SUMMARY
DNA hydroxylation catalyzed by Tet dioxygenases occurs abundantly in embryonic stem cells and neurons in mammals. However, its biological function in vivo is largely unknown. Here we demonstrate that Tet1 plays an important role in regulating neural progenitor cell proliferation in adult mouse brain. Mice lacking Tet1 exhibit impaired hippocampal neurogenesis accompanied by poor learning and memory. In adult neural progenitor cells deficient in Tet1, a cohort of genes involved in progenitor proliferation were hypermethylated and down-regulated. Our results indicate that Tet1 is positively involved in the epigenetic regulation of neural progenitor cell proliferation in the adult brain.
INTRODUCTION
Neurogenesis from neural progenitor cells (NPCs) in adult brains gives rise to functional neurons, which integrate into neuronal circuits and modulate neural plasticity (Deng et al., 2010). The potential significance of this remodeling process has been illustrated in memory, depression, and neurodegenerative disorders (Zhao et al., 2008). Sustained neurogenesis throughout life occurs mainly in two specific brain regions: the subventricular zone of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus. The maintenance and differentiation of neural progenitors in these two regions are regulated by many molecular players and signaling pathways, including niche signals, neurotransmitters, growth factors, transcriptional factors, and epigenetic regulators (Mu et al., 2010; Zhao et al., 2008).
DNA methylation at cytosines (5-methylcytosine, 5mC) plays an important role in adult neurogenesis by regulating the proliferation and survival of neural progenitors, as well as dendritic growth of newborn neurons in both embryonic and adult brains (Fan et al., 2005; Hutnick et al., 2009; Ma et al., 2009; Wu et al., 2010). A new form of DNA modification, 5-hydroxymethylcytosine (5hmC) was discovered recently in mammalian pluripotent stem cells and brains (Kriaucionis and Heintz, 2009). This modification is derived from the hydroxylation of 5mC and the conversion is catalyzed by the Tet family of dioxygenases in an Fe (II) and α-ketoglutarate-(α-KG)-dependent manner (Tahiliani et al., 2009; Tan and Shi, 2012). Despite reports about the implications of Tet1 and 5hmC in mouse ES cells and meiosis (Ito et al., 2010; Koh et al., 2011; Xu et al., 2011; Yamaguchi et al., 2012), little is known about their biological role in mammalian development and in the neural system. Tet1 knockout and even Tet1/2 double knockout appear to be compatible with embryonic and postnatal development (Dawlaty et al., 2013a, b; Dawlaty et al., 2011). Considering high levels of 5hmC and Tet1 expression in adult brain (Wu and Zhang, 2011), we speculate a potential role of Tet1 in the regulation of neural function. Although a recent Tet1 knockdown study implicates DNA hydroxylation in neural activity-induced demethylation in mature neurons of adult brain (Guo et al., 2011b), the physiological significance of Tet1-mediated DNA demethylation in other neural processes remains to be examined by using knockout mouse models.
RESULTS
Tet1 Deficiency is Compatible with Brain Development but Causes Impaired Spatial Learning and Memory
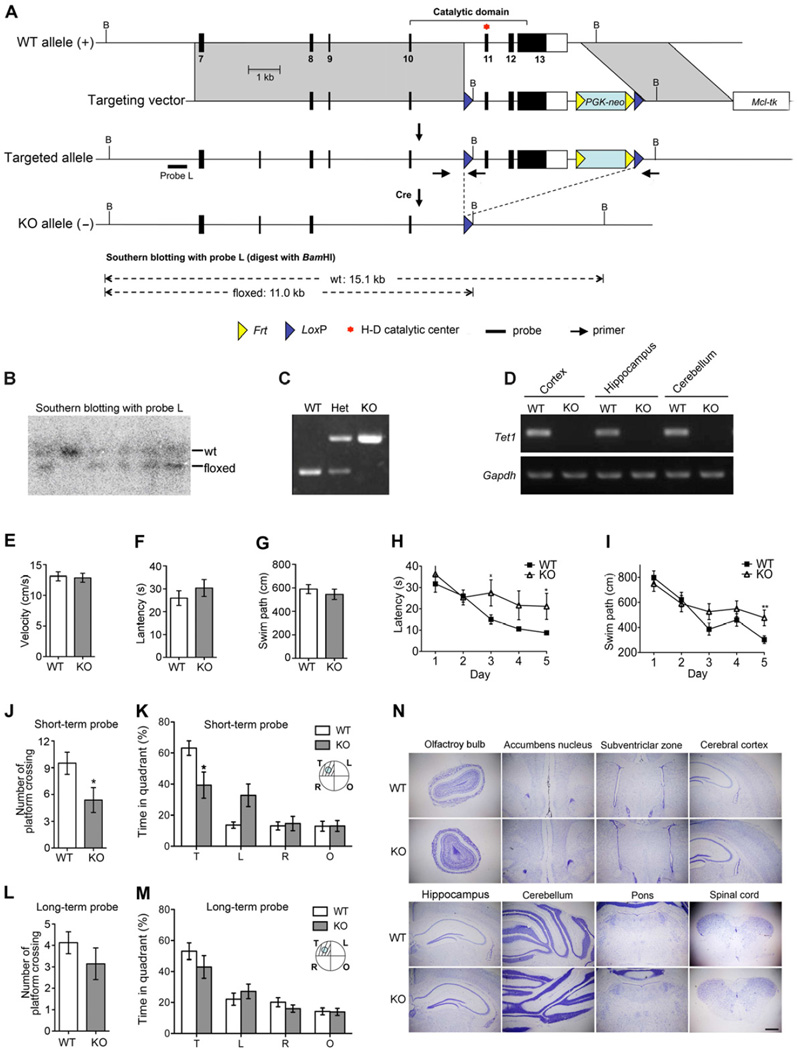
To study the biological function of Tet1, we generated mutant mice in which the Tet1 gene was disrupted by deletion of the exons 10 to 12 encoding the critical catalytic domain in dioxygenases (Figures 1A–1D). Consistent with the previous report (Dawlaty et al., 2011), homozygous mice deficient in Tet1 catalytic activity were viable and fertile, with no discernible morphological and growth abnormality.
