Abstract
Purpose of review
We review accumulating evidence that nonalcoholic steatohepatitis (NASH), a more advanced form of nonalcoholic fatty liver disease (NAFLD), predisposes patients to the risk of developing hepatocellular carcinoma (HCC), and we summarize recent advances in the elucidation of cancer-promoting pathways in NASH. We highlight the potential role of progenitor cells and hepatic stellate cells (HSCs) in promoting the early events that could culminate in cancer, as well as the emerging contribution of the gut–liver axis in promoting inflammation, senescence, and tumor growth in NASH and HCC. Finally, we review the role of bile acid receptors, vitamin D, and protective cellular pathways such as autophagy.
Recent findings
Studies have recently uncovered roles for gut microbiota, bile acid receptors and vitamin D in regulating the progression from NAFLD to HCC. Intriguing findings linking senescence and autophagy in hepatic stellate cells to HCC have also been discovered, as well as a link between dysregulated progenitor cell regulation and HCC.
Summary
NAFLD is the most common cause of chronic liver disease in the United States and Western Europe. The lack of definitive mechanisms underlying development of NASH among patients with NAFLD and its progression to HCC limit diagnosis and management, but new findings are paving the way for better biomarkers and therapies.
Keywords: bile acids, hepatic stellate cells, hepatocellular carcinoma, microbiome, nonalcoholic fatty liver disease
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of pathologies beginning with simple triglyceride accumulation in hepatocytes to fibrosis, then cirrhosis, which markedly enhances the risk of hepatocellular carcinoma (HCC). NAFLD is defined as an accumulation of lipids inside hepatocytes exceeding 5% of the liver weight in the absence of secondary causes such as hepatitis B or C viral infection, recent or ongoing excessive ethanol intake (<20 g/day in women, <30 g/day in men), use of steatogenic medication, or hereditary disorders. Those NAFLD cases in which the liver also contains inflammation and hepatocyte injury are further classified as having nonalcoholic steatohepatitis (NASH) [1].
NAFLD prevalence ranges from 6.3% to 33% in the general population, with the prevalence of NASH estimated at 3–5% [2]. In high-risk populations, for example, the severely obese and populations with diabetes, incidence can be as high as 60–90% [3–6].
Reliable biomarkers and treatment options for NAFLD are very limited. Lifestyle modification with improved diet and exercise form the mainstay of treatment but are rarely effective long term. In obese patients, weight loss leads to improved histology [7,8], but many patients fail to achieve enough weight loss to yield sustained benefit. Other treatment approaches, including insulin sensitizers, antioxidants, and anti-inflammatory medications, have yielded mixed success [9–11]. NAFLD is sometimes associated with elevated serum alanine aminotransferases (ALT and AST), but these tests are unreliable biomarkers for disease severity, and definitive diagnosis requires liver biopsy. Reliable noninvasive biomarkers for disease stage and progression are being pursued [12–16].
Evidence linking NASH to cirrhosis and liver cancer has accumulated steadily over the past 10 years and a recent meta-analysis concluded that there was a causal link between NAFLD and HCC [17▪]. Data are confounded by the asymptomatic nature of the early stages of the disease making the true prevalence uncertain, together with difficulties in tracking NAFLD progression to NASH. The risk of HCC in patients with NASH and cryptogenic cirrhosis (typically this represents end-stage NASH) range between 2.4% over 7 years [18] and 12.8% over 3 years [19]. Intriguingly, case reports have also appeared identifying HCC in NASH prior to the onset of overt cirrhosis, challenging the notion that cirrhosis must be present to give rise to HCC [20–22]. This suggests that pathways activated in fatty liver and inflammation may contribute to the premalignant transformation of hepatocytes. Indeed, clues for this link have arisen from the identification of pathways activated both in steatotic liver and HCC. To date, however, there are no well-defined prospective studies that confirm the link between NAFLD and HCC in patients without cirrhosis.
NONALCOHOLIC FATTY LIVER DISEASE AND INFLAMMATION
In many tissues, there is a strong correlation between chronic inflammation and increased cancer risk [23], for example, in inflammatory bowel disease, which predisposes to colorectal cancer. Disease progression in NAFLD occurs against a complex backdrop of metabolic and endocrine derangements that, together with genetic and environmental factors, promote persistent subclinical activation of the innate immune system. In linking NAFLD to inflammation, studies have focused on the role of adipokines, metabolites (e.g., free fatty acids), and cellular components released by damaged cells and metabolically stressed organs – especially adipose tissue and the liver – in contributing to a state of ‘sterile inflammation’ (Fig. 1) [24,25].
