Abstract
A well-characterized neural network is associated with motor learning, involving several brain regions known to have functional and structural deficits in persons with multiple sclerosis (PwMS). However, it is not known how MS affects postural motor learning or the neural networks involved. The aim of this study was to gain a better understanding of the neural networks underlying adaptation of postural responses within PwMS. Participants stood on a hydraulically driven, servo-controlled platform that translated horizontally forward and backward in a continuous sinusoidal pattern across multiple trials over two consecutive days. Our results show similar postural adaptation between PwMS and age-matched control participants despite overall deficits in postural motor control in PwMS. Moreover, PwMS demonstrated better retention the following day. PwMS had significantly reduced functional connectivity within both the cortico-cerebellar and cortico-striatal motor loops; neural networks that subserve implicit motor learning. In PwMS, greater connectivity strength within the cortico-cerebellar circuit was strongly related to better baseline postural control, but not to postural adaptation as it was in control participants. Further, anti-correlated cortico-striatal connectivity within the right hemisphere was related to improved postural adaptation in both groups. Taken together with previous studies showing a reduced reliance on cerebellar- and proprioceptive-related feedback control in PwMS, we suggest that PwMS may rely on cortico-striatal circuitry to a greater extent than cortico-cerebellar circuitry for the acquisition and retention of motor skills.
Highlights
-
•
Postural motor learning is poorly characterized in people with multiple sclerosis (PwMS).
-
•
PwMS showed reduced communication within both the cortico-cerebellar and cortico-striatal motor loops.
-
•
Anti-correlated cortico-striatal connectivity was strongly related to better postural adaptation in PwMS.
-
•
PwMS may preferentially rely on feed-forward strategies for motor control and learning.
1. Introduction
Mobility impairments are often the most visible symptom of multiple sclerosis and serve as the clinical hallmark of the disease. The decline of mobility in persons with MS (PwMS) is associated with a multitude of adverse outcomes including disability, falls and mortality (Prosperini et al., 2011; Zwibel, 2009). Of utmost importance, loss of mobility has been identified as the primary concern related to functional independence and quality of life in PwMS (Heesen et al., 2008). While a variety of pharmacological therapies are successful at reducing the number of relapses and delaying disease progression, effective rehabilitation to address the existing and evolving sensorimotor deficits remains elusive.
The acquisition and retention of motor skills are pervasive to daily life and are required to perform basic and complex motor behaviors. Implicit learning refers to the “effortless acquisition” of information without explicit knowledge of what has been absorbed (Reber, 1989). Although recent work demonstrated that deficits in sequence-specific motor learning for PwMS were more pronounced during implicit conditions (Tacchino et al., 2014), motor sequence learning in general has been poorly characterized in PwMS. A diminutive literature suggests that PwMS maintain the ability for motor skill acquisition, despite impaired overall motor performance (Bonzano et al., 2011; Tomassini et al., 2011). These studies have principally focused on upper extremity tasks, thus the extrapolation to whole body motor learning remains unclear.
While multiple neural regions likely underlie this process, experimental (Doyon and Benali, 2005; Seitz et al., 1990) and theoretical (Doyon et al., 2003; Hikosaka et al., 2002) studies have demonstrated substantial involvement of cortico-striatal and cortico-cerebellar circuits that appear to work in parallel to mediate implicit learning (Seidler et al., 2013). Specifically, the cortico-cerebellar network is recruited during the early stages of learning when movement kinematics must be adapted via sensory feedback (Doyon et al., 2003; Hikosaka et al., 2002). When performance is more automated in the later stages of learning, neuronal activity is shifted to cortico-striatal circuits where the striatum is thought to play a critical role in storage and retention of motor programs (Doyon et al., 2009; Penhune and Steele, 2012). Our previous work showed that postural response latencies in PwMS were related to impaired scaling response magnitude based on feedback control, but improved scaling of response magnitude based on feed-forward control (Cameron et al., 2008). As a result, we suggest that PwMS may preferentially rely on feed-forward strategies for motor control.
The implication of functional brain alterations on disease progression, motor deficits, and overall quality of life in clinical populations is a topic of great interest. Mounting evidence reveals that reduced structural connectivity and functional neural connectivity are related to movement deficits in PwMS (Bonzano et al., 2008; Bonzano et al., 2015; Fling et al., 2014b; Petsas et al., 2014). Several studies report altered functional connectivity of the motor network in PwMS (Janssen et al., 2013; Lowe et al., 2002), however; little is known about how these differences in functional communication contribute to lower limb dysfunction. The current study provides an improved understanding of the neural networks underlying postural response adaptation within PwMS. We hypothesized that PwMS would demonstrate similar postural adaptation to age-matched controls despite overall deficits in postural motor control. Further, we hypothesized that PwMS would have significantly reduced functional connectivity within the cortico-cerebellar network underlying motor learning, contributing to an increased reliance on feed-forward postural control.
2. Materials and methods
2.1. Participants
Twenty-four individuals with relapsing–remitting MS [21 females; 48.4 years old (11.0); disease duration = 12.2 (7.5)] were recruited through the Multiple Sclerosis Center of Oregon at Oregon Health & Science University (OHSU). Fourteen age-matched healthy controls [11 females; 46.9 years old (13.4)] were also recruited from the surrounding Portland, OR area. Participants were excluded if they could not: safely walk 20 feet without walking aids, had a joint replacement, musculoskeletal or vestibular disorder, dementia, claustrophobia, severe tremor or had metal in their body. The Expanded Disability Status Scale (EDSS) was utilized to assess clinical disability in PwMS, all participants were required to have a value less than 4.0 indicating that the individual was fully ambulatory and could walk at least 500 m without aid [median EDSS = 4 (range: 2–4)]. Thus our cohort of PwMS was relatively mobile, experienced mild to moderate functional limitations, and performed similar levels of physical activity (4.6 h per week) compared to the control group (4.8). OHSU's Institutional Review Board approved this study and all participants gave their informed written consent prior to beginning the experiment.
2.2. Postural motor adaptation task
Using procedures similar to previous work from our laboratory, participants stood on a hydraulically driven, servo-controlled platform that could be translated horizontally forward and backward (Van Ooteghem et al., 2009). To prevent falls without restricting motion, participants wore an industrial safety harness tethered to a sliding hook on an overhead rail (Fig. 1A). They were instructed to maintain balance while standing with eyes focused on a poster approximately 2-m straight ahead and arms crossed at the chest. Trials were conducted over 48-s of a platform oscillation to induce implicit learning. The same sequence was repeated on each trial. The platform oscillated at a fixed frequency of 0.5 Hz in a sinusoidal fashion of variable amplitudes with the largest amplitude scaled to the maximum that participants could withstand without taking a step as determined by a 20-s, constant amplitude practice trial (range: 7–15 cm) (Van Ooteghem et al., 2010) (Fig. 1B). To decrease the likelihood of a step or fall, the platform was offset forward by 6 cm at the start of each trial and the first movement of the platform was in the backward direction. Testing consisted of five blocks of five trials with rest periods provided between blocks and was performed in the morning to mitigate fatigue. To separate temporary performance effects from more permanent changes in behavior reflecting learning, participants returned for two, five-trial blocks approximately 24 h following practice to assess long-term adaptation.