Figure 1. Tet1 Deficient Mice Show Normal Brain Development but Impaired Spatial Learning and Memory.
(A) Gene targeting strategy. Coding exons are shown as filled boxes and the 3’ non-coding part of exon 13 is shown as a blank box.
(B) Southern blot confirmation of targeted ES clones. Fragments corresponding to the wild-type allele (wt) and the floxed allele are indicated.
(C) PCR genotyping of mutant mice. Primer locations are indicated in panel A and their sequences are listed in the method.
(D) Loss of Tet1 mRNA confirmed by RT-PCR assay. Gapdh was used for sample normalization.
(E) – (G) Visible platform screening showed no significant differences of visual acuity and mobility between WT and the whole-body knockout (KO) mice. (E) Average swim speed. (F) Latency to the visible platform. (G) The length of swim path to the visible platform.
(H) and (I) Spatial acquisition performances were recorded every day during 5-day training. (H) Escape latency. (I) Swim path to the hidden platform.
(J) and (K) Probe trial for short-term memory retention was carried out 24 hours after the last training. (J) Platform crossing. (K) Time in the target quadrant (T) and other quadrants (L, R &O).
(L) and (M) Probe for long-term memory retention was carried out 3 weeks after the last training. (L) Platform crossing. (M) Time in the target quadrant (T) and other quadrants (L, R &O).
(N) Tet1 deficiency is compatible with brain development. Shown are Nissl-stained coronal sections from Tet1 whole-body knockout mice at postnatal day 60. Scale bar, 500 µm.
For behavioral tests, 9 pairs of 4-month-old male mice were examined; For (E) – (G) and (J) – (M), two-tailed t-test was used in statistics; For (H) and (I), two-way ANOVA was used in statistics; * P < 0.05, **P < 0.01; All data are presented as mean ±s.e.m.
Although Tet1 mutant mice have no detectable defect during early development, the potential role for Tet1 in adult function remains to be investigated. Given the high level of 5hmC in WT adult brain (Munzel et al., 2010; Ruzov et al., 2011; Szwagierczak et al., 2010), Tet1-mediated DNA hydroxylation might have a role in the regulation of neural plasticity in which dynamic DNA methylation has been implicated (Ma et al., 2010). To determine whether Tet1 deficiency affects neural plasticity, we compared the performance of 4-month-old WT and Tet1 whole-body knockout mice in the Morris water maze. Both WT and Tet1 KO mutants exhibited similar escape latency and swim path to the visible platform, suggesting comparable vision and motivation between the two groups (Figures 1E–1G). 5-day acquisition trials were then conducted in a hidden platform version. Delay in learning was detected among the KO mice from day 3 by comparisons of escape latency and swim path (Figures 1H and 1I). Short-term memory retention was tested 24 hours after the 5-day training. In this probe, the mutant group showed significant deficiency in reaching the virtual platform, measured by both the platform crossing and the time spent in the target quadrant (Figures 1J and 1K). However, long-term memory retention examined 3 weeks after the last training session did not show statistically significant differences between WT and Tet1 mutants (Figures 1L and 1M). These data indicate that Tet1 deficiency can lead to impairment in spatial learning and short-term memory.
To identify potential neural defects associated with the poor learning and memory observed in Tet1 mutants, we examined the brain structure by Nissl staining. No obvious morphological abnormality was observed in brains of adult Tet1 KO mice (Figure 1N). Because brain development of Tet1 null mice is normal and adult neurogenesis has been associated with spatial learning and memory (Deng et al., 2009; Zhang et al., 2008; Zhao et al., 2003), we speculate that the memory impairment is likely to reflect a function of Tet1 in adult neurogenesis.
