Abstract
Arguably one of the most complex joints in the body, the temporomandibular joint (TMJ) presents one of the most difficult problems in modern medicine. Tissue engineering, for the TMJ disc in particular, has been proposed as a potential breakthrough treatment strategy for TMJ disorders. Central to tissue engineering is understanding growth factor effects on TMJ disc cells, and to the best of our knowledge, this is the first 3D growth factor study for these cells. The purpose was to examine the effects of high and low concentrations of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF), and transforming growth factor-β1 (TGF-β) on porcine TMJ disc cells. Cells were seeded onto non-woven PGA scaffolds (95% porosity) in spinner flasks, then cultured with a growth factor for 6 weeks. Constructs were analyzed for mechanical and structural integrity, cell number, and matrix biosynthesis. All growth factors improved mechanical and structural integrity compared to the control. IGF and TGF-β were most effective at promoting collagen synthesis, although there were no significant differences in glycosaminoglycan synthesis or cell number between any groups. After considering the economic advantage of IGF over TGF-β, the conclusion of this study is to use IGF in future TMJ disc tissue engineering experiments.
Keywords: Temporomandibular joint, TGF-β, bFGF, IGF, Maxillofacial, Craniofacial, Orofacial pain
Introduction
The multifactorial and complex disorders often exhibited by the temporomandibular joint (TMJ), also known as the jaw joint, constitute some of the most difficult problems to address in modern musculoskeletal medicine. These disorders include arthritis, ankylosis, traumas, and internal derangements, all of which can be extremely painful and severely restrict the range of motion in mouth opening. Patients suffering from disorders of this nature find agony in simple everyday activities most people take for granted, such as yawning, talking, or eating. In addition, clinicians have a daunting task of restoring function, as from a biomechanical perspective, with its ginglymodiarthrodial motion and rigid endpoint (the dentition), the TMJ is a highly complex joint. Central to the motion and function of the TMJ is a fibrocartilaginous disc, situated between the mandible and cranium. The disc is of particular interest, as it is known that approximately 70% of TMJ disorder cases are attributed to TMJ disc displacement.11 It has been proposed that tissue engineering the TMJ disc may lead to treatment of advanced cases of disc displacement,6 or even to treatment of a wide variety of other TMJ disorders as part of a larger tissue engineered TMJ disc/mandibular condyle construct.5
Fortunately, the field of TMJ disc tissue engineering is now rapidly emerging. Characterization studies have been performed to define construct validation criteria and guide strategies for experimental design.1,3,7 – 10,13 Furthermore, valuable early efforts in TMJ disc tissue engineering12,21,23 have been followed by more recent strategic approaches,2,22 providing a solid foundation upon which we can now build. Effects of cell-seeding methodology2 and choice of scaffold2,22 have been investigated, and it is now time for growth factor selection to enter into the equation. However, the precious few studies that have investigated the influence of growth factors on TMJ disc cells have all been performed in monolayer culture.5,15,16 In non-confluent monolayer culture, a 250% increase in proliferation was reported for bovine TMJ disc cells as a result of transforming growth factor-beta (TGF-β).15 In addition, it was discovered that platelet-derived growth factor (PDGF), tumor necrosis factor-alpha (TNF-α) and basic fibroblast growth factor (bFGF) were able to upregulate specific pathways involved in cellular responses, although IL-1 had less of an effect and TGF-β had no effect on these pathways.16
