Abstract
Objective
Motor abnormalities, including impaired balance and increased postural sway, are commonly reported in children with ADHD, but have yet to be investigated in adults with ADHD. Furthermore, although these abnormalities are thought to stem from cerebellar deficits, evidence for an association between the cerebellum and these motor deficits has yet to be provided for either adults or children with ADHD.
Method
In this study, we measured postural sway in adults with ADHD and controls, examining the relationship between sway and regional cerebellar gray matter volume. Thirty-two ADHD and 28 control participants completed various standing-posture tasks on a Wii balance board.
Results
Postural sway was significantly higher for the ADHD group compared to the healthy controls. Higher sway was positively associated with regional gray matter volume in the right posterior cerebellum (lobule VIII/IX).
Conclusion
These findings show that sway abnormalities commonly reported in children with ADHD are also present in adults, and for the first time show a relationship between postural control atypicalities and the cerebellum in this group. Our findings extend the literature on motor abnormalities in ADHD and contribute to our knowledge of their neural substrate.
Keywords: Attention-deficit hyperactivity disorder (ADHD), Postural balance, Cerebellum
Highlights
-
•
Balance abnormalities in ADHD observed in children also occur in adults.
-
•
Sway is positively associated with gray matter volume in the posterior cerebellum.
-
•
We provide first evidence of link between balance and cerebellar morphology in ADHD.
-
•
Findings support cerebellar involvement in motor abnormalities observed in ADHD.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a developmental neurobehavioral disorder characterized by symptoms of inattentiveness, hyperactivity, and impulsiveness. It affects up to 10% of children and 5% of adults worldwide (Faraone et al., 2003) and has been associated with high levels of morbidity, distress, and disability across the lifecycle (Spencer et al., 2007).
Although not included in the diagnostic criteria for ADHD, up to 50% of children with ADHD have been shown to have motor control problems (Pitcher et al., 2003). One such motor deficit, postural sway, has been reported in children with ADHD in a number of studies. Zang and colleagues (2002) demonstrated that children with ADHD had significant balance dysfunction compared to control children under various testing conditions, including standing with eyes open on a foam platform, or with eyes closed on firm and foam platforms. Additionally, while balance deficits in children with ADHD have been reported in simple standing postures such as standing on a fixed platform with eyes open (Bucci et al., 2014; Hassan and Azzam, 2012; Kooistra et al., 2009; Shorer et al., 2012), postural deficits are even more pronounced in more difficult standing conditions (Buderath et al., 2009; Hassan and Azzam, 2012; Shum et al., 2009; Zang et al., 2002). For example, when sensory signals are disrupted by having subjects close their eyes or by manipulating the angle of the platform and visual surround in response to the child's sway (i.e., sway-referenced), children with ADHD perform poorly relative to more standard conditions (Hassan and Azzam, 2012; Shum et al., 2009). Since sensorimotor integration and balance regulation rely on the cerebellum, these results suggest that poor balance in children with ADHD could stem from suboptimal cerebellar function (Shum et al., 2009).
Studies showing sway abnormalities in children with ADHD support the idea that cerebellar mechanisms contribute to balance dysfunction. In a study of children with ADHD, children with fetal alcohol spectrum disorder (FASD), and controls, postural stability was impaired in the ADHD and FASD groups. The authors suggested that the increased sway might stem from the cerebellar abnormalities seen in both conditions (Kooistra et al., 2009). In another study, children with ADHD had mild postural impairments similar to children with cerebellar lesions, further supporting the role of cerebellar dysfunction in balance deficits in ADHD (Buderath et al., 2009).
Substantial evidence now shows that alterations in cerebellar structure are involved in the pathophysiology of ADHD (e.g., Castellanos et al., 2002; Seidman et al., 2005; Valera et al., 2007). Relative to controls, decreased cerebellar volume has been observed in individuals with ADHD including children (Mostofsky et al., 1998; Valera et al., 2007), adolescents (Castellanos et al., 2002) and adults (Makris et al., 2013). Relatively smaller cerebellar volumes have also been associated with increased symptom severity (Castellanos et al., 2002), suggesting the importance of the cerebellum in the pathophysiology of ADHD. However, postural sway and its relation to cerebellar volume have not been assessed in adults with ADHD. Assessing balance and other motor abilities in adults with ADHD is important, as motor difficulties are linked to social and emotional problems in children (Schoemaker and Kalverboer, 1994), and perhaps to an increased risk of injuries in children with ADHD (Discala et al., 1998; Rowe et al., 2007) and adults with ADHD (Kaya et al., 2008). Also, examining the relationship between balance and cerebellar volume is important to our understanding of the neural substrates of such associated features in ADHD.
