Abstract
Permeability edema is a life-threatening complication accompanying acute lung injury (ALI), severe pneumonia and the acute respiratory distress syndrome (ARDS), which can be associated with a reduced alveolar liquid clearance (ALC) capacity, a disruption of the alveolar epithelial barrier, and an increased capillary endothelial permeability. Bacterial and viral infections can directly promote pulmonary endothelial hyperpermeability and indirectly decrease the function and/or expression of ion transporters regulating ALC in type II alveolar epithelial cells, by means of inducing a strong inflammatory and oxidative stress response in the infected lungs. Apart from ventilation strategies, no standard treatment exists for permeability edema, making the search for novel regulators of endothelial and epithelial hyperpermeability and dysfunction important. Here, we present an overview of recently identified substances that inhibit and/or reverse endothelial barrier disruption and permeability or alveolar epithelial dysfunction: 1) zinc chelators, which were shown to attenuate the effects of oxidative stress on the pulmonary endothelium; 2) peroxisome proliferator activated receptor (PPAR) ligands, which have been shown to exert antiinflammatory effects, by decreasing the expression of pro-inflammatory genes; 3) extracellular ATP, produced during inflammation, which induces a rapid and dose-dependent increase in transendothelial electrical resistance (TER) across pulmonary endothelial cells; 4) the lectin-like domain of TNF, which is spatially distinct from the receptor binding sites and which protects from hydrostatic and permeability edema and 5) Hsp90 inhibitors, which prevent and repair toxin-induced hyperpermeability. Unraveling the mechanism of action of these agents could contribute to the development of novel therapeutic strategies to combat permeability edema.
Introduction
Pulmonary permeability edema is a major complication of acute lung injury (ALI), severe pneumonia and ARDS. This pathology can be accompanied by 1) a reduction of alveolar liquid clearance capacity, caused by an inhibition of the expression of crucial sodium transporters, such as the epithelial sodium channel (ENaC) and the Na+-K+-ATPase, 2) an epithelial and endothelial hyperpermeability and 3) a disruption of the epithelial and endothelial barriers, caused by increased apoptosis or necrosis. Since, apart from ventilation strategies, no standard treatment exists for permeability edema, the following chapters will review a selection of novel approaches aiming to improve these parameters in the capillary endothelium and the alveolar epithelium.
Role of Apoptotic Pathways in the development of ALI/ARDS
Apoptosis is an essential physiological process for the selective elimination of cells. However, the dysregulation of apoptotic pathways is thought to play an important role in the pathogenesis of ALI. Both delayed neutrophil apoptosis and enhanced endothelial/epithelial cell apoptosis have been identified in ALI/ARDS. In the case of neutrophils, which contribute significantly to ALI/ARDS, studies in both animals and ARDS patients suggest that apoptosis is inhibited during the early stages (< 2h) of inflammation. Although this is likely due to the action of anti-apoptotic cytokines on the neutrophil population, there is no correlation between the levels of these cytokines and the severity of ALI in humans. There is more compelling evidence that increased epithelial/endothelial cell apoptosis contributes to the endothelial and epithelial injury that is characteristic of ALI/ARDS in humans. Studies have shown that ALI is associated with increased cell death in humans, while apoptosis inhibitors showed increased survival rodent models of ALI [1]. However, the mechanisms responsible for increased apoptosis in ALI/ARDS are poorly understood.
Although studies have provided strong evidence that the extrinsic apoptosis pathway is upregulated in ALI/ARDS, its role in ALI is still unclear. For example, Albertine et al found increased expression of soluble Fas/FasL in ALI/ARDS patients, compared to controls [2], while Fas/FasL-induced apoptosis has been implicated in alveolar repair through reversal of reparative hyperplasia of type II alveolar epithelial cells seen following lipopolysaccharide-induced ALI in rat lungs [3]. With respect to the intrinsic apoptotic pathway in ALI, a variety of factors have been shown to induce apoptosis in the lung including ventilator-induced mechanical stress [4], hypoxia [5], oxidative stress [6], and NO generated from inducible nitric oxide synthase (iNOS) [1]. The role of iNOS is unclear, as studies using iNOS knockout mice and iNOS inhibitors indicated that iNOS-derived NO was detrimental. However, studies at extended time points (24 h) found that iNOS inhibition enhanced alveolar and airway epithelial cell death, suggesting that iNOS may inhibit apoptosis in later phases of the disease [1], which could explain the unexpected decrease in patient survival seen during clinical trials with iNOS inhibitors. Thus, although it is clear that aberrant apoptotic signaling endothelial and epithelial cells likely contributes to the impairment of the barrier function of pulmonary endothelium and epithelium and development of pulmonary edema, the roles played by the extrinsic and intrinsic pathways are unclear. Nor is it clear how the intrinsic apoptotic pathways become dysregulated. We have previously shown that acute increases in both oxidative and nitrosative stress in endothelial cells (similar to that occurring in ALI) led to increases in labile Zn2+ and that this disruption in Zn2+ homeostasis preceded the disruption of mitochondrial function and resulted in the induction of apoptosis [7, 8]. Furthermore, the apoptotic death process is dependent upon Zn2+ release, as the chelation of free Zn2+ led to a reduction in apoptosis [7, 8].
Disruption of Zn2+ homeostasis in ALI/ARDS
Many physiological, nutritional, and biochemical functions have been attributed to Zn2+ [9]. There is evidence that Zn2+ requirements of the vascular endothelium increase during inflammatory conditions, such as atherosclerosis, where apoptotic cell death is prevalent [9]. Further, zinc deficiency has been shown to increase the lung injury associated with hyperoxia [10], while the addition of exogenous Zn2+ can reduce the injury [11]. This has led to the suggestion that Zn2+ is a cytoprotective agent, defending cells against oxidative insults and apoptotic events. However, cells have a very tight regulatory apparatus in place for labile Zn2+ and we have previously shown that the disruption of Zn2+ homeostasis causes cell death via apoptosis [7, 8], or at high enough concentrations, necrosis [7, 8]. There also is also in vivo evidence showing that excessive dietary zinc intake can induce pathological conditions that have been associated with oxidative stress [12]. There are also situations resulting in ALI in which the lung can be exposed to high concentrations of labile Zn2+, such as accidental inhalation of zinc chloride from smoke bombs [13]. In addition, zinc appears to be involved in the lung injury associated with particulate matter toxicity [14]. Thus, in the same manner as Ca2+, loss of intracellular Zn2+ homeostatic regulation can be equally damaging as situations of Zn2+ deficiency.
Labile Zn2+ levels are maintained in the low pico-nano-molar range, due to the presence of a group of the heavy metal binding proteins, called the metallothioneins (MT) [15]. MT is a multi-gene family of at least three members (I, II, III) [15]. MT expression in the lung is normally much less than in the liver but its transcription can be stimulated by a number of agents such as Zn2+, cadmium, as well as cytokines, reactive oxygen and nitrogen species metals themselves and proinflammatory molecules [15]. In addition, MT expression is enhanced in a variety of ALI models. As the main role of MT is to bind heavy metals like Zn2+, the fact that MT over-expression can limit the injury associated with these types of injury suggests that the loss of Zn2+ homeostasis may play a significant role in the cell death associated with ALI.
Persoxisome proliferators activated receptor (PPAR) signaling in the lung
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily, that includes receptors for steroid hormones, thyroid hormones, retinoic acid, and fat-soluble vitamins. Since their discovery in 1990, increasing data has been published on the role of PPARs in diverse processes, including lipid and glucose metabolism, diabetes and obesity, atherosclerosis, cellular proliferation and differentiation, neurological diseases, inflammation and immunity. PPARs have both gene-dependent and gene-independent effects. Gene-dependent functions involve the formation of heterodimers with the retinoid X-receptor. Activation by PPAR ligands results in the binding of the heterodimer to peroxisome proliferator response elements, located in the promoter regions of PPAR-regulated genes. Gene-independent effects involve the direct binding of PPARs to transcription factors, such as NF-κB, which then alters their binding to DNA promoter elements. PPARs can also bind and sequester various cofactors for transcription factors, and thus further alter gene expression. Importantly, the precise effects of PPARs vary greatly between cell types. To date, three subtypes of PPAR have been identified: α, β, and γ. There is increasing data suggesting that PPAR signaling may play an important role in the pathobiology of systemic vascular disease. However, there is less data implicating PPAR signaling in diseases of the lung. Levels of the γ isoform of PPAR have been shown to be decreased in lung tissues and cells from patients with advanced pulmonary vascular disease [16].