Tet1 Deficiency Diminishes the Adult SGZ Neural Progenitor Pool
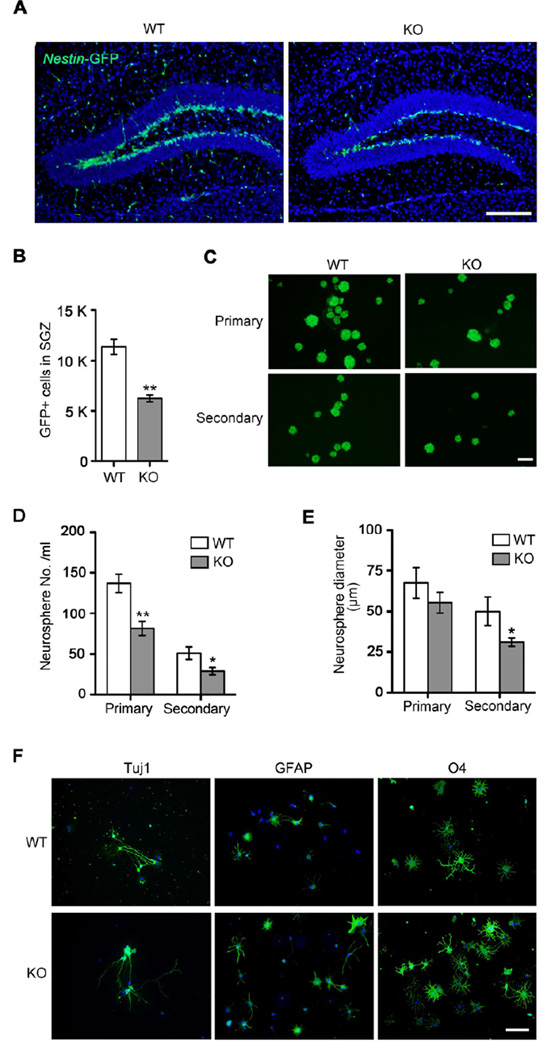
To determine the role of Tet1 in adult neurogenesis, we first looked into adult neural progenitor cell properties in Tet1 mutant brains since Tet1 is highly expressed in neural progenitor cells (NPCs) (Figure S1A and S1B). In order to facilitate the identification of NPCs in the brain, Tet1 whole-body knockout mice were crossed into the Nestin-GFP transgenic background (Mignone et al., 2004). Notably, the number of GFP-positive cells decreased by 45% in the SGZ in 4-month-old mutant mice compared to that in the wild-type controls (Figures 2A and 2B), suggesting that Tet1 is essential for the maintenance of the neural progenitor cell pool in the hippocampal dentate gyrus of adult mouse brains.
Figure 2. Reduction of Nestin-GFP Positive Neural Progenitor Cells in the Tet1 Deficient Dentate Gyrus and Their Impaired Proliferation in Vitro.
(A) Observation of neural progenitor cells in the adult SGZ using Nestin-GFP transgenic mice. Shown are coronal images of the SGZ in 4-month-old WT (Tet1+/+; Nestin-GFP) and KO (Tet1−/−; Nestin-GFP) mice captured at the same exposure. Scale bar, 100 µm.
(B) Quantification analysis of Nestin-GFP positive cells in the subgranular cell layer of dentate gyrus (n = 3 pairs of mice).
(C) Isolation and culture of Nestin-GFP positive progenitors from 2-month-old WT and KO dentate gyrus by FACS. Scale bar, 100 µm.
(D) Quantification of primary and secondary neurospheres (The initial seeding is 20,000 cells/ml, n = 3 cases).
(E) Average diameters of primary and secondary neurospheres (n = 3 cases).
(F) The tripotent differentiation capacity of Tet1-deficient NPCs. Neurons, astrocytes and oligodendrocytes were induced by in vitro differentiation of neurospheres isolated from WT and Tet1 KO DG. Cell lineage markers used: Tuj1 for neurons, GFAP for astrocytes and O4 for oligodendrocytes. Scale bar, 100 µm.
All quantifications are presented as mean ± s.e.m. and analyzed by two-tailed t-test. ** P < 0.01, * P < 0.05.
See also Figure S1.
To further investigate the proliferation capacity of neural progenitors, we derived neurospheres from adult DG in vitro. NPCs isolated from Tet1 KO DG yielded fewer and smaller primary neurospheres compared to those from WT controls (Figures 2C–2E). To determine the self-renewal capability of these neurospheres, primary neurospheres were individually dissociated into single cells and plated at clonal density. Tet1-null secondary neurospheres also exhibited significant reduction in both number and size compared to the WT spheres (Figures 2C–2E). Consistently, acute deletion of Tet1 from adult NPCs isolated from 2-month-old Tet1f/f mice by the lentiviral Cre expression also led to reduction in neurosphere growth (Figure S1C and S1D). Therefore, Tet1 deficiency compromised the self-renewal capability of NPCs.
In contrast, the differentiation potential of NPCs did not appear to be affected by Tet1 deletion. Upon differentiation in vitro, the neurospheres from mutant mice were generally tripotent with comparable potential in generation of neurons, astrocytes, and oligodendrocytes to that of wild-type neurospheres, as evidenced by immunostaining of Tuj1, GFAP and O4 markers (Figure 2F).