Fig. 1.
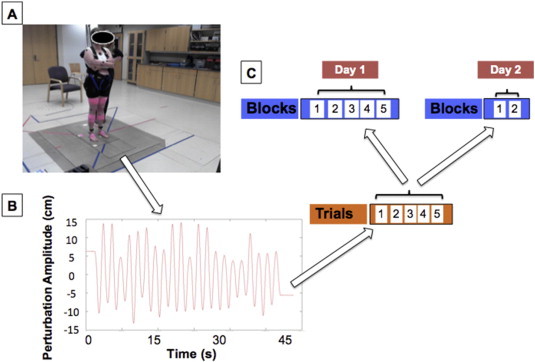
Experimental setup depicting a participant ready to begin the postural adaptation paradigm (A). The continuous, sinusoidal pattern of oscillation for the force platform in the anterior–posterior direction comprising one, 45-s trial (B). Data collection structure for postural adaptation over two consecutive days (C).
2.2.1. Data recording
A Motion Analysis System (Santa Rosa, CA, USA) with eight cameras captured three-dimensional spatial coordinate information regarding body segment displacements and platform motion. Reflective markers were placed bilaterally on the following anatomical landmarks: fifth metatarsophalangeal, lateral malleolus, lateral femoral condyle, greater trochanter, anterior superior iliac spine, iliac crest, styloid process, olecranon, acromium process, lateral mandibular joint, cervical 7th and lumbar 5th levels and above the sternal notch. A marker was also placed on the back of the platform. Data were sampled at 60 Hz and low pass filtered using a 2nd order, dual pass Butterworth filter with a cut-off frequency of 5 Hz. The position of the center of mass (COM) of each body segment in the antero-posterior (AP) direction was calculated using kinematic data and anthropometric data (Winter, 2009). Whole body COM position (in space) in the AP direction was derived from the weighted sum of the individual segment COM locations. Relative phase lag of the COM (COM time peak/platform time peak) was calculated using the time values of the peaks to compute a point estimate of maximum COM relative to maximum platform position on a cycle-by-cycle basis (Zanone and Kelso, 1992). These values were averaged for each segment within a trial to determine mean relative phase lag. In line with previous work (Van Ooteghem et al., 2009) we considered reduced phase lag of the body COM relative to platform motion as an indication of an improved, and more predictive, postural control strategy.
2.2.2. Data analysis
Relative phase lag was compared across all blocks to assess postural adaptation performance using a two-way [Group (2) × Block (6)] repeated measures ANOVA. Because no within-subject differences were observed via a paired t-test (P = 0.10) between the adaptation blocks on day two, data were averaged to provide one value of long-term adaptation. Post-hoc analyses were conducted with independent sample t-tests. To assess short- and long- term adaptation within each group we performed two planned comparisons: relative phase lag from the initial block of day one with i) relative phase lag during the final block of day one (short-term) and ii) the averaged phase lag during retention testing on day two (long-term) via independent sample t-tests and Bonferroni-corrected for multiple comparisons (α = 0.05/2). For all tests, an acceptable significance level was 0.05 unless otherwise noted.
2.3. Image acquisition and processing
Within 2 weeks of behavioral testing participants were scanned on a 3.0 Tesla Siemens Magnetom Tim Trio scanner with a 12-channel head coil at Oregon Health and Science University's Advanced Imaging Research Center. One high-resolution T1-weighted MP-RAGE sequence (orientation = sagittal, echo time = 3.58 ms, repetition time = 2300 ms, 256 × 256 matrix, resolution 1.0 × 1.0 × 1.1 mm, total scan time = 9 min 14 s) was acquired. One BOLD-weighted functional image was acquired with a T2*-weighted EPI (repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, field of view = 240 mm, 33 slices covering the whole brain, resolution = 3.8 mm3, total volumes = 300, total scan time = 10 min 6 s). Steady-state magnetization was assumed after three volumes (~6 s). Subjects were instructed to remain still and fixate on a standard fixation cross, projected in the center of their visual field. To minimize variability all imaging was performed in the morning.
Functional images were preprocessed using methods shown to alleviate known types of artifacts (Miezin et al., 2000). These steps included 1) removal of central spike caused by MR signal offset, 2) correction of odd versus even slice intensity differences due to interleaved acquisition without gaps, 3) correction for head movement within the run, and 4) within-run intensity normalization to a whole brain mode value of 1000. Atlas transformation of the functional data was computed for each individual via the MP-RAGE scan (Talairach and Tournoux, 1988). The functional data were then resampled into atlas space on an isotropic 3-mm grid, combining movement correction and atlas transformation in one interpolation (Lancaster et al., 1995). All subsequent operations were performed on the atlas-transformed volumetric time series.
Connectivity preprocessing followed prior methods from our laboratory (Fling et al., 2014a). These steps included: 1a) a temporal band-pass filter (0.009 Hz < f < 0.08 Hz), 1b) spatial smoothing (6 mm full width at half maximum), 2) regression of six parameters obtained by rigid body head motion correction, 3) regression of the whole brain signal averaged over the whole brain, 4) regression of ventricular signal averaged from ventricular region of interest (ROI), and 5) regression of white matter signal averaged from white matter ROI. Regressions of the first order derivatives from steps 3–5 were also included in the correlation preprocessing. These preprocessing steps are thought to best reduce variance that is unlikely to reflect neuronal activity (Fox and Raichle, 2007).
2.3.1. Motion correction
To further account for inter-acquisition subject motion that could potentially be problematic for correlation analysis, an additional motion correction step was implemented as previously described (Power et al., 2012). This method, titled framewise displacement (FD), calculates a time series of volume-to-volume motion from the movement measures created by the aforementioned rigid body motion correction. This is calculated for all six parameters by the equation FDi = |Δdix| + |Δdiy| + |Δdiz| + |Δαi| + |Δβi| + |Δγi|, where Δdix = d(i − 1)x − dix, (the same holds true for the other five parameters [dix diy diz αi βi γi]). Essentially, this formula sums the absolute values of the volume-to-volume changes in six directions. The rotational measures were first converted to millimeters by calculating surface displacement on a sphere of radius 50 mm, which is the approximate distance from the cerebral cortex to the center of the head. This method ‘scrubs’ the data by removing any volume that exceeds a set threshold (in this case 0.5 mm) from the time series. The correlation analysis was performed on the remaining concatenated volumes. If greater than 50% of the functional volumes (>150 out of 300) exceeded the threshold, participants were excluded from analysis. No participants in the current study were excluded due to excessive movement (mean volumes exceeding threshold = 8.3 ± 4.6%).