Anti-Inflammatory effects of PPAR signaling
A role for PPARs in the control of inflammation was first evidenced for PPARa, where mice deficient in PPARa exhibited an increased duration of ear-swelling in response to the pro-inflammatory mediator, LTB4 [17]. More recently, a number of studies in mice and in humans have shown that PPAR agonists exhibit anti-inflammatory effects under a wide range of conditions. There are two main mechanisms by which PPARs exert their antiinflammatory effect. The first involves complex formation, and the inhibition of transcription factors that positively regulate the transcription of pro-inflammatory genes. These include nuclear factor-κB (NF-κB), signal transducers and activators of transcription (STATs), nuclear factor of activated T cells (NF-AT), CAAT/enhancer-binding protein (C/EBP) and activator protein 1 (AP-1). These transcription factors are the main mediators of the major pro-inflammatory cytokines, chemokines, and adhesion molecules involved in inflammation. The second PPAR-mediated anti-inflammatory pathway is mediated by the sequestration of rate limiting, but essential, co-activators or co-repressors.
PPAR agonists and ALI
Recent studies have shown that PPAR signaling can attenuate the airway inflammation induced by LPS in the mouse. It was shown that mice treated with the PPARα agonist, fenofibrate, had decreases in both inflammatory cell infiltration and inflammatory mediators [19]. Conversely, PPARα−/− mice have been shown to have a greater number of neutrophils and macrophages, and increased levels of inflammatory mediators in bronchoalveolar lavage fluids (BALF) [20]. Other PPAR agonists, such as rosiglitazone or SB 21994 have also been shown to reduce LPS-mediated ALI in the mouse lung [21]. PPARγ signaling has also been shown to be protective in regulating pulmonary inflammation associated with fluorescein isothiocyanate (FITC)-induced lung injury, with the PPARγ ligand pioglitazone decreasing neutrophil infiltration [22]. Collectively, these data suggest that therapeutic agents that activate either or both PPARα and PPARγ could be beneficial for the treatment of ALI.
Regulation of endothelial permeability
Permeability edema is characterized by a reduced alveolar liquid clearance capacity, combined with an endothelial hyperpermeability. Various signaling pathways, such as those involving reactive oxygen species (ROS), Rho GTPases and tyrosine phosphorylation of junctional proteins, converge to regulate junctional permeability, either by affecting the stability of junctional proteins or by modulating their interactions [23]. The regulation of junctional permeability is mainly mediated by dynamic interactions between the proteins of the adherens junctions and the actin cytoskeleton. Actin-mediated endothelial cell contraction is the result of myosin light chain (MLC) phosphorylation by MLC kinase (MLCK) in a Ca2+/calmodulin-dependent manner. RhoA additionally potentiates MLC phosphorylation, by inhibiting MLC phosphatase activity through its downstream effector Rho kinase (ROCK). As such, actin/myosin-driven contraction will generate a contractile force that pulls VE-cadherin inward. This contraction will force VE-cadherin to dissociate from its adjacent partner, as such producing interendothelial gaps [23]. Another possible mechanism of adherens junctions disassembly and interendothelial gap formation involves microtubule disassembly, as has been shown to occur upon treatment of pulmonary artery endothelial cells with TNF, which will be discussed in the following chapters [24].
Regulation of endothelial permeability by extracellular purines
Extracellular purines (adenosine, ADP, and ATP) function as intercellular signaling molecules when released to extracellular compartments from different sources in the body and subsequently reach the target organs. Extracellular ATP has been detected in most tissues, including the epithelium and endothelium and the smooth muscles. Normally, the level of extracellular ATP is low (1–10 nM), due to the activity of ectonucleotidases. However, under pathological conditions, like during vascular injury or traumatic shock, the local concentration of ATP at the cell surface has been reported to reach micromolar concentrations and may temporally even exceed 25 µM [25, 26].
In particular, vascular endothelial cells can be regulated by nucleotides released from platelets. During vascular injury, broken cells are also the source of the extracellular nucleotides. Furthermore, endothelium may provide a local source of ATP within vascular beds. Primary cultures of human endothelial cells derived from multiple blood vessels release ATP constitutively and exclusively across the apical membrane under basal conditions. Hypotonic challenge or the calcium agonists (ionomycin and thapsigargin) stimulate ATP release in a reversible and regulated manner [27]. Enhanced release of pharmacologically relevant amounts of ATP was observed in endothelial cells under such stimuli as shear stress, lipopolysaccharide (LPS), and ATP itself [25, 28]. Pearson and Gordon demonstrated that incubation of aortic endothelial and smooth muscle cells with thrombin resulted in the specific release of ATP, which was converted to ADP by vascular hydrolases [29]. Yang et al. showed that endothelial cells isolated from guinea pig heart release nucleotides in response to bradykinin, acetylcholine, serotonin and ADP [30].
Nucleotide action is mediated by cell surface purinoreceptors. Once released from endothelial cells, ATP may act in the blood vessel lumen at P2 receptors on nearby endothelium downstream from the site of release. ATP is also degraded rapidly and its metabolites have also been recognized as signaling molecules, which can initiate additional receptor-mediated functions. These include ADP and the final hydrolysis product adenosine.
Purinoceptors are divided into two classes: P1 or adenosine receptors and P2, which recognize primarily extracellular ATP, ADP, UTP and UDP [31, 32]. The P2 receptors are further subdivided into two subclasses. P2X receptors are extracellular ATP-gated calcium-permeable non-selective cation channels that are modulated by extracellular Ca2+, Na+, Mg2+, Zn2+, and Cu2+ [33]. The P2Y receptors are G-protein coupled receptors. P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 are coupled to Gq and activate PLCγ. P2Y12, 13, and 14 are coupled to Gi and inhibit adenylate cyclase (reviewed in [34]). Both P2Y G protein-coupled receptors, via phospholipase-induced release of Ca2+ from intracellular stores, and P2X receptor channels, via direct influx of Ca2+ through the channel from extracellular stores, are capable of triggering calcium-dependent signal transduction cascades.
The final metabolite of ATP, adenosine, binds to P1 receptors. Four different adenosine receptors have been identified and pharmacologically characterized: A1, A2A, A2B, and A3 [35]. These receptors are coupled with G proteins. A2A and A2B receptors activate adenylate cyclase, and their stimulation increases the intracellular cAMP concentration. A1 and A3 receptors stimulation decreases cAMP concentration and raises intracellular Ca2+ by a pathway involving phospholipase C activation [35].
Several studies have demonstrated that P2X sub4 is the most abundant P2 receptor in endothelial cells [36, 37]. P2X4, P2Y11, P2Y1, and P2Y2 are the most expressed P2 receptors in human umbilical vein endothelial cells (HUVEC) [37]. RT-PCR showed expression of P2Y1, P2Y2, and P2Y4 receptors, but not P2Y6 receptors in rabbit pulmonary artery EC [38].
Data regarding the role of purines in maintenance and alteration of EC barrier are contradictory. Barrier-protective properties of ATP have been reported [39, 40]. On the other hand, the P2Y1-receptor agonists 2-methylthio ATP (2meS-ATP) and ADP decreased cell size and enhanced permeation of FITC-labeled dextran through HUVEC monolayers [41]. ATP was found to increase paracellular permeability of microvascular endothelium in frog microvessels [42].