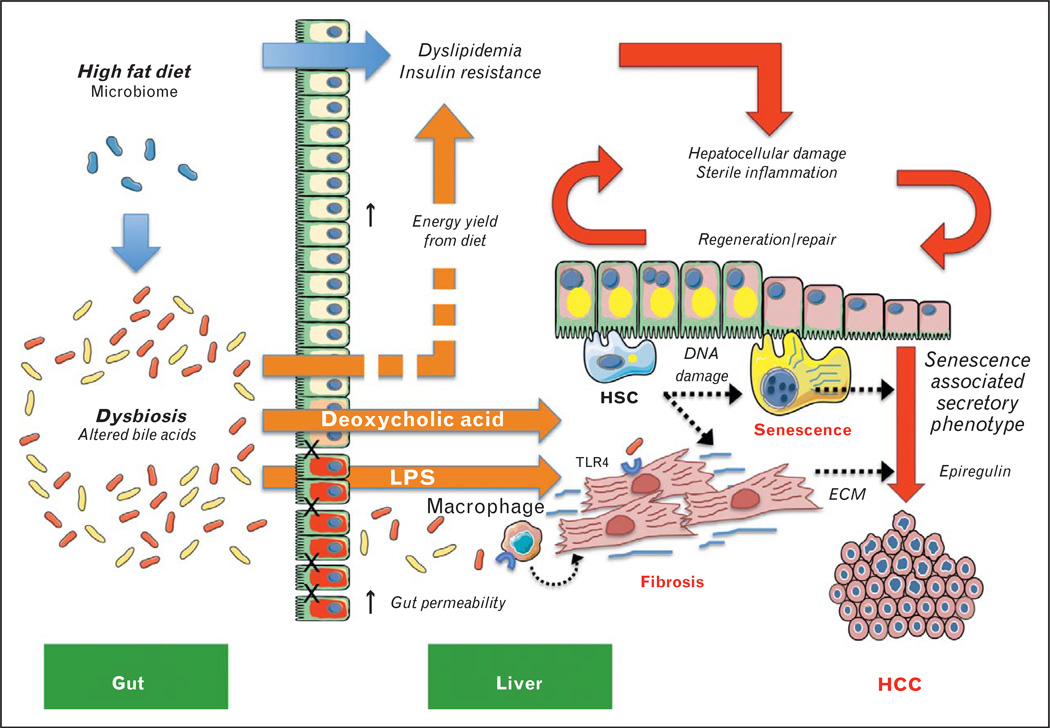
FIGURE 1.
The role of diet and the gut–liver axis in promoting inflammation and hepatocarcinogenesis in nonalcoholic steatohepatitis (NASH). The effects of a high-fat diet (HFD) (blue arrows) drive metabolic and endocrine dysregulation by promoting the uptake and accumulation of lipids in metabolically sensitive organs, especially liver, adipose tissue, and the vasculature, leading to systemic dyslipidaemia and insulin resistance (red arrows indicate downstream pathogenic processes). HFD simultaneously promotes dysbiosis and changes to the bile acid pool in the gut, contributing to hepatic inflammation and promoting hepatocarcinogenesis in NASH (orange arrows) by: enhancing energy yield from the diet, which exacerbates the effects of increased caloric intake that drive chronic hepatocellular damage; sterile inflammation; liver regeneration/repair; production of toxic bile acids, especially deoxycholic acid (DCA); and inflammasome-mediated changes in gut permeability leading to the influx of TLR agonists including lipopolysaccharide (LPS). Hepatic stellate cells (HSCs) are emerging as key players in the gut–liver axis and new evidence implicates the cells in NASH-associated hepatocellular carcinoma (HCC) (black dotted arrows) by: DCA-mediated DNA damage in HSCs that promotes a ‘senescence-associated secretory phenotype’; endotoxin (LPS)-mediated TLR4 activation in both activated HSCs and macrophages, which enhance fibrosis through the secretion of extracellular matrix (ECM) and mitogenic factors (e.g., epiregulin), which stimulate tumor growth.
