Abstract
The majority of intermediate risk Rhabdomyosarcoma (RMS) patients have gross residual disease (Group III) after their first operative procedure. It is currently not known if local control rates can be maintained when, following induction chemotherapy, the radiation therapy (RT) dose is decreased after a delayed primary excision (DPE). To answer this question we evaluated patients enrolled on COG D9803 (1999-2005) who had Group III tumors of the bladder dome, extremity, or trunk (thorax, abdomen, pelvis) were candidates for DPE at week 12 if the primary tumor appeared resectable. RT dose was then adjusted by the completeness of DPE: no evidence of disease (NED) 36 Gy, microscopic residual (MR) 41.4 Gy, and gross residual disease (GRD) 50.4 Gy. A total of 161 Group III patients were evaluated (24 bladder dome, 63 extremity, and 74 trunk). Seventy-three patients (45%) underwent DPE which achieved removal of all gross disease in 61 (84%) who were then eligible for reduced RT dose [43/73 received 36 Gy, 19/73 received 41.4 Gy]. The local 5-year failure rate (0% for bladder dome, 7% for extremity and 20% for trunk) was similar to IRS-IV, which did not encourage DPE and did not allow for DPE adapted RT dose reduction. In conclusion, DPE was performed in 45% of Group III RMS patients with tumors at select anatomic sites (bladder dome, extremity and trunk), and 84% of those who had DPE were eligible for RT dose reduction. Local control outcomes were similar to historic results with RT alone.
Keywords: Bladder, Trunk, Extremity, Second look operation (SLO), Pediatric
Introduction
Rhabdomyosarcoma (RMS) comprises 2.9% of all pediatric cancers with an annual incidence in the United States of 4.3 per million children.1,2 Comprehensive reviews of RMS biology and therapy have recently been published. The survival for children with RMS has improved over the last five decades.3 Optimal treatment of RMS includes chemotherapy and local control, with surgery and/or radiation therapy (RT). However, the late effects among survivors are a matter of concern. Approximately half of survivors of childhood sarcoma have at least one major adverse outcome in their health status illustrating the need to develop less toxic treatments that are still effective.4-6
Local control of RMS may be attained through surgery, definitive RT, individually or in combination. A combined modality approach may optimize local control yet minimize the potential morbidity associated with each individual modality. Previous Intergroup Rhabdomyosarcoma Study (IRS) protocols used radiation doses based upon the extent of tumor present prior to starting chemotherapy: 41.4 Gy for patients with microscopic disease, and 50.4 Gy for gross residual tumor.7 The excellent local control results obtained in these trials presented the opportunity to investigate selectively decreasing the RT dose for patients with minimal residual disease achieved by delayed primary excision (DPE).
For RMS, primary excision should be attempted only when complete resection can be achieved without significant functional or cosmetic sequelae. In most cases of large and/or invasive tumors the initial procedure is limited to biopsy alone. After induction chemotherapy local tumor control may also be achieved with RT alone or in conjunction with DPE. RT can be associated with significant late effects, particularly in young children.8-12 Surgery also can be associated with significant late effects, particularly if organs are sacrificed.13 In an effort to minimize RT toxicity, many European RMS trials have utilized RT selectively.14-15 In a Children's Oncology Group (COG) trial of low risk RMS patients, reduction of RT dose based upon the completeness of surgical resection of the primary tumor prior to chemotherapy did not compromise local control, failure free survival (FFS) or overall survival (OAS).16 The COG Soft Tissue Sarcoma Committee (STS)tested the local control strategy of DPE and RT dose reduction in select intermediate risk RMS patients to assess the ability of DPE to achieve complete resection at anatomic sites likely to be amenable to DPE without loss of function and to evaluate local control rates. This local control paradigm has potential benefit to the patient since DPE may facilitate local control. In addition, patients that have DPE may be able to receive decreased RT dosing in an effort to minimize the long term complications associated with higher dosed RT. Our hypothesis is that the combination DPE and modulated RT may maintain local control yet reduce the incidence of chronic health conditions and second malignancies associated with current treatment paradigms.
Patients and Methods
Eligibility/Patient Classification
COG D9803, a trial for intermediate-risk RMS, has previously been described in detail.15 Patients were randomly assigned to 1 of 2 treatment regimens: vincristine, dactinomycin, and cyclophosphamide (VAC) without or with topotecan (VAC/VTC). Since the chemotherapy regimens did not result in significant differences in outcome, patients from both treatment algorithms were combined for this analysis. Informed consent was obtained from patients, their parents, or both, according to guidelines of the National Cancer Institute (NCI). Patients were assigned a presurgical stage 18 and a postsurgical clinical group 19. Stage and group were reviewed by surgical members of the STS Committee. Evaluation of DPE pathology specimens occurred at the treating institution.