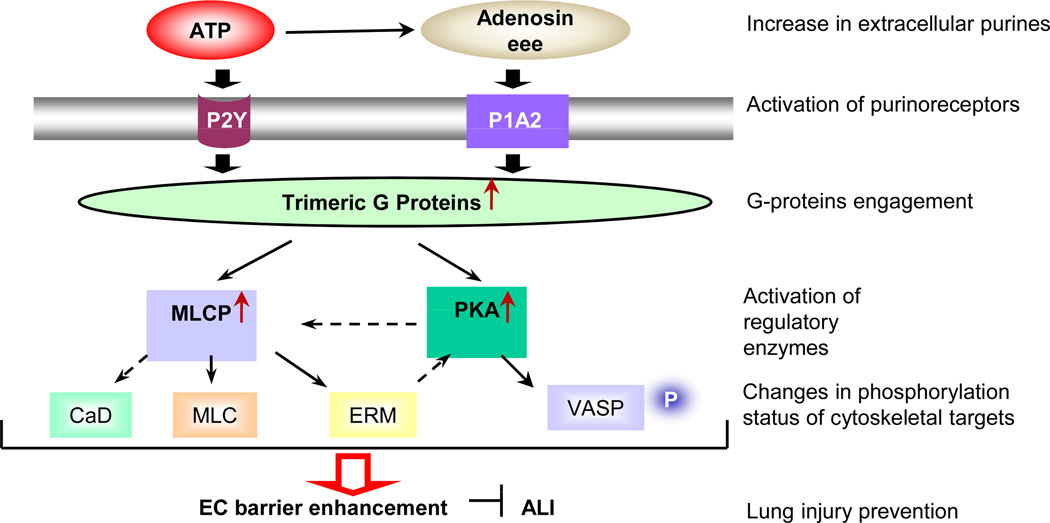
Our recently published data [43] demonstrate that ATP increases the transendothelial electrical resistance (TER) of human pulmonary artery EC (HPAEC) monolayers in a concentration-dependent manner, indicating barrier enhancement. Stable ATP analogs ATP-γ-S and 2-MeS-ATP also increase TER. In contrast, AMP-CCP, which is more specific for the P2X1 and P2X3 receptors, was completely inactive [43] suggesting that P2X receptors are unlikely to be involved in ATP-mediated EC barrier enhancement. Human pulmonary artery, human lung microvascular and bovine pulmonary artery EC demonstrate similar responses to ATP stimulation, characterized by increased TER [43]. Importantly, simultaneous addition of ATP and thrombin significantly attenuates thrombin-induced EC permeability, indicating that ATP has a barrier-protective effect [40]. ATP-induced barrier enhancement involves remodeling of intercellular junctions, but not increases in cytosolic free Ca2+ and ERK activation [43]. Specific depletion of a subunits of the trimeric G proteins Gq and Gi2, but not G12 and G13, significantly attenuates the ATP-induced increase in TER, indicating the involvement of Gq and Gi2 in ATP-induced EC barrier enhancement [43].
The ATP-induced increase in TER is tightly linked to a decrease in myosin light chain (MLC) phosphorylation and an increase in MLC phosphatase (PPase) 1 (MLCP) activity. In addition, ATP induced activation of protein kinase A (PKA), which usually has a barrier-protective effect [43]. PKA inhibition attenuates both ATP-induced increases in TER and phosphorylation of its cytoskeletal target, VASP, which in the phosphorylated form inhibits stress fiber formation, supporting the involvement of the PKA/VASP pathway in ATP-induced EC barrier enhancement. Finally, we have recently shown that EC barrier enhancement by ATP is mediated by the small GTPase Rac and the regulatory cytoskeletal protein cortactin [44]. Moreover, our data also demonstrated that ATPγS has a barrier-protective and antiinflammatory effects in vivo in a murine model of LPS-induced lung injury [45]. Based on these observations, we speculate that ATP may be added to a select list of agonists which promote the integrity of the vascular bed. Further studies are needed, however, to fully characterize molecular mechanisms of ATP-induced EC barrier enhancement/protection.
Dichotomous activities of TNF during ALI
During the course of ALI, the alveolar space, as well as the interstitium, are sites of intense inflammation, leading to the local production of pro-inflammatory cytokines, such as IL-1β, TGF-β and TNF. The latter pleiotropic cytokine is a 51 kD homotrimeric protein, binding to two types of receptors, i.e. TNF-R1 and TNF-R2 and which is mainly produced by activated macrophages and T cells. Soluble TNF, as well as the soluble TNF receptors 1 and 2, are generated upon cleavage of membrane TNF or of the membrane-associated receptors, respectively, by the enzyme TNF-alpha convertase (TACE). TNF-R1, but not TNF-R2, contains a death domain, which signals apoptosis upon the formation of the Death Inducing Signaling Complex (DISC) [46]. In spite of its lack of a death domain, TNF-R2 can nevertheless be implicated in apoptosis induction, since its activation causes degradation of TNF Receptor Associated Factor 2 (TRAF2), an inhibitor of the TNF-R1-induced DISC formation [47]. Moreover, apoptosis induction of lung microvascular endothelial cells by TNF was shown to require activation of both TNF receptors [48]. TNF-R2 was also shown to be important for ICAM-1 upregulation in endothelial cells in vitro and in vivo, an activity important in the sequestration of leukocytes in the microvessels [49]. Moreover, lung microvascular endothelial cells isolated from ARDS patients express significantly higher levels of TNF-R2 and of ICAM-1 than cells isolated from patients who had undergone a lobectomy for lung carcinoma, used as controls [50]. These findings therefore suggest that ICAM-1 and TNF-R2 may have a particular involvement in the pathogenesis of acute lung injury.
Recent results have indicated a dichotomous role of TNF in ALI and pulmonary edema. With regard to activities contributing to the generation of permeability edema, the cytokine has been proposed to be involved in the induction of apoptosis of lung microvascular endothelial cells, which can contribute to the disruption of the endothelial barrier during ALI and ARDS [48, 51]. TNF can also indirectly promote edema formation by means of inducing the production of ROS [52]. ROS have been shown to increase MLC phosphorylation in the vascular endothelium [23] and moreover decrease the expression of ENaC and the Na+-K+-ATPase [53].
TNF can also directly increase endothelial permeability in pulmonary artery endothelial cells, by means of destabilizing microtubules, which in turn induces barrier dysfunction in a RhoA/ROCK-dependent, but MLC kinase-independent manner [24]. As such, TNF-mediated microtubule destabilization can amplify endothelial cell contraction by a Rho-dependent MLC phosphatase inhibition, thereby inducing a profound permeability increase. Microvascular endothelial cells were reported to respond to TNF by altering their F-actin cytoskeleton and junctional permeability, through mechanisms that include protein kinase C (PKC) and p38 MAPK. In these cells, TNF induces Ezrin, radixin and moesin phosphorylation, accompanied by cytoskeletal changes, paracellular gap formation, and increased permeability to dextran and albumin [54].
TNF has moreover been shown to inhibit epithelial sodium uptake, which is crucial for alveolar liquid clearance, by means of reducing the expression of at least 3 subunits (alpha, beta, gamma) of the epithelial sodium channel ENaC [55], most likely by means of a TNF-R1 and ceramide-dependent mechanism [56]. In sharp contrast to its effects described above, which promote pulmonary edema, TNF has also been shown to increase alveolar fluid clearance in a rat pneumonia model [57]. Taken together, these results thus point towards a dichotomous role of TNF in the development of pulmonary edema during ALI.
The lectin-like domain of TNF reduces pulmonary edema formation
In order to explain the apparently contradictory effects of TNF in models of pulmonary edema, as discussed in the previous paragraph, we propose that functionally distinct domains of the cytokine, i.e. the receptor binding sites versus the lectin-like domain, account for the cytokine’s dichotomous activity during pulmonary edema [58]. Spatially distinct from its receptor binding sites, TNF carries a lectin-like domain, recognizing specific oligosaccharides, such as N,N’-diacetylchitobiose and branched trimannoses [59]. Apart from exerting a lytic activity towards bloodstream forms of African trypanosomes [60], the lectin-like domain of TNF was also shown to increase sodium uptake in lung microvascular endothelial cells [61], that were recently shown to express ENaC [62], as well as in alveolar epithelial cell lines [63]. Interestingly, the activities of the lectin-like domain of TNF cannot be inhibited by the soluble TNF receptors [60].