2.3.2. fcMRI region of interest selection
Because we were interested in the ability to learn adaptive postural responses to continuous postural perturbations we focused our connectivity analysis on the motor network, with a specific emphasis on regions of the brain underlying lower extremity control. All fcMRI time-series analyses were performed using fidl (v. 2.64). The primary motor cortex serves as the principal cortical output region and has been repeatedly shown to be involved in motor sequence learning and the retention of motor memories (Karni et al., 1995; Muellbacher et al., 2002). Whole brain group comparisons were performed to identify differences in functional connectivity strength from the right and left leg areas of the primary motor cortex (M1). In both the right and left hemispheres, a 10-mm sphere was created in Talairach space consistent with the leg region of the M1, centered at x = ±20, y = 25, z = 57 (Saisanen et al., 2008). Groups were compared via a two sample; random effects t-test and were Monte-Carlo corrected for multiple comparisons and were subsequently thresholded at Z > 2.25; P < 0.05.
2.3.3. Associations between motor fcMRI and postural adaptation
For neural regions showing group differences that have previously been implicated in implicit sequence learning we performed post-hoc regression analyses to identify associations between functional connectivity strength of leg motor networks and performance on postural adaptation. This included regions within the bilateral caudate nuclei and the anterior cerebellum. Regression analysis was performed between the described neural regions and performance during I) the first block of postural adaptation (baseline; Block 1), II) short-term postural adaptation (Δ Block 5–Block 1) and III) long-term postural adaptation (Δ Retention–Block 1). Regressions were Bonferonni-corrected for multiple comparisons α = 0.05/3. Similar regression analysis was performed for a control ROI, the left parietal lobe, where group differences in connectivity strength were found, but an area not traditionally associated with implicit learning or motor control.
3. Results
3.1. Postural motor adaptation task
As hypothesized, both groups showed substantial improvement across the five blocks of postural perturbation on day one. We report a main effect for Block (F(4,144) = 57.8; P < 0.001) and Group (F(1,36) = 45.9; P < 0.001), but no Block × Group interaction (F(4,144) = 0.2; P > 0.9). Post hoc t-tests demonstrate a significantly greater relative phase lag for PwMS across all five blocks of adaptation, as well as on retention tests (t(1,36) > 2.5; P < 0.02 for all comparisons). Nearly all participants improved performance for both short-term (Fig. 2A) and long-term adaptation (Fig. 2B). Thus, we report no group differences in short- (t(1,36) > −0.3; P > 0.77) or long-term postural adaptation (t(1,36) > −2.0; P > 0.05), although PwMS did tend to demonstrate slightly better retention, relative to their baseline performance (Fig. 2C and D). Taken together, these behavioral results suggest that absolute performance is impaired in PwMS, but not the relative capacity for adaptation.
Fig. 2.
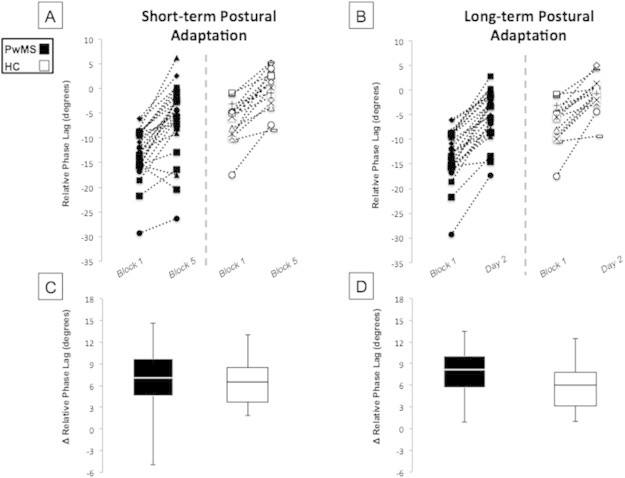
Performance of the implicit postural motor adaptation task in PwMS and HC. Data points are shown for each participant with their performance connected by a dashed line for both short (A) and long-term (B) adaptation. Box and whisker plots depicting mean group performance for short- (C) and long-term (D) adaptation. Relative phase lag was different between groups at Block 1, Block 5 and Day two, but no significant group differences were found for either short- or long-term adaptation. PwMS tended to display greater retention for long-term adaptation (P = 0.06).
3.2. Leg motor network connectivity strength
Consistent with the literature, we report a well-defined functional motor network in both PwMS and age-matched controls when seeded in the leg region(s) of M1 (Fig. 3A–F). As traditionally noted, this network is defined by strong cortical intra- and inter-hemispheric communication between the supplementary motor areas, pre-motor areas, as well as the primary motor and somatosensory cortices. In addition, functional connectivity between the motor cortices and the thalamus is well-defined within our populations.
Fig. 3.
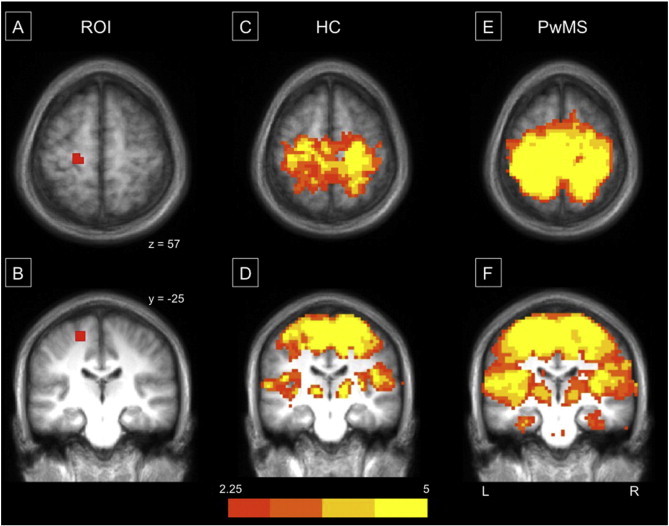
Motor connectivity maps identified from seed regions within the leg region of the left primary motor cortex (M1). Representation of the seed region placed in the left hemisphere's leg region of M1 (A & B). Panels C–F reflect group connectivity maps from HC (C & D) and PwMS (E & F), respectively. All group maps are Monte Carlo corrected and thresholded at Z > 2.25. Data are overlaid on an averaged 1 mm Talairach anatomical template for display. An identical ROI was placed in the right M1, data not shown.
When comparing whole-brain connectivity strength of the left leg M1 between groups, several regions of reduced connectivity in PwMS were found including the right caudate nucleus as well as the anterior, midline cerebellum (anterior lobe V; Fig. 4A) and multiple regions in the right cerebellar hemisphere, compared to HC (Table 1). Reduced connectivity was also observed in the right hemisphere of PwMS between the right M1 and bilateral caudate nuclei (Fig. 5A), bilateral posterior cerebellar lobes, left precuneus and the left pallidum. While no regions demonstrated greater connectivity within PwMS compared to HC, a qualitative view of the thresholded and multiple-comparison corrected group maps suggests that the specificity of the motor network appears to be substantially degraded in PwMS (Fig. 3E & F). This is reflected by similar connectivity strength across the entire motor network in PwMS as opposed to the more nuanced network observed in HC (Fig. 3C & D).