The patients considered for DPE, as specified in the protocol, were group III patients with: alveolar RMS (ARMS), head/neck (non-orbital, non-parameningeal) primaries, superficial sites, negative regional nodes; ARMS, vagina/vulva/uterus primaries, negative regional nodes; embryonal RMS (ERMS) or ARMS, bladder dome, extremity or trunk. These anatomic sites were identified prospectively as having the potential to be amenable to DPE with acceptable surgical morbidity.
Primary Tumor Treatment
The primary tumor treatment algorithm is outlined in Figure 1. Patients were evaluated for response to induction chemotherapy at week 12. Oncologic resection of the tumor was then encouraged for the candidate anatomic sites if organ preservation without loss of form or function was likely. The decision to perform DPE was entirely the decision of the treating institution and surgeon. The reasons why DPE was not performed in individual patients is unknown. The study was approved at over 150 institutions and no single institution or surgeon contributed a disproportionate share of the patients to this DPE analysis. Post-operative RT dose was determined according to the amount of residual tumor following DPE (36 Gy if the tumor was completely resected with negative margins (NED), 41.4 Gy for microscopic residual tumor (MR) or clinical complete remission by imaging criteria with biopsy confirmation, or 50.4 Gy for those without DPE or with DPE in which gross residual disease (GRD) remained post-operatively. RT began 2-3 days after completion of week 12 chemotherapy if no biopsy or DPE was performed or 2-3 weeks after surgery for those who underwent DPE. Three-dimensional conformal RT was recommended using megavoltage photon and/or electron beams. The irradiated volume was determined by the initial, pre-chemotherapy extent of disease plus a 1.5 to 2 cm margin. Volume reduction was permitted for patients whose total dose was 50.4 Gy. The initial planning volume was reduced to the original gross tumor volume plus 5 mm after a tumor dose of 36 Gy (if node-negative) or 41.4 Gy (if node-positive).
Figure 1.
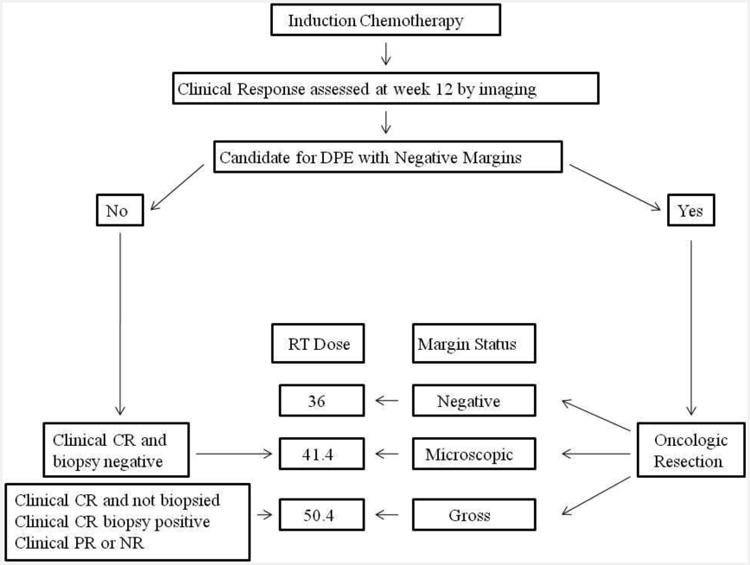
Local control algorithm for COG D9803. Delayed Primary Excision (DPE), complete response (CR), partial response (PR) radiation therapy (RT).
Radiotherapy Quality Assurance
All RT materials including diagnostic imaging and dosimetry were reviewed by members of the STS radiation oncology committee. The quality criteria have previously been described.16 Appropriate RT was defined as RT that did not have major deviations.
Statistical Methods
Data are presented as counts and percentages. The distributions of categorical patient characteristics were compared based on DPE status and based on RT delivery using Fisher's exact test. Time to local recurrence was estimated using cumulative incidence curves. Local recurrence was defined as the time from DPE to any recurrence of disease that included the primary site. Local recurrence that occurred in combination with regional or distant recurrence as a first progression event were included in the local recurrence rate. Outcome data are reported through June 30, 2008, at which point, follow-up ranged from 0 to 9.2 years with a median of 5.5 years. A p-value < 0.05 was considered statistically significant.
Results
Patient Population
A total of 164 (of 451 total Group III) patients (36%) were eligible for study inclusion based upon the site of primary tumor. The number of patients at each tumor site who underwent a DPE is summarized in Table 1. A total of 73 of 164 patients (45%) underwent DPE. None of the three patients with head/neck or vagina/vulva/uterus primary sites had DPE performed; these 3 patients were excluded from further analysis, leaving 161 patients in the study population. The patient demographics and disease characteristics for this cohort are presented in Table 2. There were no disease or patient characteristics that predicted which patients would receive optimal local therapy (DPE results of NED or appropriate RT) although it should be noted that patients who were classified as CR at week 12 were more likely to be NED and receive appropriate RT. The same patient and disease characteristics did not show any differences between patients who underwent DPE vs. no DPE (data not shown).
Table 1.
