ABSTRACT
Control of human cytomegalovirus (HCMV) requires a continuous immune surveillance, thus HCMV is the most important viral pathogen in severely immunocompromised individuals. Both innate and adaptive immunity contribute to the control of HCMV. Here, we report that peripheral blood natural killer cells (PBNKs) from HCMV-seropositive donors showed an enhanced activity toward HCMV-infected autologous macrophages. However, this enhanced response was abolished when purified NK cells were applied as effectors. We demonstrate that this enhanced PBNK activity was dependent on the interleukin-2 (IL-2) secretion of CD4+ T cells when reexposed to the virus. Purified T cells enhanced the activity of purified NK cells in response to HCMV-infected macrophages. This effect could be suppressed by IL-2 blocking. Our findings not only extend the knowledge on the immune surveillance in HCMV—namely, that NK cell-mediated innate immunity can be enhanced by a preexisting T cell antiviral immunity—but also indicate a potential clinical implication for patients at risk for severe HCMV manifestations due to immunosuppressive drugs, which mainly suppress IL-2 production and T cell responsiveness.
IMPORTANCE Human cytomegalovirus (HCMV) is never cleared by the host after primary infection but instead establishes a lifelong latent infection with possible reactivations when the host′s immunity becomes suppressed. Both innate immunity and adaptive immunity are important for the control of viral infections. Natural killer (NK) cells are main innate effectors providing a rapid response to virus-infected cells. Virus-specific T cells are the main adaptive effectors that are critical for the control of the latent infection and limitation of reinfection. In this study, we found that IL-2 secreted by adaptive CD4+ T cells after reexposure to HCMV enhances the activity of NK cells in response to HCMV-infected target cells. This is the first direct evidence that the adaptive T cells can help NK cells to act against HCMV infection.
INTRODUCTION
Human cytomegalovirus (HCMV) infects and establishes a persistent infection in the majority of humans worldwide. Postnatally it rarely causes severe complications in healthy individuals. However, HCMV is a significant cause of morbidity and mortality in severely immunocompromised individuals (1). This indicates the importance of immune surveillance in the control of HCMV. While T cells and HCMV antibodies are considered to be the main effectors of protective immunity, recent evidence supports the idea that NK cells also play an important role in the control of HCMV (2–4) and that NK cells degranulate after contact with HCMV-infected cells (5, 6). We have shown before that humoral antiviral immunity enhanced the degranulation and gamma interferon (IFN-γ) production of NK cells in response to HCMV-infected macrophages (6). In this article, we show that NK cell activity can also be enhanced by the T cell-mediated antiviral immunity against HCMV.
The activity of NK cells is regulated by (i) a balance of signals from activating and inhibitory receptors and (ii) cytokine stimulation (7). Furthermore, many studies suggest that antigen-presenting cells and other accessory cells are required to generate a primary triggering signal for the immune response of lymphocytes. Costimulatory and coinhibitory receptors might be involved in the generation of the signal (8). HCMV is the only virus known so far that shapes the receptor repertoire in human NK cells (9). Myeloid cells are an important site of HCMV latency and reactivation (10). Macrophages can act as antigen-presenting cells upon HCMV infection and can secrete cytokines leading to T- and NK cell activation (11, 12). It has been shown that the activating receptors NKp46, 2B4, and DNAM-1 contributed to the NK cell response to HCMV-infected macrophages (12). The role of the accessory cells and cytokines for the NK cell activity in HCMV infection is largely unknown. By using an autologous cell model (13), we compared the peripheral blood NK cell (PBNK) activities of HCMV-seropositive and -seronegative donors. Furthermore, we compared the activities of PBNKs and purified NK cells. We provide evidence that IL-2 secreted by T cells is important for NK cell activity in HCMV infection.
MATERIALS AND METHODS
Study subjects and cells.
Buffy coats from 12 HCMV-seropositive and 13 seronegative donors were purchased from the Transfusion Center of the Ulm University Hospital (Institut für Klinische Transfusionsmedizin und Immungenetik Ulm GmbH, Ulm, Germany) obtained from healthy blood donors. Human erythroleukemia cell line K562 (DMSZ), ex vivo macrophages, peripheral blood mononuclear cells (PBMCs), and purified NK cells were cultured in RPMI 1640 medium (GIBCO/Invitrogen) containing 10% fetal calf serum (FCS) (GIBCO/Invitrogen). Human foreskin fibroblasts (HFFs) were cultured in minimal essential medium (MEM) (GIBCO/Invitrogen) containing 10% FCS (GIBCO/Invitrogen). Granulocyte-macrophage colony-stimulating factor (GM-CSF) and M-CSF (R&D Systems) polarized M1 and M2 macrophages were obtained from human monocytes as previously described (11). NK cells were enriched by negative selection from PBMCs (Miltenyi). CD3 microbeads were used for positive selection or depletion of CD3+ T cells from PBMCs (Miltenyi). The purities of the NK cells, T cells, and T cell-depleted PBMCs are more than 95% as determined by flow cytometry after CD3 and CD56 staining.
Preparation of viral stocks and infection of macrophages.