A recent study by our group examined the effects of insulin-like growth factor (IGF), bFGF, and PDGF on proliferation and biosynthesis with TMJ disc cells in confluent monolayer culture.5 It was determined that bFGF was most beneficial, increasing glycosaminoglycan (GAG) production and proliferation to the greatest extent while also significantly increasing collagen synthesis. IGF was deemed more beneficial than PDGF due to its ability to promote collagen synthesis, despite producing lower levels of GAGs than PDGF. However, while monolayer culture may be an effective screening tool, it has been shown with fibrochondrocytes that key differences exist in growth factor effects between 2D and 3D cultures.19,20 Therefore, the current study endeavored to examine in 3D culture, in terms of proliferation and biosynthesis, the effects of prime candidates IGF and bFGF identified in monolayer culture, along with TGF-β, long known for its beneficial effects in cartilage tissue engineering.4
Materials and Methods
Cells were obtained from a hog (P.I.C. Genetic Breed) in the body weight range of 70–80 kg (150–180 lbs). Both TMJs were removed en bloc with capsule intact, placed in ethanol for approximately 20 min, air dried, and scrubbed with sterile iodine pads. In a laminar flow hood, the disc of each TMJ was removed aseptically and minced with a scalpel. The discs were then digested for 24 h in 40 ml of 2 mg/ml collagenase (Type 2, 405 U/mg; Worthington Biochemical, Lakewood, NJ). Approximately 19 million cells were obtained from each disc. The TMJ disc cells were plated for expansion in 2D, and fed every 2 days with a cell culture medium consisting of Dulbecco's Modified Eagle Medium (Biowhittaker, East Rutherford, NJ), 10% fetal bovine serum (Biowhittaker, East Rutherford, NJ), 50 μg/ml ascorbic acid (Sigma, St. Louis, MO), 1% non-essential amino acids (Life Technologies, Carlsbad, CA) and 1% penicillin–streptomycin–fungizone (Biowhittaker, East Rutherford, NJ). During this 10–day expansion phase, the cells were passaged twice.
A poly(glycolic acid) (PGA) non-woven mesh (Albany International, Albany, NY) was used as the scaffolding material. According to the manufacturer, the PGA was 45–55% crystalline and 95% porous. An elliptical-shaped leather punch and scalpel were used to cut 5 mm × 7 mm scaffolds from a PGA sheet with a thickness of 2.0 mm. Cells were seeded onto these scaffolds at a density of 4 million cells per milliliter of scaffold using spinner flasks, as previously described.2 Briefly, two spinner flasks (including stir bars) were autoclaved, then scaffolds were skewered onto a thin wire in a spinner flask. The scaffolds in the spinner flasks were then sterilized together with ethylene oxide and aired for one day. The scaffolds were then wetted by a sequence of sterile-filtered ethanol, two washes of sterile PBS, and then left overnight with 250 ml of cell culture medium at 37°C. Equivalent cell numbers were added to each flask, and stir bars were set to rotate at 55 rpm. Stirring was stopped after 3 days, and cells were given an additional 5 days in the spinner flask to firmly adhere to the scaffolds. The medium was not changed during the seeding period, although an additional 1% penicillin–streptomycin–fungizone was added to the flasks 4 days after cells were initially added to the flasks. Scaffolds from the spinner flask were transferred to individual wells in 24-well untreated plates for culture. Wells were pre-coated with 600 μl of 2% agarose to further prevent cell migration from the scaffolds. A total of 1 ml of cell culture medium with growth factor was added to each well.
A total of six growth factor combinations were examined, consisting of three growth factors tested at two concentrations each. Combinations of growth factors were not investigated. All growth factors were obtained from Peprotech Inc. (Rocky Hill, NJ). Recombinant human transforming growth factor-β1 (TGF-β) was examined at 5 and 30 ng/ml. Recombinant human bFGF and recombinant human IGF were each examined at 10 and 100 ng/ml. In addition, a control group with no growth factors was examined. Half of the media was changed every other day for a period of 6 weeks. An additional 2D control (no growth factors) was also run in parallel. When cells were added to the spinner flasks, cells were added to a 12-well tissue culture treated plate with 2 ml of cell culture medium. The 2D cell cultures were seeded at confluence, with 360,000 cells per well. The medium was not changed during this seeding period while the 3D constructs were in the spinner flasks. Afterwards, half of the media was changed every other day for a period of 6 weeks.