The primary objectives of this study were to assess standing sway under different conditions in adults with ADHD, and determine whether sway was related to regional cerebellar volume. We also explored whether sway was associated with ADHD symptoms. We predicted that sway would be greater in adults with ADHD relative to controls and related to regional cerebellar volume.
2. Materials and methods
2.1. Participants
Thirty-two adults with ADHD (18 females) and 28 healthy controls (HC; 15 females) comparable in age (ADHD mean age = 26.2 years; SD = 7.4; HC mean age = 27.3 years; SD = 8.0; p > .6) participated in the experiment. Two additional participants were excluded due to excessively high sway (>2.5 SDs from the mean; 1 ADHD and 1 control participant). All participants provided written informed consent, and the study was approved by the Partners Human Research Institutional Review Board.
Exclusion criteria for all subjects included: any current DSM-IV Axis I mood, psychotic, or generalized anxiety disorder; full scale IQ < 80; any major sensorimotor handicaps or neurological disorders; current alcohol or substance abuse or dependence or a chronic history of abuse or dependence as defined by review of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID); or current use of psychotropic medications (other than short-acting psychostimulants). Participants taking psychostimulants for ADHD (n = 13) underwent a 24-hour washout period.
To assess psychopathology, all participants underwent the Structured Clinical Interview for DSM-IV (SCID) Axis I Disorders (First et al., 1997). As in previous studies (Valera et al., 2010a, 2010b) to assess ADHD, we used a module modified for adults from the Schedule for Affective Disorders and Schizophrenia for School Age Children (Orvaschel, 1994). The ADHD participants met DSM-IV criteria for ADHD with childhood onset and persistence into adulthood. We included two ADHD participants who had an age-of-onset after age 7 (ages of onset were 9 and 11 years). This decision was based on studies supporting the validity of ADHD in subjects with onset of symptoms later than the 7-year cutoff (Faraone et al., 2006). A child and adult psychiatrist resolved diagnostic uncertainties. Previous work in our laboratory has shown that retrospective diagnoses can be made in a reliable and valid manner (Biederman et al., 1990). ADHD subtypes included 18 participants with combined-type, 12 with inattentive-type, and 2 with hyperactive-type. Vocabulary and matrix reasoning were used to calculate an IQ estimate (Wechsler, 1999). Full-scale IQ estimates did not differ between the ADHD (M = 118.7) and control groups (M = 117.1), p > .6.
In addition to the clinical interview, participants completed the Adult ADHD Self-Report Scale (ASRS) (Kessler et al., 2005) to assess ADHD symptoms. The ASRS is a common symptom checklist that contains questions consistent with the DSM-IV criteria for ADHD, and has been shown to be a valid and reliable scale for evaluating ADHD (Adler et al., 2006). It can be used to derive dimensional measures of hyperactive and inattentive symptoms and total symptomatology.
2.2. Postural sway task acquisition and analysis
Postural sway was assessed while standing on a Wii balance board. The Wii provides a valid and reliable assessment of postural control (Clark et al., 2010). Participants completed four standing postures while balancing on two feet. The four conditions were: eyes open with feet apart (approximately shoulder width), eyes closed with feet apart, eyes open with feet together, and eyes open with feet apart and shoes off. During each trial participants were instructed to remain as still as possible for the duration of the 30 s trial. The first 5 s of each trial were not analyzed. Postural sway was quantified as the path length (in cm) of the center of pressure (COP) (e.g., Bucci et al., 2014; Shorer et al., 2012). Data from a small number of trials (4 of 240 total trials) were missing due to technical failure and their estimated values were imputed using multiple regression (Gelman and Hill, 2006). Differences between conditions and between groups were tested using a mixed-model ANOVA. Spearman bivariate correlations were used to assess the relationship between sway and symptom scores. Analyses were performed with SPSS 21.
2.3. Brain imaging acquisition and analysis
Whole-brain structural MR images were collected on a Siemens Tim Trio 3 Tesla MRI system at the MGH Martinos Center (Charlestown, MA). A T1-weighted multi-echo MPRAGE sagittal scan was collected (TR = 2.54 s; TE = 1.64/3.5/5.36/7.22 ms; TI: 1.2s; FoV = 256 mm; 176 slices; voxel size = 1.0 × 1.0 × 1.0 mm). Scans were acquired for 29 ADHD and 25 control participants.
Images were first inspected by a neurologist for structural abnormalities, image artifacts or excessive motion. None were excluded. Subsequent image preprocessing and modeling were done using SPM12 software (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm).