Mutant bacterial artificial chromosomes (BACs) were generated by markerless mutagenesis (14). HCMV strain TB40/E and viruses reconstituted from TB40/E BACs (15) were propagated in HFFs. For preparation of virus stocks, infected cells were scraped from culture flasks and harvested together with supernatants at 5 to 7 days postinfection. Cellular debris was removed by centrifugation at 2,800 × g for 10 min, and virus particles were precipitated from the supernatants by ultracentrifugation (70,000 × g for 70 min at 10°C). Then, pellets were suspended in RPMI 1640 medium with 10% FCS. The infectious titer of HCMV preparations was determined as previously described (13). Macrophages were infected using a multiplicity of infection (MOI) of 5 PFU/macrophage for 24 h.
Antibodies and cytokines.
The following monoclonal antibodies (MAbs) were used for flow cytometry: peridinin chlorophyll protein (PerCP)-Cy5.5–anti-CD3 (UCHT1), allophycocyanin–anti-CD56 (B159), phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)–anti-CD107a (H4A3), PE–anti-IFN-γ (B27) (BD Biosciences), FITC–anti-IL-2 (MQ1-17H12 [Biolegend]), AF488- or PE–anti-NKG2C (134591 [R&D Systems]), and allophycocyanin–anti-CD4 (EDU-2; [Immunotools]). Cells were analyzed using a FACSCalibur (BD Biosciences) fluorescence-activated cell sorter. Purified anti-IL-2 (MQ1-17H12 [Biolegend]) and the isotype control (RTK2758 [Biolegend]) were used at a concentration of 20 μg/ml for antibody blocking experiments. Recombinant human IL-2 (R&D Systems) was used at concentration of 100 IU/ml for stimulation. A commercial enzyme-linked immunosorbent assay (ELISA) kit determined the IL-2 concentration (Biolegend). Pooled Ig intravenous Gamunex (Talecris Biotherapeutics) was purchased commercially and used at 1:200 dilutions.
NK cell degranulation and IFN-γ production assay.
After coculture of effector and target cells, monensin (GolgiStop, 2 μM [BD]), brefeldin A (5 mg/ml [Sigma]), and anti-CD107amAb (20 μl/ml) were added to the cultures for the last 5 h. After being gated on NK cells, CD3− CD56+ and CD107a surface expression and IFN-γ production were analyzed by flow cytometry.
Statistical analysis.
The nonparametric Kruskal-Wallis test was performed for multigroup comparison. Results were considered significant at the two-sided P level of 0.05.
RESULTS
Enhanced activity of seroposi-PBNKs in response to HCMV-infected autologous macrophages.
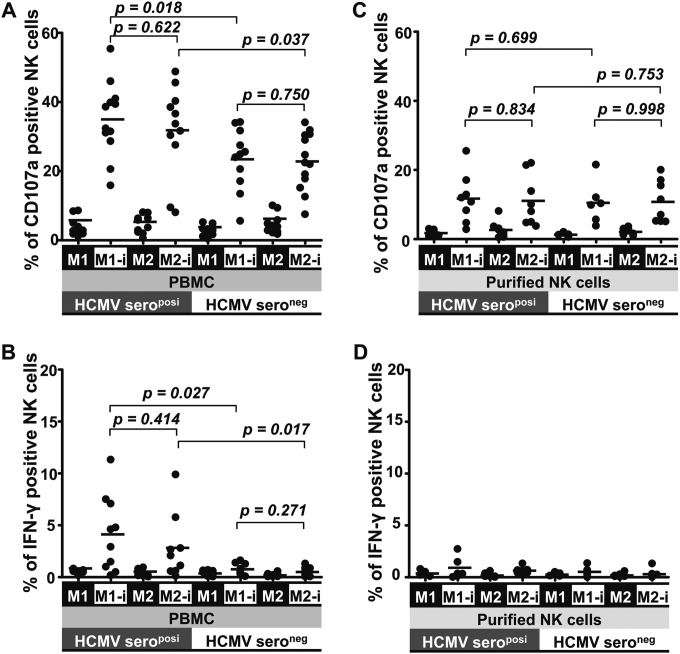
To compare the responses to an HCMV infection between NK cells from HCMV-seropositive and -seronegative healthy donors, we first applied unfractionated PBMCs as effectors. PBMCs were cocultured with HCMV-infected autologous M1 or M2 macrophages for 48 h, and then CD107a degranulation and intracellular IFN-γ production of peripheral blood NK cells (PBNKs) were assessed. Interestingly, PBNKs from HCMV-seropositive donors (seroposi-PBNKs) demonstrated an enhanced activity in response to the HCMV infection. They showed significant degranulation, with a mean value of 35%, and IFN-γ production (mean of 3%) in response to HCMV-infected autologous macrophages (Fig. 1A and B), in contrast to PBNKs from HCMV-seronegative donors (seroneg-PBNKs). As control of the HCMV infection rates of macrophages, the numbers of NK cells and the CD107a degranulation capacity of the PBNKs toward K562 cells proved to be comparable between HCMV-seropositive and -seronegative donors (data not shown). Intracellular IFN-γ production was not detected in seroneg-PBNKs, whereas it could clearly be found within most of the seroposi-PBNKs (Fig. 1B). Seroposi-PBNKs did not respond to uninfected macrophages. The activity of seroposi-PBNKs in response to HCMV-infected M1 macrophages seemed to be higher than that with HCMV-infected M2 macrophages, but there was no statistically significant difference (Fig. 1A).
FIG 1.