Patients eligible for Delayed Primary Excision (DPE) at each primary tumor site. Alveolar rhabdomyosarcoma (ARMS), embryonal rhabdomyosarcoma (ERMS), parameningeal (PM)
| Anatomic Site | Number Eligible For DPE | Number Undergoing DPE During Phase 1 of Therapy (percent) |
|---|---|---|
| I. ARMS, Group III, Head/Neck (non-orbital, non-PM) primaries, superficial sites, negative nodes | 2 | 0 (0%) |
| II. ARMS, Group III, Vagina/Vulva/Uterus primaries, negative nodes | 1 | 0 (0%) |
| III. ERMS or ARMS, Group III, Bladder Dome | 24 | 12 (50%) |
| IV. ERMS or ARMS, Group III, Extremity | 63 | 31 (49%) |
| V. ERMS or ARMS, Group III, Trunk/Retroperitoneum/Perineal/Perianal/Intra-thoracic/GI/Biliary | 74 | 30 (41%) |
| Total | 164 | 73 (45%) |
Table 2.
Patient demographics for study cohort comparing the total cohort to those that achieved optimal DPE (NED) and those that achieved optimal RT (Appropriate). p-values refer to the comparison of the distribution of patient characteristic between those that achieved NED or appropriate RT and those that did not. N-0, no nodal disease; N-1 nodal disease; T-1 not tumor invasion of adjacent structures; T-2 tumor invasion of adjacent structures.
| Baseline Characteristic | Total (n=161) | NED (n=43) | Appropriate RT (n=38) | p-value comparing NED (n=43) to other patients (n=118) | p-value comparing appropriate RT (n=38) to other patients (n=123) |
|---|---|---|---|---|---|
| Count (%) | Count (%) | Count (%) | |||
| Age (years) | |||||
| <1 | 5 (3%) | 2 (5%) | 1 (3%) | ||
| 1 to 9 | 118 (73%) | 29 (67%) | 29 (76%) | ||
| 10+ | 38 (24%) | 12 (28%) | 8 (21%) | 0.49 | 0.93 |
| Histology | |||||
| Embryonal | 84 (52%) | 20 (49%) | 22 (61%) | ||
| Alveolar | 65 (40%) | 21 (51%) | 14 (39%) | 0.27 | 0.57 |
| Tumor Size | |||||
| ≤ 5 cm | 39 (24%) | 12 (28%) | 10 (26%) | ||
| >5 cm | 121 (76%) | 31 (72%) | 28 (74%) | 0.54 | 0.83 |
| Tumor Stage | |||||
| 2 | 33 (20%) | 8 (19%) | 9 (24%) | ||
| 3 | 128 (80%) | 35 (81%) | 29 (76%) | 0.83 | 0.65 |
| Tumor Invasion | |||||
| T-1 | 88 (55%) | 25 (58%) | 26 (68%) | ||
| T-2 | 72 (45%) | 18 (42%) | 12 (32%) | 0.72 | 0.06 |
| Anatomic Sites | |||||
| Extremity | 63(39%) | 23 (53%) | 17 (45%) | ||
| Bladder Dome | 24 (15%) | 6 (14%) | 6 (13%) | ||
| Trunk/Retroperitoneum/Perineal/Perianal/Intra-thoracic/GI/Biliary | 74 (46%) | 14 (33%) | 16 (42%) | 0.07 | 0.75 |
| Response at week 12 | |||||
| NR | 30/149 (20%) | 6 (14%) | 5 (16%) | ||
| PR | 101/149 (68%) | 23 (53%) | 17 (45%) | ||
| CR | 18/149 (12%) | 14 (33%) | 16 (42%) | <0.001 | <0.001 |
Local Management by Site of Primary Tumor
Bladder dome
DPE at week 12 was performed in 12/24 (50%) of patients. Tumor response at week 12 was similar between those with and without DPE. Operative procedures included 7 partial cystectomies and 5 cystoscopies with either tumor resection/fulguration (2 patients) or biopsy (3). Nine of the 12 pathology specimens (75%) contained viable tumor. Post operatively 6 patients were categorized as NED and 2 still had MR. None of the operative procedures at week 12 resulted in loss of function. Therefore, 8 (67%) of patients who underwent DPE were eligible for a reduced dose of RT (6 patients at 36 Gy and 2 at 41.4 Gy). Of the patients that underwent DPE and who had complete information regarding the RT administered 6/11 (54%) received appropriate RT compared to 3/8 (38%) that did not have a DPE.
Extremity
A total of 31/63 (49%) patients underwent DPE at week 12. Patients that underwent DPE were less likely to have a CR by imaging (p=0.0012) and a tumor that invaded adjacent structures (p=0.04). DPE procedures included three amputations, one excisional biopsy and 27 wide excisions (87% of patients), resulting in 23 patients (74%) achieving NED, 5 (16%) MR, and 3 (10%) with GRD. Five DPE procedures resulted in loss of function, including the 3 amputations. These amputations were all planned and not a result of surgical complications. In total, 28 (90%) patients were eligible for a reduced dose of RT (36 Gy [n=23 patients] or 41 Gy [n=5]). Of the patients that underwent DPE and who had complete information regarding the RT administered 18/28 (64%) received appropriate RT compared to 10/28 (36%) that did not have a DPE.