2.3.1. Preprocessing
Voxel-based morphometry (VBM) analysis was performed on the final sample of 54. VBM is a voxel-wise method for comparing regional gray matter (GM) volume in different groups or experimental conditions (Ashburner and Friston, 2000, 2001). The T1-weighted images were first segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) probability maps using a generative model (Ashburner and Friston, 2005). Then, rigid body alignment was used to achieve approximate spatial alignment. Next DARTEL (Ashburner, 2007) was used with the resulting gray and WM images from each subject to iteratively estimate the nonlinear deformations resulting in the best group alignment and a series of progressively more accurate group average templates. Finally, the resulting individual deformation fields were combined with affine atlas alignment to generate spatially normalized and smoothed (8 mm 3D FWHM) Jacobian-scaled GM images in MNI space at 1.5 × 1.5 × 1.5 mm resolution. The resulting regional GM volume maps were subject to a quality assurance review using the VBM8 tools. The final DARTEL group template was transformed to MNI space using an affine transformation and used as a background image for structural identification.
2.3.2. Statistical analysis
Following preprocessing, statistical analysis was performed using general linear models. To correct for inter-subject variation in global brain size, the GM images were proportionally scaled using individual total brain volumes. To restrict the model fit to voxels containing brain tissue, the GM images were masked using an absolute GM threshold of 0.2.
Separate multiple regression models were used to test associations of regional GM volume with both total sway and the individual sway conditions (i.e., eyes open/feet apart, eyes open/feet together, eyes closed/feet apart and eyes open/shoes off). For each of these models, the center-of-pressure path length for each measure and subject were entered as predictors. The critical threshold for whole-brain analyses was p < 0.05, using family-wise error (FWE) correction. To facilitate future research, we also graphically report associations at an uncorrected critical threshold of p < 0.01.
3. Results
3.1. Sway
A 4 (Condition) × 2 (Group) mixed-model ANOVA revealed significantly higher sway for the ADHD group (M = 44.4 cm) compared to the healthy control group (M = 39.5 cm), F(1,58) = 5.47, p = .023, (Fig. 1; see Table 1 for group and condition means). This represents 12.4% greater sway for the ADHD group. Sway also differed between conditions, as indicated by a main effect of Condition, F(3, 174) = 23.15, p < .001. Consistent with previous data, higher sway was observed in the eyes-open/feet-together and eyes-closed/feet-apart conditions compared to the eyes-open/shoes-off and eyes-open/feet-apart conditions (ps < .001). The two conditions with the highest sway (eyes-open/feet-together and eyes-closed/feet-apart) did not differ from each other (p > .15); and the two conditions with the lowest sway (eyes-open/shoes-off and eyes-open/feet-apart) did not differ from each other (p > .25). The Group × Condition interaction was not significant (p > .4). In an exploratory analysis, sway did not differ between ADHD-combined type and ADHD-inattentive type (p > .2).
Fig. 1.
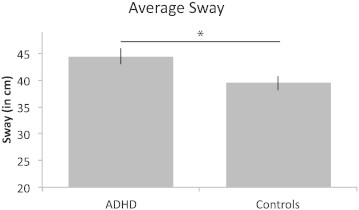
Average sway for ADHD and control groups. Error bars depict ±SEM. Adults with ADHD show greater sway than the controls. *Significantly different at p < .05.
Table 1.
Average sway (path length of COP in cm ± SD) for the ADHD and control groups and effect size (Cohen's d) for total sway and for each condition separately.
| Condition | ADHD group (n = 32) | Control group (n = 28) | Effect size (Cohen's d) |
|---|---|---|---|
| Total sway | 44.4 ± 9.0 | 39.5 ± 7.2 | .60 |
| Eyes open, feet apart | 41.4 ± 10.5 | 36.4 ± 8.7 | .52 |
| Eyes closed, feet apart | 49.6 ± 11.5 | 43.7 ± 10.5 | .54 |
| Eyes open, feet together | 46.1 ± 10.5 | 43.7 ± 7.9 | .25 |
| Eyes open, shoes off | 40.5 ± 12.5 | 34.3 ± 8.0 | .59 |
3.2. Sway and ADHD symptoms
Sway did not correlate significantly with ASRS scores within the ADHD or control groups, ps > .3.
3.3. Associations between sway and cerebellar regional volume
In an examination of associations between sway and cerebellar volume, the average sway showed a positive association with regional gray matter volume in the right posterior cerebellum, with a peak in lobule VIII (x = 17, y = −60, z = −51; cluster size = 1442 voxels; r = .43; FWE corrected, p < .05), (Fig. 2). The left posterior cerebellum showed a similar trend, r = .38, p > .05. When examining associations between each sway condition separately with cerebellar volume, the more difficult conditions, eyes closed/feet apart and eyes open/feet together, showed similar regional associations as seen with the average sway (Fig. 3). However, effects of sway were not seen in the biomechanically most stable conditions, including eyes open/feet apart with either shoes on or off.