Enhanced activity of seroposi-PBNKs in HCMV infection. Unfractionated PBMCs (1 × 106) (A and B) and purified NK cells (1 × 105) (C and D) from HCMV-seropositive (n = 12) and -seronegative (n = 13) donors were cocultured with mock- or HCMV-infected autologous M1 or M2 macrophages (1 × 105) for 48 h. Then surface expression of CD107a and intracellular IFN-γ production of NK cells were assessed. “M1” and “M2” indicate uninfected macrophages, and “M1-I” and “M2-I” indicate infected macrophages. The horizontal lines represent the mean value for each group.
To elucidate whether this enhanced activity was an intrinsic property of NK cells from HCMV-seropositive donors, we also included purified NK cells as effectors in the same experimental settings. Purified NK cells responded poorly to HCMV-infected macrophages, demonstrating only a lower-level degranulation, with a mean value of 11%, but no induction of intracellular IFN-γ production, without any difference between HCMV-seropositive and -seronegative donors (Fig. 1C and D). Furthermore, the low degranulation of purified NK cells was independent from the HCMV serostatus of the donors (Fig. 1C). Clearly, the enhanced activity of seroposi-PBNKs was supported by other accessory cells present in unfractionated PBMCs.
seroposi-PBMCs produce IL-2 following HCMV reexposure, and CD4+ T cells are the source of IL-2.
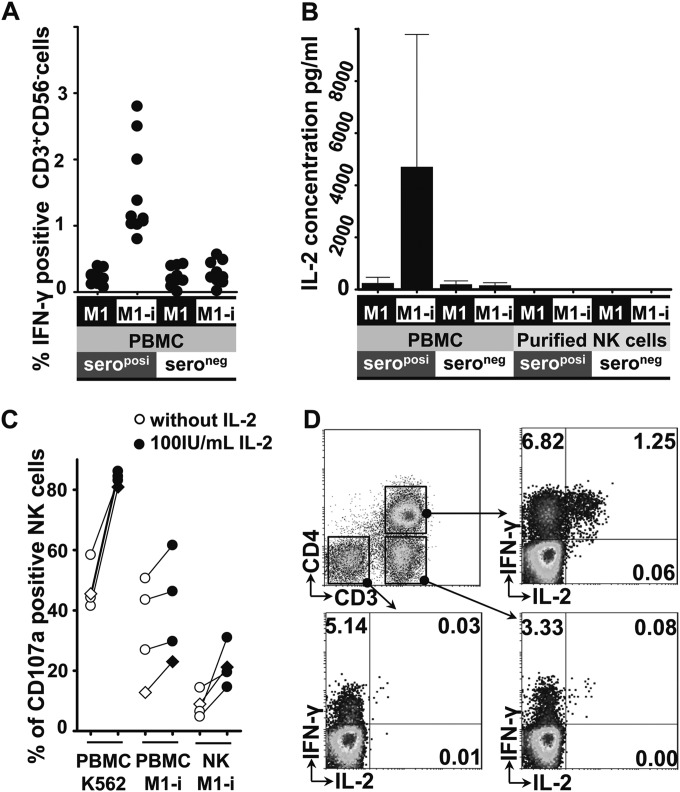
When analyzing in parallel T cells in the seroposi-PBMCs, as expected, these cells exhibited the typical memory activity after HCMV reexposure. They produced IFN-γ in response to HCMV-infected macrophages, whereas this could not be found in HCMV-seronegative donors (Fig. 2A). A previous study demonstrated that T cells could produce IL-2 in response to HCMV antigens (16), and IL-2 has been shown to enhance NK cell-dependent reduction of HCMV yield (17). Furthermore, IL-2 played an important role in NK cell proliferation and cytotoxic capacity (18). Importantly, NK cells expressed CD25 (IL-2 receptor α [IL-2Rα] chain) when cocultured with HCMV-infected macrophages (12). Since we hypothesized that IL-2 might play a critical role for the enhanced activity of seroposi-PBNKs, we measured the level of IL-2 from supernatants of cocultures. IL-2 production could only be detected in cultures that contained seroposi-PBMCs as effector cells, whereas IL-2 remained undetectable when seroneg-PBMCs were used (Fig. 2B). The level of IL-2 in the individual supernatants was highly variable, but the lowest level of IL-2 (425 pg/ml) from seroposi-PBMC cocultures was still much higher than that from seroneg-PBMC cocultures. When purified NK cells from both HCMV-seropositive and -seronegative donors were used as effectors, no IL-2 production could be detected (less than 10 pg/ml) (Fig. 2B). Furthermore, CD107a degranulation of PBNKs and purified NK cells after contact with HCMV-infected macrophages could be enhanced by addition of exogenous IL-2 in all donors tested (Fig. 2C). Degranulation of PBNKs to K562 was also enhanced by IL-2, which served as a control. However, exogenous addition of IL-2 alone was insufficient to rescue the degranulation of purified NK cells to the level of PBNKs as effectors (Fig. 2C).
FIG 2.
