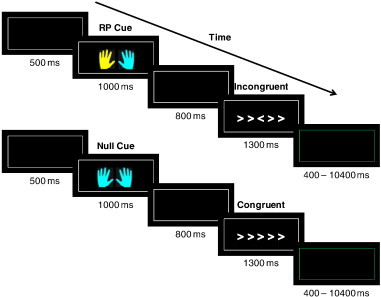
Two trials from our modified flanker task. In this example yellow is the informative color. The top trial demonstrates the response preparation cue. The left hand in yellow indicates that a left hand response will be required to the following flanker. The bottom trial demonstrates the null cue. The null cue provides no information about the nature of the following flanker or the response that will be required. All stimuli were surrounded by a white border and participants were instructed to restrict their gaze within it; the inter-trial interval was indicated by a color change of the surrounding border from white to green.
Following the cue and a fixation (800 ms), participants were presented with the flanker stimulus (1300 ms). Participants were instructed to identify the orientation of the central arrow symbol while ignoring the orientation of the flanking arrows, and they indicated their response by pressing a button with the corresponding hand. The instruction to the participants emphasized both speed and accuracy. Trials could be congruent (five arrows all facing the same direction), incongruent (five arrows in which the central arrow is facing the opposite direction of the four flanking arrows), or neutral (an arrow in the middle with four flanking plus signs). Trials were separated by a variable inter-trial interval (400–10,400 ms).
There were a total of 110 neutral, 110 congruent and 160 incongruent stimuli. Null cues preceded 76.4% of neutral and 76.4% of congruent trials; the remaining neutral and congruent trials were preceded by response preparation cues. For incongruent trials, 47.5% were preceded by null cues and response preparation and 26.25% by warning cues. No repetitions of identical stimuli occurred on adjacent trials to diminish potential priming effects (Mayr et al., 2003). Participants were trained on the task prior to the imaging session to ensure that they understood all the conditions. The training also included an auditory cue which encouraged speedy responding (tone if the RT exceeded 600 ms). We included this manipulation in the practice to maximize response conflict during the flanker paradigm. Both speed and accuracy were emphasized during the imaging task.
2.4. Statistical analysis
2.4.1. Behavioral and clinical data
Independent t-tests examined any potential differences between the ADHD and TD groups on clinical variables.
Behavioral data analysis focused on the incongruent flanker stimuli. Analyses of clinical and behavioral data were performed using SPSS version 22 (IBM, Armonk, NY). A 2 (group: TD; ADHD) × 3 (cue type: null; response preparation; warning) ANOVA examined the main effect of cues on performance (separate ANOVAs for correct responses and RT) as well as any potential group × cue type interactions.
2.4.2. Imaging data
fMRI data analyses were performed using AFNI (Cox, 1996). The first three volumes from each scan were discarded by the scanner to allow for equilibrium effects. For each functional run, volumes were slice time corrected and registered to a representative volume of the fourth run (closest in time to the structural scan) to compensate for small head movements. The representative volume was identified by the following criteria: the median volume of the longest window of time points with the lowest number of outlier voxels. Each run underwent non-brain removal using brain extraction tool (BET; Smith, 2002) before being aligned to each individual's T1-weighted structural MR image and transformed to Montreal Neurological Institute (MNI) space. Registration was carried out using FMRIB's Linear Image Registration Tool (FLIRT; Jenkinson et al., 2002; Jenkinson and Smith, 2001). Each run was then aligned to each individual's T1-weighted structural MR image and transformed to Montreal Neurological Institute (MNI) space. A 4-mm full-width at half-maximum (FWHM) Gaussian filter was applied to each functional dataset and data were normalized to a mean of 100.
Following preprocessing, general linear model analyses fit hemodynamic responses with a boxcar activation function with onset given by trial the cue onset and duration of 4 s (the length of each trial incorporating the cue and stimulus periods). Regressors were included for: (1) correct null cued incongruent trials, (2) correct response preparation cued incongruent trials, (3) correct warning cued incongruent trials, (4) correct congruent and neutral trials collapsed across cue type and (5) error trials collapsed across cue and flanker type. Due to the nature of the task (providing cues to prepare an upcoming response) and based upon the literature and usual activation patterns for this type of paradigm (Chambers et al., 2007; Fassbender et al., 2006; Vaidya et al., 2005; van 't Ent et al., 2009; Wager et al., 2005) we used a mask comprised of the bilateral superior parietal lobule (SPL), agranular premotor cortex, caudate and thalamus and performed a region of interest (ROI) analysis. Cortical regions were determined based on the Jülich Histological Atlas (Geyer, 2004; Scheperjans et al., 2008) and subcortical regions were based on the Harvard–Oxford Subcortical Structural Atlas (Frazier et al., 2005), both in FSL (Jenkinson et al., 2012). We used small volume cluster-threshold correction to maintain the overall p value at 0.05 and determine any significant effects within this mask. This method utilizes Monte-Carlo simulations to determine the minimum cluster size required within the volume of interest, at the voxel-wise threshold, in order to maintain an overall probability of 0.05 of a significant cluster surviving by chance. We used t-tests in AFNI to investigate differences between ADHD versus TD participants for the following contrasts: (1) correct null cued incongruent trials versus fixation, (2) correct response preparation cued trials versus fixation and (3) correct warning cued incongruent trials versus fixation.