Fig. 2.
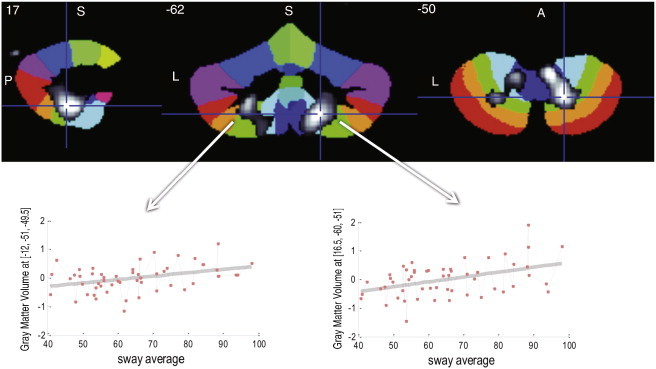
The average of the four sway conditions shows a positive association with regional gray matter volume in the right posterior cerebellum, including bilateral lobules VIIIa, VIIIb and IX. A similar trend is shown in the left posterior cerebellum. Overlay maps are shown in gray scale with t = 2.3–3.5, superimposed on the SUIT Atlas background (Diedrichsen, 2006). A = anterior; L = left; P = posterior; S = superior.
Fig. 3.
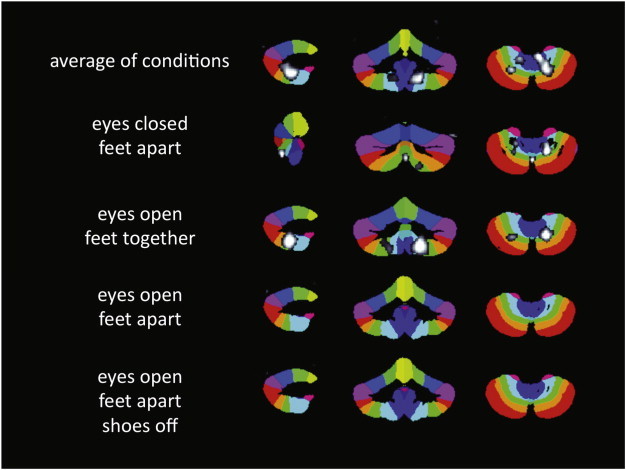
Regional cerebellar volume in posterior motor areas has a linear association with sway. The more difficult conditions, eyes closed/feet apart and eyes open/feet together, show similar regional distributions, with lobules VIIIa, VIIIb, IX and posterior vermis showing effects. Effects of sway are not seen in the biomechanically most stable conditions, eyes open/feet apart with shoes on or off. The overlay maps are shown in grayscale with t = 2.3–3.5, superimposed on the SUIT Atlas background.
4. Discussion
In this study, we observed higher postural sway in adults with ADHD relative to controls, and showed that sway was associated with regional cerebellar volume. These data extend to adults a motor control phenomenon previously documented in children with ADHD and provide further evidence for its neural substrates. Motor abnormalities have been associated with affective disturbances and the potential for greater injuries in children (Discala et al., 1998; Schoemaker and Kalverboer, 1994). Consequently, it is important to better understand these abnormalities in adults so that we can begin to assess and fully understand their clinical significance in the daily lives of ADHD patients across the lifespan.
Previous research has reported higher postural sway in children with ADHD during minimally demanding conditions such as standing on a fixed platform with eyes open. Even greater impairments have been seen in challenging conditions that disrupt sensory channels, such as standing with eyes closed or with sway-referenced platforms or visual surrounds (Buderath et al., 2009; Hassan and Azzam, 2012; Shum et al., 2009; Zang et al., 2002). As such, we expected to see atypical sway in adults with ADHD, as well as greater sway in the eyes closed and feet together conditions, as they have less sensory feedback and a smaller base of support relative to the other conditions. Our results demonstrate that overall sway was greater in adults with ADHD than in controls. They also showed that sway was greater in the more difficult conditions relative to the others, but only across groups, as there was no group by condition interaction. These results mapped nicely onto the findings with the structural imaging data, as discussed below.