A simplistic ‘two-hit’ model was initially proposed to explain NAFLD progression by suggesting that steatosis (the first ‘hit’) is followed by proinflammatory signaling coupled with insulin resistance leading to further hepatic injury. Fat accumulation in hepatocytes results in the generation of reactive oxygen species (ROS) that can interfere with endoplasmic reticulum and mitochondrial function and can generate toxic lipid peroxides that can lead to DNA damage [26]. Damaged hepatocytes also activate prosurvival and cell stress pathways such as c-Jun N-terminal kinase (JNK), and release factors called damage-associated molecular patterns (DAMPs), which promote a cascade of proinflammatory events mediated by tumor necrosis factor (TNF)-α, nuclear factor (NF) -κB and toll-like receptor (TLR) signaling, extensively reviewed elsewhere [24,27].
Both DAMPs and pathogen-associated molecular patterns activate a scaffold protein complex known as the inflammasome, which is responsible for initiating inflammation by inducing cleavage of interleukin-1β to its mature form, release of cytokines and recruitment of immune cells [24]. TLR activation leads to secretion of proinflammatory signals (TNFα, interleukin-1β, interleukin-6, interleukin-12, interleukin-18) and reactive oxygen intermediates that exacerbate insulin resistance and metabolic dysregulation [28].
Inflammation and hepatocellular damage drive the activation of regenerative pathways as well as the proliferation of fibrogenic cells, principally hepatic stellate cells (HSCs), whose ability to remodel extracellular matrix (ECM) places them in a strategic position to foster a microenvironment favoring cellular survival and proliferation. In this milieu of persistent regeneration and repair, some hepatocytes may evolve adaptive mechanisms of cell survival and proliferation, which may in turn promote premalignant transformation and/or tumor growth [29].
EMERGING PATHOGENIC PATHWAYS
Recently, new insights have clarified potential mechanisms underlying the relationship among diet, fatty liver disease and HCC, in particular the role of the gut–liver axis and bile acid metabolism, as well as the contributions of progenitor cells, HSC senescence, and autophagy.
The gut–liver axis and bile acid metabolism
Diet has a direct effect on an individual’s energy uptake, storage, hormonal milieu, and adiposity, which together can promote disease progression in NAFLD, for example, by exacerbating a state of subthreshold ‘sterile inflammation’ caused by the release of DAMPs from metabolically sensitive tissues [24]. However, indirect consequences of diet are also of potential significance to both NAFLD and liver cancer (Fig. 1) and include effects on: the commensal bacteria of the gut, the so-called ‘microbiome’, and the composition of the bile acid pool. Changes in the microbiome and bile acid turnover both have profound effects on the absorption and uptake of nutrients and therefore on organismal metabolism. However, their dysregulation can also lead to the generation of hepatotoxic and potentially mutagenic compounds reaching the liver. Gut bacteria metabolize bile acids, which contribute to cross-talk among inflammatory and fibrogenic pathways between gut and liver. Moreover, liver disease, as well as diet-induced and genetic forms of obesity, can alter both the microbiome and bile acid metabolism, rendering the gut epithelium increasingly permeable to extrinsic pathogens that exacerbate hepatic inflammation. These elements comprise the ‘gut–liver axis,’ discussed below.
Microbiome
Advances in the capacity to fully sequence the genome of microorganisms have shed new light on the complexity of symbiotic bacteria in the gut and how the microbiome can influence health and disease [30]. The gut microbiota play a key role in the development of obesity, insulin resistance, and NAFLD by: affecting caloric extraction from the diet and the bioavailability of essential nutrients such as choline [31]; activation of TLRs and inflammation in the gut, the vasculature, and the liver; and production of alcohol, injurious bile acids, and toxins that can lead to DNA damage (summarized in Fig. 1) [32,33].
Mediators of the innate immune response play a major role in the cross-talk between gut and liver by maintaining normal gut-microbiome homeostasis, but may promote hepatocarcinogenesis resulting from dietary factors and/or chronic liver injury. For example, genetically obese ob/ob mice develop intestinal bacterial overgrowth, overexpress TNF-α, and have a compromised intestinal mucosal barrier, leading to HSC activation and a more pronounced lipopolysaccharides (LPS)-mediated proinflammatory response [34,35] (Fig. 1). The microbiome is also altered in TLR5 deficient mice, which develop obesity and insulin resistance that can be transmitted through feces to co-housed nonmutant littermates [35]. In similar co-housing studies, inflammasome-deficient Asc−/− or NLRP6/NLRP3−/− mice promote disease progression in NAFLD through sharing of the microbiome with littermates [36▪▪]. Thus, not only is there essential cross-talk between the gut microflora and innate immune sensors that serve to protect an organism from metabolic disease, but changes in the microbiome associated with loss of these sensors can lead to metabolic and liver disease that is transmissible to healthy animals. It is not yet known whether transmission of gut microbiota among humans can promote liver disease as well.