Trunk
A total of 30/74 (41%) underwent DPE at week 12. The imaging response at week 12 was similar between those who had DPE and those who did not. DPE procedures included two biopsies and 28 resections, 2 of which resulted in removal of a vital structure and loss of function (1 hysterectomy and 1 abdominal perineal resection). DPE resulted in 14 patients (47%) achieving NED, 11 (37%) with MR, and 5 (16%) in GRD. Of the 30 patients 25 (83%) of patients were eligible for a reduced dose of RT (14 patients to 36 Gy and 11 to 41 Gy). The frequency of appropriate RT was similar between patients who did or did not receive DPE.
Summary of DPE at Week 12 for All Sites
Of the 161 patients eligible at study entry, 73 underwent DPE (46%). The distribution of surgical results (GRD, MR, NED) is similar across extremity, bladder and trunk. (p=0.10, Table 3). Overall, 10% had loss of function as a result of DPE. A total of 58 (79%) pathology specimens contained viable tumor. There was no correlation between imaging response at week 12 and the presence of viable tumor (p=0.1156). As a result of DPE, 84% of patients were eligible to receive a reduced dose of RT, including 59% who were eligible to receive 36Gy. Although not specifically recommended, additional surgical procedures later in therapy were performed in 5/73 (7%) of patients who had DPE and 32/88 (36%) who did not have DPE at week 12.
Table 3.
Results of DPE for each primary tumor site. GRD, gross residual disease; MR, microscopic residual disease; NED, no evidence of disease.
| Primary Site | Total DPE (%) | DPE Outcome | ||
|---|---|---|---|---|
| GRD (%) | MR (%) | NED (%) | ||
| Bladder | 12/24 (50%) | 4/12 (33%) | 2/12 (17%) | 6/12 (50%) |
| Extremity | 31/63 (49%) | 3/31 (10%) | 5/31 (16%) | 23/31 (74%) |
| Trunk | 30/74 (41%) | 5/30 (16%) | 11/30 (37%) | 14/30 (47%) |
| Total | 73/161 (46%) | 12/73 (16%) | 18/73 (25%) | 43/73 (59%) |
Summary of RT for All Sites
The majority of patients (77%) received RT as part of their therapy. For patients that underwent week 12 DPE, 81% received RT, with 70% considered appropriately administered. (Table 4)Administered RT was least likely to be considered appropriate for patients with bladder tumors. The appropriateness of RT administration was not dependent upon the RT dose required. Among both DPE and no DPE patients, RT was omitted in 21% of cases and was more common in extremity lesions.
Table 4.
Results of RT after DPE for each tumor site. There was one patient in bladder, 3 patients in extremity, and 6 patients in trunk for whom RT information was incomplete and therefore could not be classified by RT administered.
| Primary Site | Total Eligible for RT Dose Reduction (%) | RT Administered | ||
|---|---|---|---|---|
| Appropriate RT (%) | Major Deviation (%) | No RT (%) | ||
| Bladder | 8/12 (67%) | 6/11 (55%) | 1/11 (9%) | 4/11 (36%) |
| Extremity | 28/31 (90%) | 18/28 (64%) | 2/28 (7%) | 8/28 (29%) |
| Trunk | 25/30 (83%) | 20/24 (83%) | 3/24 (13%) | 1/24 (4%) |
| Total | 61/73 (84%) | 44/63 (70%) | 6/63 (9%) | 13/63 (21%) |
Local control rates
The 5 year cumulative local failure rate at each tumor site was 0% for bladder dome, 7% (95% CI, 0-21%) for extremity and 20% (95% CI, 0-44%) for trunk. For comparison, a similar but not identical population of Group III RMS patients treated on IRS-IV had 5 year cumulative local failure rates of 19% for bladder/prostate (not exclusively bladder dome), 7% for extremity, and 14% for “other” sites.20
Discussion
We evaluated the feasibility, acute morbidity, and local control success of DPE at selected anatomic sites, with the subsequent dose of RT being determined based upon the completeness of resection. Our results show that: 1) 45 % of the patients eligible for DPE underwent the procedure, 2) 79% of pathology specimens obtained at DPE contained viable tumor, 3) 84% of DPE patients were eligible to receive a reduced dose of RT as a result of gross total tumor resection, 4) RT was appropriately administered in 70% of patients, and 5) local control rates at these selected anatomic sites were similar to IRS-IV, a study that did not include planned DPE and used higher doses of RT.