We conducted a conjunction analysis to examine regions of overlap between the TD and ADHD maps of all the correct incongruent trials. The conjunction analysis examined brain regions which were activated by all three cue (response preparation, null and warning cue) conditions for each group separately and was derived by performing an intersection operation on the individual brain maps for each of the conditions, implemented using the 3dcalc function in AFNI. All individual condition maps were generated using an overall p value of 0.05 within our volume of interest paired with a cluster size criterion that sets the overall p value of 0.05 for each condition prior to the conjunction analysis.
We then conducted correlational analyses with SCT scores and inattentive scores from the ADHD rating scales for these same three contrasts. We used multiple regression analysis via AFNI's 3dRegAna script to examine correlations between brain activity and SCT symptoms. Analyses were carried out with and without controlling for inattentive symptoms. Maps were thresholded and corrected for multiple comparisons using identical cluster-threshold correction to those described above.
3. Results
3.1. Behavioral and clinical data
TD adolescents did not differ from the ADHD group in terms of accuracy on the flanker paradigm across different cues and flanker conditions (see Supplementary Table 1). The ADHD group did display significantly longer RTs across most conditions (see Supplementary Table 2). Thus the ADHD group responded more slowly in general despite the absence of group differences in correct responses.
3.1.1. Cue effect on performance
An ANOVA revealed no main effect of cue type for percent correct responses (p = 0.38) but a main effect for RT (F(2,54) = 41.90, p < 0.0001; see Fig. 2). There was no significant incongruent type × group interaction for correct responses (p = 0.2) or RT (p = 0.09). There was a main effect of group for RT (F(1,27) = 7.61, p = 0.01) but not percent correct (p = 0.30), such that the TD group was faster than the ADHD group in general. As Fig. 2 shows, the response preparation cue resulted in the fastest RTs in both TD and ADHD groups. Thus, the response preparation cues resulted in a similar RT benefit in both groups.
Fig. 2.
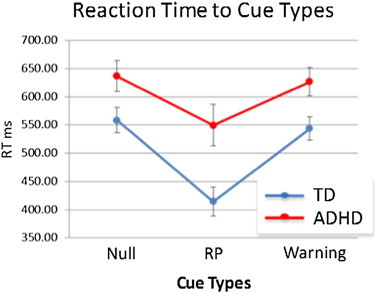
Reaction times to null, response preparation and warning cues. Both groups benefited from response preparation (RP) cues in the form of faster RT.
3.2. fMRI data
3.2.1. Cognitive control-related activity in TD and ADHD groups
Within-group analysis in the TD group in null cued, response preparation cued and warning cued conditions revealed a number of somewhat overlapping brain regions (see Fig. 3 for conjunction map). Activated regions include regions commonly observed in the flanker paradigm (e.g., Fassbender et al., 2006), such as the bilateral lateral frontal and parietal regions, supplementary motor area (SMA) and basal ganglia. Additional activity associated with the null cued incongruents was observed in extended areas of the left caudate and the bilateral thalamus.
Fig. 3.
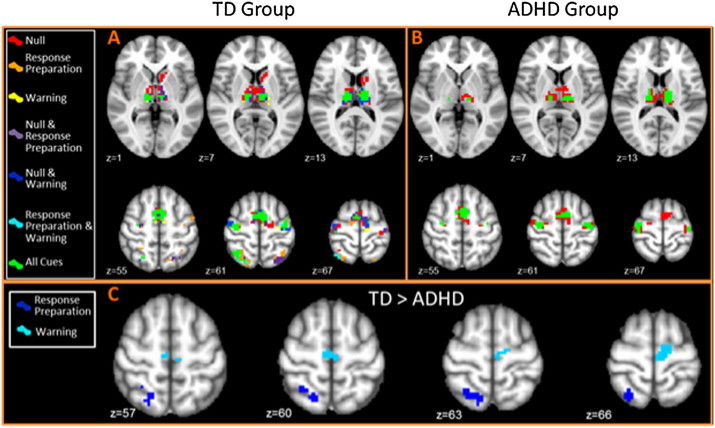
Top panels: conjunction maps displaying brain activity during correct incongruent trials. A) TD group and B) ADHD group different red, orange and yellow colors represent the activation to correct incongruent trials during the different cue conditions; red = null cues; orange = response preparation cues; and yellow = warning cues. The remaining colors represent regions of overlap between these conditions and overlap between them. Green represents areas of overlap between activation during correct incongruent stimuli during all the cue types. Less differentiation between cue types is observed in the ADHD group. Regions of the basal ganglia, bilateral frontal and medial frontal regions were associated with uncued trials only (red) with activation in similar regions all cue types (in green). Bottom panel: C) between group activation: the TD group displayed greater activity than the ADHD group during response preparation cued incongruents in R SPL (dark blue) and during warning cued trials in the SMA (light blue).