The gut microbiome also alters the function of HSCs to promote liver inflammation and tumorigenesis. Changes in the gut microbiota are associated with inflammation, fibrosis [37▪], and NASH, which have been linked to changes in gut permeability and the influx of TLR4 and TLR9 agonists into the portal circulation (Fig. 1). These agonists enhance hepatic TNF-α expression [36▪▪]. Interestingly, in a mouse model, translocation of LPS from the gut can promote liver tumorigenesis through TLR4 signaling on HSCs, leading to increased production of epiregulin, an hepatic mitogen [38▪▪] (Fig. 1). In this study, sterilization with antibiotics ameliorated cancer progression but did not affect tumor initiation, leading to the conclusion that the gut-liver-axis promotes tumor growth by perpetuating inflammation [38▪▪]. A separate study has also linked gut-dysbiosis to hepatocarcinogenesis in both genetically obese and high-fat diet (HFD)-fed mice via increased production of deoxycholic acid (DCA), combined with promotion of liver tumorigenesis in mice primed with a single neonatal injection of a chemical carcinogen [39▪▪]. Intriguingly, this effect was independent of TLR4 activation, but involved HSCs as mediators of enhanced tumorigenesis through activation of a p21/interleukin-1β-dependent senescence associated secretory phenotype (see below and Fig. 1).
Bile acid receptors
Bile acids are potential carcinogens whose alterations may contribute to NAFLD development and progression and have also been linked to HCC [40]. Bile acids are produced by hepatocytes, stored in the gallbladder for release into the gut to promote fat digestion, after which they are reabsorbed in the ileum to enter the enterohepatic circulation. Bile acids regulate cholesterol, energy homeostasis, glucose storage and release, and their own homeostasis via interactions with their cognate nuclear receptors, including farnesoid X receptor (FXR), pregnane X receptor (PXR), vitamin D receptor (VDR), constitutive androstane receptor (CAR), and TGR5. FXR is an inhibitor of de-novo bile acid synthesis in liver through regulation of the cytochrome P450 enzyme that regulates bile acid production from cholesterol (Cyp7a1), and plays roles in regulating lipid metabolism in hepatocytes as well as regulating NF-kB activity. Loss of FXR in mice leads to elevated plasma triglycerides and cholesterol, steatohepatitis [41], and hepatocarcinogenesis [42–44], an effect that is mediated in part by increased Wnt/β-catenin signaling [43]. FXR−/− mice develop liver cancer more rapidly in animals with diabetes [42], pointing to a synergy in tumor formation between obesity-related inflammation through increased expression of the pro-inflammatory interleukin-1β and FXR suppression. HFD-induced obese mice have upregulated expression of Yin Yang 1 (YY1), which promotes steatosis via suppression of FXR [45]. Other bile acid receptors also influence NAFLD and HCC, including PXR [46] and CAR [47,48]. The G protein-coupled bile acid receptor TGR5 also regulates steatosis and inflammation (reviewed in [49]), and mice lacking Tgr5 have increased carcinogen-induced liver cancer [50].
Vitamin D receptor
Also a bile acid sensor, the VDR acts as a receptor for the bile acid lithocholic acid, which is hepatotoxic and a potential enteric carcinogen [51]. Vitamin D deficiency is rising in Western countries and has been epidemiologically linked to NAFLD [52] and HCC [53], but a causative relationship is not yet established. Supplementation with exogenous vitamin D improves glycemic indices in patients with insulin resistance [54] and, interestingly, the insulin gene contains a VDR response element in its promoter and is transcriptionally regulated by vitamin D ligand-dependent binding [55,56]. Vitamin D deficiency is also associated with low adiponectin in type 2 diabetics, and vitamin D supplementation increases serum adiponectin levels [52]. Moreover, rats fed a vitamin D-deficient ‘Westernized’ high-fat/high-fructose corn syrup diet have significantly worsened steatosis and more lobular inflammation than animals on a low-fat diet with normal vitamin D content. Vitamin D deficiency has also been correlated with upregulation of genes involved in oxidative stress and inflammation, including TLRs 2, 4, and 9 [57]. Vitamin D analogs have antigrowth effects on HCC cells in culture, and exhibit chemopreventive properties in a mouse of spontaneous HCC [53]. Recent work has uncovered a mechanism linking vitamin D to antifibrotic activity in HSCs, in which ligand-bound VDR inhibits transforming growth factor (TGF)-β1-mediated HSC activation by reducing Smad3 occupancy on the promoters of profibrotic genes [58].