Constructs and 2D cultures were analyzed for proliferation and biosynthesis at 0, 3, and 6 weeks. Mechanical integrity was evaluated at 0 and 6 weeks, and histology was performed at 6 weeks (constructs washed off of slides at week 0). We refer to time points here as they pertain to the time after being seeded, so that t = 0 refers to the day the constructs were removed from the spinner flask. Week 0 mechanical testing refers to empty and seeded constructs, corresponding to before and after seeding, respectively. Proliferation and biosynthesis were measured as previously described.5 Briefly, scaffolds were lyophilized for 2 days, then digested overnight in 1.5 ml papain at 60°C. Proliferation was then assessed with a fluorescence plate reader to detect a reaction between picogreen and DNA (Molecular Probes, Eugene, OR). A conversion factor of 7.7 pg DNA per cell for TMJ disc cells was reported previously.5 Biosynthesis was evaluated by quantifying total glycosaminoglycan (GAG) and collagen quantities produced. GAG content was tested with a dimethylmethylene blue (DMMB) dye binding assay kit using a chondroitin sulfate standard (Bio-color, Newtownabbey, Northern Ireland). Collagen content was measured with a hydroxyproline assay using a collagen standard (Accurate Chemical and Scientific Corporation, Westbury, NY).
Mechanical integrity of 3D constructs was evaluated by measuring the construct creep response under compression, as previously described for the native TMJ disc.13 Briefly, a constant feedback-controlled force of 0.5 g was applied to constructs in normal saline with protease inhibitors using a porous indenter tip with a diameter of 1.66 mm. A tare load of 0.2 g was applied to determine contact, then the 0.5 g test load was applied and creep continued until constructs reached equilibrium (maximum time = 45 min). The aggregate modulus, shear modulus, Poisson's ratio, and permeability were calculated using a numerical algorithm following the linear biphasic theory.13,14,17,18 Histology was performed on one sample per group with 14 μm frozen sections using a modified Picro-sirius Red collagen stain. Weigart's hematoxylin was first applied for 3 min, followed by analine blue for 5 min and Picro-sirius Red for 60 min. Slides were then washed with 0.5% acetic acid, dehydrated in graded ethanol, and mounted. A Picro-sirius stain was performed on 2D cultures as described earlier, except the analine blue stain was omitted.
Statistical analyses were performed with ANOVAs, and when significance was detected with ANOVA (p < 0.05), comparisons among groups were made with a Fisher's Protected Least Significant Difference post-hoc test. A twofactor ANOVA was performed for biochemical tests (proliferation and biosynthesis) to account for the effect of time points (3 weeks versus 6 weeks) with the effect of growth factor treatments. Sample sizes were n = 4 for mechanical testing and n = 4 for biochemical testing. A total of 100 scaffolds and 12 monolayer wells were required for the study. Comparisons to mechanical properties of the native TMJ disc and to 2D proliferation and biosynthesis were not included in the statistical analysis.
Results
In 2D controls, only slight staining for collagen occurred (Fig. 1), although staining was more frequent and intense at 6 weeks than at 3 weeks (times represent post-seeding period). Biochemical testing supported this finding, with 9.9 ± 7.5 μg of collagen detected at 6 weeks compared to undetectable amounts (0 ± 0) at 3 weeks (p < 0.05). There was no significant difference in GAG biosynthesis or cell numbers between 3 and 6 weeks. GAG content was measured at 1.8 ± 1.3 and 1.3 ± 1.6 μg at 3 and 6 weeks, respectively. Cell numbers per well were determined to be 2.9 ± 0.3 × 105 and 2.6 ± 0.9 × 105 at 3 weeks and 6 weeks, respectively.
Figure 1.

Histology with a Picro-sirius Red stain for 2D cultures. Cells were obtained from porcine TMJ discs. The top micrograph was taken after 3 weeks in culture and the bottom panel after 6 weeks (post-plating; plating period: 1 week). The red color is indicative of collagen production. The staining in the top panel (3 weeks) was an isolated example of a rare occurrence, whereas the bottom panel represents one of several stained areas.
Immediately after initial seeding of the 3D scaffolds, cells appeared to be preferentially located around the periphery of the scaffolds. The gross morphology of constructs did not appear to change drastically until about four and a half weeks after seeding, whereupon the constructs began to contract from the original 5 mm × 7 mm elliptical shape to a more circular 2-mm diameter. Under low magnification, constructs became opaque at about the same time. Fibers could be clearly identified at 3 weeks after seeding, but at 6 weeks the constructs appeared to be an opaque, homogeneous mass.