Several observations point towards a positive activity of the lectin-like domain in hydrostatic edema reabsorption: 1) the ALC-activating effect of mTNF in C57BL6 mice is as strong in wild type as in double TNF receptor knock out mice, indicating a TNF receptor independent activity of the cytokine [63]; 2) the T104A-E106A-E109A Triple mouse TNF mutant, which has a significantly reduced lectin-like activity, but retains TNF-R1 and TNF-R2-mediated activities [64], fails to activate ALC in flooded rat lungs in situ and no longer stimulates Na+ uptake in A549 cells in vitro, in contrast to wt mTNF [63]; 3) the lectin-like domain of the cytokine, mimicked by the mouse TIP peptide, was shown to activate alveolar liquid clearance in a blood-perfused isolated flooded rat lung model ex vivo [65]; 4) the human TIP peptide was shown to activate ALC in a flooded rat lung model in situ and lung liquid clearance in vivo when applied intratracheally, but not intravenously, to the same extent as the beta2-adrenergic agonist terbutaline [58]; 5) the neutral effect of hTNF on ALC in flooded rat lungs in situ can be shifted towards a positive effect upon complexing the cytokine with a soluble TNF-R1 construct, and this positive activity can be inhibited upon adding N,N-diacetylchitobiose, an oligosaccharide binding to the lectin-like domain of TNF, to the complex [58]. Taken together, these results indicate that in flooded rat lungs the receptor binding sites of TNF can inhibit, whereas its lectin-like domain can activate edema reabsorption.
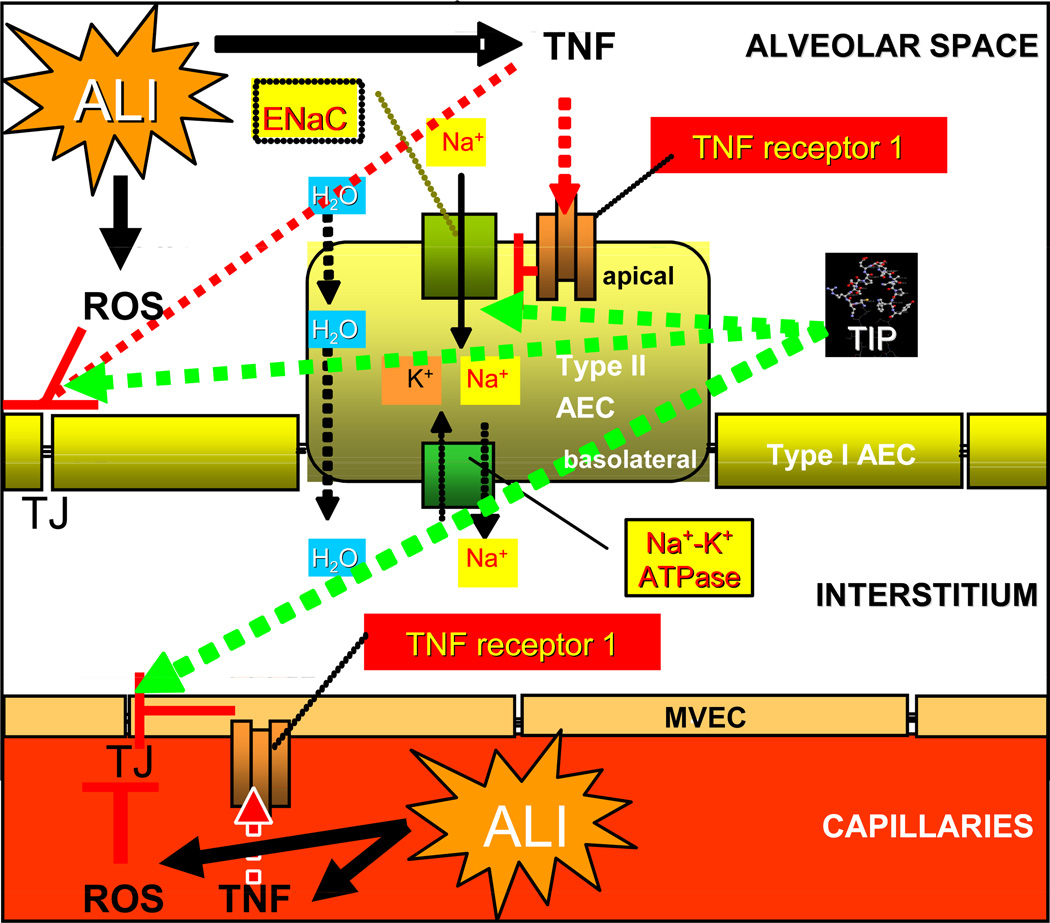
In contrast to the situation in hydrostatic edema, during acute lung injury, such as in ARDS, severe pneumonia or upon lung transplantation-induced ischemia-reperfusion damage, the alveolar-capillary barriers can be disrupted, leading to an infiltration of e.g. neutrophils and factors contained in the blood into the alveoli. Other’s recent data have indicated that also in LPS/S. aureus alpha-toxin-treated isolated perfused rabbit lungs ex vivo the TIP peptide significantly increases fluid reabsorption and moreover reduces microvascular permeability, by means of a still unknown mechanism [66]. Our recent data have moreover demonstrated that the TIP peptide also has positive effects in ischemia-reperfusion induced acute lung injury. Indeed in a rat left lung isotransplantation model in vivo, the TIP peptide significantly improved lung parameters, upon clamping of the right lung, indicating that it ameliorates lung function upon transplantation (Hamacher et al., submitted). As indicated in scheme 2, acute lung injury is accompanied by an increased ROS and TNF production. Stimulation of TNF-R1 leads to a reduced expression of ENaC [55, 56], whereas increased extracellular and intracellular ROS production leads to a reduced expression of both the Na+-K+-ATPase and ENaC [53]. Both TNF and ROS moreover directly increase endothelial permeability. The TIP peptide, mimicking the lectin- like domain of TNF, is able to upregulate sodium uptake in type II alveolar epithelial cells, as such increasing alveolar fluid clearance capacity. The peptide moreover protects endothelial barrier integrity, by means of a still unknown mechanism. Taken together, these results indicate the potential therapeutic use of the TIP peptide, mimicking the lectin-like domain of TNF, for the treatment of permeability edema.
Scheme 2.
Dichotomous activity of TNF in alveolar liquid clearance and barrier protection during ALL TNF, which is induced during ALI, causes a downregulation of ENaC expression in type II alveolar epithelial cells, upon activating TNF-R1 [55, 56]. Moreover, TNF increases permeability, by means of interfering with tight junctions (TJ) in both alveolar epithelial (AEC) and capillary endothelial cells (MVEC). ROS, the generation of which is frequently increased during ALI, were also shown to downregulate ENaC and Na+-K+-ATPase expression [53] and moreover also lead to decreased endothelial barrier integrity. The TIP peptide, mimicking the lectin-like domain of TNF, is able to increase sodium uptake in alveolar epithelial cells and to restore endothelial barrier integrity, as such providing a significant protection against the development of permeability edema (red lines: inhibition, green arrows: activation).