All cancer therapy is associated with significant short and long term morbidity and mortality.5 Depending upon the site and dose as well as patient age, RT can be associated with a variety of late-effects in survivors of RMS.6,11,12 Therefore, the goal of treatment protocols for patients with traditionally good outcomes is to reduce the overall burden of therapy.4
In an effort to decrease RT related morbidity by decreasing RT dosing yet maintaining good local control rates, two recent COG STS studies including the current D9803 and low risk study D9602 provided the option of adjusted RT dose based upon the extent of resection at DPE.16-17 D9602 and D9803 RT doses were based on studies suggesting efficacy in controlling local disease with 30-36 Gy for microscopic disease.21-23 European multicenter trials also supported this approach.14,24 However, eliminating radiotherapy for group II/III patients has been shown to result in high local failure rates.4,14,25 A lower RT dose could result in decreased late tissue injury especially in infants, although this remains speculative.26-30 Currently we lack metrics sufficient to detect clinically relevant differences in late effects in patients receiving doses of 36 vs. 50.4 Gy. Although it is reasonable to accept that any reduction in RT dosing, so long as adequate local control is maintained, is beneficial and desirable. In addition, it is not clear what the combined long-term morbidity of DPE and reduced-dose RT will be and this will be further evaluated in upcoming studies.
The results of radiologic imaging can be misleading about the presence of viable tumor. Prior IRS/COG studies have failed to show a correlation between outcome and imaging response after induction chemotherapy 31 or at the completion of planned therapy.32 These findings are in contrast to the predictive value of imaging in the Cooperative Soft Tissue Sarcoma Studies.24 There are several possible explanations for this apparent discrepancy that have been discussed previously.32 Reductions in RT dose based on observing a radiographic CR must therefore be considered cautiously.
In our series, 79% of pathology specimens contained viable tumor after 12 weeks of systemic therapy. This is consistent with previous reports of delayed surgery in IRS-IV patients where only 59% of tumors contained viable tumor, although these patients had received RT.33 Patients with no viable tumor had better FFS rates than patients with viable tumor at DPE. Viable tumor was present in 50% of patients who underwent biopsy or resection of residual masses after completing all protocol specified treatment.32 These results differ from IRS-III, in which DPE was suggested after RT and 20 weeks of chemotherapy, where 12% and 25% of patients in CR and PR had viable tumor, respectively.34, 35 The value of histological verification of CR is uncertain.36 In addition, this requires distinguishing viable tumor from benign differentiating rhabdomyoblasts.37
In D9803 45% of Group III patients eligible for DPE received an operative procedure, most commonly a resection. This frequency of DPE is similar to previous IRS protocols where DPE was encouraged. IRS-III (1984-1991) recommended DPE in patients whose mass had not disappeared after 20 weeks of chemotherapy and RT. In IRS- III, 90/153 patients (59%) with Group III disease underwent a DPE.34-35 In this cohort, 46% of patients with a PR by imaging criteria were in complete pathologic remission and another 28% were converted to NED by excision of residual tumor. In addition, 30% of patients with NR were in complete pathologic remission and another 43% were converted to NED. A much lower rate of DPE was observed in the RMS low risk study D9602. Thirty-nine Group III patients at sites other than orbit or vagina were eligible for DPE but only 7 (18%) underwent DPE.16 This lower rate of DPE is most likely related to the high frequency of CR by week 12, the favorable outcome in low-risk patients, and the higher morbidity associated with resection at these sites. The reasons that DPE are not performed will be evaluated in the next intermediate risk study.
As attempts are made to modify local tumor therapy to better reflect the severity of disease for the patient, the rate of local recurrence becomes a good indicator of efficacy. Our approach in D9803 demonstrated local control rates similar to IRS-IV (which used similar systemic chemotherapy and had similar outcomes) for these select sites of disease.17, 34 Local control was least effective in trunk lesions as expected since these are frequently large and invasive tumors involving major vital organs thus limiting RT and resection.38 However, it is important to note that direct statistical comparison to IRS-IV is not possible. IRS-IV did not distinguish bladder dome from all bladder/prostate patients, and the category of “other” primary site is the closest to the trunk category used for the present analysis. We did not compare DPE local control to no DPE local control within D9803 given the small number of patients would preclude any meaningful statistical conclusions to be reached and, since the treatment was not randomized, a significant amount of bias would have entered into the analysis. Nonetheless, even without a direct statistical comparison to IRS IV our data support the conclusion that for patients with primary sites amenable to surgery, DPE with adjusted RT dose results in local control rates similar to that achieved with conventional RT doses. There is other supporting evidence to substantiate our conclusions. Similar findings and conclusions were reached in low risk RMS patients (D9602), in which the 5 year cumulative local failure rate was 15% in the 62 patients treated with the same local control paradigm utilized in D9803 (week 12 DPE and modulated RT).16, 39 This local failure rate in low risk patients was similar to expected results based on IRS-III and IV. In addition, the FFS (89%) and OAS (97%) were similar to IRS III, suggesting that RT dose reduction did not adversely impact outcomes. Our conclusions are further supported by contemporary European studies. The CWS-81 Protocol utilized a treatment paradigm similar to D9803.40 The local failure rate in these 87 patients was 18% for Stage 2-3 patients. Although these results are similar to our own, this patient population is very different in that all patients were eligible for DPE, rather than selected sites, and other non-RMS soft tissue sarcoma histologies were included.