HCMV-specific IL-2 secretion follows HCMV reexposure. (A) Unfractionated PBMCs from HCMV-seropositive (n = 11) and -seronegative (n = 9) donors were cocultured with HCMV-infected autologous M1 macrophages for 48 h, and then the percentage of IFN-γ-producing T cells (CD3+ CD56−) was assessed. (B) Unfractionated PBMCs and purified NK cells from HCMV-seropositive (n = 6) and -seronegative (n = 6) donors were cocultured with HCMV-infected autologous M1 macrophages for 48 h, and the IL-2 concentrations in supernatants of cocultures were determined by ELISA in duplicate. (C) Unfractionated PBMCs and purified NK cells from the same donor were cocultured with TB40/E-infected autologous M1 macrophages for 48 h in the presence or absence of 100 IU/ml recombinant IL-2, and surface CD107a expression of NK cells was assessed. Unfractionated PBMCs (1 × 106) cocultured with K562 cells (1 × 105) for 5 h served as a positive control. Three donors were HCMV seropositive (circles), and one donor was HCMV seronegative (diamonds). (D) Unfractionated PBMCs from HCMV-seropositive donors were cocultured with HCMV-infected M1 macrophages for 6 h, and then intracellular IL-2 and IFN-γ production of the different lymphocyte populations was analyzed by flow cytometry. Results from one representative experiment for the five donors is shown.
We next examined the source of the IL-2 production. We performed intracellular staining of IL-2 at different times, gating on different lymphocytes. We found that IL-2 was mainly produced by CD4+ T cells in seroposi-PBMCs after 6 h of coculture (Fig. 2D). This was in agreement with the fact that mainly HCMV-specific CD4+ T cells secreted IFN-γ and IL-2 after HCMV antigen restimulation (19). CD4+ T cells secrete the IL-2 immediately after HCMV reexposure, which might play a critical role for NK cell priming and activation. The intracellular IL-2 staining could no longer be detected after 48 h of coculture (data not shown). The exact mechanism for this IL-2 production kinetic is still unknown, but the IL-2 production of CD4+ T cells after reexposure seems to be tightly controlled. IL-2 production could not be detected in HCMV-seronegative donors at any time.
T cell-dependent IL-2 production is critical for NK cell activity in response to HCMV infection.
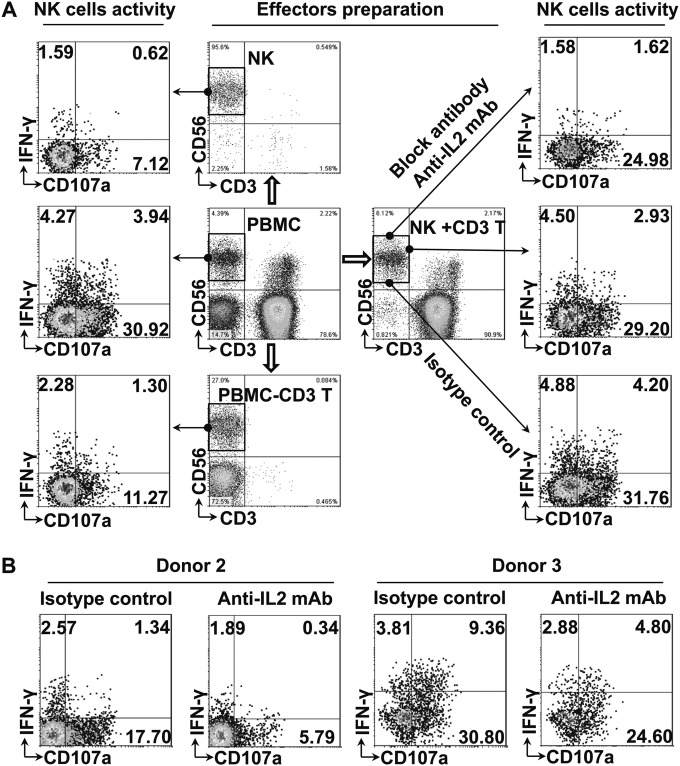
To further elucidate the contribution of T cells and IL-2 to the NK cell response, we prepared different effectors from HCMV-seropositive donors. Unfractionated PBMCs, purified NK cells, T cell-depleted PBMCs, and purified NK cells plus purified T cells were prepared from the same donor and were cocultured with the HCMV-infected macrophages using the same experimental settings. NK cell activity was again assessed as CD107 degranulation and intracellular IFN-γ production. As shown in Fig. 3A, NK cell activity was highest when unfractionated PBMCs were used as effectors. Purified NK cells proved to be the poorest effectors. T cell-depleted PBMCs slightly enhanced NK cell degranulation and IFN-γ production. When purified T cells were added to purified NK cells, the NK cell activity in response to HCMV-infected macrophages nearly reached the level with PBNKs as effectors. Furthermore, when IL-2 was blocked by antibodies, the IFN-γ production was clearly inhibited, and degranulation of NK cells was also inhibited compared with that of the isotype control in each donor (Fig. 3B).
FIG 3.
T cell-dependent IL-2 production enhances NK cell activity in HCMV infection. Different effectors, including unfractionated PBMCs (1 × 106), purified NK cells (NK cell percentage × 106), T cell-depleted PBMCs (1 × 106 − T cell percentage × 106), and purified NK cells plus purified T cells (NK cell percentage × 106 + T cell percentage × 106) from the same HCMV-seropositive donor were cocultured with HCMV-infected autologous macrophages (1 × 105) for 48 h, and then activity of NK cells (gated on CD3− CD56+ cells under all conditions) was assessed by surface CD107a expression and intracellular IFN-γ production. For IL-2 blocking experiments, purified anti-IL-2 monoclonal antibody and an isotypic antibody control were added at the beginning of coculturing. (A) One representative experiment out of three from HCMV-seropositive donors is shown. (B) Percentages of IFN-γ and CD107a-positive NK cells with IL-2 blocking antibody or isotypic antibody control from another two HCMV-seropositive donors are shown.