Anti-inflammatory and barrier-protective effects of hsp90 inhibitors
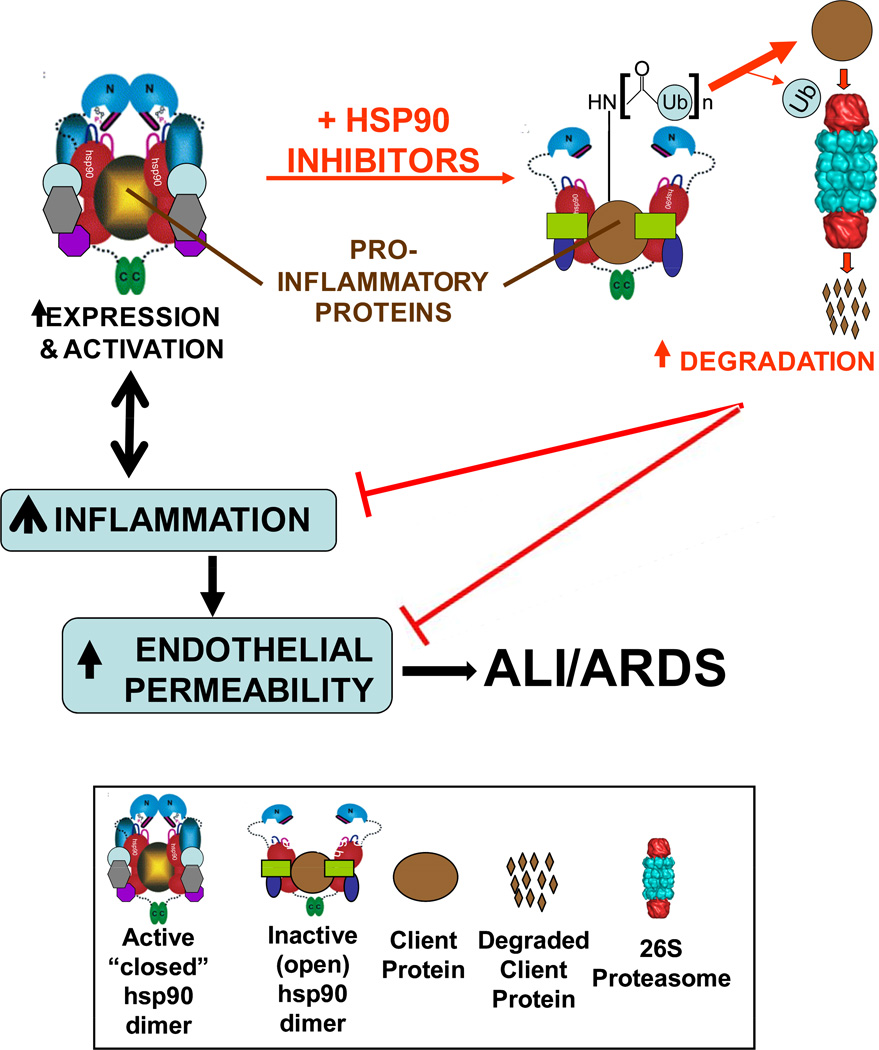
Inflammation is a causative factor in most major cardiovascular diseases, including acute lung injury (ALI) and its most severe form, the acute respiratory distress syndrome (ARDS). Single-target anti-inflammatory agents (e.g., COX inhibitors) lack serious side effects but are void of broad-spectrum anti-inflammatory activity. Clearly, the availability of multi-targeted, strong anti-inflammatory agents with limited side effects would be of great significance in the prevention and management of ALI and ARDS. Emerging data suggest that heat shock protein 90 (hsp90) inhibitors may fit this profile. Hsp90, a ubiquitous molecular chaperone constituting 1–3% of total cellular protein, is involved in the conformational regulation of more than 100 client proteins. The hsp90 homodimer exists in a flux between an open and a closed conformation. In the closed conformation, hsp90 promotes client protein folding, stability and –in certain cases- increased activity, whereas in the open conformation, it promotes protein degradation. The so-called “hsp90 inhibitors” lock the dimer to an open conformation and promote client protein degradation. We and others have shown that hsp90 inhibitors block the expression and activity of certain proinflammatory mediators in vitro. Recently, we demonstrated that pretreatment with either of two hsp90 inhibitors dramatically protects septic mice by greatly prolonging survival, reducing or abolishing systemic and end organ inflammation, attenuating capillary hyper-permeability and restoring normal end organ function. Furthermore, cell culture studies indicated that these hsp90 inhibitors prevent as well as restore endothelial hyper-permeability induced by direct application of any of several pro-inflammatory mediators. The mechanism(s) behind these effects remain unclear. Since hsp90 inhibitors have recently completed Phase I and II trials for cancer, demonstrating low incidence and severity of side effects, they represent an exciting new possibility as clinically useful anti-inflammatory drugs.
Hsp90 is an abundant molecular chaperone (constituting 1–3% of total cytosolic protein), highly conserved from prokaryotes to eukaryotes. The multi-chaperone heat shock protein (hsp) 90 complexes mediate the maturation and stability of a variety of proteins, many of which are involved in the regulation of cell survival, apoptosis, motility and migration. These proteins are referred to as ‘clients’ of hsp90 and include Akt/PKB, Raf, p53, cSrc, all vertebrate steroid receptors, EGF-R, VEGFR2, Apaf-1, eNOS, vimentin, F-actin and many others [67]. Acting as a scaffold, hsp90 facilitates client protein interactions and optimizes cell response to stimuli. This response can be beneficial or injurious. Hsp90 exists as a homodimer (namely α/α and β/β); each monomer consisting three distinct domains- a 25kDa N-terminal “ATP-binding domain”, a 35kDa middle domain and a 12kDa C-terminal dimerization domain. The ADP-bound hsp90, which corresponds to an “open” conformation, first binds to its client proteins with the assistance of different co-chaperones. Replacement of ADP by ATP results in transient association of the N-terminal domains giving rise to a “closed” structural conformation, which now effectively clamps the client protein and aids in its proper folding, stabilization and maturation [68]. The hsp90 chaperone machinery is therefore in a constant flux between two different conformations, which in turn, specifies its interaction with a defined set of co-chaperones [69].
Geldanamycin (a benzoquinone antibiotic) and radicicol (a macrolactone) are two chemically distinct compounds that interact with the N-terminal domain of hsp90 and result in the destabilization and degradation of many client proteins [70]. Unlike geldanamycin, the solid state conformation of radicicol when bound to the ATPase site of hsp90 is identical to that when un-bound to hsp90 [71], making it one of the strongest known inhibitors of hsp90 (the Kd for radicicol is 19 nM as compared to 1.2 µM for geldanamycin). Geldanamycin-bound hsp90 resembles the chaperone’s ADP-bound “open” conformation, and this results in recruitment events of other hsp90-interacting proteins such as hsp70 and E3 ubiquitin ligases (e.g. CHIP) which interact with hsp90/hsp70 through the TPR motifs and promote ubiquitination and subsequent degradation of client proteins [72]. Hsp90 inhibitors are unique in that, although they are directed towards a specific molecular target, they simultaneously inhibit multiple, interdigitating, hsp90-requiring signaling pathways. Since many of hsp90 client proteins are involved in survival and growth of tumor cells, selective inhibition of hsp90 has shown extremely promising results in vitro and in vivo in inducing tumor cell death/apoptosis. Hsp90 inhibitors are now being utilized to investigate association of hsp90 with members of pro-inflammatory signaling pathways, such as NFκB [73]. A second generation geldanamycin analog, which is less hepatoxic and more stable than geldanamycin, 17-allylamino-17-demethoxy-geldanamycin (17-AAG), recently completed Phase II clinical trials against various malignancies [74] and has so far revealed promising outcomes. There is a second ATP binding site located in the C-terminal region of hsp90. Novobiocin [75] and cisplatin [76] bind only to this particular C-terminal ATPase pocket, thereby inhibiting hsp90 function [75, 77]. Hsp90 requires several distinct co-chaperone proteins to perform its functions, such as hsp70, hsp40 and hsp27 [78], hop (hsp90/hsp70-organizing protein), hip (hsp interacting protein), BAG-1, and CHIP (c-terminus of hsp70 interacting protein), etc. [79–81]. Furthermore, the energy-dependent ubiquitin-proteasome pathway (UPP) eliminates proteins that fail to attain their native conformation, as presented to it by the hsp90 complex in the open conformation [82]. Several lines of evidence indicate that there are functional relationships between the UPP and hsp90 [83]. For example, inhibition of the UPP results in up-regulation of heat shock proteins [84] and hsp90 or hsp70 are required for ubiquitination and degradation of substrates [85]. Hsp90 inhibitors convert the hsp90 function from protein folding to protein degradation through ubiquitination of the client proteins [86, 87]. Thus, chaperones and UPP appear to form a cellular surveillance system that monitors protein quality. Among the several co-chaperones, CHIP is the only one (at least so far known) which also is one of the U-box E3 of the UPP, thus enabling it to act as a bridge that links the chaperones and the UPP [88].