Fig. 4.
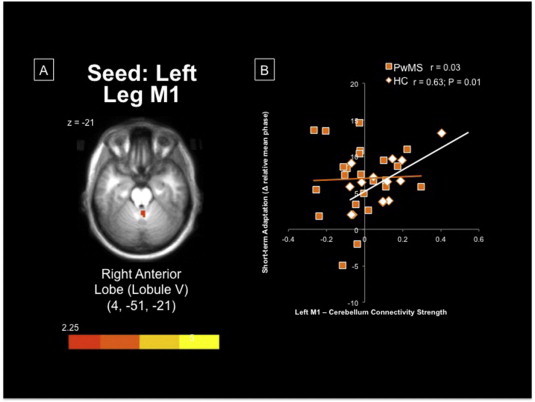
(A) Midline cerebellar region demonstrating significantly greater connectivity with left M1 within HC, compared to PwMS. (B) Greater connectivity between left leg M1 and midline, anterior lobe of the cerebellum was associated with better adaptation, particularly within HC. Imaging data are overlaid on an averaged 1 mm Talairach anatomical template for display. M1 = primary motor cortex; PwMS = persons with multiple sclerosis; HC = healthy controls.
Table 1.
Regions showing group differences in fcMRI strength to the leg region of right and left M1, respectively. HC show greater connectivity than PwMS from between M1 and multiple regions. No regions of greater connectivity for PwMS were found within either hemisphere.
| x | y | z | Z | P (2-tailed) | ||
|---|---|---|---|---|---|---|
| Right leg M1 | HC > MS | |||||
| L parietal lobe (precuneus) | −44 | −74 | 35 | 3.86 | 0.00011 | |
| R caudate | 8 | 12 | 11 | 3.48 | 0.00051 | |
| L caudate | −9 | 13 | 8 | 2.79 | 0.0052 | |
| L globus pallidum interior | −19 | 1 | −6 | 2.75 | 0.006 | |
| R cerebellum | ||||||
| Posterior lobe (semi-lunar lobule) | 45 | −73 | −35 | 2.77 | 0.0056 | |
| L cerebellum | ||||||
| Posterior lobe (pyramis) | −40 | −74 | −32 | 3.03 | 0.0024 | |
| Left leg M1 | HC > MS | |||||
| R caudate | 8 | 5 | 17 | 2.47 | 0.013 | |
| R cerebellum | ||||||
| Anterior lobe (V) | 4 | −51 | −21 | 2.46 | 0.014 | |
| Anterior lobe | 11 | −47 | −30 | 2.50 | 0.013 | |
| Anterior lobe (nodule) | 10 | −59 | −30 | 2.77 | 0.0055 | |
| Posterior lobe (tonsil) | 34 | −45 | −37 | 3.58 | 0.00034 | |
Fig. 5.
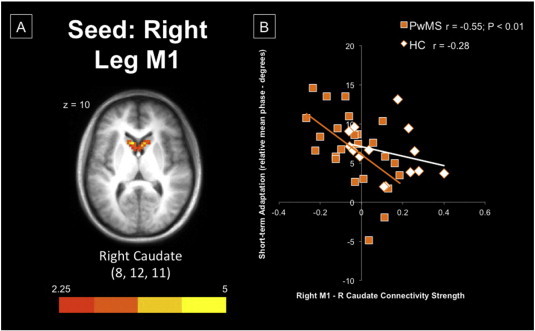
(A) Bilateral caudate regions demonstrating significantly greater connectivity with right M1 within HC, compared to PwMS. (B) Greater connectivity within the right hemisphere's cortico-striatal loop was associated with poorer adaptation, particularly within PwMS. Imaging data are overlaid on an averaged 1 mm Talairach anatomical template for display. M1 = primary motor cortex; PwMS = persons with multiple sclerosis; HC = healthy controls.
3.3. Associations between motor fcMRI and postural motor adaptation
We performed regression analyses between postural motor adaptation performance and those areas showing group differences in connectivity strength between the leg M1 ROIs and neural regions previously implicated in implicit motor learning. Thus we analyzed LM1 — the anterior, midline cerebellum, LM1 — right caudate, and RM1 — bilateral caudate nuclei (Table 2). It is important to note that the midline cerebellar region identified in the current study is nearly identical to the region identified in a recent ALE meta-analysis that underlies implicit motor learning (Bernard and Seidler, 2013).
Table 2.
Associations between I) baseline, II) short- and, III) long-term postural adaptation with functional connectivity strength of leg motor network.
| HC |
PwMS |
|||||||
|---|---|---|---|---|---|---|---|---|
| LM1-Cb | LM1-RCaud | RM1-RCaud | RM1-LCaud | LM1-Cb | LM1-RCaud | RM1-RCaud | RM1-LCaud | |
| Baseline | −0.35 | 0.13 | 0.52 | 0.44 | 0.62** | −0.15 | −0.05 | 0.09 |
| Short-term adaptation | 0.63* | 0.24 | −0.28 | −0.28 | 0.10 | −0.36 | −0.55** | −0.05 |
| Long-term adaptation | 0.48 | 0.04 | −0.41 | −0.32 | −0.19 | −0.14 | −0.20 | −0.006 |
Cb = cerebellum; M1 = primary motor cortex; Caud = caudate.
Significant correlations are highlighted in bold.
P < 0.05.
P < 0.01.
In PwMS, cortico-cerebellar connectivity was positively related to baseline performance (r = 0.62; P = 0.013; Fig. 4B), but not correlated with either short- or long-term postural adaptation. Conversely, within HC we report a strong positive correlation between cortico-cerebellar connectivity and short-term postural adaptation (r = 0.63; P = 0.012), and to a lesser extent with long-term postural adaptation (r = 0.48; P = 0.06). There was relatively weak correlation strength between postural motor adaptation and motor cortico-striatal connectivity. Anti-correlated communication along the cortico-striatal loop within the right hemisphere was strongly related to better short-term postural adaptation in PwMS (r = −0.55; P = 0.017; Fig. 5B). It is interesting to note that right cortico-striatal connectivity was positively correlated to baseline performance (r = 0.52), but negatively related to short- and long-term adaptation in HC, similar to the association in PwMS.
Lastly, connectivity strength between the right leg M1 region and the left parietal lobe showed no association with postural adaptation for baseline, short-term, or long-term adaptation for either HC or PwMS (r < 0.11; P > 0.5 for all comparisons).