Studies of postural control in ADHD have suggested cerebellar involvement (Buderath et al., 2009), but until now, have not actually demonstrated a relationship between postural control and cerebellar structure. Here, we demonstrated a relationship between postural sway and regional volume in the right cerebellar hemisphere, lobules VIII/IX. Additionally, when looking at the individual conditions, only the more difficult conditions showed the same pattern of association within lobules VIII/IX as did total sway, suggesting that these more difficult conditions are largely driving this effect.
Of note is the positive relationship between sway and cerebellar volume, such that lower sway was associated with smaller volume. These findings are consistent with a number of other studies showing that better performance in various motor-related tasks is related to smaller cerebral and cerebellar GM volumes. For example, cerebellar GM density in lobules VIII/IX (similar to our region) was smaller in trained ballet dancers relative to controls and was negatively correlated with dancing experience (Nigmatullina et al., 2015). Smaller cerebellar volumes were also observed in early trained musicians compared to later trained musicians and controls; furthermore, smaller volumes [of right lobule VI] were associated with better motor timing (Baer et al., 2015). Also, musical expertise was associated with lower GM density in brain regions associated with sensorimotor function, possibly reflecting more efficient and automated sensorimotor processes; whereas musical expertise was associated with greater GM density in regions associated with cognitive functions (James et al., 2014). It has been suggested that highly automated low-level sensorimotor processes could demand fewer neural resources and less sensory feedback (Baer et al., 2015; James et al., 2014). Given that postural sway is a highly automatic process and that lobule VIII is considered the secondary motor representation in the cerebellum (Bushara et al., 2001; Grodd et al., 2001; Rijntjes et al., 1999; Stoodley et al., 2012; Stoodley and Schmahmann, 2009, 2010; Woolsey, 1952), our findings are consistent with smaller volumes being associated with more efficient sensorimotor processes.
Considering our results in the context of the cognitive findings of James et al. (2014), who showed relatively greater GM density in regions associated with cognitive function, is interesting since mounting evidence indicates that most of the cerebellum is associated with non-motor or cognitive functions (see Buckner et al., 2011; Habas et al., 2009; O'Reilly et al., 2010; Schmahmann, 2010; Schmahmann and Sherman, 1998). It is tempting to speculate that one might expect impairments in ADHD cognitive functions to be associated with relatively smaller GM volume in cerebellar regions associated with cognitive processes. This could explain the often observed smaller cerebellar volumes in children with ADHD (Mostofsky et al., 1998; Valera et al., 2007). It could also explain why studies of ADHD samples with high IQs, equivalent to those of controls, have failed to show significant differences in total cerebellar volume (e.g., Seidman et al., 2006). Specifically examining such questions would be useful for future studies.
One could ask if the increased sway simply captures increased movement from hyperactivity or the inability to attend to and perform the task successfully. A number of factors argue against these explanations. First, sway did not correlate with ADHD symptoms within either group. This suggests that sway is not simply a consequence of inattentiveness or hyperactivity. Second, a recent study showed that sway was actually decreased in both children with ADHD and control children when not attending to a balance task, a condition which might allow for more automatic control of posture (Shorer et al., 2012). This suggests that increased sway in the ADHD group is unlikely to result from an inability to focus attention on the balance task. Previous research also shows that sway impairments were more pronounced when sensory signals are disrupted, suggesting abnormalities above and beyond hyperactivity issues (Buderath et al., 2009; Hassan and Azzam, 2012; Shum et al., 2009; Zang et al., 2002). Finally, other evidence has shown that children with ADHD respond to an applied force with higher (and shorter latency) muscle activity, indicating an inability to generate appropriate muscle force modulations for balance control (Kooistra et al., 2009). In sum, a number of reported abnormalities in children with ADHD as well as our current results suggest that sway likely reflects more foundational balance dysfunction in ADHD.
Overall, these data show that adults with ADHD manifest subtle abnormalities in postural sway. Other reports have linked hospital admissions from accidents with the presence of adult ADHD, and many of these patients were referred to as “clumsy” (Kaya et al., 2008). Though the reasons for such accidents are unknown, poor balance combined with ADHD inattention and impulsivity could play a role. These data could motivate further exploration of this explanation. Increasing awareness of this relatively subtle motor control abnormality could be of practical significance in helping to reduce accident rates in ADHD individuals. Also, given the potential of motor training and rehabilitation programs in other domains, such as Parkinson's disease (Nombela et al., 2013), an improved understanding of the motor impairments and their neural substrates in ADHD could encourage new treatment strategies that reduce the negative consequences of living with ADHD.