Deficit in Adult Neurogenesis due to Reduced Progenitor Proliferation in Tet1 Deficient Brain
To further study the role of Tet1 in hippocampal neurogenesis, we next examined progenitor cell proliferation and differentiation in Nestin-Cre neural tissue-specific KO mice. Examination of the gross morphology of P0 and P60 KO hippocampi showed no obvious defect in DG development (Figure S2A). The progenitor reduction could not be ascribed to decreased cell survival as evidenced by the lack of apoptosis signal in TUNEL assay (Figure S2B). We then traced cell proliferation in DG at different developmental stages by 5-bromodeoxyuridine (BrdU) incorporation. The numbers of BrdU-positive cells in the KO and WT DG were comparable during early postnatal stages of P14 and P30 (Figures S2C–S2F). However, a significant reduction of BrdU/Ki67 positive proliferating cells and BrdU/DCX positive newborn neurons was observed in the SGZ of hippocampal dentate gyrus in 4-month-old Tet1 KO mice (Figures S3A–S3D). To determine whether Tet1 has a direct role in regulating the proliferation of adult neural progenitors in the hippocampus, we next performed localized knockdown of Tet1 in the dentate gyrus of adult wild type mice. A Tet1 shRNA-expressing lentiviral vector was introduced into the dentate gyrus of adult wild type mouse brains by stereotaxic lentiviral transduction. Viral expression of Tet1 shRNA was monitored by co-expressed GFP and cell proliferation was monitored by BrdU labeling. Intracranial injection of Tet1 shRNA-expressing lentiviruses led to considerable decrease of cell proliferation in the DG, as revealed by a marked decrease in the percentage of BrdU-positive and GFP-positive cells in all GFP-labeled cells, compared to that in scrambled control RNA-transduced DG (Figures S3E and S3F). These results together indicate that Tet1 deficiency in the nervous system negatively regulates adult NPC proliferation in the hippocampal dentate gyrus.
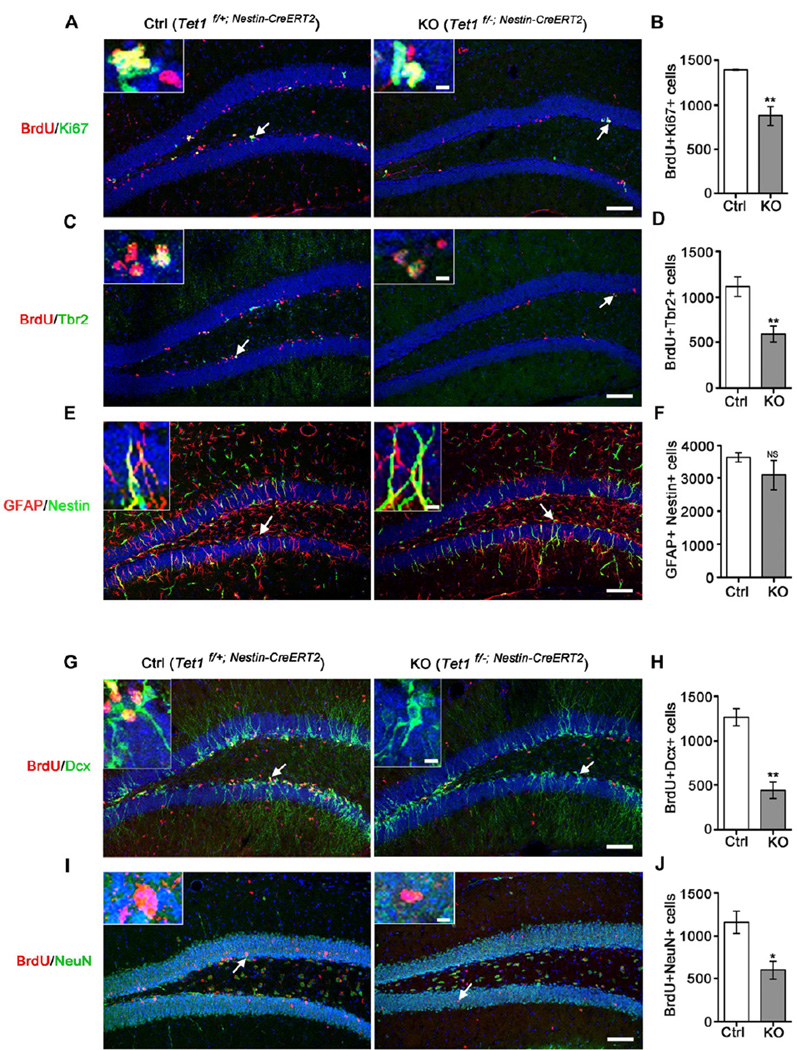
To further delineate the regulatory function of Tet1 in adult hippocampal NPCs, we generated Tet1 floxed mice carrying an inducible Nestin-Cre ERT2 gene (Lagace et al., 2007), in which the Tet1 gene can be deleted selectively in neural progenitors at adult stage by administration of tamoxifen. We treated 2-month-old mice with tamoxifen for 7 days and then with 7-day BrdU labeling for dividing cells after 6 weeks of interval. We found that the proliferating cell population represented by BrdU and Ki67 double positive cells was decreased by 35% in tamoxifen-treated Tet1f/− mice compared with tamoxifen-treated Tet1f/+ littermates (Figures 3A and 3B), which was consistent with our previous observations in whole-body and neural tissue-specific Tet1 KO adults. Next we used specific cell lineage markers to test the effect of Tet1 deletion on the population of radial glia-like stem cells and nonradial progenitors. Immunostaining assay showed a significant reduction of Tbr2-positive non-radial progenitors (Figures 3C and 3D), while there was no obvious change in the radial glia-like stem cells double positive for GFAP and Nestin (Figures 3E and 3F). These results provided in vivo evidence that NPC proliferation in the DG of adult brain is regulated by Tet1 in a cell-autonomous manner. To determine whether the proliferative defect of progenitors is accompanied by reduced cell population undergoing differentiation and maturation, adult tamoxifen-treated Tet1f/+ and Tet1f/− mice were injected daily with BrdU for 7 days and sacrificed for examination one or three weeks later. Consistently, both newborn neurons represented by BrdU+ Dcx+ cells (Figures 3G and 3H) and mature neurons represented by BrdU+ NeuN+ cells (Figures 3I and 3J) were markedly decreased in the Tet1f/− SGZ. Taken together, our results reveal impaired neurogenesis in Tet1 deficient adult brain.