NFκB is an important pro-inflammatory transcription factor which mediates up-regulated expression of several pro-inflammatory cytokines and chemokines, such as TNF-α, IL-6, IL-8, IL-1β etc., critical for amplifying the inflammatory insult in ALI and ARDS. Although these mediators are important for host defense against the invading bacteria, their uncontrolled and excessive production ultimately contributes to multiple organ injury. Activation of NFκB requires phosphorylation of, and subsequent dissociation from, its associated inhibitory IκB that mask its nuclear localization signal sequence, by IκB kinase (IKK) [89]. IKK exists in complexes with hsp90, required for IKK stabilization and function [90]. Consequently, hsp90 inhibitors inhibit NFκB activation in various cell lines, in vitro [91]. Other hsp90 client proteins include STAT3 [92] and pp60c-Src [93], who also play important roles in augmenting the proinflammatory response in inflammation [94, 95] and may thus be additional targets for the protective effects of hsp90 inhibitors. Prior induction of stress proteins by “heat shock” protects against LPS-induced vascular leakage [96] and ischemia/reperfusion and ventilator induced lung injury [97, 98]. It was hypothesized that induction of hsp70 by heat shock is the principal mediator of the observed cytoprotective effect. Hsp90 inhibitors have been shown repeatedly to upregulate hsp70 expression. Hsp70 is an important component of the open-conformation hsp90 dimer complex, necessary for the recruitment of ligases and subsequent ubiquitination and proteasomal degradation of many pro-inflammatory client proteins of hsp90. Taking all these findings together, it is very likely that the protective effects of hsp70 are mediated through the degradation of one or (more likely) multiple proinflammatory hsp90 client proteins.
Recently, we demonstrated that hsp90 inhibitors prolong survival, attenuate inflammation and reduce lung injury in a murine model of LPS-induced inflammation [99]. C57BI/6 mice received either one of two hsp90 inhibitors, radicicol or 17-AAG before receiving a high dose of LPS. Outcomes included survival and parameters of systemic inflammation (plasma neutrophil, cytokine, chemokine and nitrite/nitrate levels), pulmonary inflammation (lung NFκB and myeloperoxidase activities, iNOS expression, iNOS-hsp90 complex formation, leukocyte infiltration), and lung injury (pulmonary capillary leak, lung function). Mice pre-treated with vehicle and receiving endotoxin exhibited 100% 24-h lethality, dramatic increase in all parameters of systemic and pulmonary inflammation, increased capillary leak and reduced lung function. Compared to them, mice receiving either radicicol or 17-AAG prior to LPS, exhibited prolonged survival, reduced or abolished increases in systemic and pulmonary inflammatory parameters, attenuated capillary leak and restored, normal lung function [99]. Further experiments [100, 101] suggest that a major mechanism of the antiinflammatory, organ-protective effects of hsp90 inhibitors is their ability to prevent and restore endothelial barrier function, possibly via their targeting of pp60c-Src. Thus, pulmonary endothelial cell hyperpermeability induced by a number of agents (LPS, TGFβ1, PMA, thrombin, VEGF) was prevented as well as repaired by hsp90 inhibitors [100]. The LPS-induced endothelial hyperpermeability was associated with activation of pp60c-Src, which was completely prevented by hsp90 inhibitors [101]. Preliminary studies suggest that the actin cytoskeleton, perhaps through its association with hsp27, is involved in the barrier protective actions of hsp90 inhibitors [100].
Conclusion
Permeability edema represents a life-threatening complication of acute lung injury, severe pneumonia and ARDS, characterized by a combined dysregulation of pulmonary epithelial and endothelial apoptosis, endothelial barrier integrity and alveolar liquid clearance capacity. As such, it is likely that several of these parameters have to be targeted in order to obtain a successful therapy. This review focuses on a selection of recently discovered substances and mechanisms that might improve ALI therapy. As such, we have discussed the inhibition of apoptosis and necrosis occurring during ALI, by means of the restoration of Zn2+ homeostasis. PPARα and γ agonists can represent therapeutically promising molecules, since they inhibit transcription factors as well as essential co-activators involved in the activation of pro-inflammatory cytokines, chemokines and adhesion molecules, all of which are implicated in ALI. Apart from inducing a potent inhibition of inflammation upon interfering with NF-κB activation, hsp 90 inhibitors were shown to prevent and restore endothelial barrier integrity. These agents are able to significantly improve survival and lung function during LPS-induced ALI. A restoration of endothelial barrier integrity during ALI can also be obtained upon increasing extracellular levels of ATP or adenosine, which activate the purinoreceptors P2Y and P1A2, respectively, leading to a decrease in myosin light chain phosphorylation and an increase in MLC phosphatase 1 activity. The pro-inflammatory cytokine TNF is involved in endothelial apoptosis and hyperpermeability, as well as in the reduction of alveolar liquid clearance, upon activating its receptors. However, apart from its receptor-binding sites, TNF harbors a lectin-like domain, which can be mimicked by the TIP peptide. This peptide has been shown to increase alveolar liquid clearance and moreover induces endothelial barrier protection. As such, TNF can be considered as a moonlighting cytokine, combining both positive and negative activities for permeability edema generation within one molecule.
Since each of the discussed strategies inhibit only a selection of the parameters implicated in the generation of permeability edema, this suggests that combination therapies might prove to be more efficient than single target strategies.
Scheme 1.
Signal transduction pathways implicated in ATP-mediated endothelial barrier enhancement.
Scheme 3.
Proposed mechanism of action for the anti-inflammatory and barrier-protective actions of hsp90 inhibitors.
Scheme 4.
Frequently used hsp 90 inhibitors.
Non-standard abbreviations
- ALC
alveolar liquid clearance
- ALI
acute lung injury
- ARDS
Acute respiratory distress syndrome
- BALF
broncholaveolar lavage fluid
- DISC
Death Inducing Signaling Complex
- Hsp90
Heat shock protein 90
- iNOS
inducible nitric oxide synthase
- MLC
Myosin Light Chain
- MT
metallothioneins
- NF-AT
nuclear factor of activated T cells
- PPAR
Peroxisome proliferator-activated receptor
- STATs
signal transducers and activators of transcription
- TACE
TNF-Alpha Converting Enzyme
- TER
transendothelial electrical resistance
- TRAF2
TNF Receptor Associated Factor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rudkowski JC, Barreiro E, Harfouche R, Goldberg P, Kishta O, D’Orleans-Juste P, et al. Roles of iNOS and nNOS in sepsis-induced pulmonary apoptosis. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L793–L800. doi: 10.1152/ajplung.00266.2003. [DOI] [PubMed] [Google Scholar]
- 2.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Pathol. 2002;161(5):1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Young RS, Sun NN, Witten ML. In vitro cytokine release from rat type II pneumocytes and alveolar macrophages following exposure to JP-8 jet fuel in co-culture. Toxicology. 2002;173(3):211–219. doi: 10.1016/s0300-483x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 4.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Current opinion in critical care. 2005;11(1):82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Liu D, Zeng BX, Zhang SH, Wang YL, Zeng L, Geng ZL, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces acute lung injury in endotoxemic rats. Crit. Care Med. 2005;33(10):2309–2316. doi: 10.1097/01.ccm.0000183161.81503.7d. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Liu L, Fletcher BS, Visner GA. Novel action of indoleamine 2,3-dioxygenase attenuating acute lung allograft injury. Am. J. Respir. Crit. Care Med. 2006;173(5):566–572. doi: 10.1164/rccm.200509-1413OC. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L165–L177. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol. 2006;291(3):555–568. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]
- 9.Beattie JH, Kwun IS. Is zinc deficiency a risk factor for atherosclerosis? Br. J. Nutr. 2004;91(2):177–181. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 10.Taylor CG, Towner RA, Janzen EG, Bray TM. MRI detection of hyperoxia-induced lung edema in Zn-deficient rats. Free Radic. Biol. Med. 1990;9(3):229–233. doi: 10.1016/0891-5849(90)90033-f. [DOI] [PubMed] [Google Scholar]