NKG2C-positive NK cells and HCMV immunomodulatory genes US2, US3, US6, US11, UL18, and UL40 do not contribute to the enhanced activity of seroposi-PBNKs against infected macrophages.
In humans, it has been shown that HCMV infection selectively expanded NKG2C-positive NK cells in healthy individuals (6, 20, 21). Even in coinfections of HCMV with HIV (22, 23), hantavirus (24), hepatitis B virus, and hepatitis C virus (25), the expansion of NKG2C-positive NK cells was exclusively dependent on the HCMV infection. We have shown that NKG2Chi CD57hi NK cells in the absence of HCMV antibodies are poor effectors of natural cytotoxicity compared to NKG2C-negative NK cells when using the same experimental settings (6). The low responsiveness of NKG2C-positive NK cells was also observed in response to HCMV-infected dendritic cells and fibroblasts (5, 26). As shown in Fig. 1A, we tested 12 HCMV-seropositive donors. The mean of PBNK degranulation in response to infected M1 macrophages was 34.9%. We checked that 4 of them contained NKG2C-positive NK cells. (The percentages were 6.03%, 6.95%, 13.2%, and 25.6%). The mean degranulation level of these 4 donors was 27.7%, whereas the mean degranulation level of the other 8 HCMV-seropositive donors without NKG2C-positive NK cells was 38.6%. Thus, the enhanced activity of NK cells from HCMV-seropositive donors is not due to NKG2C-positive NK cells.
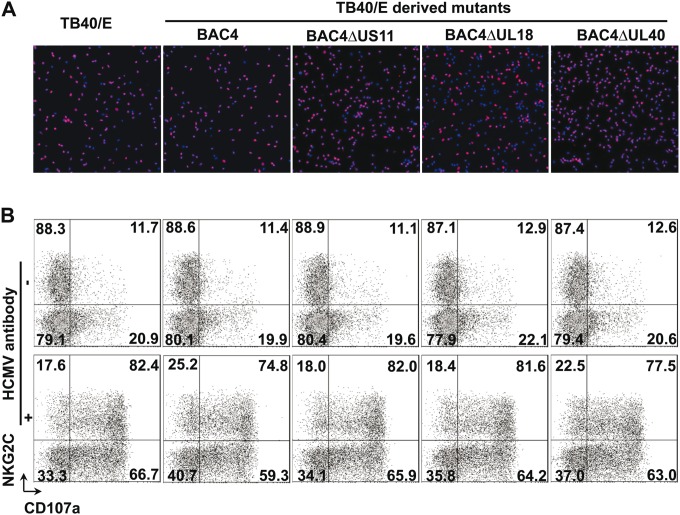
To investigate whether some specific HCMV genes are involved in the response of the NK cell in our experimental setting, we used the HCMV TB40/E-derived BAC cloned viruses lacking the viral genes US2 to -6 (15) and mutants thereof with additional deletion of US11 (27), UL18, and UL40. HCMV unique short (US) genes have been extensively characterized for their capacity to dampen the surface expression of major histocompatibility complex class I (MHC-I) in infected cells (28). HCMV encodes the glycoprotein UL40, which can upregulate the cell surface expression of HLA-E, which in turn is recognized by the NK cell receptor complex CD94/NKG2A, -B, and -C (29). The MHC-I homologue glycoprotein UL18 can directly bind to NKG2C with low affinity and LIR-1 with high affinity (30, 31). Macrophages were infected with the four TB40/E-derived mutants and then cocultured with autologous NK cells. We subsequently assessed the NKG2Chi NK cell and NKG2C-negative NK cell responses toward HCMV-infected macrophages in the absence or presence of HCMV antibodies. As shown in Fig. 4A, all virus mutants exhibited a comparable infectivity in macrophages, as determined by immediate early antigen (IEA)-positive cells at 24 h postinfection (red fluorescence). Additionally, no differences were observed between degranulation of NKG2Chi NK cells and NKG2C-negative NK cells cocultivated with macrophages infected by the HCMV TB40/E wild-type virus or by the different recombinant viruses lacking these so-called “immunomodulatory” genes (Fig. 4B, upper panel). The antibody enhancement of NK degranulation was also not affected by the presence or absence of these immunomodulatory genes (Fig. 4B, lower panel). These data indicate that the HCMV genes US2, US3, US6, US11, UL18, and UL40 on the background of TB40/E do not contribute to NK cell-mediated response against infected autologous macrophages in our experimental setting.
FIG 4.
Degranulation of NKG2Chi NK cells and NKG2C-negative NK cells in response to macrophages infected with TB40/E-derived mutants. (A) A total of 105 macrophages were infected with TB40/E- and TB40/E-derived mutants at an MOI of 5, and infection rates were determined 24 h postinfection. The presence of HCMV IEAs (red fluorescence) indicates infected macrophages, and nuclei are shown in blue (DAPI [4′,6-diamidino-2-phenylindole]). (B) Thawed PBMCs (1 × 106) were cocultured with autologous macrophages (1 × 105) infected with TB40/E or the TB40/E-derived mutants for 48 h, and then surface expression of CD107a on the NK cells was assessed in the absence or after addition of HCMV antibodies for an additional 5 h. One representative experiment out of four from NKG2Chi NK cell-positive donors is shown.