Several methodological limitations should be considered. First, participants did not perform highly challenging balance tasks such as standing on a sway-referenced platform or with a sway-referenced visual surround. Tasks that disrupt sensory information have yielded stronger balance deficits in children with ADHD. Thus, it is possible that the relatively unchallenging standing postures employed here did not sufficiently tax the postural maintenance system in a way that would expose maximal balance abnormalities. Second, although we focus on the cerebellum and more automatic postural control, balance control involves attentional resources and cognitive factors (Woollacott and Shumway-Cook, 2002), which we did not explicitly manipulate or measure. The attentional demands of balance control can be tested using dual task paradigms, and future work that varies task complexity could help dissociate attentional factors from more automatic balance control. Finally, while we quantified postural sway as the path length of the COP (as is often done), a more dynamic measure of postural instability could also be informative. For example, analyzing the center of mass' time-to-contact with the ‘stability margin’ could better capture stability threats relevant to falling (Forth et al., 2007).
Despite these considerations, our findings extend to adults with ADHD, observations previously documented only for children with ADHD. Thus, sway abnormalities in ADHD do not simply indicate delayed motor development, but instead represent enduring phenomena across the lifecycle. Additionally, we show that higher sway is associated with larger cerebellar regional volume in posterior motor areas, providing additional support for cerebellar involvement in the pathophysiology of ADHD. By increasing our understanding of motor abnormalities and their neural mechanisms in adults with ADHD, we provide a more complete understanding of the disorder, and how it manifests in adulthood.
Conflicts of interest
Dr. Eve Valera has received travel support and/or honoraria from Galenea, Eli Lilly, Shire Pharmaceuticals, and divisions of Ortho-McNeil Janssen Pharmaceuticals (McNeil Pediatrics and Janssen Pharmaceuticals), Remedica Medical Education and Publishing, MGH Psychiatry Academy for a tuition-funded CME course and consulting, Reed Exhibitions and Veritas Institute.
Dr. Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, AACAP, Alcobra, Forest Research Institute, Ironshore, Lundbeck, Magceutics Inc., Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion, Vaya Pharma/enzymotec, and NIH. In 2014, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2013, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy for a tuition-funded CME course. He received research support from APSARD, ElMindA, McNeil, and Shire. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Shire and Sunovion; these royalties were paid to the Department of Psychiatry at MGH.
Dr. Schmahmann receives royalties for book publishing from Oxford University Press, Elsevier, Academic Press and MacKeith Press; research funding from the National Institutes of Mental Health, the National Ataxia Foundation, the National Organization for Rare Disorders, and the Ataxia Telangiectasia Children's Project; medical consulting fees from Takeda Pharmaceuticals and Ataxion; and honoraria from academic institutions for visiting professorships and lectures. The Brief Ataxia Rating Scale is licensed to Dr. Schmahmann and the Massachusetts General Hospital. Dr. Schmahmann has no conflicts with this manuscript.
The other authors have no interests to declare.
Acknowledgments
We thank Dr. Vladimir Ivkovic for his insightful and helpful comments on versions of this manuscript. This work was supported by grants from the National Institutes of Health T32 MH16259 (to MJH) and R01 HD067744-01A1 (to EMV) and NCRR P41RR14075. This research was carried out in whole or in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant numbers 1S10RR023043 and 1S10RR023401.
References
- Adler L.A., Spencer T., Faraone S.V., Kessler R.C., Howes M.J., Biederman J., Secnik K. Validity of pilot Adult ADHD Self-report Scale (ASRS) to rate adult ADHD symptoms. Ann. Clin. Psychiatry. 2006;18(3):145–148. doi: 10.1080/10401230600801077. 16923651 [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. 17761438 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry — the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. 10860804 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Why voxel-based morphometry should be used. Neuroimage. 2001;14(6):1238–1243. doi: 10.1006/nimg.2001.0961. 11707080 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. 15955494 [DOI] [PubMed] [Google Scholar]