Within-group analyses in the ADHD group revealed a group of similar activations, with the exception of the bilateral parietal cortex; the ADHD group did not display any significant clusters of activation in this region. Furthermore, the ADHD group displayed more overlap of their activation between incongruent conditions, with a relative failure to display any specialization of functional activity between the response preparation and warning cue conditions at this threshold (see Fig. 3, conjunction map).
Between-group analyses revealed no significant between-group differences in the null cued condition. The TD group displayed significantly greater activity in the right SPL in the response preparation cued condition and in the supplementary motor area (SMA) in the warning cued incongruent stimuli compared to the ADHD group. The ADHD group revealed no regions of greater activity compared to the TD group.
3.2.2. Correlations between cognitive control-related activity and SCT and inattentive symptoms in the ADHD group alone
Activation in the left SPL to null cued trials correlated negatively with SCT symptoms such that individuals with more SCT symptoms displayed less activity in this region. This was found with and without controlling for inattention (although the area was slightly larger when controlling for inattention; see Fig. 4).
Fig. 4.
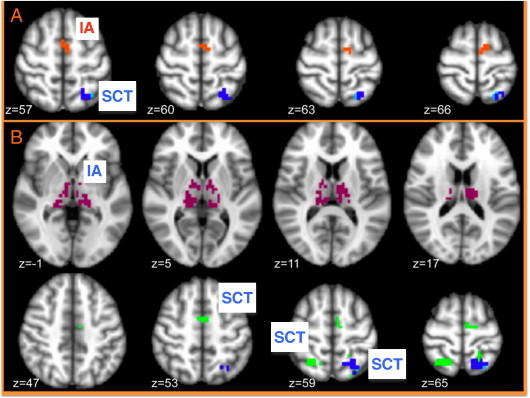
Significant correlations with symptoms. IA = inattentive symptoms, SCT = sluggish cognitive tempo symptoms. Red text labels indicate positive correlations, blue text labels indicate negative correlations. A) Significant correlations with symptoms for null cue trials. Dark blue cluster indicates a negative correlation with SCT in L SPL with or without controlling for inattentive symptoms. Light blue voxels are only significant when we control for inattentive symptoms. The orange cluster represents a positive correlation with inattentive symptoms in SMA. B) Significant correlations with symptoms for cued trials. Green clusters indicate negative correlations with SCT in bilateral SPL and SMA when not controlling for inattentive symptoms. Dark blue is a negative correlation with SCT in L SPL with or without controlling for inattentive symptoms. Light blue voxels represent a negative correlation with SCT when controlling for inattentive symptoms in L SPL. The violet clusters are negative correlations with inattentive symptoms in bilateral thalamus.
A region in the SMA displayed a positive correlation with inattentive symptoms, such that individuals with more inattentive symptoms displayed greater activity in the SMA to null cued trials.
For response preparation cued trials, there was a negative correlation between activity in the bilateral SPL and SMA and SCT symptoms without controlling for inattention. When controlling for inattention only the left SPL remained and again the area was slightly larger (see Fig. 4).
Finally, for response preparation cued trials, activity in the bilateral thalamus correlated positively with inattentive symptoms.
4. Discussion
In this study we examined the unique contributions of SCT symptoms separate from inattentive symptoms to brain activity associated with cognitive control in a group of adolescents with ADHD. We examined cue-related impairments associated with the preparation of an up-coming motor response and the recruitment of attention resources in anticipation of response conflict (Correa et al., 2009; Sohn et al., 2007). In both the ADHD and control groups response preparation cues improved performance in the form of faster RT. Incongruent stimuli activated brain regions including the bilateral lateral frontal cortex, supplementary motor area (SMA) and basal ganglia in both groups. However conjunction analyses suggested more specialization of brain activity to the different cue conditions in the TD group. The TD group displayed significantly greater activity than the ADHD group in the right SPL to the response preparation incongruent stimuli and in the SMA to warning cued incongruents. Correlational analyses suggested that adolescents in the ADHD group with more SCT symptoms had less activity in the left SPL during null and response preparation cued incongruent stimuli when controlling for inattention. Individuals with more inattentive symptoms displayed more activity in the SMA to null cued incongruents and in the bilateral thalamus during response preparation cued incongruents.