DISCUSSION
NK cells play an important role in the control of HCMV, and HCMV is able to shape the NK cell receptor repertoire (2, 4, 9). We previously could show that the activity of NK cells in response to HCMV infection is enhanced by the humoral antiviral immunity (6). Our actual results further confirm the importance of the adaptive antiviral immunity in the regulation of NK cell activity in HCMV infection. Our study extends the knowledge on the mechanisms of the immune surveillance in HCMV infection with potential clinical relevance.
Activated NK cells are the main source of IFN-γ, which plays a pivotal role in the antiviral response (32). NK cells from PBMCs have been shown to produce IFN-γ in response to influenza virus, Plasmodium falciparum-infected red blood cells (RBCs), and hepatitis C virus-infected hepatoma cells (JFH-1/HuH7.5). However, purified NK cells could not produce IFN-γ in the same experimental setting (33–35). This already suggested the need of accessory cells for IFN-γ secretion of NK cells during infection (36). During influenza virus and Plasmodium falciparum in vitro infection studies, T cells secreted IL-2 after primary in vitro infection and enhanced the IFN-γ activity of NK cells (33, 34). In our study, the seroneg-PBNKs did not produce any significant amount of intracellular IFN-γ in response to HCMV-infected autologous macrophages. However, the seroneg-PBNKs degranulated after cell-to-cell contact with HCMV-infected macrophages at a median level (22%). This indicated that IFN-γ production and degranulation of NK cells were differentially activated and regulated in response to infected cells. An in vitro study using Plasmodium falciparum infection also showed that IFN-γ production and degranulation of NK cells may be regulated separately (37). In our assay, we detected IL-2 production in PBMCs from seropositive donors after HCMV reexposure, and we found that purified T cells enhanced the IFN-γ production of purified NK cells, which was highly dependent on the IL-2 secreted by CD4+ T cells after HCMV reexposure. It is of note that the low degranulation of purified NK cells compared with PBNKs was independent from the HCMV serostatus of the donors (Fig. 1C), and exogenous addition of IL-2 alone was insufficient to rescue the degranulation of purified NK cells to the level of PBNKs as effectors (Fig. 2C). This suggests that T cells might provide additional cell contact-dependent signals that contribute to the activity of NK cells. In our study, when CD3+ T cells were added to purified NK cells, NK cell IFN-γ production nearly reached that of PBNKs. To this point, we could not fully exclude the role of a low level of contamination by natural killer T (NKT) cells (CD3+ CD56+) in our experimental setting due to the limitations of cell isolation. However, NKT cells are not involved in the IL-2-enhanced activation of NK cells since we could not detect IL-2-positive NKT cells in our experiments. In addition to T cells, we also observed that T cell-depleted PBMCs also slightly enhanced the NK cell activity in HCMV infection compare to purified NK cells (Fig. 3A). We hypothesize that additional accessory cells (e.g., monocytes and B cells) might also contribute to the NK cells' response to HCMV-infected cells.
We have extensively studied the effect of HCMV infection on the expression of costimulatory and HLA molecules on macrophages (11). Although M1 and M2 macrophages independent of the HCMV serostatus of the donor express different levels of costimulatory and HLA molecules on their surface, and these molecules were differently modulated by HCMV infection, we could show that NK cells are equally activated by infected M1 and M2 macrophages. Furthermore, M1 and M2 macrophages equally stimulated autologous T cell proliferation from HCMV-seropositive donors (11). This indicates that these molecules might not play a specific role for the enhanced activity of NK cells described in our study. This is further supported by our data on virus mutants, namely, that the HCMV US2, -3, -6, and -11 genes do not play a role in the NK cells' activity in response to infected autologous macrophages. We further tested the cytokine and chemokine production of HCMV-infected M1 and M2 macrophages. It is noted that M1 secretes 10-fold more IL-12 (100 pg/ml) than M2 macrophages independent from the HCMV serostatus of the donor. We could not find a difference in NK cell activation in response to infected M1 or M2, which gives a hint that IL-12 might not be involved in the enhanced activity of seroposi-PBNKs, but we cannot exclude that other cytokines synergize with IL-2 in enhancing the activity of NK cells in our experimental setting. These aspects need further investigation.
Strikingly, HCMV-specific T cells comprise approximately 5% of all circulating T cells and 10% of all memory T cells during persistent HCMV infection and remain functional in healthy individuals (38). Pathogen-experienced CD4+ T cells have unique features and respond more rapidly and effectively during reexposure to the virus (39). We suggest that the early activation of NK cells by IL-2 production from CD4+ T cells serves to enhance immune surveillance in HCMV-seropositive individuals. We found that IL-2 significantly enhances NK cell control of HCMV transmission in different cell types in vitro (4). This could efficiently prevent virus replication and dissemination in the host. Enhanced NK cell activity induced by cellular immunity was also shown after rabies virus, Plasmodium falciparum, and HIV vaccination (40–42). In these studies, IL-2 from reexposed CD4+ T cells again played a key role in enhancement of NK cell responses. IL-2-producing CD4+ T cells contributed not only to NK cell activity during infection but also to a homeostatic and antitumor activity in vivo (43–45). A recent study showed that “missing-self” responses of NK cells were increased in a CD4+ T cell- and IL-2-dependent manner after depletion of regulatory T cells. Furthermore, IL-2 from CD4+ T cells rapidly boosted the capacity of NK cells to engage target cells and enabled NK cell responses (43). NK cells preactivated by IL-12/15/18 (44) and immature CD127+ NK cells (45) are potent effectors in an IL-2-dependent manner, requiring the presence of CD4+ T cells.