4. Discussion
Consistent with our hypotheses, we report similar postural adaptation between PwMS and age-matched healthy control participants despite overall deficits in postural motor control for PwMS. Moreover, PwMS demonstrated noticeably better retention/long-term adaptation than their healthy counterparts. In addition, PwMS had reduced specificity of the motor network as well as significantly reduced functional connectivity within both the cortico-cerebellar and cortico-striatal motor loops; neural networks subserving implicit motor learning. In PwMS, greater connectivity strength within the cortico-cerebellar circuit was strongly related to better baseline postural control, but not to adaptation as it was in control participants, suggesting that PwMS may rely on alternate neural circuitry for postural motor learning. Finally, cortico-striatal connectivity was negatively related to postural adaptation in both groups, indicating that implicit learning is maximized when the supporting motor and cognitive circuits remain weakly associated, or anti-correlated at rest.
The literature regarding motor learning in PwMS is sparse and conflicting. Interventions of variable length in PwMS demonstrate improvements in upper (Casadio et al., 2008) and lower limb performance (Baram and Miller, 2006; Hatzitaki et al., 2006) comparable to those observed in age-matched controls. These studies suggest that the ability of MS patients to learn motor skills is preserved across a wide range of disability (Tomassini et al., 2011). Conversely, more recent work suggests deficits in sequence-specific motor learning of the upper extremities for PwMS with more pronounced impairment during implicit conditions (Tacchino et al., 2014) or on tasks requiring complex sensory integration (Leocani et al., 2007). In the current work, we report similar implicit postural motor learning in PwMS despite substantial deficits in postural motor control at baseline. However, PwMS vary widely in their ability to learn to optimize their postural control, potentially as a result of the differential brain–behavior relationships observed between PwMS and control participants.
A well-known neural network underlies motor learning with activity in the motor cortices and the cerebellum decreasing as learning progresses, whereas striatal activity increases in the later stages of learning (Lehéricy et al., 2005; Seidler et al., 2005; Steele and Penhune, 2010). Excitatory stimulation of the M1 facilitates motor learning (Nitsche et al., 2003; Schambra et al., 2011) and retention (Galea et al., 2011; Reis et al., 2009), whereas inhibitory stimulation disrupts consolidation during the early stages of learning (Muellbacher et al., 2002; Wilkinson et al., 2010). While no regions demonstrated significantly greater connectivity among PwMS, the specificity of the motor network appears to be substantially degraded in PwMS. Similar results, often termed “dedifferentiation”, have been reported in the healthy aging literature with older adults demonstrating a loss of motor network specificity with limited compensatory effects (Langan et al., 2010; Seidler et al., 2010). This increased activation/connectivity may be the result of decreased interhemispheric inhibition via the corpus callosum. Transcranial magnetic stimulation studies have shown that healthy older adults (Fling and Seidler, 2012) and PwMS (Manson et al., 2006; Wahl et al., 2011) display less excitability of intra- and interhemispheric inhibitory circuits than young adults. Thus, increased connectivity/activation within the motor network may not be related to functional task demands and does not necessarily reflect reorganization to compensate for the neurobiological changes of disease or aging. While there was an overall loss of nuanced motor connectivity within PwMS, we also report significantly reduced connectivity strength within the cortico-cerebellar and cortico-striatal circuitry of PwMS.
The cerebellum has been hypothesized to serve a variety of roles in motor and cognitive performance (Bernard and Seidler, 2013), but a general consensus agrees that it is relevant for motor learning via the formation of internal models (Wolpert et al., 1998) as well as the generation of prediction errors (Ohyama et al., 2003). Recent work has shown that the cortico-cerebellar loop plays a distinctive role in implicit motor learning (Celnik, 2015; Tzvi et al., 2014). Activating Purkinje cells in the anterior lobe of the cerebellum inhibits the deep cerebellar nuclei's disynaptic excitatory connection with M1 resulting in the temporary inhibition of M1 (Werhahn et al., 1996). Locomotor learning has been associated with a decrease in the magnitude of the inhibitory tone the cerebellum exerts over M1, i.e. individuals learning the most experienced the largest reduction in cerebellar inhibitory output (Jayaram et al., 2011). Bonzano and colleagues (2015) recently showed that greater (motor) cortico-cerebellar connectivity (i.e. less inhibition) was correlated with learning on a finger-tapping task in PwMS. This is in agreement with our current results demonstrating that greater cortico-cerebellar connectivity strength was associated with better short-term postural adaptation in healthy participants. Greater cortico-cerebellar connectivity was predictive of baseline performance in PwMS, but was not related to their short- or long-term adaptation.
Pathological changes in the cerebellar cortex of PwMS are well characterized (Anderson et al., 2009; Gilmore et al., 2009; Kutzelnigg et al., 2007). Dysfunctional neural integration in the cerebellum of PwMS is supported by positron emission tomography studies reporting bilateral reductions in cerebellar resting-state glucose metabolism in early relapsing–remitting MS (Blinkenberg et al., 2000; Derache et al., 2006). Moreover, fMRI studies have reported decreased connectivity during motor tasks involving cerebellar regions in PwMS (Rocca et al., 2009; Saini et al., 2004). Recent work also indicates a reduced reliance on slowed, somatosensory feedback (Cameron et al., 2008) along the spinocerebellar pathways in PwMS. Taken together, these results indicate that while cortico-cerebellar circuitry is essential for maintaining postural control and balance, it does not appear to be a neural loop relied upon by PwMS for postural motor learning. Reduced and/or delayed somatosensory feedback may result in an increased reliance on feed-forward control via the basal ganglia in PwMS.
The caudate nuclei, and the striatum in general, play a key role in implicit learning. This likely involves learning predictive associations between the individual movements in the sequence (Penhune and Steele, 2012), as well as gradually fragmenting repeated and convergent sequence information into chunks (Bo and Seidler, 2010; Boyd et al., 2009), a mechanism known to be highly efficient for information processing and memory. In the current study, anti-correlated cortico-striatal connectivity and short-term adaptation were strongly related in PwMS. Although we did not expect to find negative correlations between motor connectivity and postural adaptation, our results are in agreement with recent work revealing that connectivity between the dorsal caudate and both the pre- and post-central gyrus was negatively correlated with sequence learning in college-aged adults (Stillman et al., 2013). The authors speculated that the superior learning observed in individuals with anti-correlated resting-state connectivity may reflect that motor learning is maximized when the supporting motor and cognitive circuits remain disassociated at rest, allowing for segregation of motor and cognitive networks and facilitating more efficient adaptation (Stillman et al., 2013). An alternative, though not mutually exclusive interpretation can be drawn from recent literature demonstrating a strong positive association between (motor) cortico-striatal connectivity and fatigue severity in PwMS (Finke et al., 2014), further strengthening the suggestion that anti-correlated cortico-striatal connectivity may signal a compensatory mechanism for PwMS.
We suggest that PwMS may rely on cortico-striatal circuitry to a greater extent than cortico-cerebellar circuitry. This hypothesis of cortico-striatal substitution for cortico-cerebellar motor learning in PwMS requires further testing as volumetric MRI studies have shown that the striatum is susceptible to neurodegeneration (Bergsland et al., 2012; Ceccarelli et al., 2012) and microstructural gray matter damage of the caudate in PwMS has been associated with impaired motor function (Cavallari et al., 2014; Hasan et al., 2009). Similar to the current work, this association is specific to the caudate and not the putamen (Cavallari et al., 2014), an unexpected finding given the putamen's relevance within the sensorimotor loop.