A distinct difference between the growth factor groups and the control group (no growth factor) was identified with histological staining (Fig. 2). The entire interior of the control scaffolds was completely degraded, with cells, matrix and polymer present only around the periphery. In general, in all six growth factor groups, the matrix and polymer were distributed in what appeared to be a much more uniform manner. However, the matrix and polymer were slightly more sparse in the TGF-β groups compared to the other growth factor groups. Since the PGA picked up the stains and only slightly de-stained in graded ethanol dehydration, it was difficult to discern in some locations between PGA fibers alone and PGA fibers coated in matrix.
Figure 2.

Histology with a modified Picro-sirius Red stain for 3D constructs at 6 weeks post seeding (seeding period: 1 week). The left column represents the low concentration of each growth factor (10, 5 and 10 ng/ml for IGF, TGF-β and bFGF, respectively), and the right column represents the high concentration (100, 30 and 100 ng/ml for IGF, TGF-β and bFGF, respectively). Scale bars represent 500 μm. Note the complete degradation of the interior of the control scaffold compared to the more uniform distribution of matrix/polymer throughout scaffolds in the growth factor groups. Lower quantities of collagen at 6 weeks for TGF-β's high concentration and the control (total amount: Fig. 4, content per total dry weight: Table 2) are consistent with the sparse matrix composition observed here with that TGF-β group and the dense peripheral matrix with the control.
Mechanical testing and manipulation of the constructs by hand provided another distinction between the growth factor groups and the control. The control group constructs had no mechanical integrity and were not testable for mechanical evaluation. This group was thus assigned zero values for aggregate and shear moduli. Aggregate moduli are displayed in Fig. 3 alongside the aggregate modulus for the center of the native porcine TMJ disc, obtained using the same testing approach in a previous study by our group.13 While there were no significant differences in aggregate modulus among growth factor groups and the initial groups (empty and seeded), each one of these groups had higher moduli compared to the week 6 control (p < 0.01 for empty PGA and TGF-β at 5 ng/ml, p < 0.005 for all others). Values obtained for the shear modulus, permeability, and Poisson's ratio are listed in Table 1, also juxtaposed with native disc values. With the shear modulus, again all groups were significantly greater than the control (p < 0.05 for IGF at 10 ng/ml, p < 0.005 for all others). The largest shear modulus belonged to the freshly seeded constructs at 3.1 ± 1.5 kPa, which was significantly larger than 2.0 ± 0.4 kPa for empty PGA (p < 0.05) and 1.3 ± 0.3 kPa for IGF at 10 ng/ml (p = 0.005). There were no other statistically significant differences in shear moduli between groups. With the lowest shear modulus, IGF at 10 ng/ml exhibited the highest Poisson's ratio at 0.38 ± 0.17. IGF at 100 ng/ml had the next highest Poisson's ratio at 0.26 ± 0.06, while all other groups fell in the range of 0.09–0.19. Although there was no statistical significance for differences among groups for the Poisson's ratio as determined by ANOVA, significant differences were detected for permeability. The empty PGA at 180 ± 66 × 10−14 m4/N/s was the most permeable, and was more permeable than all growth factor groups at 6 weeks (p < 0.05 for TGF-β at 5 ng/ml, p < 0.002 for all others). The two lowest permeabilities belonged to the low concentrations of IGF and bFGF groups with values of 19 ± 17 × 10−14 and 25 ± 19 × 10−14 m4/N/s, respectively. These two values were also significantly less permeable than the second most permeable group, which was the freshly seeded constructs at 112 ± 79 × 10−14 m4/N/s (p < 0.05). However, it should be noted that the permeability of the native disc is an order of magnitude less than the engineered constructs from the growth factor groups.
Figure 3.