Role of HSCs and senescence
Hepatic stellate cells are a source of proinflammatory mediators and ECM that promote progression from NAFLD to HCC. HSCs activated in response to liver injury express TLR4, which promotes the activation of IκB kinase/NF-κB and JNK pathways in addition to the secretion of interleukin-6, TGF-β1, and MCP-1. Senescent HSCs are associated with a more pronounced ‘inflammatory’ but less ‘fibrogenic’ phenotype (i.e., senescence-associated secretory phenotype), which facilitates the removal of HSCs by NK cells, leading to the resolution of fibrosis [59]. This has led to the speculation that senescence in HSCs could be antitumorigenic; indeed, blocking senescence by ablation of HSC p53 leads to increased fibrosis, inflammation, and a protumorigenic microenvironment in the liver, in which M2-type macrophages promote proliferation of preneoplastic hepatocytes [60▪▪].
In contrast to these findings, more recent evidence implicates senescent HSCs in promoting hepatocarcinogenesis in mice on an HFD and treated with a carcinogen [39▪▪]. In this study, HSCs expressing high levels of p21 surrounded tumors, and depletion of senescent HSCs in this model using liposomes containing siRNAs to heat shock protein 47 (HSP47) has led to reduced tumorigenesis.
Progenitor cells
The chronic state of regeneration and repair in NASH closely correlates with activation of progenitor cell populations and reactivation of pathways more commonly associated with development, angiogenesis, and cancer, including hedgehog, canonical Wnt signaling and Notch. Progenitor cell activation has recently been demonstrated both in adult [61▪] and pediatric NAFLD [62], raising the possibility that these cells may contribute to HCC initiation. Portal inflammation and fibrosis correlate with expansion of the progenitor cell marker and Hh target gene Gli2 [61▪,63]. This finding has been linked to increased expression of Hh ligand by ballooned hepatocytes and bile duct cells. Hedgehog mediates epithelial–mesenchymal transition (EMT) in ductular cells [64] and may be a driver of the progenitor cell and/or fibrotic responses [65]. Other ligands that may direct progenitor cell fate in the liver, such as the Notch ligand Jagged and Wnt3a, are produced by myofibroblasts and macrophages, respectively, in the context of chronic liver injury [66] and may also contribute to the differentiation and expansion of progenitors in NAFLD. Wnt/β-catenin plays an important role in liver development, and mutations in β-catenin are common in HCC [67,68].
Regenerative pathways activated in NAFLD yield cells that are exposed to intense selection pressures imposed by high circulating free fatty acid and glucose, insulin resistance, inflammation, and cytostatic factors such as TGF-β. These pressures drive cellular escape and transformation that may lead to HCC. Localized insulin/IGF signaling by niche cells can regulate progenitor cell proliferation and coordinate the organismal response to changes in systemic insulin and metabolic fluctuations under normal conditions [69]. However, escape from insulin resistance by loss of PTEN (phosphatase and tensin homolog) or activation of the insulin signaling pathway components is common in HCC, and in a drosophila model of obesity, Wnt signaling mediates insulin resistance escape in tumor cells [70]. TLR4 activation can also promote expression of the pluripotency gene NANOG in HCC tumor-initiating cells [71]. Interestingly, NANOG mediates escape from cytostatic TGFβ signaling by suppression of SMAD3 signaling, which mediates TGFβ’s effects [72].
Autophagy defects
The accumulation of ROS and DNA damage in metabolically stressed cells is ameliorated by the activation of (macro)autophagy – a lysosomedependent process of ‘self-eating’ that increases the turnover of damaged proteins, peroxisomes, and mitochondria [73]. Autophagy is a key regulator of lipid turnover [74], insulin sensitivity [75], inflammation [76], and fibrosis [77] and there is growing evidence that it contributes to NAFLD progression [78]. Global inhibition of autophagy in liver leads to steatosis [79], and both genetic and dietary forms of obesity cause decreased hepatic expression of key autophagy effectors including Atg7, Atg5, and beclin 1 [75]. Autophagy has been implicated in tumor suppression in liver [80]. Consistent with this notion, autophagy is impaired in HCC cell lines, and reduced beclin I expression in human HCC samples correlates with survival and tumor differentiation [81]. Also, autophagy promotes EMT of HCC cells in response to TGF-β, suggesting that its inhibition may prevent HCC invasion [82].