5. Conclusions
The current work supplements a growing consensus implicating the necessary interaction and appropriate balance of cognitive and motor circuitry for optimum movement control and adaptation. Results regarding PwMS should be interpreted with the knowledge that impairments in the relapsing–remitting population tested in the current study were relatively mild and results may translate differently to those with more progressive and/or advanced stages of MS. The results of this study provide two distinct, testable hypotheses for future studies regarding mildly impaired PwMS: can 1) postural motor sequence training and/or 2) dual task postural training alter connectivity strength within either of the neural networks principally responsible for motor adaptation (cortico-cerebellar or cortico-striatal) to facilitate gait and balance rehabilitation in PwMS?
Acknowledgements
We thank the volunteers for participating in this study. We are also grateful to Heather Schlueter for assistance in participant recruitment and data collection. This work was principally supported by grants from the National Multiple Sclerosis Society (BWF: RG 5273A1/T, GGD: FG 2058-A-1 and FBH: MB0011). Additional support was provided by the Medical Research Foundation of Oregon (BWF & GGD) and the N.L. Tartar Research Fund (BWF). The authors declare no competing financial interests.
References
- Anderson V.M., Fisniku L.K., Altmann D.R., Thompson A.J., Miller D.H. MRI measures show significant cerebellar gray matter volume loss in multiple sclerosis and are associated with cerebellar dysfunction. Mult. Scler. 2009;15(7):811–817. doi: 10.1177/1352458508101934. 19465449 [DOI] [PubMed] [Google Scholar]
- Baram Y., Miller A. Virtual reality cues for improvement of gait in patients with multiple sclerosis. Neurology. 2006;66(2):178–181. doi: 10.1212/01.wnl.0000194255.82542.6b. 16434649 [DOI] [PubMed] [Google Scholar]
- Bergsland N., Horakova D., Dwyer M.G., Dolezal O., Seidl Z.K., Vaneckova M., Krasensky J., Havrdova E., Zivadinov R. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing–remitting multiple sclerosis. A.J.N.R. Am. J. Neuroradiol. 2012;33(8):1573–1578. doi: 10.3174/ajnr.A3086. 22499842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Seidler R.D. Cerebellar contributions to visuomotor adaptation and motor sequence learning: an ALE meta-analysis. Front. Hum. Neurosci. 2013;7:27. doi: 10.3389/fnhum.2013.00027. 23403800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkenberg M., Rune K., Jensen C.V., Ravnborg M., Kyllingsbaek S., Holm S., Paulson O.B., Sørensen P.S. Cortical cerebral metabolism correlates with MRI lesion load and cognitive dysfunction in MS. Neurology. 2000;54(3):558–564. doi: 10.1212/wnl.54.3.558. 10680783 [DOI] [PubMed] [Google Scholar]
- Bo J., Seidler R.D. Spatial and symbolic implicit sequence learning in young and older adults. Exp. Brain Res. 2010;201(4):837–851. doi: 10.1007/s00221-009-2098-5. 19949778 [DOI] [PubMed] [Google Scholar]
- Bonzano L., Palmaro E., Teodorescu R., Fleysher L., Inglese M., Bove M. Functional connectivity in the resting-state motor networks influences the kinematic processes during motor sequence learning. Eur. J. Neurosci. 2015;41(2):243–253. doi: 10.1111/ejn.12755. 25328043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzano L., Tacchino A., Roccatagliata L., Abbruzzese G., Mancardi G.L., Bove M. Callosal contributions to simultaneous bimanual finger movements. J. Neurosci. 2008;28(12):3227–3233. doi: 10.1523/JNEUROSCI.4076-07.2008. 18354026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzano L., Tacchino A., Roccatagliata L., Mancardi G.L., Abbruzzese G., Bove M. Structural integrity of callosal midbody influences intermanual transfer in a motor reaction-time task. Hum. Brain Mapp. 2011;32(2):218–228. doi: 10.1002/hbm.21011. 20336657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd L.A., Edwards J.D., Siengsukon C.S., Vidoni E.D., Wessel B.D., Linsdell M.A. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol. Learn. Mem. 2009;92(1):35–44. doi: 10.1016/j.nlm.2009.02.009. 19249378 [DOI] [PubMed] [Google Scholar]
- Cameron M.H., Horak F.B., Herndon R.R., Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens. Mot. Res. 2008;25(2):113–122. doi: 10.1080/08990220802131127. 18570015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio M., Sanguineti V., Morasso P., Solaro C. Abnormal sensorimotor control, but intact force field adaptation, in multiple sclerosis subjects with no clinical disability. Mult. Scler. 2008;14(3):330–342. doi: 10.1177/1352458507085068. 18208874 [DOI] [PubMed] [Google Scholar]
- Cavallari M., Ceccarelli A., Wang G.Y., Moscufo N., Hannoun S., Matulis C.R., Jackson J.S., Glanz B.I., Bakshi R., Neema M., Guttmann C.R. Microstructural changes in the striatum and their impact on motor and neuropsychological performance in patients with multiple sclerosis. PLOS One. 2014;9(7) doi: 10.1371/journal.pone.0101199. 25047083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli A., Jackson J.S., Tauhid S., Arora A., Gorky J., dell'Oglio E., Bakshi A., Chitnis T., Khoury S.J., Weiner H.L., Guttmann C.R., Bakshi R., Neema M. The impact of lesion in-painting and registration methods on voxel-based morphometry in detecting regional cerebral gray matter atrophy in multiple sclerosis. A.J.N.R. Am. J. Neuroradiol. 2012;33(8):1579–1585. doi: 10.3174/ajnr.A3083. 22460341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P. Understanding and modulating motor learning with cerebellar stimulation. Cerebellum. 2015;14:171–174. doi: 10.1007/s12311-014-0607-y. 25283180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derache N., Marié R.M., Constans J.M., Defer G.L. Reduced thalamic and cerebellar rest metabolism in relapsing–remitting multiple sclerosis, a positron emission tomography study: correlations to lesion load. J. Neurol. Sci. 2006;245(1–2):103–109. doi: 10.1016/j.jns.2005.09.017. 16647086 [DOI] [PubMed] [Google Scholar]
- Doyon J., Bellec P., Amsel R., Penhune V., Monchi O., Carrier J., Lehéricy S., Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. 19061920 [DOI] [PubMed] [Google Scholar]
- Doyon J., Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005;15(2):161–167. doi: 10.1016/j.conb.2005.03.004. 15831397 [DOI] [PubMed] [Google Scholar]
- Doyon J., Penhune V., Ungerleider L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. 12457751 [DOI] [PubMed] [Google Scholar]