- 11.Taylor CG, McCutchon TL, Boermans HJ, DiSilvestro RA, Bray TM. Comparison of Zn and vitamin E for protection against hyperoxia-induced lung damage. Free Radic. Biol. Med. 1997;22(3):543–550. doi: 10.1016/s0891-5849(96)00390-5. [DOI] [PubMed] [Google Scholar]
- 12.Yanagisawa H, Sato M, Nodera M, Wada O. Excessive zinc intake elevates systemic blood pressure levels in normotensive rats-potential role of superoxide-induced oxidative stress. J. Hypertens. 2004;22(3):543–550. doi: 10.1097/00004872-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Homma S, Jones R, Qvist J, Zapol WM, Reid L. Pulmonary vascular lesions in the adult respiratory distress syndrome caused by inhalation of zinc chloride smoke: a morphometric study. Hum. Pathol. 1992;23(1):45–50. doi: 10.1016/0046-8177(92)90010-z. [DOI] [PubMed] [Google Scholar]
- 14.Kodavanti UP, Schladweiler MC, Ledbetter AD, Hauser R, Christiani DC, Samet JM, et al. Pulmonary and systemic effects of zinc-containing emission particles in three rat strains: multiple exposure scenarios. Toxicol. Sci. 2002;70(1):73–85. doi: 10.1093/toxsci/70.1.73. [DOI] [PubMed] [Google Scholar]
- 15.Vasto S, Mocchegiani E, Malavolta M, Cuppari I, Listi F, Nuzzo D, et al. Zinc and inflammatory/immune response in aging. Ann. N. Y. Acad. Sci. 2007;1100:111–122. doi: 10.1196/annals.1395.009. [DOI] [PubMed] [Google Scholar]
- 16.Ameshima S, Golpon H, Cool CD, Chan C, Vandivier RW, Gardai SJ, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ. Res. 2003;92(10):1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 17.Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384(6604):39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 18.Gervois P, Vu-Dac N, Kleemann R, Kockx M, Dubois G, Laine B, et al. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor alpha agonists via inhibition of CCAAT box/enhancer-binding protein beta. J. Biol. Chem. 2001;276(36):33471–33477. doi: 10.1074/jbc.M102839200. [DOI] [PubMed] [Google Scholar]
- 19.Ward JE, Gould H, Harris T, Bonacci JV, Stewart AG. PPAR gamma ligands, 15-deoxy-delta12,14-prostaglandin J2 and rosiglitazone regulate human cultured airway smooth muscle proliferation through different mechanisms. Br. J. Pharmacol. 2004;141(3):517–525. doi: 10.1038/sj.bjp.0705630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respiratory research. 2005;6:91–98. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Zeng BX, Zhang SH, Yao SL. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm. Res. 2005;54(11):464–470. doi: 10.1007/s00011-005-1379-0. [DOI] [PubMed] [Google Scholar]
- 22.Standiford TJ, Keshamouni VG, Reddy RC. Peroxisome proliferator-activated receptor-γ as a regulator of lung inflammation and repair. Proceedings of the American Thoracic Society. 2005;2(3):226–231. doi: 10.1513/pats.200501-010AC. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 24.Petrache I, Birukova A, Ramirez SI, Garcia JG, Venn AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol. 2003;28(5):574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- 25.Bodin P, Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm. Res. 1998;47(8):351–354. doi: 10.1007/s000110050341. [DOI] [PubMed] [Google Scholar]
- 26.Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch. 1998;437(1):31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- 27.Schwiebert LM, Rice WC, Kudlow BA, Taylor AL, Schwiebert EM. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am J Physiol Cell Physiol. 2002;282(2):C289–C301. doi: 10.1152/ajpcell.01387.2000. [DOI] [PubMed] [Google Scholar]
- 28.Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol. 1996;27(6):872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74(3):401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–697. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson KA, Gao ZG. Adenosine Receptors as therapeutic targets. Nature Reviews Drug Discovery. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 34.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y–receptor activating molecules. Mol Pharmacol. 2003;64(4):785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 35.Fredholm BB, Uzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279(1):H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, et al. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40(6):841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Konduri GGI, Bakhutashvili Frenn R, Chandrasekhar I, Jacobs ER, Khanna AK. P2Y purine receptor responses and expression in the pulmonary circulation of juvenile rabbits. Am J Physiol Heart Circ Physiol. 2004;287(1):H157–H164. doi: 10.1152/ajpheart.00617.2003. [DOI] [PubMed] [Google Scholar]
- 39.Noll T, Holschermann H, Koprek K, Gunduz D, Haberbosch W, Tillmanns H, et al. ATP reduces macromolecule permeability of endothelial monolayers despite increasing [Ca2+]i. Am J Physiol. 1999;276(6 Pt 2):H1892–H1901. doi: 10.1152/ajpheart.1999.276.6.H1892. [DOI] [PubMed] [Google Scholar]
- 40.Gunduz D, Hirche F, Hartel FV, Rodewald CW, Schafer M, Pfitzer G, et al. ATP antagonism of thrombin-induced endothelial barrier permeability. Cardiovasc Res. 2003;59(2):470–478. doi: 10.1016/s0008-6363(03)00427-9. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka N, Kawasaki K, Nejime N, Kubota Y, Nakamura K, Kunitomo M, et al. P2Y receptor-mediated Ca2+ signaling increases human vascular endothelial cell permeability. J Pharmacol Sci. 2004;95(2):174–180. doi: 10.1254/jphs.fpj03036x. [DOI] [PubMed] [Google Scholar]
- 42.He P, Curry FE. Differential actions of cAMP on endothelial [Ca2+]i and permeability in microvessels exposed to ATP. Am J Physiol. 1993;265(3 Pt 2):H1019–H1023. doi: 10.1152/ajpheart.1993.265.3.H1019. [DOI] [PubMed] [Google Scholar]
- 43.Kolosova IA, Mirzapoiazova T, Adyshev D, Usatyuk P, Romer LH, Jacobson JR, et al. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res. 2005;97(2):115–124. doi: 10.1161/01.RES.0000175561.55761.69. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L289–L295. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- 45.Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, Verin AD. Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L319–L324. doi: 10.1152/ajplung.00283.2007. [DOI] [PubMed] [Google Scholar]
- 46.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Yang Y, Ashwell JD. TNF-RII and C-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416(6878):345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 48.Lucas R, Garcia I, Donati YR, Hribar M, Mandriota SJ, Giroud C, et al. Both TNF receptors are required for direct TNF-mediated cytotoxicity in microvascular endothelial cells. Eur J Immunol. 1998;28(11):3577–3586. doi: 10.1002/(SICI)1521-4141(199811)28:11<3577::AID-IMMU3577>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Lucas R, Lou J, Morel DR, Ricou B, Suter PM, Grau GE. TNF receptors in the microvascular pathology of acute respiratory distress syndrome and cerebral malaria. J Leukoc Biol. 1997;61(5):551–558. doi: 10.1002/jlb.61.5.551. [DOI] [PubMed] [Google Scholar]
- 50.Grau GE, Mili N, Lou JN, Morel DR, Ricou B, Lucas R, et al. Phenotypic and functional analysis of pulmonary microvascular endothelial cells from patients with acute respiratory distress syndrome. Lab Invest. 1996;74(4):761–770. [PubMed] [Google Scholar]
- 51.Hamacher J, Lucas R, Lijnen HR, Buschke S, Dunant Y, Wendel A, et al. Tumor necrosis factor and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. AJRCCM. 2002;166:651–656. doi: 10.1164/rccm.2109004. [DOI] [PubMed] [Google Scholar]
- 52.Faggioni R, Gatti S, Demitri MT, Delgado R, Echtenacher B, Gnocchi P, et al. Role of xanthine oxidase and reactive oxygen intermediates in LPS- and TNF-induced pulmonary edema. J Lab Clin Med. 1994;123(3):394–399. [PubMed] [Google Scholar]
- 53.Dada LA, Sznajder Jl. Hypoxic inhibition of alveolar fluid reabsorption. Adv Exp Med Biol. 2007;618:159–168. doi: 10.1007/978-0-387-75434-5_12. [DOI] [PubMed] [Google Scholar]
- 54.Koss M, Pfeiffer GR2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, et al. Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol. 2006;176(2):1218–1227. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- 55.Dagenais A, Frechette R, Yamagata Y, Yamagata T, Carmel JF, Clermont ME, et al. Downregulation of ENaC activity and expression by TNF-alpha in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L301–L311. doi: 10.1152/ajplung.00326.2002. [DOI] [PubMed] [Google Scholar]
- 56.Bao HF, Zhang ZR, Liang YY, Ma JJ, Eaton DC, Ma HP. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-alpha through protein kinase C. Am J Physiol Renal Physiol. 2007;293(4):F1178–F1186. doi: 10.1152/ajprenal.00153.2007. [DOI] [PubMed] [Google Scholar]