These and related findings have led to the suggestion that pharmacological activators of autophagy may treat NASH by ameliorating lipid accumulation, insulin resistance, and possibly tumorigenesis. However, such effects would clearly need to be targeted specifically to hepatocytes because there is strong evidence that activation of autophagy in HSCs and cancer cells may lead to increased fibrosis, cell survival, and EMT that could instead promote tumorigenesis. This is because the role of autophagy diverges between hepatic parenchymal and nonparenchymal cells. Autophagy in HSCs promotes fibrosis, which may indirectly contribute to a carcinogenic microenvironment [77,83–85].
Unmet needs
Epidemiologic evidence links NAFLD to cirrhosis and cancer, but underlying mechanisms are still uncertain. There is an urgent need for well-tolerated and effective treatments for NAFLD and NASH, which if successful could attenuate the risk of HCC. Potential new therapies based on recent advances might include probiotics [86,87], vitamin D repletion, and bile acid intermediates. Better biomarkers of disease stage and progression are also urgently required for early and reliable detection and treatment monitoring. A number of studies have identified genetic polymorphisms and micro- RNAs correlating with NAFLD development and progression (reviewed in [88] and [89]), which could prove valuable as future biomarkers.
CONCLUSION
A picture is crystallizing in which NAFLD, the hepatic manifestation of metabolic syndrome, combined with its systemic metabolic and endocrine derangements, generates a state of chronic inflammation and hepatocellular damage. These events trigger the activation of regenerative pathways as well as the proliferation of HSCs that promote fibrosis and cancer [90]. However, given our fragmentary understanding of HCC pathogenesis in NAFLD, greater clarification of underlying mechanisms remains a high priority and will provide a more rational template for establishing new therapies to prevent this fatal cancer in a disease (NAFLD) that already has reached epidemic proportions.
KEY POINTS.
Disease progression from the bland steatosis of early NAFLD to the more advanced steatohepatitis stage (NASH) can promote HCC; however, a mechanistic link between NASH and HCC is not yet fully established.
Obesity and NASH are associated with changes in the gut microflora and bile acid metabolism, which may promote liver tumorigenesis.
The innate immune system and hepatic stellate cells are key sensors of perturbations in the gut–liver axis.
Molecular mediators of the gut–liver axis, especially the inflammasome and bile acid receptors, are potential therapeutic targets for HCC in NASH, but a greater understanding of disease pathogenesis is essential to uncover new therapies beyond these approaches.
Acknowledgements
None.
L.A.N. is supported by a Marie Curie People Cofund Fellowship of the Seventh Framework Program of the European Commission under grant agreement number 267248: DIATRAIN. The work in Dr Friedman’s laboratory is supported by NIH Grants DK56621, AA018408 and AA020709.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–826. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol. 2012;32:1766–1770. doi: 10.1161/ATVBAHA.111.241927. [DOI] [PubMed] [Google Scholar]
- 5.Leite NC, Salles GF, Araujo AL, et al. Prevalence and associated factors of nonalcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 6.Prashanth M, Ganesh HK, Vima MV, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–210. [PubMed] [Google Scholar]
- 7.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 8.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalasani NP, Sanyal AJ, Kowdley KV, et al. Pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013;92:114–118. doi: 10.1016/j.lfs.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 12.Feldstein AE, Alkhouri N, De Vito R, et al. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013;108:1526–1531. doi: 10.1038/ajg.2013.168. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick E, Mitry RR, Quaglia A, et al. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr. 2010;51:500–506. doi: 10.1097/MPG.0b013e3181e376be. [DOI] [PubMed] [Google Scholar]
- 14.Tsutsui M, Tanaka N, Kawakubo M, et al. Serum fragmented cytokeratin 18 levels reflect the histologic activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels. J Clin Gastroenterol. 2010;44:440–447. doi: 10.1097/MCG.0b013e3181bdefe2. [DOI] [PubMed] [Google Scholar]
- 15.Malik R, Chang M, Bhaskar K, et al. The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:564–568. doi: 10.1111/j.1440-1746.2008.05731.x. [DOI] [PubMed] [Google Scholar]
- 16.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 17. White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. e2. doi: 10.1016/j.cgh.2012.10.001. This is a systematic meta-analysis of 17 cohort, 18 case–control/cross-sectional studies, and 26 case series showing epidemiologic support for an association between NAFLD and increased risk of HCC.