Although the rates of appropriate RT was lower in the bladder and extremity patients they still maintained good local control rates. This should not be interpreted as minimizing the importance of RT. Instead it should illustrate the importance of a collaborative approach utilizing both operative resection and RT in varying amounts to achieve local control. There is data from IRS IV to suggest that patients who did not receive RT benefited from resection (unpublished data). Conversely, the importance of RT, even after gross total resection initially, to optimize local control is illustrated by a recent review of 83 Group II (microscopic residual disease) patients who had local recurrence; most (55%) did not receive appropriate RT.25 Similar findings are observed when RT is omitted after DPE. In 125 patients with Group III intraabdominal RMS, progression-free survival was 73% in patients who had complete surgical resection and RT, compared to 40-48% in patients with complete resection but no RT.41 Similar findings were described in 27 patients who underwent DPE but no RT of whom 30% had a local relapse, compared to only 8.3% that received RT.15
Our series has several limitations. A minority of all Group III RMS patients were prospectively considered candidates for DPE due to anatomic site constraints. Extrapolation of these results to other anatomic sites is unproven. Future studies are needed to explore the role of DPE at more surgically challenging primary sites. Patients were non-randomly selected for DPE and although there were no differences in patient or disease characteristics between those that received DPE compared to no DPE the rationale for not performing DPE are unknown. The ability to resect tumors is surgeon-dependent and may have varied. Although the short-term morbidity of DPE (as measured by function loss) was low, it is not clear that the late effects of the combination of DPE and reduced dose RT will be less than standard dose RT alone.
In conclusion, for carefully selected Group III RMS patients at candidate anatomic sites, DPE is feasible, often permits an RT dose reduction, and this RT dose reduction after DPE does not compromise local control compared to treatment with conventional dose RT alone. Based upon our results upcoming intermediate risk RMS studies will again be utilizing this local control paradigm to achieve good local tumor control with the anticipation of decreased long term health conditions in RMS survivors.
Novelty/Impact.
In pediatric patients with rhabdomyosarcoma (RMS) we showed that delayed primary excision (DPE) of RMS at selected anatomic sites, with the subsequent dose of radiotherapy (RT) being determined based upon the completeness of resection is feasible, often permits an RT dose reduction, and this RT dose reduction after DPE does not compromise local control compared to treatment with conventional dose RT alone.
Abbreviations
- RMS
rhabdomyosarcoma
- RT
radiation therapy
- DPE
delayed primary excision
- NED
no evidence of disease
- MR
microscopic residual
- GRD
gross residual disease
- FFS
failure free survival
- OAS
overall survival
- VAC
vincristine, dactinomycin, and cyclophosphamide
- VAC/VTC
without or with topotecan
- ARMS
alveolar RMS
- ERMS
embryonal RMS
- COG
Children's Oncology Group
- STS
COG Soft Tissue Sarcoma Committee
Contributor Information
Moody D. Wharam, Email: wharamo@jhmi.edu.
Elizabeth R. Lyden, Email: elyden@unmc.edu.
Julie A. Stoner, Email: julie-stoner@ou.
Kenneth Brown, Email: kbrown@mail.ubc.ca.
Suzanne L. Wolden, Email: woldens@mskcc.org.
Charles N. Paidas, Email: cpaidas@health.usf.edu.
Sarah S. Donaldson, Email: sarah2@stanford.edu.
Douglas S. Hawkins, Email: doug.hawkins@seattlechildrens.org.
Sheri L. Spunt, Email: sheri.spunt@stjude.org.
Carola A. Arndt, Email: carndt@mayo.edu.