- 57.Rezaiguia S, Garat C, Delclaux C, Meignan M, Fleury J, Legrand P, et al. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J Clin Invest. 1997;99(2):325–335. doi: 10.1172/JCI119161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braun C, Hamacher J, Morel DR, Wendel A, Lucas R. Dichotomal role of TNF in experimental pulmonary edema reabsorption. J Immunol. 2005;175(5):3402–3408. doi: 10.4049/jimmunol.175.5.3402. [DOI] [PubMed] [Google Scholar]
- 59.Hession C, Decker JM, Sherblom AP, Kumar S, Yue CC, Mattaliano RJ, et al. Uromodulin (Tamm-Horsfall glycoprotein): a renal ligand for lymphokines. Science. 1987;237(4821):1479–1484. doi: 10.1126/science.3498215. [DOI] [PubMed] [Google Scholar]
- 60.Lucas R, Magez S, De Leys R, Fransen L, Scheerlinck JP, Rampelberg M, et al. Mapping the lectin-like activity of tumor necrosis factor. Science. 1994;263(5148):814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- 61.Hribar M, Bloc A, van der Goot FG, Fransen L, De Baetselier P, Grau GE, et al. The lectin-like domain of tumor necrosis factor-alpha increases membrane conductance in microvascular endothelial cells and peritoneal macrophages. Eur J Immunol. 1999;29(10):3105–3111. doi: 10.1002/(SICI)1521-4141(199910)29:10<3105::AID-IMMU3105>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 62.Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, et al. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch. 2008;455(5):849–857. doi: 10.1007/s00424-007-0341-0. [DOI] [PubMed] [Google Scholar]
- 63.Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, et al. Mechanisms of TNF-alpha stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1258–L1265. doi: 10.1152/ajplung.2001.280.6.L1258. [DOI] [PubMed] [Google Scholar]
- 64.Lucas R, Echtenacher B, Sablon E, Juillard P, Magez S, Lou J, et al. Generation of a mouse tumor necrosis factor mutant with antiperitonitis and desensitization activities comparable to those of the wild type but with reduced systemic toxicity. Infect Immun. 1997;65(6):2006–2010. doi: 10.1128/iai.65.6.2006-2010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elia N, Tapponnier M, Matthay MA, Hamacher J, Pache JC, Brundler MA, et al. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor-alpha. Am J Respir Crit Care Med. 2003;168(9):1043–1050. doi: 10.1164/rccm.200206-618OC. [DOI] [PubMed] [Google Scholar]
- 66.Vadasz I, Schermuly RT, Ghofrani HA, Rummel S, Wehner S, Muhldorfer I, et al. The lectin-like domain of tumor necrosis factor-alpha improves alveolar fluid balance in injured isolated rabbit lungs. Crit Care Med. 2008;36(5):1543–1550. doi: 10.1097/CCM.0b013e31816f485e. [DOI] [PubMed] [Google Scholar]
- 67.Blagg BS, Kerr TD. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev. 2005 doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- 68.Chadli A, Bouhouche I, Sullivan W, Stensgard B, McMahon N, Catelli MG, et al. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc Natl Acad Sci U S A. 2000;97(23):12524–12529. doi: 10.1073/pnas.220430297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3(3):213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 70.Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, et al. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell. 2003;11(3):647–658. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 71.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42(2):260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 72.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19(6):4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeo M, Park HK, Lee KM, Lee KJ, Kim JH, Cho SW, et al. Blockage of HSP 90 modulates Helicobacter pylori-induced IL-8 productions through the inactivation of transcriptional factors of AP-1 and NF-kappaB. Biochem Biophys Res Commun. 2004;320(3):816–824. doi: 10.1016/j.bbrc.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 74.Pacey S, Banerji U, Judson I, Workman P. Hsp90 inhibitors in the clinic. Handb Exp Pharmacol. 2006;172:331–358. doi: 10.1007/3-540-29717-0_14. [DOI] [PubMed] [Google Scholar]
- 75.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275(47):37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 76.Itoh H, Ogura M, Komatsuda A, Wakui H, Miura AB, Tashima Y. A novel chaperone-activity-reducing mechanism of the 90-kDa molecular chaperone HSP90. Biochem J. 1999;343(Pt 3):697–703. [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenhagen MC, Soti C, Schmidt U, Wochnik GM, Hartl FU, Holsboer F, et al. The heat shock protein 90-targeting drug cisplatin selectively inhibits steroid receptor activation. Mol Endocrinol. 2003;17(10):1991–2001. doi: 10.1210/me.2003-0141. [DOI] [PubMed] [Google Scholar]
- 78.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun. 2004;72(12):7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frydman J, Hohfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22(3):87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- 80.Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, et al. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16(16):4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3(1):93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 82.Wickner S, Maurizi MR, Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999;286(5446):1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- 83.Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158(6):1883–1889. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 84.Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18(1):30–38. doi: 10.1128/mcb.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 2003;278(31):28490–28500. doi: 10.1074/jbc.M209682200. [DOI] [PubMed] [Google Scholar]
- 86.Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, et al. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A. 1996;93(25):14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitesell L, Cook P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol. 1996;10(6):705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- 88.McClellan AJ, Frydman J. Molecular chaperones and the art of recognizing a lost cause. Nat Cell Biol. 2001;3(2):E51–E53. doi: 10.1038/35055162. [DOI] [PubMed] [Google Scholar]
- 89.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 90.Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene. 2004;23(31):5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- 91.Malhotra V, Shanley TP, Pittet JF, Welch WJ, Wong HR. Geldanamycin inhibits NF-kappaB activation and interleukin-8 gene expression in cultured human respiratory epithelium. Am J RespirCell Mol Biol. 2001;25(1):92–97. doi: 10.1165/ajrcmb.25.1.4384. [DOI] [PubMed] [Google Scholar]
- 92.Sato N, Yamamoto T, Sekine Y, Yumioka T, Junicho A, Fuse H, et al. Involvement of heat-shock protein 90 in the interleukin-6-mediated signaling pathway through STAT3. Biochem Biophys Res Commun. 2003;300(4):847–852. doi: 10.1016/s0006-291x(02)02941-8. [DOI] [PubMed] [Google Scholar]
- 93.Oppermann H, Levinson W, Bishop JM. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci USA. 1981;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao H, Guo RF, Speyer CL, Reuben J, Neff TA, Hoesel LM, et al. Stat3 activation in acute lung injury. J Immunol. 2004;172(12):7703–7712. doi: 10.4049/jimmunol.172.12.7703. [DOI] [PubMed] [Google Scholar]
- 95.Severgnini M, Takahashi S, Tu P, Perides G, Homer RJ, Jhung JW, et al. Inhibition of the Src and Jak Kinases Protects Against Lipopolysaccharide-induced Acute Lung Injury. Am J Respir Crit Care Med. 2005;171(8):858–867. doi: 10.1164/rccm.200407-981OC. [DOI] [PubMed] [Google Scholar]
- 96.Suganuma T, Irie K, Fujii E, Yoshioka T, Muraki T. Effect of heat stress on lipopolysaccharide-induced vascular permeability change in mice. J Pharmacol Exp Ther. 2002;303(2):656–663. doi: 10.1124/jpet.102.035758. [DOI] [PubMed] [Google Scholar]
- 97.Godzich M, Hodnett M, Frank JA, Su G, Pespeni M, Angel A, et al. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in a model of lung ischemia-reperfusion injury in rats. FASEB J. 2006;20(9):1519–1521. doi: 10.1096/fj.05-4708fje. [DOI] [PubMed] [Google Scholar]
- 98.Ribeiro SP, Rhee K, Tremblay L, Veldhuizen R, Lewis JF, Slutsky AS. Heat stress attenuates ventilator-induced lung dysfunction in an ex vivo rat lung model. Am J Respir Crit Care Med. 2001;163(6):1451–1456. doi: 10.1164/ajrccm.163.6.9908076. [DOI] [PubMed] [Google Scholar]
- 99.Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, et al. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med. 2007;176(7):667–675. doi: 10.1164/rccm.200702-291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Antonov A, Snead C, Gorshkov B, Antonova GN, Verin AD, Catravas JD. Heat Shock Protein 90 Inhibitors Protect and Restore Pulmonary Endothelial Barrier Function. Am J Respir Cell Mol Biol. 2008;39(5):551–559. doi: 10.1165/rcmb.2007-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD. Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L755–L763. doi: 10.1152/ajplung.00350.2007. [DOI] [PubMed] [Google Scholar]