Aggregate moduli of engineered constructs under compression, means ± standard deviations. Low concentrations were 5,10 and 10 ng/ml for TGF-β, IGF and bFGF, respectively. High concentrations were 30,100 and 100 ng/ml for TGF-β, IGF and bFGF, respectively. The aggregate modulus of the native disc represents the center of the porcine TMJ disc, obtained in a previous study using the same testing approach.13 Every control construct at 6 weeks had no mechanical integrity, disintegrating under gentle manipulation, and were thus assigned zero values for aggregate modulus. There were no other statistically significant differences between groups, except that all constructs were significantly stiffer than the control at week 6 control (p < 0.01). The seeded constructs at week 0 refer to constructs after a seeding period of 1 week, and the “Week 6” constructs refer to 6 weeks beyond that.
Table 1. Mechanical properties of engineered constructs under compression.
| Group | Week | G(kPa) | k(10−14 m4/N/s) | ν |
|---|---|---|---|---|
| Empty* | 0 | 2.0 ± 0.4 | 180 ± 66 | 0.17 ± 0.10 |
| Seeded* | 0 | 3.1 ± 1.5 | 112 ± 79 | 0.17 ± 0.21 |
| Control | 6 | 0 | n/a | n/a |
| TGF-β, 5 ng/ml | 6 | 2.1 ± 0.3 | 90 ± 59 | 0.09 ± 0.17 |
| TGF-β, 30 ng/ml | 6 | 2.5 ± 0.4 | 37 ± 34 | 0.19 ± 0.17 |
| IGF, 10 ng/ml | 6 | 1.3 ± 0.3 | 19 ± 17 | 0.38 ± 0.17 |
| IGF, 100 ng/ml | 6 | 2.2 ± 0.8 | 34 ± 56 | 0.26 ± 0.06 |
| bFGF, 10 ng/ml | 6 | 2.2 ± 1.1 | 25 ± 19 | 0.19 ± 0.17 |
| bFGF, 100 ng/ml | 6 | 2.2 ± 0.6 | 33 ± 14 | 0.19 ± 0.17 |
| Native TMJ disc** | n/a | 8.8 ± 2.5 | 2.3 ± 1.0 | 0.06 ± 0.06 |
Note. G: shear modulus, k: permeability, ν: Poisson's ratio. Reported values are means ± standard deviations.
Empty: PGA scaffold only, Seeded: scaffolds after 8 days in spinner flask with cells.
Obtained from center of the porcine TMJ disc in a previous study.13
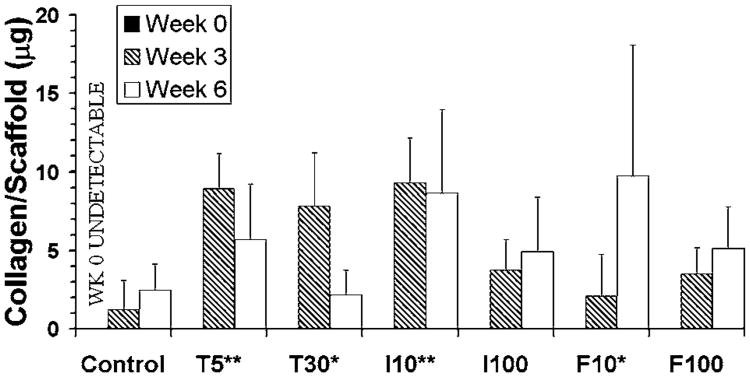
While histology and mechanical testing results highlighted differences between collective growth factor groups and the control group, testing of collagen biosynthesis was able to identify statistically significant differences among these growth factor groups (Fig. 4). Overall, IGF at 10 ng/ml was the largest producer of collagen, outperforming bFGF at 10 ng/ml and TGF-β at 30 ng/ml (p < 0.05), bFGF and IGF at 100 ng/ml (p < 0.01), and the control (p < 0.0001). Only TGF-β at 5 ng/ml was not statistically different from IGF at 10 ng/ml. However, while these two groups had similar values (within 5%) at 3 weeks, IGF produced 51% more collagen at 6 weeks. The only other significant difference in collagen synthesis between growth factor groups was between TGF-β at 5 ng/ml and bFGF at 100 ng/ml, with the former producing more collagen (p < 0.05). There were additional significant differences, however, between growth factor groups and the control group. Specifically, the control group produced less collagen than bFGF at 10 ng/ml and TGF-β at 30 ng/ml (p < 0.05), TGF-β at 5 ng/ml (p < 0.001), and IGF at 10 ng/ml (p < 0.0001). Differences between the control group and the high concentrations of IGF and bFGF were not statistically significant. Examination of individual growth factors over time demonstrated that the TGF-β groups appeared to lose collagen, whereas IGF groups appeared to maintain collagen levels and bFGF groups apparently increased collagen production.