It has been shown that NKG2C-positive NK cells are induced by HCMV infection and has been speculated that they function as memory-like cells. We could show that the NKG2C-positive NK cells are not responsible for the enhanced PBNK activity described in our study. MHC-I downregulation induced by the HCMV genes US2, -3, -6, and 11 was supposed to render infected cells vulnerable to an NK cell attack. However, our data in accordance with data from others who used infected fibroblasts as target cells did not show a higher vulnerability of cells infected by the mutant viruses (28). Glycoprotein UL18 (gpUL18) was identified to bind the NK cell inhibitory receptor LIR-1 with high affinity (31). Target cells expressing gpUL18 were observed to inhibit LIR-1+ NK cells but to stimulate LIR-1− NK cells (46). Although a high percentage of NKG2Chi NK cells are LIR-1+ (6), these cells did not show the anticipated better response to the UL18 deletion mutant in our assay. We also did not observe a different response using the UL40 deletion mutant. Taken together, these data provide evidence that these viral genes on the background of TB40/E do not modulate the NK cell response to HCMV-infected macrophages and these viral genes do not contribute to the enhanced activity of seroposi-PBNKs in our experiments.
In conclusion, the important finding of our study is that T cells enhance the NK cell activity in HCMV infection via an IL-2-dependent pathway. Together with our previous finding that NK cell activity can be enhanced by the antiviral humoral immunity (6), our studies demonstrate that NK cells are also effectors of the adaptive cellular immunity in the control of HCMV. This mechanism might have clinical implications for patients at risk for severe HCMV manifestations who received immunosuppressive drugs, which mainly suppress IL-2 production and T cell responsiveness. After bone marrow transplantation, NK cells are one of the first reconstituted lymphocyte populations while adaptive immunity is not yet available, and in cases of T cell- and/or B-cell-deficient SCID patients, NK cells may exert an antiviral activity to some extent (2). Thus, therapeutic strategies should be considered by enhancing the NK cell activity against HCMV.
ACKNOWLEDGMENTS
We thank Ingrid Bennett for help in preparing the manuscript and Christian Sinzger for HCMV strain TB40/E and BAC-4 mutants.
This work was supported by grants from the DFG-Program “International Graduate School in Molecular Medicine, Ulm University.”
Z.W. designed and performed research, analyzed data, and wrote the paper. G.F. provided reagents. C.B. and T.S. performed research and analyzed data. T.M. designed research, analyzed data, and wrote the paper.
The authors declare they have no financial or commercial conflicts of interest.
REFERENCES
- 1.Britt W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 2.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. 2008. Human NK cells can control CMV infection in the absence of T cells. Blood 112:914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 3.Biron CA, Byron KS, Sullivan JL. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Sinzger C, Reichel JJ, Just M, Mertens T. 2015. Natural killer cells can inhibit the transmission of human cytomegalovirus in cell culture by using mechanisms from innate and adaptive immune responses. J Virol 89:2906–2917. doi: 10.1128/JVI.03489-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magri G, Muntasell A, Romo N, Saez-Borderias A, Pende D, Geraghty DE, Hengel H, Angulo A, Moretta A, Lopez-Botet M. 2011. NKp46 and DNAM-1 NK-cell receptors drive the response to human cytomegalovirus-infected myeloid dendritic cells overcoming viral immune evasion strategies. Blood 117:848–856. doi: 10.1182/blood-2010-08-301374. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, Schirmbeck R, Mertens T. 2013. Human cytomegalovirus-induced NKG2Chi CD57hi natural killer cells are effectors dependent on humoral antiviral immunity. J Virol 87:7717–7725. doi: 10.1128/JVI.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pak-Wittel MA, Yang L, Sojka DK, Rivenbark JG, Yokoyama WM. 2013. Interferon-gamma mediates chemokine-dependent recruitment of natural killer cells during viral infection. Proc Natl Acad Sci U S A 110:E50–E59. doi: 10.1073/pnas.1220456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Yao S, Chen L. 2011. Cell surface signaling molecules in the control of immune responses: a tide model. Immunity 34:466–478. doi: 10.1016/j.immuni.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, Retiere C, Sverremark-Ekstrom E, Traherne J, Ljungman P, Schaffer M, Price DA, Trowsdale J, Michaelsson J, Ljunggren HG, Malmberg KJ. 2013. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair J, Sissons P. 2006. Latency and reactivation of human cytomegalovirus. J Gen Virol 87:1763–1779. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- 11.Bayer C, Varani S, Wang L, Walther P, Zhou S, Straschewski S, Bachem M, Soderberg-Naucler C, Mertens T, Frascaroli G. 2013. Human cytomegalovirus infection of M1 and M2 macrophages triggers inflammation and autologous T-cell proliferation. J Virol 87:67–79. doi: 10.1128/JVI.01585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romo N, Magri G, Muntasell A, Heredia G, Baia D, Angulo A, Guma M, Lopez-Botet M. 2011. Natural killer cell-mediated response to human cytomegalovirus-infected macrophages is modulated by their functional polarization. J Leukoc Biol 90:717–726. doi: 10.1189/jlb.0311171. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Frascaroli G, Mertens T. 2013. Assessment of natural killer cell responses to human cytomegalovirus-infected macrophages. Methods Mol Biol 1064:289–298. doi: 10.1007/978-1-62703-601-6_21. [DOI] [PubMed] [Google Scholar]
- 14.Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol 634:421–430. doi: 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