The response preparation cue resulted in better performance in both groups in that all participants responded more quickly to the response preparation cued incongruent stimuli. We interpret this as indicating a performance benefit following the response preparation cue as our previous work in a larger group using the same paradigm showed a significant speeding of RT accompanied by more percent correct responses to the response preparation compared to null cued incongruents (Mazaheri et al., 2014). The effect of the warning cues appears more ambiguous with RTs being more similar to the null cued incongruents. This suggests that the response preparation cue resulted in a performance benefit for both groups whereas the warning cue may have resulted in a marginal benefit.
Brain activity associated with the incongruent cues in general included activation associated with cognitive control and motor preparation in regions such as the bilateral premotor cortex, SMA and bilateral basal ganglia in both groups. In the TD group, within-group analyses also revealed additional activations in the bilateral parietal cortex. Between-group analyses revealed that the TD group had significantly more activity in the right SPL during response preparation cues. The parietal cortex is a brain region frequently implicated in ADHD impairments (Castellanos and Proal, 2012; Makris et al., 2009). Altered activity in the parietal lobe in adults with ADHD has been associated with impaired attention (Cubillo et al., 2010; Rubia et al., 2007; Schneider et al., 2010; Tamm et al., 2006), attention shifting (Morein-Zamir et al., 2014), response inhibition (Dickstein et al., 2006; Rubia, 2011), and working memory (Burgess et al., 2010) and is linked to an increase in hyperactive symptoms (Congdon et al., 2014). In children with ADHD, less fronto-parietal activity compared to typically developing peers is the most common functional imaging finding (Cortese et al., 2012).
Between-group analysis also revealed a region of significantly greater activity in the SMA to the warning cued incongruents in the TD group compared to the ADHD group. Increased activity in the medial PFC has previously been noted in preparation for response conflict (Sohn et al., 2007); however this activity was in the ACC and not the SMA. The SMA has been associated with response inhibition (Nee et al., 2007; Simmonds et al., 2008), motor preparation (Deiber et al., 1996; Lee et al., 1999; Romo and Schultz, 1992) and motor planning (Braver et al., 2001; Kawashima et al., 1996; Nagahama et al., 1999). In a recent study, adults with ADHD displayed hypo-active cue-related preparatory activity in the SMA compared to their peers (Clerkin et al., 2013). However, a meta-analysis of response inhibition studies showed SMA hypoactivity only in children with ADHD and not in adults (Hart et al., 2013). Data from the ADHD-200 multisite sample, a large-scale resting-state data acquisition project, revealed that in 757 participants, there was altered connectivity, in the form of inadequate anti-correlation between the posterior cingulate cortex of the default attention network and the SMA and right anterior insula of the ventral attention network (Sripada et al., 2014). The authors suggested that this weakened anti-correlation might be a key locus of dysfunction in ADHD.
Aside from the hypoactivity in the parietal cortex and the SMA in the ADHD group compared to the control group, the ADHD group also tended to display less specialization of brain activity. Our prior research (Fassbender et al., 2011) also suggests a lack of specificity of brain activation in ADHD in response to differing cognitive demands. This may suggest a lack of cognitive flexibility in this group, in the context of this task, a lack of differentiation between differing cue conditions, although here we did not observe any impact on behavioral performance.
When examining SCT symptoms in the ADHD group, results suggested that in response to null and response preparation cued incongruents, adolescents with more SCT symptoms tended to display less activity in the left SPL, controlling for inattentive symptoms. The parietal cortex has also been associated with reorienting attention (Corbetta et al., 2008), a process that has shown to be disrupted in ADHD (Morein-Zamir et al., 2014; Stevens et al., 2007). During a counting Stroop paradigm, children with ADHD displayed less activity in the left SPL than their typically developing peers (Fan et al., 2014). We found that activity in the left SPL during null and response preparation cued incongruents was associated with SCT symptoms. This suggests that the relationship is not necessarily limited to the preparation of a motor response but in response to the incongruent stimuli in general. In a previous study examining preparatory activity in a flanker paradigm in healthy adults, the left SPL was active both during the cue periods and during correct responses to the incongruents themselves, suggesting that this area serves a general attention role, rather than something specific to preparation of a response (Fassbender et al., 2006). We suggest that reorienting of attention may be impaired in individuals with elevated SCT ratings, however with the caveat that this did not translate to poor performance. It is possible that our inclusion of “catch” trials within the current paradigm somewhat improved performance in the adolescents with ADHD.
There was a positive correlation between activity in the SMA to null cued incongruents and a negative correlation in the bilateral thalamus to response preparation cued incongruents. It is possible that in adolescents with greater inattention, the SMA must be strongly engaged to compensate for poor attention to the incongruent stimuli. Reduced cue-related activity in the thalamus has been observed in adults with ADHD (Clerkin et al., 2013). Hypoactivity in the thalamus, associated with target detection and directed attention, has been noted in adolescents with ADHD (Tamm et al., 2006). Therefore it is possible that in individuals with more inattentive symptoms, less attention is given to the response preparation cue. This is in line with our previous research, which shows reduced processing of the response preparation cue in adolescents with ADHD inattentive subtype (Mazaheri et al., 2014). Treatment for ADHD with stimulant medication (i.e., methylphenidate) is also associated with increased activity in the thalamus during cognitive tasks (Schweitzer et al., 2004), and thus, stimulants may partially improve general attention via the thalamus. The thalamus, along with the medial prefrontal cortex and basal ganglia has also been associated with integrating information from different sources (Bosch-Bouju et al., 2013; Nagano-Saito et al., 2014) and mediating motivation, planning and goal-directed behavior (Haber and Calzavara, 2009).