- 18.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 20.Nkontchou G, Tran Van Nhieu J, Ziol M, et al. Peripheral intrahepatic cholangiocarcinoma occurring in patients without cirrhosis or chronic bile duct diseases: epidemiology and histopathology of distant nontumoral liver in 57 White patients. Eur J Gastroenterol Hepatol. 2013;25:94–98. doi: 10.1097/MEG.0b013e328357cdd7. [DOI] [PubMed] [Google Scholar]
- 21.Guzman G, Brunt EM, Petrovic LM, et al. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- 22.Ertle J, Dechene A, Sowa JP, et al. Nonalcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 24.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 27.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 28.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stickel F, Hellerbrand C. Nonalcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303–1307. doi: 10.1136/gut.2009.199661. [DOI] [PubMed] [Google Scholar]
- 30.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and nonalcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 33.Compare D, Coccoli P, Rocco A, et al. Gut–liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 35.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. This is the key study providing evidence that inflammasome deficiency leads to a transmissible dysbiosis, which can exacerbate disease progression in co-housed mouse models of obesity and NAFLD leading to NASH.
- 37. De Minicis S, Rychlicki C, Agostinelli L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury. Hepatology. 2013 Aug 19; doi: 10.1002/hep.26695. [Epub ahead of print] This is the study showing that dysbiosis contributes to fibrogenesis during the course of chronic liver injury.
- 38. Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. This is an elegant study demonstrating a causal link between the gut microbiota and tumor growth and survival in the context of chronic liver injury via LPS-mediated fibrogenic activation of HSCs.
- 39. Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. This is the first evidence highlighting the potential role of HSC senescence in promoting liver tumorigenesis resulting from changes in the microbiome. Also demonstrates how the gut–liver axis may have the potential to promote tumorigenesis in NAFLD independently of fibrosis and obesity.
- 40.Wang X, Fu X, Van Ness C, et al. Bile acid receptors and liver cancer. Curr Pathobiol Rep. 2013;1:29–35. doi: 10.1007/s40139-012-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarrinpar A, Loomba R. Review article: the emerging interplay among the gastrointestinal tract, bile acids and incretins in the pathogenesis of diabetes and nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;36:909–921. doi: 10.1111/apt.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Ge X, Heemstra LA, et al. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272–280. doi: 10.1210/me.2011-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfe A, Thomas A, Edwards G, et al. Increased activation of the Wnt/beta-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338:12–21. doi: 10.1124/jpet.111.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang F, Huang X, Yi T, et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y, Ma Z, Zhang Z, et al. Yin Yang 1 promotes hepatic steatosis through repression of farnesoid X receptor in obese mice. Gut. 2013 Jan 24; doi: 10.1136/gutjnl-2012-303150. [DOI] [PubMed] [Google Scholar]
- 46.Sookoian S, Castano GO, Burgueno AL, et al. The nuclear receptor PXR gene variants are associated with liver injury in nonalcoholic fatty liver disease. Pharmacogenet Genomics. 2010;20:1–8. doi: 10.1097/FPC.0b013e328333a1dd. [DOI] [PubMed] [Google Scholar]
- 47.Moreau A, Vilarem MJ, Maurel P, Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm. 2008;5:35–41. doi: 10.1021/mp700103m. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa D, Kakizaki S, Horiguchi N, et al. Constitutive active/androstane receptor promotes hepatocarcinogenesis in a mouse model of nonalcoholic steatohepatitis. Carcinogenesis. 2011;32:576–583. doi: 10.1093/carcin/bgq277. [DOI] [PubMed] [Google Scholar]
- 49.Stepanov V, Stankov K, Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J Recept Signal Transduct Res. 2013;33:213–223. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- 50.Chen WD, Yu D, Forman BM, et al. Deficiency of G-protein-coupled bile acid receptor Gpbar1 (TGR5) enhances chemically induced liver carcinogenesis. Hepatology. 2013;57:656–666. doi: 10.1002/hep.26019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 52.Kwok RM, Torres DM, Harrison SA. Vitamin D and nonalcoholic fatty liver disease (NAFLD): Is it more than just an association? Hepatology. 2013 Mar 16;58:1166–1174. doi: 10.1002/hep.26390. [DOI] [PubMed] [Google Scholar]
- 53.Chiang KC, Yeh CN, Chen MF, Chen TC. Hepatocellular carcinoma and vitamin D: a review. J Gastroenterol Hepatol. 2011;26:1597–1603. doi: 10.1111/j.1440-1746.2011.06892.x. [DOI] [PubMed] [Google Scholar]
- 54.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maestro B, Davila N, Carranza MC, Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223–230. doi: 10.1016/s0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 56.Maestro B, Molero S, Bajo S, et al. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D. Cell Biochem Funct. 2002;20:227–232. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 57.Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55:1103–1111. doi: 10.1002/hep.24737. [DOI] [PubMed] [Google Scholar]
- 58.Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schnabl B, Purbeck CA, Choi YH, et al. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 60. Lujambio A, Akkari L, Simon J, et al. Noncell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. This is the study demonstrating the role of p53-mediated senescence in HSCs as a tissue homeostatic mechanism limiting fibrosis and tumorigenesis by promoting clearance of hepatic stellate cells by macrophages.