- 15.Sinzger C, Hahn G, Digel M, Katona R, Sampaio KL, Messerle M, Hengel H, Koszinowski U, Brune W, Adler B. 2008. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J Gen Virol 89:359–368. doi: 10.1099/vir.0.83286-0. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, Maino VC. 2004. Analyzing T-cell responses to cytomegalovirus by cytokine flow cytometry. Hum Immunol 65:493–499. doi: 10.1016/j.humimm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Iversen AC, Norris PS, Ware CF, Benedict CA. 2005. Human NK cells inhibit cytomegalovirus replication through a noncytolytic mechanism involving lymphotoxin-dependent induction of IFN-beta. J Immunol 175:7568–7574. doi: 10.4049/jimmunol.175.11.7568. [DOI] [PubMed] [Google Scholar]
- 18.Becknell B, Caligiuri MA. 2005. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 19.Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, Kern F. 2012. Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J Virol 86:1001–1009. doi: 10.1128/JVI.00873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. 2004. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. 2011. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A 108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guma M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, Lopez-Botet M. 2006. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis 194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- 23.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. 2010. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS 24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. 2011. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med 208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debre P, Bjorkstrom NK, Malmberg KJ, Marcellin P, Vieillard V. 2012. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol 42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Scott JM, Hwang I, Kim S. 2013. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schempp S, Topp M, Kessler T, Sampaio KL, Dennehy KM, Einsele H, Hahn G, Grigoleit GU, Jahn G. 2011. Deletion mutant of human cytomegalovirus lacking US2-US6 and US11 maintains MHC class I expression and antigen presentation by infected dendritic cells. Virus Res 155:446–454. doi: 10.1016/j.virusres.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson GW, Tomasec P, Stanton RJ, Armstrong M, Prod'homme V, Aicheler R, McSharry BP, Rickards CR, Cochrane D, Llewellyn-Lacey S, Wang EC, Griffin CA, Davison AJ. 2008. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol 41:206–212. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser BK, Pizarro JC, Kerns J, Strong RK. 2008. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc Natl Acad Sci U S A 105:6696–6701. doi: 10.1073/pnas.0802736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willcox BE, Thomas LM, Bjorkman PJ. 2003. Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat Immunol 4:913–919. doi: 10.1038/ni961. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, Walter MR, Nagabhushan TL, Trotta PP, Pestka S. 1998. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res 58:2489–2499. [PubMed] [Google Scholar]
- 33.He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, Arvin AM, Parham P, Greenberg HB. 2004. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest 114:1812–1819. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. 2010. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 184:6043–6052. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Saha B, Kodys K, Szabo G. 2013. IFN-gamma production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells. J Hepatol 59:442–449. doi: 10.1016/j.jhep.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman KC, Riley EM. 2007. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol 7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 37.Korbel DS, Newman KC, Almeida CR, Davis DM, Riley EM. 2005. Heterogeneous human NK cell responses to Plasmodium falciparum-infected erythrocytes. J Immunol 175:7466–7473. doi: 10.4049/jimmunol.175.11.7466. [DOI] [PubMed] [Google Scholar]
- 38.Snyder CM. 2011. Buffered memory: a hypothesis for the maintenance of functional, virus-specific CD8(+) T cells during cytomegalovirus infection. Immunol Res 51:195–204. doi: 10.1007/s12026-011-8251-9. [DOI] [PubMed] [Google Scholar]
- 39.Swain SL, McKinstry KK, Strutt TM. 2012. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol 12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. 2010. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol 185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P, Cohen J, Vekemans J, Villafana T, Corran PH, Bejon P, Drakeley CJ, von Seidlein L, Riley EM. 2012. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J Immunol 188:5054–5062. doi: 10.4049/jimmunol.1102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jost S, Tomezsko PJ, Rands K, Toth I, Lichterfeld M, Gandhi RT, Altfeld M. 2014. CD4+ T-cell help enhances NK cell function following therapeutic HIV-1 vaccination. J Virol 88:8349–8354. doi: 10.1128/JVI.00924-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasteiger G, Hemmers S, Firth MA, Le Floc'h A, Huse M, Sun JC, Rudensky AY. 2013. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med 210:1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. 2012. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. 2013. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med 210:1179–1187. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prod'homme V, Griffin C, Aicheler RJ, Wang EC, McSharry BP, Rickards CR, Stanton RJ, Borysiewicz LK, Lopez-Botet M, Wilkinson GW, Tomasec P. 2007. The human cytomegalovirus MHC class I homolog UL18 inhibits LIR-1+ but activates LIR-1− NK cells. J Immunol 178:4473–4481. doi: 10.4049/jimmunol.178.7.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]