There are a number of potential limitations that may somewhat limit the generalizability of our findings. We did not enroll subjects based upon SCT symptoms alone; we enrolled subjects based upon ADHD diagnoses. However, we do find differential brain activity related to inattention symptoms and SCT symptoms, even in this sample. This makes the results from this study a compelling first step in investigating the neural substrates of SCT. It is possible that our correlation analyses in such a relatively small sample may have led to somewhat inflated p values due to limited power therefore the results should be interpreted keeping that in mind. This sample may have fewer affective disorder symptoms than the typical SCT population, as volunteers with other Axis I disorders were excluded, however, this may be an advantage in this study as the authors are more likely to identify neural patterns associated with the SCT symptoms than affective symptoms. Future investigations should focus on examining neural activity related to SCT symptoms independent of an ADHD diagnosis and compare this group to ADHD inattentive and combined subtypes. This would shed further light on the question as to whether or not SCT represents an independent disorder.
5. Conclusion
The results from this study suggest that cognitive-control-related brain activity is distinctly related to SCT symptoms and inattentive symptoms within a group of adolescents with ADHD. We argue that this represents a first step towards defining SCT as having a distinct neural signature from more traditionally defined ADHD symptomatology. A tendency in participants with more SCT symptoms to activate the left SPL less may reflect impaired reorientation or shifting of attention. An association between inattentive symptoms and more activity in the SMA and less in the bilateral thalamus during response preparation may suggest a general deficit in response preparation with increasing inattention; however this may be due to impoverished processing of the cue itself. Alternatively, it may represent impairment in integrating information or in mediating task set maintenance. This study is the first to demonstrate dissociation between inattention- and SCT-related symptoms in ADHD in terms of cue-related brain activity.
Acknowledgments
The authors would like to thank all the participants and their families for their time, and Tadeus Hartanto, Lauren Boyle, Erin Calfee and Dorothy Yip for their assistance with running the research participants. Thanks to J. Faye Dixon and Ruby Bhangoo for the assessment of the research participants. We also thank Russell Barkley for input on the manuscript and Cameron Carter, G. Ron Mangun and Silvia Bunge for advice on the paradigm design. Funding for this study was provided by the Klingenstein Third Generation Foundation, ADHD fellowship (C.F.), the Children's Miracle Network (J.B.S.) and NIMH 5 R01 MH066310 (J.B.S).
Appendix A. Supplementary data
Supplementary tables.
References
- Barkley R.A. Distinguishing sluggish cognitive tempo from attention-deficit/hyperactivity disorder in adults. J. Abnorm. Psychol. 2012;121(4):978–990. doi: 10.1037/a0023961. 21604823 [DOI] [PubMed] [Google Scholar]
- Barkley R.A. Distinguishing sluggish cognitive tempo from ADHD in children and adolescents: executive functioning, impairment, and comorbidity. J. Clin. Child Adolesc. Psychol. 2013;42(2):161–173. doi: 10.1080/15374416.2012.734259. 23094604 [DOI] [PubMed] [Google Scholar]
- Barkley R.A. Sluggish cognitive tempo (concentration deficit disorder?): current status, future directions, and a plea to change the name. J. Abnorm. Child Psychol. 2014;42(1):117–125. doi: 10.1007/s10802-013-9824-y. 24234590 [DOI] [PubMed] [Google Scholar]
- Bauermeister J.J., Barkley R.A., Bauermeister J.A., Martínez J.V., McBurnett K. Validity of the sluggish cognitive tempo, inattention, and hyperactivity symptom dimensions: neuropsychological and psychosocial correlates. J. Abnorm. Child Psychol. 2012;40(5):683–697. doi: 10.1007/s10802-011-9602-7. 22179974 [DOI] [PubMed] [Google Scholar]
- Becker S.P., Marshall S.A., McBurnett K. Sluggish cognitive tempo in abnormal child psychology: an historical overview and introduction to the special section. J. Abnorm. Child Psychol. 2014;42(1):1–6. doi: 10.1007/s10802-013-9825-x. 24272365 [DOI] [PubMed] [Google Scholar]
- Bosch-Bouju C., Hyland B.I., Parr-Brownlie L.C. Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Front. Comput. Neurosci. 2013;7:163. doi: 10.3389/fncom.2013.00163. 24273509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11(9):825–836. doi: 10.1093/cercor/11.9.825. 11532888 [DOI] [PubMed] [Google Scholar]