- Finke C., Schlichting J., Papazoglou S., Scheel M., Freing A., Soemmer C., Pech L., Pajkert A., Pfüller C., Wuerfel J., Ploner C., Paul F., Brandt A. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult. Scler. 2014 doi: 10.1177/1352458514555784. 25392321 [DOI] [PubMed] [Google Scholar]
- Fling B.W., Cohen R.G., Mancini M., Carpenter S.D., Fair D.A., Nutt J.G., Horak F.B. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLOS One. 2014;9(6) doi: 10.1371/journal.pone.0100291. 24937008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling B.W., Dutta G.G., Schlueter H., Cameron M.H., Horak F.B. Associations between proprioceptive neural pathway structural connectivity and balance in people with multiple sclerosis. Front. Hum. Neurosci. 2014;8:814. doi: 10.3389/fnhum.2014.00814. 25368564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling B.W., Seidler R.D. Fundamental differences in callosal structure, neurophysiologic function, and bimanual control in young and older adults. Cereb. Cortex. 2012;22(11):2643–2652. doi: 10.1093/cercor/bhr349. 22166764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. 17704812 [DOI] [PubMed] [Google Scholar]
- Galea J.M., Vazquez A., Pasricha N., de Xivry J.J., Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb. Cortex. 2011;21(8):1761–1770. doi: 10.1093/cercor/bhq246. 21139077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore C.P., Donaldson I., Bö L., Owens T., Lowe J., Evangelou N. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J. Neurol. Neurosurg. Psychiatry. 2009;80(2):182–187. doi: 10.1136/jnnp.2008.148767. 18829630 [DOI] [PubMed] [Google Scholar]
- Hasan K.M., Halphen C., Kamali A., Nelson F.M., Wolinsky J.S., Narayana P.A. Caudate nuclei volume, diffusion tensor metrics, and T(2) relaxation in healthy adults and relapsing–remitting multiple sclerosis patients: implications for understanding gray matter degeneration. J. Magn. Reson. Imaging. 2009;29(1):70–77. doi: 10.1002/jmri.21648. 19097116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzitaki V., Koudouni A., Orologas A. Learning of a novel visuo-postural co-ordination task in adults with multiple sclerosis. J. Rehabil. Med. 2006;38(5):295–301. doi: 10.1080/16501970600680247. 16931459 [DOI] [PubMed] [Google Scholar]
- Heesen C., Böhm J., Reich C., Kasper J., Goebel M., Gold S.M. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult. Scler. 2008;14(7):988–991. doi: 10.1177/1352458508088916. 18505775 [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Nakamura K., Sakai K., Nakahara H. Central mechanisms of motor skill learning. Curr. Opin. Neurobiol. 2002;12(2):217–222. doi: 10.1016/s0959-4388(02)00307-0. 12015240 [DOI] [PubMed] [Google Scholar]
- Janssen A.L., Boster A., Patterson B.A., Abduljalil A., Prakash R.S. Resting-state functional connectivity in multiple sclerosis: an examination of group differences and individual differences. Neuropsychologia. 2013;51(13):2918–2929. doi: 10.1016/j.neuropsychologia.2013.08.010. 23973635 [DOI] [PubMed] [Google Scholar]
- Jayaram G., Galea J.M., Bastian A.J., Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb. Cortex. 2011;21(8):1901–1909. doi: 10.1093/cercor/bhq263. 21239392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A., Meyer G., Jezzard P., Adams M.M., Turner R., Ungerleider L.G. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–158. doi: 10.1038/377155a0. 7675082 [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A., Faber-Rod J.C., Bauer J., Lucchinetti C.F., Sorensen P.S., Laursen H., Stadelmann C., Brück W., Rauschka H., Schmidbauer M., Lassmann H. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17(1):38–44. doi: 10.1111/j.1750-3639.2006.00041.x. 17493036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Glass T.G., Lankipalli B.R., Downs H., Mayberg H., Fox P.T. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum. Brain Mapp. 1995;3(3):209–223. [Google Scholar]
- Langan J., Peltier S.J., Bo J., Fling B.W., Welsh R.C., Seidler R.D. Functional implications of age differences in motor system connectivity. Front. Syst. Neurosci. 2010;4:17. doi: 10.3389/fnsys.2010.00017. 20589101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S., Benali H., van de Moortele P.F., Pélégrini-Issac M., Waechter T., Ugurbil K., Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc. Natl. Acad. Sci. U S A. 2005;102(35):12566–12571. doi: 10.1073/pnas.0502762102. 16107540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L., Comi E., Annovazzi P., Rovaris M., Rossi P., Cursi M., Comola M., Martinelli V., Comi G. Impaired short-term motor learning in multiple sclerosis: evidence from virtual reality. Neurorehabil. Neural Repair. 2007;21(3):273–278. doi: 10.1177/1545968306294913. 17351084 [DOI] [PubMed] [Google Scholar]
- Lowe M.J., Phillips M.D., Lurito J.T., Mattson D., Dzemidzic M., Mathews V.P. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224(1):184–192. doi: 10.1148/radiol.2241011005. 12091681 [DOI] [PubMed] [Google Scholar]
- Manson S.C., Palace J., Frank J.A., Matthews P.M. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp. Brain Res. 2006;174(4):728–733. doi: 10.1007/s00221-006-0517-4. 16944115 [DOI] [PubMed] [Google Scholar]
- Miezin F.M., Maccotta L., Ollinger J.M., Petersen S.E., Buckner R.L. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6 1):735–759. doi: 10.1006/nimg.2000.0568. 10860799 [DOI] [PubMed] [Google Scholar]
- Muellbacher W., Ziemann U., Wissel J., Dang N., Kofler M., Facchini S., Boroojerdi B., Poewe W., Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415(6872):640–644. doi: 10.1038/nature712. 11807497 [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Schauenburg A., Lang N., Liebetanz D., Exner C., Paulus W., Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003;15(4):619–626. doi: 10.1162/089892903321662994. 12803972 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Nores W.L., Murphy M., Mauk M.D. What the cerebellum computes. Trends Neurosci. 2003;26(4):222–227. doi: 10.1016/S0166-2236(03)00054-7. 12689774 [DOI] [PubMed] [Google Scholar]
- Penhune V.B., Steele C.J. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 2012;226(2):579–591. doi: 10.1016/j.bbr.2011.09.044. 22004979 [DOI] [PubMed] [Google Scholar]
- Petsas N., Tomassini V., Filippini N., Sbardella E., Tona F., Piattella M.C., Pozzilli C., Wise R.G., Pantano P. Impaired functional connectivity unmasked by simple repetitive motor task in early relapsing–remitting multiple sclerosis. Neurorehabil. Neural Repair. 2014 doi: 10.1177/1545968314558600. 25416740 [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. 22019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperini L., Kouleridou A., Petsas N., Leonardi L., Tona F., Pantano P., Pozzilli C. The relationship between infratentorial lesions, balance deficit and accidental falls in multiple sclerosis. J. Neurol. Sci. 2011;304(1–2):55–60. doi: 10.1016/j.jns.2011.02.014. 21402386 [DOI] [PubMed] [Google Scholar]