References
- 1.Hawkins DS, Spunt SL, Skapek SX COG Soft Tissue Sarcoma Committee. Children's Oncology Group's 2013 blueprint for research: Soft tissue sarcomas. Pediatric blood & cancer. 2013;60:1001–1008. doi: 10.1002/pbc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkins DS, Gupta AA, Rudzinski ER. What is new in the biology and treatment of pediatric rhabdomyosarcoma? Current opinion in pediatrics. 2014;26:50–56. doi: 10.1097/MOP.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ognjanovic S, Linabery AM, Charbonneau B, Ross JA. Trends in childhood rhabdomyosarcoma incidence and survival in the united states, 1975-2005. Cancer. 2009 Sep 15;115(18):4218–26. doi: 10.1002/cncr.24465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens MC. Treatment for childhood rhabdomyosarcoma: The cost of cure. Lancet Oncol. 2005 Feb;6(2):77–84. doi: 10.1016/S1470-2045(05)01733-X. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006 Oct 12;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Punyko JA, Mertens AC, Gurney JG, Yasui Y, Donaldson SS, Rodeberg DA, et al. Long-term medical effects of childhood and adolescent rhabdomyosarcoma: A report from the childhood cancer survivor study. Pediatr Blood Cancer. 2005 Jun 15;44(7):643–53. doi: 10.1002/pbc.20310. [DOI] [PubMed] [Google Scholar]
- 7.Crist W, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, et al. The third intergroup rhabdomyosarcoma study. J Clin Oncol. 1995 Mar;13(3):610–30. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 8.Heyn R, Haeberlen V, Newton WA, Ragab AH, Raney RB, Tefft M, et al. Second malignant neoplasms in children treated for rhabdomyosarcoma. intergroup rhabdomyosarcoma study committee. J Clin Oncol. 1993 Feb;11(2):262–70. doi: 10.1200/JCO.1993.11.2.262. [DOI] [PubMed] [Google Scholar]
- 9.Raney RB, Asmar L, Vassilopoulou-Sellin R, Klein MJ, Donaldson SS, Green J, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the intergroup rhabdomyosarcoma studies (IRS)-II and - III. IRS group of the children's cancer group and the pediatric oncology group. Med Pediatr Oncol. 1999 Oct;33(4):362–71. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Raney RB, Anderson JR, Kollath J, Vassilopoulou-Sellin R, Klein MJ, Heyn R, et al. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: Report from the intergroup rhabdomyosarcoma study (IRS)-III, 1984-1991. Med Pediatr Oncol. 2000 Jun;34(6):413–20. doi: 10.1002/(sici)1096-911x(200006)34:6<413::aid-mpo6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Sung L, Anderson JR, Donaldson SS, Spunt SL, Crist WM, Pappo AS, et al. Late events occurring five years or more after successful therapy for childhood rhabdomyosarcoma: A report from the soft tissue sarcoma committee of the children's oncology group. Eur J Cancer. 2004 Aug;40(12):1878–85. doi: 10.1016/j.ejca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Spunt SL, Sweeney TA, Hudson MM, Billups CA, Krasin MJ, Hester AL. Late effects of pelvic rhabdomyosarcoma and its treatment in female survivors. J Clin Oncol. 2005 Oct 1;23(28):7143–51. doi: 10.1200/JCO.2005.12.096. [DOI] [PubMed] [Google Scholar]
- 13.Alexander N, Lane S, Hitchcock R. What is the evidence for radical surgery in the management of localized embryonal bladder/prostate rhabdomyosarcoma? Pediatr Blood Cancer. 2012 Jun;58(6):833–5. doi: 10.1002/pbc.24087. [DOI] [PubMed] [Google Scholar]
- 14.Dantonello TM, Int-Veen C, Harms D, Leuschner I, Schmidt BF, Herbst M, et al. Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol. 2009 Mar 20;27(9):1446–55. doi: 10.1200/JCO.2007.15.0466. [DOI] [PubMed] [Google Scholar]
- 15.Cecchetto G, Carretto E, Bisogno G, Dall'Igna P, Ferrari A, Scarzello G, et al. Complete second look operation and radiotherapy in locally advanced non-alveolar rhabdomyosarcoma in children: A report from the AIEOP soft tissue sarcoma committee. Pediatr Blood Cancer. 2008 Nov;51(5):593–7. doi: 10.1002/pbc.21702. [DOI] [PubMed] [Google Scholar]
- 16.Raney RB, Walterhouse DO, Meza JL, Andrassy RJ, Breneman JC, Crist WM, et al. Results of the intergroup rhabdomyosarcoma study group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: A report from the soft tissue sarcoma committee of the children's oncology group. J Clin Oncol. 2011 Apr 1;29(10):1312–8. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arndt CA, Stoner JA, Hawkins DS, Rodeberg DA, Hayes-Jordan AA, Paidas CN, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's oncology group study D9803. J Clin Oncol. 2009 Nov 1;27(31):5182–8. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence W, Jr, Gehan EA, Hays DM, Beltangady M, Maurer HM. Prognostic significance of staging factors of the UICC staging system in childhood rhabdomyosarcoma: A report from the intergroup rhabdomyosarcoma study (IRS-II) J Clin Oncol. 1987 Jan;5(1):46–54. doi: 10.1200/JCO.1987.5.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Pappo AS, Shapiro DN, Crist WM, Maurer HM. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995 Aug;13(8):2123–39. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson SS, Meza J, Breneman JC, Crist WM, Laurie F, Qualman SJ, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma--a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001 Nov 1;51(3):718–28. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 21.Mandell L, Ghavimi F, Peretz T, LaQuaglia M, Exelby P. Radiocurability of microscopic disease in childhood rhabdomyosarcoma with radiation doses less than 4,000 cGy. J Clin Oncol. 1990 Sep;8(9):1536–42. doi: 10.1200/JCO.1990.8.9.1536. [DOI] [PubMed] [Google Scholar]