- 61. Guy CD, Suzuki A, Zdanowicz M, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. This study provides key evidence supporting a relationship between Hh-pathway activation and progenitor cell expansion in NASH.
- 62.Nobili V, Carpino G, Alisi A, et al. Hepatic progenitor cells activation, fibrosis and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012 Mar 29;56:2142–2153. doi: 10.1002/hep.25742. [DOI] [PubMed] [Google Scholar]
- 63.Swiderska-Syn M, Suzuki A, Guy CD, et al. Hedgehog pathway and pediatric nonalcoholic fatty liver disease. Hepatology. 2013;57:1814–1825. doi: 10.1002/hep.26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman SL. Liver fibrosis in 2012: Convergent pathways that cause hepatic fibrosis in NASH. Nat Rev Gastroenterol Hepatol. 2013;10:71–72. doi: 10.1038/nrgastro.2012.256. [DOI] [PubMed] [Google Scholar]
- 66.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lade AG, Monga SP. Beta-catenin signaling in hepatic development and progenitors: which way does the WNT blow? Dev Dyn. 2011;240:486–500. doi: 10.1002/dvdy.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43:1021–1029. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheetham SW, Brand AH. Cell biology. Insulin finds its niche. Science. 2013;340:817–818. doi: 10.1126/science.1238525. [DOI] [PubMed] [Google Scholar]
- 70.Hirabayashi S, Baranski TJ, Cagan RL. Transformed Drosophila cells evade diet-mediated insulin resistance through wingless signaling. Cell. 2013;154:664–675. doi: 10.1016/j.cell.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Machida K, Tsukamoto H, Mkrtchyan H, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen CL, Tsukamoto H, Liu JC, et al. Reciprocal regulation by TLR4 and TGF-beta in tumor-initiating stem-like cells. J Clin Invest. 2013;123:2832–2849. doi: 10.1172/JCI65859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 74.Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang L, Li P, Fu S, et al. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takamura A, Komatsu M, Hara T, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding ZB, Shi YH, Zhou J, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–9175. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Yang B, Zhou Q, et al. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 2013;34:1343–1351. doi: 10.1093/carcin/bgt063. [DOI] [PubMed] [Google Scholar]
- 83.Hernandez-Gea V, Hilscher M, Rozenfeld R, et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol. 2013 Feb 25;59:98–104. doi: 10.1016/j.jhep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hernandez-Gea V, Friedman SL. Autophagy fuels tissue fibrogenesis. Autophagy. 2012;8:849–850. doi: 10.4161/auto.19947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 86.Iacono A, Raso GM, Canani RB, et al. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem. 2011;22:699–711. doi: 10.1016/j.jnutbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Darnaud M, Faivre J, Moniaux N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J Hepatol. 2013;58:385–387. doi: 10.1016/j.jhep.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 88.Hooper AJ, Adams LA, Burnett JR. Genetic determinants of hepatic steatosis in man. J Lipid Res. 2011;52:593–617. doi: 10.1194/jlr.R008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo J, Friedman SL. The expression patterns and clinical significance of microRNAs in liver diseases and hepatocellular carcinoma. Curr Pharm Des. 2013;19:1262–1272. doi: 10.2174/138161213804805667. [DOI] [PubMed] [Google Scholar]
- 90.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]