- Burgess G.C., Depue B.E., Ruzic L., Willcutt E.G., Du Y.P., Banich M.T. Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;67(7):632–640. doi: 10.1016/j.biopsych.2009.10.036. 20060961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C.L., Mann M. Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, predominantly inattentive type. J. Clin. Child Adolesc. Psychol. 2002;31(1):123–129. doi: 10.1207/S15374424JCCP3101_14. 11845644 [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn. Sci. (Regul. Ed.) 2012;16(1):17–26. doi: 10.1016/j.tics.2011.11.007. 22169776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.D., Bellgrove M.A., Gould I.C., English T., Garavan H., McNaught E., Kamke M., Mattingley J.B. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J. Neurophysiol. 2007;98(6):3638–3647. doi: 10.1152/jn.00685.2007. 17942624 [DOI] [PubMed] [Google Scholar]
- Clerkin S.M., Schulz K.P., Berwid O.G., Fan J., Newcorn J.H., Tang C.Y., Halperin J.M. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am. J. Psychiatry. 2013;170(9):1011–1019. doi: 10.1176/appi.ajp.2013.12070880. 24030612 [DOI] [PubMed] [Google Scholar]
- Congdon E., Altshuler L.L., Mumford J.A., Karlsgodt K.H., Sabb F.W., Ventura J., McGough J.J., London E.D., Cannon T.D., Bilder R.M., Poldrack R.A. Neural activation during response inhibition in adult attention-deficit/hyperactivity disorder: preliminary findings on the effects of medication and symptom severity. Psychiatry Res. 2014;222(1–2):17–28. doi: 10.1016/j.pscychresns.2014.02.002. 24581734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K. Manual for the Conners' Rating Scales-Revised. Multi-Health Systems; North Tonawanda: 1997. [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. 18466742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A., Rao A., Nobre A.C. Anticipating conflict facilitates controlled stimulus-response selection. J. Cogn. Neurosci. 2009;21(8):1461–1472. doi: 10.1162/jocn.2009.21136. 18823248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012;169(10):1038–1055. doi: 10.1176/appi.ajp.2012.11101521. 22983386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. 8812068 [DOI] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Ecker C., Giampietro V., Taylor E., Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J. Psychiatr. Res. 2010;44(10):629–639. doi: 10.1016/j.jpsychires.2009.11.016. 20060129 [DOI] [PubMed] [Google Scholar]
- Deiber M.P., Ibañez V., Sadato N., Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J. Neurophysiol. 1996;75(1):233–247. doi: 10.1152/jn.1996.75.1.233. 8822554 [DOI] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. 17073984 [DOI] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16(1):143–149. [Google Scholar]
- Fan L.Y., Gau S.S., Chou T.L. Neural correlates of inhibitory control and visual processing in youths with attention deficit hyperactivity disorder: a counting Stroop functional MRI study. Psychol. Med. 2014;44(12):2661–2671. doi: 10.1017/S0033291714000038. 24451066 [DOI] [PubMed] [Google Scholar]
- Fassbender C., Foxe J.J., Garavan H. Mapping the functional anatomy of task preparation: priming task-appropriate brain networks. Hum. Brain Mapp. 2006;27(10):819–827. doi: 10.1002/hbm.20223. 16541457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Schweitzer J.B., Cortes C.R., Tagamets M.A., Windsor T.A., Reeves G.M., Gullapalli R. Working memory in attention deficit/hyperactivity disorder is characterized by a lack of specialization of brain function. PLOS One. 2011;6(11):e27240. doi: 10.1371/journal.pone.0027240. 22102882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Chiu S., Breeze J.L., Makris N., Lange N., Kennedy D.N., Herbert M.R., Bent E.K., Koneru V.K., Dieterich M.E., Hodge S.M., Rauch S.L., Grant P.E., Cohen B.M., Seidman L.J., Caviness V.S., Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry. 2005;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. 15994707 [DOI] [PubMed] [Google Scholar]
- Garner A.A., Marceaux J.C., Mrug S., Patterson C., Hodgens B. Dimensions and correlates of attention deficit/hyperactivity disorder and sluggish cognitive tempo. J. Abnorm. Child Psychol. 2010;38(8):1097–1107. doi: 10.1007/s10802-010-9436-8. 20644992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A.A., Peugh J., Becker S.P., Kingery K.M., Tamm L., Vaughn A.J., Ciesielski H., Simon J.O., Loren R.E., Epstein J.N. Does sluggish cognitive tempo fit within a bi-factor model of ADHD? J. Atten. Disord. 2014 doi: 10.1177/1087054714539995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv. Anat. Embryol. Cell Biol. 2004;174:I–VIII. doi: 10.1007/978-3-642-18910-4. 14750415 [DOI] [PubMed] [Google Scholar]