- 22.Etcubanas E, Rao BN, Kun LE, Horowitz ME, Parham DM, Hustu HO, et al. The impact of delayed surgery on radiotherapy dose and local control of rhabdomyosarcoma. Arch Surg. 1987 Dec;122(12):1451–4. doi: 10.1001/archsurg.1987.01400240099018. [DOI] [PubMed] [Google Scholar]
- 23.Puri DR, Wexler LH, Meyers PA, La Quaglia MP, Healey JH, Wolden SL. The challenging role of radiation therapy for very young children with rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2006 Jul 15;65(4):1177–84. doi: 10.1016/j.ijrobp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Koscielniak E, Harms D, Henze G, Jurgens H, Gadner H, Herbst M, et al. Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the german cooperative soft tissue sarcoma study CWS-86. J Clin Oncol. 1999 Dec;17(12):3706–19. doi: 10.1200/JCO.1999.17.12.3706. [DOI] [PubMed] [Google Scholar]
- 25.Million L, Anderson J, Breneman J, Hawkins DS, Laurie F, Michalski J, et al. Influence of noncompliance with radiation therapy protocol guidelines and operative bed recurrences for children with rhabdomyosarcoma and microscopic residual disease: A report from the children's oncology group. Int J Radiat Oncol Biol Phys. 2011 Jun 1;80(2):333–8. doi: 10.1016/j.ijrobp.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S10–9. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P, et al. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S50–7. doi: 10.1016/j.ijrobp.2009.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S58–63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S77–85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 30.Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S94–100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke M, Anderson JR, Kao SC, Rodeberg D, Qualman SJ, Wolden SL, et al. Assessment of response to induction therapy and its influence on 5-year failure-free survival in group III rhabdomyosarcoma: The intergroup rhabdomyosarcoma study-IV experience--a report from the soft tissue sarcoma committee of the children's oncology group. J Clin Oncol. 2007 Nov 1;25(31):4909–13. doi: 10.1200/JCO.2006.10.4257. [DOI] [PubMed] [Google Scholar]
- 32.Rodeberg DA, Stoner JA, Hayes-Jordan A, Kao SC, Wolden SL, Qualman SJ, et al. Prognostic significance of tumor response at the end of therapy in group III rhabdomyosarcoma: A report from the children's oncology group. J Clin Oncol. 2009 Aug 1;27(22):3705–11. doi: 10.1200/JCO.2008.19.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raney RB, Anderson JR, Brown KL, Huh WW, Maurer HM, Meyer WH, et al. Treatment results for patients with localized, completely resected (group I) alveolar rhabdomyosarcoma on intergroup rhabdomyosarcoma study group (IRSG) protocols III and IV, 1984-1997: A report from the children's oncology group. Pediatr Blood Cancer. 2010 Oct;55(4):612–6. doi: 10.1002/pbc.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001 Jun 15;19(12):3091–102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 35.Weiner LM. Applications of gamma-interferon in cancer therapy. Mol Biother. 1991 Dec;3(4):186–91. [PubMed] [Google Scholar]
- 36.Godzinski J, Flamant F, Rey A, Praquin MT, Martelli H. Value of postchemotherapy bioptical verification of complete clinical remission in previously incompletely resected (stage I and II pT3) malignant mesenchymal tumors in children: International society of pediatric oncology 1984 malignant mesenchymal tumors study. Med Pediatr Oncol. 1994;22(1):22–6. doi: 10.1002/mpo.2950220105. [DOI] [PubMed] [Google Scholar]
- 37.Arndt CA, Hammond S, Rodeberg D, Qualman S. Significance of persistent mature rhabdomyoblasts in bladder/prostate rhabdomyosarcoma: Results from IRS IV. J Pediatr Hematol Oncol. 2006 Sep;28(9):563–7. doi: 10.1097/01.mph.0000212978.21372.97. [DOI] [PubMed] [Google Scholar]
- 38.Thomas DM, O'Sullivan B, Gronchi A. Current concepts and future perspectives in retroperitoneal soft-tissue sarcoma management. Expert Rev Anticancer Ther. 2009 Aug;9(8):1145–57. doi: 10.1586/era.09.77. [DOI] [PubMed] [Google Scholar]
- 39.Breneman J, Meza J, Donaldson SS, Raney RB, Wolden S, Michalski J, et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: A report from the children's oncology group D9602 study. Int J Radiat Oncol Biol Phys. 2012 Jun 1;83(2):720–6. doi: 10.1016/j.ijrobp.2011.06.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koscielniak E, Jurgens H, Winkler K, Burger D, Herbst M, Keim M, et al. Treatment of soft tissue sarcoma in childhood and adolescence. A report of the german cooperative soft tissue sarcoma study. Cancer. 1992 Nov 15;70(10):2557–67. doi: 10.1002/1097-0142(19921115)70:10<2557::aid-cncr2820701027>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Cecchetto G, Bisogno G, Treuner J, Ferrari A, Mattke A, Casanova M, et al. Role of surgery for nonmetastatic abdominal rhabdomyosarcomas: A report from the italian and german soft tissue cooperative groups studies. Cancer. 2003 Apr 15;97(8):1974–80. doi: 10.1002/cncr.11285. [DOI] [PubMed] [Google Scholar]