Figure 3. Decrease of Intermediate Progenitor Proliferation and Impaired Adult Neurogenesis in the Tet1 Deficient Dentate Gyrus.
(A and B) Reduction of proliferating SGZ progenitors represented by BrdU & Ki67 double positive cells in adult KO (Tet1f/−; Nestin-CreERT2) mice compared to Ctrl (Tet1f/+; Nestin-CreERT2) after tamoxifen treatment (Lagace et al.) (n = 4 pairs of mice).
(C and D) Reduction of BrdU+ Tbr2+ intermediate progenitors in KO compared to Ctrl after TM (n = 4 pairs of mice).
(E and F) Insignificant change in the number of GFAP+ Nestin+ radial glia-like stem cells in KO compared to Ctrl after TM (n = 3 pairs of mice).
(G and H) Reduction of newborn neurons represented by BrdU & Dcx double positive cells in KO compared to Ctrl after TM (n = 4 pairs of mice).
(I and J) Reduction of mature neurons chased by BrdU labeling in KO compared to Ctrl after TM (n = 4 pairs of mice).
All scale bars at lower right are 100 µm and the upper-left insets are enlarged images of the arrow-pointed cells with a scale bar of 10 µm. Quantifications are presented as mean ± s.e.m. and analyzed by two-tailed t-test. ** P < 0.01, * P < 0.05, NS, not significant.
See also Figures S2 and S3.
Altered Methylation and Expression of Genes Associated with Adult Neurogenesis in Tet1 Deficient NPCs
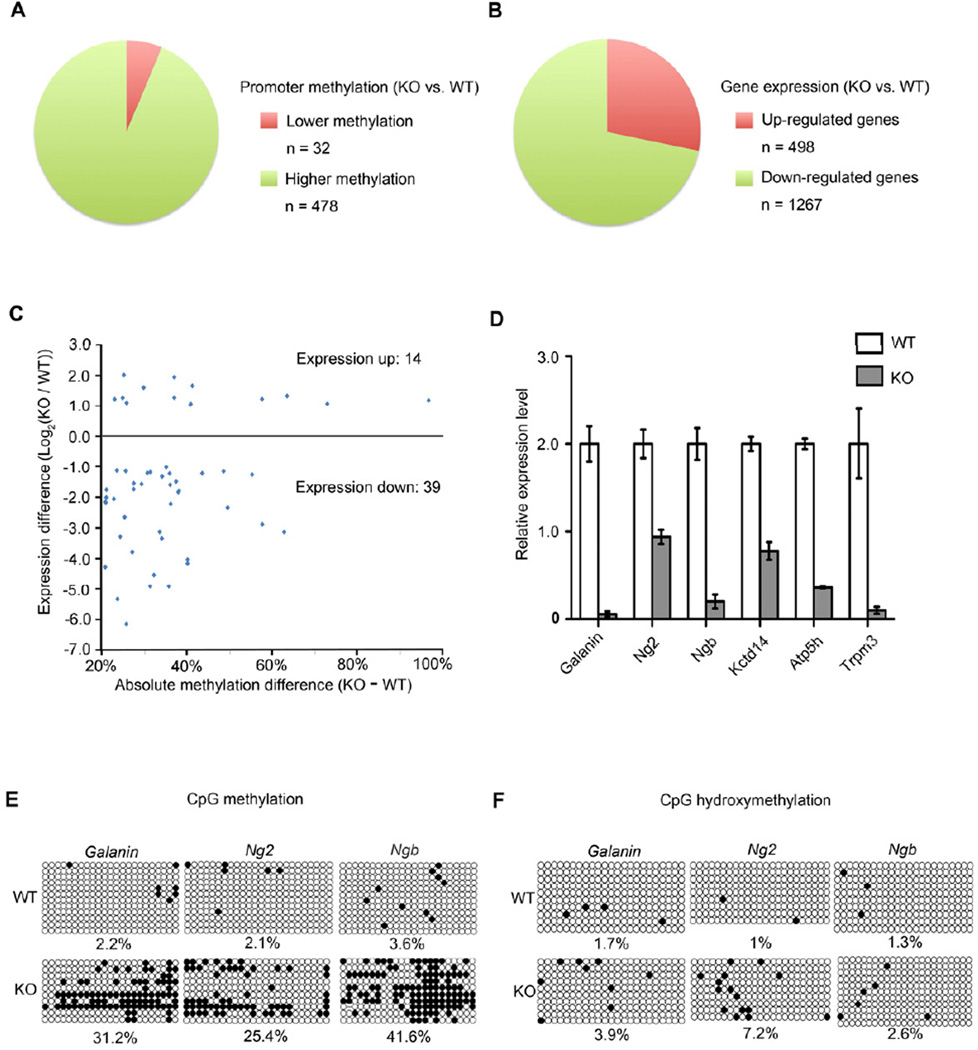
DNA methylation is involved in the regulation of many events of neural development, including neurogenesis (Guo et al., 2011a; Guo et al., 2011b; Ma et al., 2009). If Tet enzymes mediate DNA demethylation by oxidizing 5mC (Branco et al., 2012), Tet1 deficiency would lead to DNA hypermethylation in its target genomic regions. Therefore, we investigated epigenetic changes in Tet1 candidate target genes and their involvement in the regulation of neurogenesis. To identify the loci with altered DNA methylation, we conducted single-base resolution analysis of adult NPCs purified from WT and Tet1 KO Nestin-GFP mice using Reduced Representation Bisulfite Sequencing (RRBS) approach. Remarkably, 478 genes showed elevated promoter methylation levels in Tet1-null NPCs compared to the WT control, while only 32 genes had lower methylation (Figure 4A). To establish a link between altered DNA methylation pattern and transcriptional activity, we then compared gene expression profiles of the WT and KO NPCs. We identified 498 up-regulated and 1267 down-regulated genes in KO samples (Figure 4B). By combining the DNA methylation pattern with gene expression analysis, 39 genes were found to be both hypermethylated and down-regulated in NPCs isolated from the DG of adult Tet1 KO Nestin-GFP mice and FACS-sorted for the Nestin-GFP+ cells (Figure 4C and Table S1). Gene-specific qPCR and bisulfite sequencing confirmed the altered expression and methylation of several genes involved in neural progenitor proliferation, neuroprotection and mitochondria function, including Galanin, Ng2, Ngb, Kctd14 and Atp5h (Abbosh et al., 2011; Burmester et al., 2000; Calingasan et al., 2008; Kucharova and Stallcup, 2010) (Figures 4D and 4E). Considering that traditional bisulfite sequencing cannot distinguish 5mC from 5hmC (Huang et al., 2010), we examined hydroxymethylation at the promoter of these genes by Tet-assisted bisulfite sequencing (TAB- Seq) (Yu et al., 2012). 