- Haber S.N., Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res. Bull. 2009;78(2–3):69–74. doi: 10.1016/j.brainresbull.2008.09.013. 18950692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. J.A.M.A. Psychiatry. 2013;70(2):185–198. doi: 10.1001/jamapsychiatry.2013.277. 23247506 [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C.L., Nigg J.T., Carr T.H. Deficient attention is hard to find: applying the perceptual load model of selective attention to attention deficit hyperactivity disorder subtypes. J. Child Psychol. Psychiatry. 2005;46(11):1211–1218. doi: 10.1111/j.1469-7610.2005.00410.x. 16238668 [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. 12377157 [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. 21979382 [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. 11516708 [DOI] [PubMed] [Google Scholar]
- Kawashima R., Satoh K., Itoh H., Ono S., Furumoto S., Gotoh R., Koyama M., Yoshioka S., Takahashi T., Takahashi K., Yanagisawa T., Fukuda H. Functional anatomy of GO/NO-GO discrimination and response selection — a PET study in man. Brain Res. 1996;728(1):79–89. 8864300 [PubMed] [Google Scholar]
- Lee K.M., Chang K.H., Roh J.K. Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage. 1999;9(1):117–123. doi: 10.1006/nimg.1998.0393. 9918733 [DOI] [PubMed] [Google Scholar]
- Lee S., Burns G.L., Snell J., McBurnett K. Validity of the sluggish cognitive tempo symptom dimension in children: sluggish cognitive tempo and ADHD-inattention as distinct symptom dimensions. J. Abnorm. Child Psychol. 2014;42(1):7–19. doi: 10.1007/s10802-013-9714-3. 23325455 [DOI] [PubMed] [Google Scholar]
- Makris N., Biederman J., Monuteaux M.C., Seidman L.J. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev. Neurosci. 2009;31(1–2):36–49. doi: 10.1159/000207492. 19372685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U., Awh E., Laurey P. Conflict adaptation effects in the absence of executive control. Nat. Neurosci. 2003;6(5):450–452. doi: 10.1038/nn1051. 12704394 [DOI] [PubMed] [Google Scholar]
- Mazaheri A., Fassbender C., Coffey-Corina S., Hartanto T.A., Schweitzer J.B., Mangun G.R. Differential oscillatory electroencephalogram between attention-deficit/hyperactivity disorder subtypes and typically developing adolescents. Biol. Psychiatry. 2014;76(5):422–429. doi: 10.1016/j.biopsych.2013.08.023. 24120092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurnett K., Pfiffner L.J., Frick P.J. Symptom properties as a function of ADHD type: an argument for continued study of sluggish cognitive tempo. J. Abnorm. Child Psychol. 2001;29(3):207–213. doi: 10.1023/a:1010377530749. 11411783 [DOI] [PubMed] [Google Scholar]
- McBurnett K., Villodas M., Burns G.L., Hinshaw S.P., Beaulieu A., Pfiffner L.J. Structure and validity of sluggish cognitive tempo using an expanded item pool in children with attention-deficit/hyperactivity disorder. J. Abnorm. Child Psychol. 2014;42(1):37–48. doi: 10.1007/s10802-013-9801-5. 24258302 [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S., Dodds C., van Hartevelt T.J., Schwarzkopf W., Sahakian B., Müller U., Robbins T. Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Hum. Brain Mapp. 2014;35(10):5141–5152. doi: 10.1002/hbm.22539. 24819224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi S., Rijsdijk F., Battaglia M. A twin study of the relationships among inattention, hyperactivity/impulsivity and sluggish cognitive tempo problems. J. Abnorm. Child Psychol. 2014;42(1):63–75. doi: 10.1007/s10802-013-9725-0. 23435481 [DOI] [PubMed] [Google Scholar]
- Nagahama Y., Okada T., Katsumi Y., Hayashi T., Yamauchi H., Sawamoto N., Toma K., Nakamura K., Hanakawa T., Konishi J., Fukuyama H., Shibasaki H. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10(2):193–199. doi: 10.1006/nimg.1999.0451. 10417251 [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A., Martinu K., Monchi O. Function of basal ganglia in bridging cognitive and motor modules to perform an action. Front. Neurosci. 2014;8:187. doi: 10.3389/fnins.2014.00187. 25071432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee D.E., Wager T.D., Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn. Affect. Behav. Neurosci. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. 17598730 [DOI] [PubMed] [Google Scholar]
- Penny A.M., Waschbusch D.A., Klein R.M., Corkum P., Eskes G. Developing a measure of sluggish cognitive tempo for children: content validity, factor structure, and reliability. Psychol. Assess. 2009;21(3):380–389. doi: 10.1037/a0016600. 19719349 [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):59–66. doi: 10.1097/00004583-200001000-00017. 10638068 [DOI] [PubMed] [Google Scholar]
- Romo R., Schultz W. Role of primate basal ganglia and frontal cortex in the internal generation of movements. III. Neuronal activity in the supplementary motor area. Exp. Brain Res. 1992;91(3):396–407. doi: 10.1007/BF00227836. 1483514 [DOI] [PubMed] [Google Scholar]
- Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal–limbic dysfunction in conduct disorder: a review. Biol. Psychiatry. 2011;69(12):e69–e87. doi: 10.1016/j.biopsych.2010.09.023. 21094938 [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol. Psychiatry. 2007;62(9):999–1006. doi: 10.1016/j.biopsych.2007.02.024. 17585887 [DOI] [PubMed] [Google Scholar]
- Scheperjans F., Hermann K., Eickhoff S.B., Amunts K., Schleicher A., Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb. Cortex. 2008;18(4):846–867. doi: 10.1093/cercor/bhm116. 17644831 [DOI] [PubMed] [Google Scholar]
- Schneider M.F., Krick C.M., Retz W., Hengesch G., Retz-Junginger P., Reith W., Rösler M. Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults — a functional magnetic resonance imaging (fMRI) study. Psychiatry Res. 2010;183(1):75–84. doi: 10.1016/j.pscychresns.2010.04.005. 20558047 [DOI] [PubMed] [Google Scholar]
- Schweitzer J.B., Lee D.O., Hanford R.B., Zink C.F., Ely T.D., Tagamets M.A., Hoffman J.M., Grafton S.T., Kilts C.D. Effect of methylphenidate on executive functioning in adults with attention-deficit/hyperactivity disorder: normalization of behavior but not related brain activity. Biol. Psychiatry. 2004;56(8):597–606. doi: 10.1016/j.biopsych.2004.07.011. 15476690 [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Pekar J.J., Mostofsky S.H. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. 17850833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirbekk B., Hansen B.H., Oerbeck B., Kristensen H. The relationship between sluggish cognitive tempo, subtypes of attention-deficit/hyperactivity disorder, and anxiety disorders. J. Abnorm. Child Psychol. 2011;39(4):513–525. doi: 10.1007/s10802-011-9488-4. 21331639 [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. 12391568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M.H., Albert M.V., Jung K., Carter C.S., Anderson J.R. Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2007;104(25):10330–10334. doi: 10.1073/pnas.0703225104. 17563353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., Kessler D., Fang Y., Welsh R.C., Prem Kumar K., Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014;35(9):4693–4705. doi: 10.1002/hbm.22504. 24668728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Kiehl K.A. An FMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. Am. J. Psychiatry. 2007;164(11):1737–1749. doi: 10.1176/appi.ajp.2007.06050876. 17974940 [DOI] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am. J. Psychiatry. 2006;163(6):1033–1043. doi: 10.1176/ajp.2006.163.6.1033. 16741204 [DOI] [PubMed] [Google Scholar]
- Vaidya C.J., Bunge S.A., Dudukovic N.M., Zalecki C.A., Elliott G.R., Gabrieli J.D. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am. J. Psychiatry. 2005;162(9):1605–1613. doi: 10.1176/appi.ajp.162.9.1605. 16135618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Ent D., van Beijsterveldt C.E., Derks E.M., Hudziak J.J., Veltman D.J., Todd R.D., Boomsma D.I., De Geus E.J. Neuroimaging of response interference in twins concordant or discordant for inattention and hyperactivity symptoms. Neuroscience. 2009;164(1):16–29. doi: 10.1016/j.neuroscience.2009.01.056. 19409224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Sylvester C.Y., Lacey S.C., Nee D.E., Franklin M., Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–340. doi: 10.1016/j.neuroimage.2005.01.054. 16019232 [DOI] [PubMed] [Google Scholar]
- Wåhlstedt C., Bohlin G. DSM-IV-defined inattention and sluggish cognitive tempo: independent and interactive relations to neuropsychological factors and comorbidity. Child Neuropsychol. 2010;16(4):350–365. doi: 10.1080/09297041003671176. 20574866 [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence: Technical Manual. Pearson; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. fourth edition. the psychological corporation; San Antonio, TX: 2003. [Google Scholar]
- Willcutt E.G., Chhabildas N., Kinnear M., DeFries J.C., Olson R.K., Leopold D.R., Keenan J.M., Pennington B.F. The internal and external validity of sluggish cognitive tempo and its relation with DSM-IV ADHD. J. Abnorm. Child Psychol. 2014;42(1):21–35. doi: 10.1007/s10802-013-9800-6. 24122408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E.G., Nigg J.T., Pennington B.F., Solanto M.V., Rohde L.A., Tannock R., Loo S.K., Carlson C.L., McBurnett K., Lahey B.B. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J. Abnorm. Psychol. 2012;121(4):991–1010. doi: 10.1037/a0027347. 22612200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock R.W., McGrew K.S., Mather N. Woodcock-Johnson III Battery. Riverside Publications; Itasca, IL: 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.