- Baer L.H., Park M.T., Bailey J.A., Chakravarty M.M., Li K.Z., Penhune V.B. Regional cerebellar volumes are related to early musical training and finger tapping performance. Neuroimage. 2015;109:130–139. doi: 10.1016/j.neuroimage.2014.12.076. 25583606 [DOI] [PubMed] [Google Scholar]
- Biederman J., Faraone S.V., Knee D., Munir K. Retrospective assessment of DSM-III attention deficit disorder in nonreferred individuals. J. Clin. Psychiatry. 1990;51(3):102–106. 2307663 [PubMed] [Google Scholar]
- Bucci M.P., Seassau M., Larger S., Bui-Quoc E., Gerard C.L. Effect of visual attention on postural control in children with attention-deficit/hyperactivity disorder. Res. Dev. Disabil. 2014;35(6):1292–1300. doi: 10.1016/j.ridd.2014.03.029. 24691355 [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. 21795627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buderath P., Gärtner K., Frings M. Postural and gait performance in children with attention deficit/hyperactivity disorder. Gait Posture. 2009;29(2):249–254. doi: 10.1016/j.gaitpost.2008.08.016. 18963991 [DOI] [PubMed] [Google Scholar]
- Bushara K.O., Wheat J.M., Khan A., Mock B.J., Turski P.A., Sorenson J., Brooks B.R. Multiple tactile maps in the human cerebellum. Neuroreport. 2001;12(11):2483–2486. doi: 10.1097/00001756-200108080-00039. 11496134 [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Lee P.P., Sharp W. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J. Am. Med. Assoc. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. 12365958 [DOI] [PubMed] [Google Scholar]
- Clark R.A., Bryant A.L., Pua Y., Mccrory P., Bennell K., Hunt M. Validity and reliability of the Nintendo Wii Balance Board for assessment of standing balance. Gait Posture. 2010;31(3):307–310. doi: 10.1016/j.gaitpost.2009.11.012. 20005112 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33(1):127–138. doi: 10.1016/j.neuroimage.2006.05.056. 16904911 [DOI] [PubMed] [Google Scholar]
- Discala C., Lescohier I., Barthel M., Li G. Injuries to children with attention deficit hyperactivity disorder. Pediatrics. 1998;102(6):1415–1421. doi: 10.1542/peds.102.6.1415. 9832578 [DOI] [PubMed] [Google Scholar]
- Faraone S.V., Biederman J., Spencer T., Mick E., Murray K., Petty C. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am. J. Psychiatry. 2006;163(10):1720–1729. doi: 10.1176/ajp.2006.163.10.1720. 17012682 [DOI] [PubMed] [Google Scholar]
- Faraone S.V., Sergeant J., Gillberg C., Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) American Psychiatric Publications; Washington, D.C.: 1997. [Google Scholar]
- Forth K.E., Metter E.J., Paloski W.H. Age associated differences in postural equilibrium control: a comparison between EQscore and minimum time to contact (TTC(min)) Gait Posture. 2007;25(1):56–62. doi: 10.1016/j.gaitpost.2005.12.008. 16464595 [DOI] [PubMed] [Google Scholar]
- Gelman A., Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; 2006. [Google Scholar]
- Grodd W., Hülsmann E., Lotze M., Wildgruber D., Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum. Brain Mapp. 2001;13(2):55–73. doi: 10.1002/hbm.1025. 11346886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. 19571149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan D.M., Azzam H. Sensory integration in attention deficit hyperactivity disorder: implications to postural control. In: Norvilit J.M., editor. Contemporary Trends in ADHD Research. InTech; Rijeka, Croatia: 2012. pp. 1–12. [Google Scholar]
- James C.E., Oechslin M.S., Van De Ville D., Hauert C.A., Descloux C., Lazeyras F. Musical training intensity yields opposite effects on grey matter density in cognitive versus sensorimotor networks. Brain Struct. Funct. 2014;219(1):353–366. doi: 10.1007/s00429-013-0504-z. 23408267 [DOI] [PubMed] [Google Scholar]
- Kaya A., Taner Y., Guclu B. Trauma and adult attention deficit hyperactivity disorder. J. Int. Med. Res. 2008;36(1):9–16. doi: 10.1177/147323000803600102. 18230262 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Adler L., Ames M. The World Health Organization Adult ADHD Self-report Scale (ASRS): a short screening scale for use in the general population. Psychol. Med. 2005;35(2):245–256. doi: 10.1017/s0033291704002892. 15841682 [DOI] [PubMed] [Google Scholar]
- Kooistra L., Ramage B., Crawford S., Cantell M., Wormsbecker S., Gibbard B., Kaplan B.J. Can attention deficit hyperactivity disorder and fetal alcohol spectrum disorder be differentiated by motor and balance deficits? Hum. Mov. Sci. 2009;28(4):529–542. doi: 10.1016/j.humov.2009.01.007. 19345435 [DOI] [PubMed] [Google Scholar]
- Makris N., Liang L., Biederman J. Toward defining the neural substrates of ADHD: a controlled structural MRI study in medication-naïve adults. J. Atten. Disord. 2013 doi: 10.1177/1087054713506041. 24189200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky S.H., Reiss A.L., Lockhart P., Denckla M.B. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. J. Child Neurol. 1998;13(9):434–439. doi: 10.1177/088307389801300904. 9733289 [DOI] [PubMed] [Google Scholar]