5hmCs were detected at 1–7.2% of CpGs in the examined promoter regions in both WT and Tet1 KO samples (Figure 4F), indicating that most cytosine modifications in the promoters in the KO PGCs are 5mC. The gain of 5hmC in Tet1 KO NPCs might be generated by the hydroxylation of aberrantly increased 5mC by the other enzymes, Tet2 and Tet3, which are known to be also expressed in NPCs (Ito et al., 2010). Taken together, the hypomethylation and transcriptional states are maintained predominantly by Tet1 at these genes under normal conditions while hypermethylation and transcriptional down-regulation occur upon its deletion.
Figure 4. Promoter Hypermethylation and Down-regulation of Adult Neurogenesis-related Genes in Nestin-GFP Positive Progenitor Cells in Tet1 Deficient mice.
Nestin-GFP positive progenitor cells were isolated from the DG of male adult WT (Tet1+/+; Nestin-GFP) and KO (Tet1−/−; Nestin-GFP) mice.
(A) Pie representation of promoters with altered DNA methylation in Tet1 KO progenitors. A promoter was defined as −1000 to 500-bp relative to a transcription start site. The promoter methylation level was determined by calculating the average methylation level of all individual CpGs with ≥ 10 sequencing depth. A promoter was considered as differentially methylated if the absolute methylation level difference was ≥ 20% between KO and WT.
(B) Pie representation of genes with expression changes in Tet1 KO progenitors. A significant expression change was defined as 1) expression ratio between KO and WT was either ≥ 2 or ≤0.5; and 2) P < 0.01 (Fisher exact test adjusted by the Benjamini-Hochberg method).
(C) Scatter plot of genes with both promoter hypermethylation and significant expression changes in the KO progenitors. X-axis is the absolute difference of the methylation level (KO minus WT). Y-axis represents expression difference between KO and WT by log2 transformation of the expression ratios (KO / WT).
(D) Confirmation of reduced expression of adult neurogenesis-related genes in Tet1 KO progenitors. The mRNA levels were determined by RT-qPCR. Data are normalized to Gapdh. Error bars are presented as mean ± s.e.m. (n = 6 pairs of mice).
(E) Confirmation of increased promoter methylation at the Galanin, Ng2 and Ngb genes in Tet1 KO progenitors by gene specific bisulfite sequencing (n = 6 pairs of mice). Open and filled circles represent unmethylated and methylated (hydroxymethylated) CpG sites respectively.
(F) 5hmC (filled circles) profiles of the Galanin, Ng2 and Ngb promoters in Tet1 KO progenitors determined by Tet-assisted bisulfite sequencing analysis (n = 6 pairs of mice).