Figure 4.
Collagen biosynthesis, means ± standard deviations. The letters T, I, and F on the x-axis represent TGF-β, IGF, and bFGF, respectively, and the numbers represent the concentration in ng/ml. Example: I10: IGF at 10 ng/ml. Groups that produced higher collagen amounts compared to the control are marked on the x-axis with * (p < 0.05) or ** (p < 0.001). I10 produced more collagen than F10 and T30 (p < 0.05), F100 and I100 (p < 0.01), and the control (p < 0.0001). In addition, T5 produced more collagen than F100 (p < 0.05) and the control (p < 0.001).
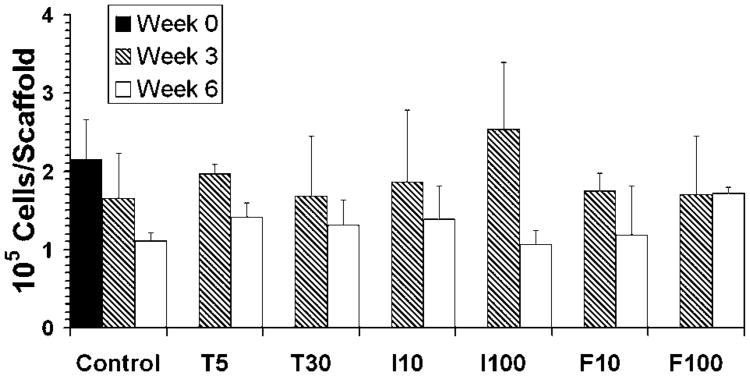
For purposes of comparison to the native TMJ disc, collagen and GAG biosynthesis are provided in Table 2 in normalized quantities of milligram matrix per gram construct. In contrast to collagen production results, there were no statistically significant differences among growth factor groups in GAG content (Fig. 5) or proliferation (Fig. 6). However, there was a statistically significant decrease between 3 and 6 weeks in GAG content (p < 0.0001) and cell number (p = 0.0002). The drop-off in GAG content was drastic, as the smallest margin of GAG loss was for the control group and TGF-β at 5 ng/ml, where the GAG content at 6 weeks was 3.7 times less than at 3 weeks for both groups. The biggest drop-offs in GAG content from 3 to 6 weeks belonged to the high concentrations of IGF and bFGF, which had 94 and 43 times less GAG at the later time point, respectively. As demonstrated by the control group, there was a steady drop in cell number over time. The only group that did not lose cells over time was bFGF at 100 ng/ml. All other groups demonstrated a consistent loss of 22–33% of cells from 3 to 6 weeks, except for the IGF group at 100 ng/ml, which demonstrated the largest drop in cell number with a 58% loss.
Table 2. Biosynthesis data expressed in terms of milligrams/gram of construct (dry weight).
| GAG synthesis (mg/g) | Collagen synthesis (mg/g) | |||
|---|---|---|---|---|
|
|
|
|||
| Treatment | Week 3 | Week 6 | Week 3 | Week 6 |
| Control | 5.6 ± 2.5 | 8.3 ± 9.2 | 0.5 ± 0.8 | 4.5 ± 3.5 |
| T5* | 7.9 ± 3.4 | 9.2 ± 6.7 | 3.7 ± 0.9 | 9.5 ± 6.0 |
| T30 | 9.8 ± 4.6 | 4.3 ± 5.4 | 3.2 ± 1.4 | 3.2 ± 2.9 |
| I10 | 8.4 ± 3.2 | 1.6 ± 1.4 | 3.0 ± 1.9 | 10.3 ± 4.7 |
| I100 | 7.3 ± 0.9 | 0.4 ± 0.8 | 1.6 ± 1.0 | 7.8 ± 7.1 |
| F10 | 7.6 ± 4.6 | 20 ± 34 | 0.9 ± 1.1 | 18 ± 16 |
| F100 | 8.4 ± 3.1 | 0.8 ± 1.7 | 1.3 ± 0.6 | 8.1 ± 6.5 |
Figure 5.