- Nigmatullina Y., Hellyer P.J., Nachev P., Sharp D.J., Seemungal B.M. The neuroanatomical correlates of training-related perceptuo-reflex uncoupling in dancers. Cereb. Cortex. 2015;25(2):554–562. doi: 10.1093/cercor/bht266. 24072889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela C., Hughes L.E., Owen A.M., Grahn J.A. Into the groove: can rhythm influence Parkinson's disease? Neurosci. Biobehav. Rev. 2013;37(10 Pt 2):2564–2570. doi: 10.1016/j.neubiorev.2013.08.003. 24012774 [DOI] [PubMed] [Google Scholar]
- O'Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20(4):953–965. doi: 10.1093/cercor/bhp157. 19684249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for Affective Disorder and Schizophrenia for School-age Children Epidemiologic Version. fifth edition. Nova Southeastern University Center for Psychological Studies; Ft Lauderdale, FL: 1994. [Google Scholar]
- Pitcher T.M., Piek J.P., Hay D.A. Fine and gross motor ability in males with ADHD. Dev. Med. Child Neurol. 2003;45(8):525–535. doi: 10.1017/s0012162203000975. [DOI] [PubMed] [Google Scholar]
- Rijntjes M., Buechel C., Kiebel S., Weiller C. Multiple somatotopic representations in the human cerebellum. Neuroreport. 1999;10(17):3653–3658. doi: 10.1097/00001756-199911260-00035. 10619661 [DOI] [PubMed] [Google Scholar]
- Rowe R., Simonoff E., Silberg J.L. Psychopathology, temperament and unintentional injury: cross-sectional and longitudinal relationships. J. Child Psychol. Psychiatry. 2007;48(1):71–79. doi: 10.1111/j.1469-7610.2006.01674.x. 17244272 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol. Rev. 2010;20(3):236–260. doi: 10.1007/s11065-010-9142-x. 20821056 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Sherman J.C. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. 9577385 [DOI] [PubMed] [Google Scholar]
- Schoemaker M., Kalverboer A. Social and affective problems of children who are clumsy: how early do they begin? Adapt. Phys. Activ. Q. 1994;11(2):130–140. [Google Scholar]
- Seidman L.J., Valera E.M., Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57(11):1263–1272. doi: 10.1016/j.biopsych.2004.11.019. 15949998 [DOI] [PubMed] [Google Scholar]
- Seidman L.J., Valera E.M., Makris N., Monuteaux M.C., Boriel D.L., Kelkar K. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol. Psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. 16876137 [DOI] [PubMed] [Google Scholar]
- Shorer Z., Becker B., Jacobi-Polishook T., Oddsson L., Melzer I. Postural control among children with and without attention deficit hyperactivity disorder in single and dual conditions. Eur. J. Pediatr. 2012;171(7):1087–1094. doi: 10.1007/s00431-012-1695-7. 22350284 [DOI] [PubMed] [Google Scholar]
- Shum S.B.M., Pang M.Y.C. Children with attention deficit hyperactivity disorder have impaired balance function: involvement of somatosensory, visual, and vestibular systems. J. Pediatr. 2009;155(2):245–249. doi: 10.1016/j.jpeds.2009.02.032. 19446843 [DOI] [PubMed] [Google Scholar]
- Spencer T.J., Biederman J., Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J. Pediatr. Psychol. 2007;32(6):631–642. doi: 10.1093/jpepsy/jsm005. 17556405 [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. 18835452 [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. 20152963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Valera E.M., Schmahmann J.D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59(2):1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. 21907811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E.M., Brown A., Biederman J., Faraone S.V., Makris N., Monuteaux M.C., Whitfield-Gabrieli S., Vitulano M., Schiller M., Seidman L.J. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry. 2010;167(1):86–94. doi: 10.1176/appi.ajp.2009.09020249. 19884224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. 16950217 [DOI] [PubMed] [Google Scholar]
- Valera E.M., Spencer R.M., Zeffiro T.A., Makris N., Spencer T.J., Faraone S.V., Biederman J., Seidman L.J. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68(4):359–367. doi: 10.1016/j.biopsych.2010.05.012. 20619827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio TX: 1999. [Google Scholar]
- Woollacott M., Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. 12127181 [DOI] [PubMed] [Google Scholar]
- Woolsey C.N. Summary of the papers on the cerebellum. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1952;30:334–336. [PubMed] [Google Scholar]
- Zang Y., Gu B., Qian Q., Wang Y. Objective measurement of the balance dysfunction in attention deficit hyperactivity disorder children. Chin. J. Clin. Rehabil. 2002;6:1372–1374. [Google Scholar]