DISCUSSION
Since Tet family proteins can catalyze hydroxylation of 5mC, their role in promoting active DNA demethylation has prompted intensive research (Branco et al., 2012; Cortellino et al., 2011; Guo et al., 2011b; He et al., 2011; Williams et al., 2011). Our study is focused on the physiological role of Tet1 in mouse models. Although the deficiency of Tet1 in mouse has no effect on development as previously reported (Dawlaty et al., 2013a; Dawlaty et al., 2011), our work presented here reveals a specific function in the positive regulation of NPC proliferation in adult brain. Loss of Tet1 compromises the maintenance of the neural progenitor pool, leading to reduced neurogenesis without affecting the tripotent differentiation capacity of NPCs. Although Tet1 has a role in mature neurons of the adult mouse brain in the process of neuronal activity-induced DNA demethylation (Guo et al., 2011b), the function of Tet1 in DG NPCs is likely cell-autonomous, based on the reduction of neurosphere growth in DG progenitors isolated and sorted from Tet1 KO Nestin-GFP mice and impaired proliferation of progenitor cells upon selective deletion of Tet1 in adult NPCs using the Nestin-Cre ERT2 system.
Previous research revealed that deficiency of hippocampal neurogenesis impairs learning and memory (Deng et al., 2010; Zhang et al., 2008; Zhao et al., 2003). Consistently, adult Tet1 KO mice displayed deficiency in adult neurogenesis and spatial learning and memory. While this observation reinforces the correlation between neurogenesis and behavior, a causal relationship suggested by previous studies (Clelland et al., 2009; Deng et al., 2010; Deng et al., 2009) remains to be further demonstrated. The Tet1KO mouse provides a useful model to further investigate the epigenetic regulation of adult neurogenesis and neural plasticity.
The previous evidence for the involvement of Tet1 in DNA demethylation is mainly based on gene hypermethylation in Tet1 knockdown ES cells (Branco et al., 2012; Ito et al., 2010; Koh et al., 2011; Williams et al., 2011) and Tet1-null germ cells (Yamaguchi et al., 2012). The role of Tet1 in adult tissues is still unclear. Our work highlights a positive role of Tet1 in promoting DNA demethylation and gene expression in adult neurogenesis, especially in the regulation of NPC proliferation. We showed a great enrichment of genes with aberrant promoter hypermethylation and decreased expression in Tet1 deficient NPCs. Among these genes, Galanin and Ng2 are best known for the regulation of neurogenesis (Abbosh et al., 2011; Deng et al., 2010; Kucharova and Stallcup, 2010); Ngb acts as hypoxia-inducible neuroprotective factor in hypoxic ischemic injury (Burmester et al., 2000; Moens and Dewilde, 2000; Sun et al., 2001); Kctd14 and Atp5h are associated with mitochondria function, which can affect adult neurogenesis indirectly (Calingasan et al., 2008). Thus, Tet1 deficiency leads to transcriptional repression of neurogenesis-related genes through promoter hypermethylation possibly due to impaired demethylation. Tet1 is important for maintaining the expression of neurogenesis-related genes in the adult NPCs by reducing DNA methylation. Since Tet1 deficiency causes epigenetic changes of multiple genes involved in neurogenesis, the phenotypic abnormality of mutant adult hippocampus is unlikely due to the expression change of a single gene. Indeed, addition of Galanin in the culture only led to a partial rescue of neurosphere growth of Tet1-null NPCs. Tet1-null NPCs with Galanin treatment could form large neurospheres, but the number did not increase significantly (Figure S12). Although the relative contribution of these genes in defective neurogenesis remains to be delineated, our findings have clearly linked Tet1-mediated DNA demethylation with the epigenetic regulation of adult neurogenesis.
Recent epigenomic studies have revealed in mature neurons an enrichment of 5hmC in gene bodies which may act as a stable active mark recognizable by MeCP2 (Hahn et al., 2013; Mellen et al., 2012; Song et al., 2011; Szulwach et al., 2011; Vorhees and Williams, 2006); In neural progenitors, our findings suggest that hydroxylation in gene promoters may act as an intermediate towards demethylation. Both of these functions of 5hmC can serve to antagonize the silencing effect of 5mC, playing a role in fine-tuning gene expression in neural progenitors and mature neurons, albeit through distinctive mechanisms. Excessive and inadequate hydroxylation may contribute to the progression of neurodegenerative disorders. The definitive role of Tet-mediated hydroxylation in the adult nervous system warrants further investigation using conditional knockout mice in which deletion of Tet genes is performed in combination and in specific developmental stages and cell types.
EXPERIMENTAL PROCEDURES
Generation of Tet1 Knockout Mice
A Tet1 targeting vector was prepared as described (Liu et al., 2003). The floxed region contains exons 10–13, which codes for the conserved Fe2+-binding motif of the catalytic domain. Male mice on a mixed 129Sv&C57BL/6J background were used in all experiments.
Morris Water Maze
The Morris water maze was conducted as described (Vorhees and Williams, 2006). The detail procedures can be found in supplemental information.
Neurosphere Assay
Nestin-GFP positive cells sorted from adult dentate gyrus were plated 20,000 cells per milliliter in neurosphere culture as described (Brewer and Torricelli, 2007). The number and diameter of neurosphere were measured using the Microcomputer Imaging Device Program. For differentiation, NeuroCult Differentiation Medium (Stem Cell) was used.
Tamoxifen Treatment and BrdU Labeling
Eight-week-old mice were injected intraperitoneally once daily with 2 mg tamoxifen per 20 g body weight for 7 consecutive days. 6 weeks after the treatment, BrdU was pulsed once daily at 50 mg per kg body weight over a 7-day period. For NPC proliferation studies, brains were fixed next day; for differentiation studies, brains were fixed 1 week (newborn neurons) or 3 weeks (mature neurons) later.