- Reber A.S. More thoughts on the unconscious: reply to Brody and to Lewicki and Hill. J Exp Psychol Gen. 1989;118(3):242–244. doi: 10.1037//0096-3445.118.3.242. 2527948 [DOI] [PubMed] [Google Scholar]
- Reis J., Schambra H.M., Cohen L.G., Buch E.R., Fritsch B., Zarahn E., Celnik P.A., Krakauer J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. U S A. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. 19164589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Absinta M., Valsasina P., Ciccarelli O., Marino S., Rovira A., Gass A., Wegner C., Enzinger C., Korteweg T., Sormani M.P., Mancini L., Thompson A.J., de Stefano N., Montalban X., Hirsch J., Kappos L., Ropele S., Palace J., Barkhof F., Matthews P.M., Filippi M. Abnormal connectivity of the sensorimotor network in patients with MS: a multicenter fMRI study. Hum. Brain Mapp. 2009;30(8):2412–2425. doi: 10.1002/hbm.20679. 19034902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S., Destefano N., Smith S., Guidi L., Amato M.P., Federico A., Matthews P.M. Altered cerebellar functional connectivity mediates potential adaptive plasticity in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2004;75(6):840–846. doi: 10.1136/jnnp.2003.016782. 15145996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säisänen L., Julkunen P., Niskanen E., Danner N., Hukkanen T., Lohioja T., Nurkkala J., Mervaala E., Karhu J., Könönen M. Motor potentials evoked by navigated transcranial magnetic stimulation in healthy subjects. J. Clin. Neurophysiol. 2008;25(6):367–372. doi: 10.1097/WNP.0b013e31818e7944. 18997630 [DOI] [PubMed] [Google Scholar]
- Schambra H.M., Abe M., Luckenbaugh D.A., Reis J., Krakauer J.W., Cohen L.G. Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J. Neurophysiol. 2011;106(2):652–661. doi: 10.1152/jn.00210.2011. 21613597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005. 19850077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R.D., Kwak Y., Fling B.W., Bernard J.A. Neurocognitive mechanisms of error-based motor learning. Adv. Exp. Med. Biol. 2013;782:39–60. doi: 10.1007/978-1-4614-5465-6_3. 23296480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R.D., Purushotham A., Kim S.G., Ugurbil K., Willingham D., Ashe J. Neural correlates of encoding and expression in implicit sequence learning. Exp. Brain Res. 2005;165(1):114–124. doi: 10.1007/s00221-005-2284-z. 15965762 [DOI] [PubMed] [Google Scholar]
- Seitz R.J., Roland E., Bohm C., Greitz T., Stone-Elander S. Motor learning in man: a positron emission tomographic study. Neuroreport. 1990;1(1):57–60. doi: 10.1097/00001756-199009000-00016. 2129858 [DOI] [PubMed] [Google Scholar]
- Steele C.J., Penhune V.B. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J. Neurosci. 2010;30(24):8332–8341. doi: 10.1523/JNEUROSCI.5569-09.2010. 20554884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman C.M., Gordon E.M., Simon J.R., Vaidya C.J., Howard D.V., Howard J.H. Caudate resting connectivity predicts implicit probabilistic sequence learning. Brain Connect. 2013;3(6):601–610. doi: 10.1089/brain.2013.0169. 24090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacchino A., Bove M., Roccatagliata L., Luigi Mancardi G., Uccelli A., Bonzano L. Selective impairments of motor sequence learning in multiple sclerosis patients with minimal disability. Brain Res. 2014;1585:91–98. doi: 10.1016/j.brainres.2014.08.031. 25148706 [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical; New York, NY: 1988. [Google Scholar]
- Tomassini V., Johansen-Berg H., Leonardi L., Paixão L., Jbabdi S., Palace J., Pozzilli C., Matthews P.M. Preservation of motor skill learning in patients with multiple sclerosis. Mult. Scler. 2011;17(1):103–115. doi: 10.1177/1352458510381257. 20834040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvi E., Münte T.F., Krämer U.M. Delineating the cortico-striatal-cerebellar network in implicit motor sequence learning. Neuroimage. 2014;94:222–230. doi: 10.1016/j.neuroimage.2014.03.004. 24632466 [DOI] [PubMed] [Google Scholar]
- Van Ooteghem K., Frank J.S., Allard F., Horak F.B. Aging does not affect generalized postural motor learning in response to variable amplitude oscillations of the support surface. Exp Brain Res. 2010;204(4):505–514. doi: 10.1007/s00221-010-2316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooteghem K., Frank J.S., Horak F.B. Practice-related improvements in posture control differ between young and older adults exposed to continuous, variable amplitude oscillations of the support surface. Exp. Brain Res. 2009;199(2):185–193. doi: 10.1007/s00221-009-1995-y. 19756552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M., Hübers A., Lauterbach-Soon B., Hattingen E., Jung P., Cohen L.G., Ziemann U. Motor callosal disconnection in early relapsing–remitting multiple sclerosis. Hum. Brain Mapp. 2011;32(6):846–855. doi: 10.1002/hbm.21071. 21495114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn K.J., Taylor J., Ridding M., Meyer B.U., Rothwell J.C. Effect of transcranial magnetic stimulation over the cerebellum on the excitability of human motor cortex. Electroencephalogr. Clin. Neurophysiol. 1996;101(1):58–66. doi: 10.1016/0013-4694(95)00213-8. 8625878 [DOI] [PubMed] [Google Scholar]
- Wilkinson L., Teo J.T., Obeso I., Rothwell J.C., Jahanshahi M. The contribution of primary motor cortex is essential for probabilistic implicit sequence learning: evidence from theta burst magnetic stimulation. J. Cogn. Neurosci. 2010;22(3):427–436. doi: 10.1162/jocn.2009.21208. 19301999 [DOI] [PubMed] [Google Scholar]
- Winter D.A. Biomechanics and Motor Control of Human Movement. John Wiley & Sons; Hoboken, NJ: 2009. [Google Scholar]
- Wolpert D.M., Goodbody S.J., Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nat. Neurosci. 1998;1(6):529–533. doi: 10.1038/2245. 10196553 [DOI] [PubMed] [Google Scholar]
- Zanone P.G., Kelso J.A. Evolution of behavioral attractors with learning: nonequilibrium phase transitions. J. Exp. Psychol. Hum. Percept. Perform. 1992;18(2):403–421. doi: 10.1037//0096-1523.18.2.403. 1593227 [DOI] [PubMed] [Google Scholar]
- Zwibel H.L. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv. Ther. 2009;26(12):1043–1057. doi: 10.1007/s12325-009-0082-x. 20082242 [DOI] [PubMed] [Google Scholar]