Immunohistochemistry and Cell Quantification
Perfused wild-type and mutant brains were sectioned for immunostaining and observation on a Olympus fluorescent microscope or a Leica confocal system. Z series stacks of confocal images were obtained. Numbers of single or double stained cells were counted using the Image Pro Plus software. Two-tailed t-test was used in statistics.
DNA Methylation, Hydroxymethylation and Gene Expression Analyses
RRBS was carried out as described (Meissner et al., 2005) and gene expression was determined by RNA-Seq. Hydroxymethylation was determined by TAB-seq.
Supplementary Material
Tet1 knockout mice show poor learning and memory
Tet1 positively regulates adult hippocampal neural progenitor cell proliferation
Tet1 maintains target gene expression by protecting them from methylation
Tet1 deletion does not affect brain development
ACKNOWLEDGEMENTS
We thank Chun-Li Zhang and Qin Shen for comments; Hongjun Song, Haikun Liu and Guoping Fan for discussions, Jianwei Jiao for Nestin-GFP and Nestin-Cre ERT2 mouse strains ; Yujiang Shi for Tet1shRNA; Desmond Ng for help with sequencing; Chenyan Ma, Ying Huang, Zelan Hu, Ningning Song, Yingyu Zhang for technical assistance. This work was supported by grants from the Ministry of Sciences and Technology of China (2012CB966903 and 2009CB941101), the National Science Foundation of China (90919061 and 31221001) and the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (XDA01010301) to G.-L. X., and grants from NIH NINDS (R01 NS059546 and RC1 NS068370) and California Institute for Regenerative Medicine (TR2-01832 and RB4-06277) to Y.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbosh C, Lawkowski A, Zaben M, Gray W. GalR2/3 mediates proliferative and trophic effects of galanin on postnatal hippocampal precursors. J Neurochem. 2011;117:425–436. doi: 10.1111/j.1471-4159.2011.07204.x. [DOI] [PubMed] [Google Scholar]
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature reviews Genetics. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nature protocols. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Ho DJ, Wille EJ, Campagna MV, Ruan J, Dumont M, Yang L, Shi Q, Gibson GE, Beal MF. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience. 2008;153:986–996. doi: 10.1016/j.neuroscience.2008.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW, Gao Q, Powell BE, Li Z, Xu M, et al. Combined Deficiency of Tet1 and Tet2 Causes Epigenetic Abnormalities but Is Compatible with Postnatal Development. Dev Cell. 2013a doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, Gao Q, Kim J, Choi SW, Page DC, et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9:166–175. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H, et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- Guo JU, Ma DK, Mo H, Ball MP, Jang MH, Bonaguidi MA, Balazer JA, Eaves HL, Xie B, Ford E, et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nature neuroscience. 2011a;14:1345–1351. doi: 10.1038/nn.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011b;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MA, Qiu R, Wu X, Li AX, Zhang H, Wang J, Jui J, Jin SG, Jiang Y, Pfeifer GP, et al. Dynamics of 5-Hydroxymethylcytosine and Chromatin Marks in Mammalian Neurogenesis. Cell Rep. 2013 doi: 10.1016/j.celrep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS One. 2010;5:e8888. doi: 10.1371/journal.pone.0008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, Silva AJ, Schweizer FE, Fan G. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Human molecular genetics. 2009;18:2875–2888. doi: 10.1093/hmg/ddp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharova K, Stallcup WB. The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience. 2010;166:185–194. doi: 10.1016/j.neuroscience.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci. 2010;13:1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Gnirke A, Bell GW, Ramsahoye B, Lander ES, Jaenisch R. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Res. 2005;33:5868–5877. doi: 10.1093/nar/gki901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. The Journal of comparative neurology. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Moens L, Dewilde S. Globins in the brain. Nature. 2000;407:461–462. doi: 10.1038/35035181. [DOI] [PubMed] [Google Scholar]
- Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Current opinion in neurobiology. 2010;20:416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel M, Globisch D, Bruckl T, Wagner M, Welzmiller V, Michalakis S, Muller M, Biel M, Carell T. Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angew Chem Int Ed Engl. 2010;49:5375–5377. doi: 10.1002/anie.201002033. [DOI] [PubMed] [Google Scholar]
- Ruzov A, Tsenkina Y, Serio A, Dudnakova T, Fletcher J, Bai Y, Chebotareva T, Pells S, Hannoun Z, Sullivan G, et al. Lineage-specific distribution of high levels of genomic 5-hydroxymethylcytosine in mammalian development. Cell research. 2011;21:1332–1342. doi: 10.1038/cr.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2011;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nature neuroscience. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwagierczak A, Bultmann S, Schmidt CS, Spada F, Leonhardt H. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic acids research. 2010;38:e181. doi: 10.1093/nar/gkq684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139:1895–1902. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell. 2011;42:451–464. doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Hong K, Liu R, Shen L, Inoue A, Diep D, Zhang K, Zhang Y. Tet1 controls meiosis by regulating meiotic gene expression. Nature. 2012;492:443–447. doi: 10.1038/nature11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li X, Dai Q, Shen Y, Park B, et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, Lein ES, Eadie BD, Willhoite AR, Muotri AR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.