Glycosaminoglycan (GAG) biosynthesis, means ± standard deviations. The letters T, I, and F on the x-axis represent TGF-β, IGF, and bFGF, respectively, and the numbers represent the concentration in ng/ml. There were no statistically significant differences among groups, although there was a statistically significant overall decrease in GAG content in constructs between 3 and 6 weeks (p < 0.0001), which may have been caused by GAGs leaching out into the culture medium or by degradation of the polymer.
Figure 6.
Cell number per scaffold, means ± standard deviations. The letters T, I, and F on the x-axis represent TGF-β, IGF, and bFGF, respectively, and the numbers represent the concentration in ng/ml. There were no statistically significant differences among groups, although there was a statistically significant overall decrease in cell numbers between 3 and 6 weeks (p= 0.0002).
Discussion
To the best of our knowledge, this is the first report of the use of growth factors on TMJ disc cells on a 3D biomaterial scaffold. A recent detailed summary has been provided related to growth factor receptors in the TMJ disc and to growth factor effects on cells from other structures in the TMJ, such as the mandibular condyle.5 However, precedent has not been set for use of growth factors with TMJ disc cells in the context of tissue engineering.
In general, the three growth factors studied appear to be beneficial. After 6 weeks in culture, constructs in the control group had virtually no mechanical or structural integrity, and had less collagen than almost every growth factor group. Constructs from the growth factor groups maintained mechanical integrity after 6 weeks in terms of compressive stiffness. In addition, all growth factor group constructs experienced significantly reduced permeability compared to empty PGA, and in two cases also compared to initially seeded PGA. This significant decrease in permeability is likely due to constructs being much denser at 6 weeks, a result of the contraction of the scaffolds occurring 4–5 weeks after seeding.
Perhaps a major contributing factor to the differences between the growth factor groups and the control groups is cell migration. Results from histological examination (Fig. 2) reveal a more homogeneous structural distribution in growth factor groups compared to a more peripheral structural arrangement with the control. Cells were initially most concentrated at the periphery from spinner flask seeding, a departure from a previous study where the spinner flask resulted in homogeneous seeding,2 which most likely can be attributed to a lower seeding density in the current study. Since there were no appreciable differences in cell number and GAG content between the control group and the growth factor groups, it stands to reason that the cells and secreted matrix were concentrated and localized at the construct periphery, to the apparent detriment of interior structural integrity. It should not be inferred from Fig. 2 that the shell around the periphery of the control scaffold had the integrity to hold its shape with a hollow interior in culture. Rather, as the interior degraded in culture, it is likely that the upper layer collapsed in on the lower layer, and the visible hollow center is most likely a result of the scaffold swelling with embedding medium prior to freezing and sectioning during histology processing.
Biochemical testing demonstrated a drop-off in GAG content and cellularity over time. The drop in cellularity may be attributed to degradation of the polymer, with attached cells leaving along with the fibers that are dislodged from the construct. Another possibility is that fluid shear during the feeding process detached cells from the constructs. The one growth factor group that did not experience a drop in cell number from 3 to 6 weeks was bFGF at 100 ng/ml. A similar result was observed in a previous study with TMJ disc cells in confluent monolayer culture, where bFGF (tested at 10 and 100 ng/ml) considerably outperformed other growth factors in promoting proliferation, especially at its higher concentration.5
In the current study, the decrease in total GAG content from 3 to 6 weeks after the initial increase from 0 to 3 weeks may have been a result of GAGs leaching out, or tied to the same factor resulting in loss in cell number. Interestingly, the collagen content of scaffolds did not follow the same pattern of drop-off over time in most groups, with TGF-β groups being the only exceptions. This may be because the collagen framework is what was essentially holding the constructs together after 6 weeks, as collagen thereby would not be as affected by polymer degradation. If collagen can be produced at a faster rate, it is possible that loss of GAG content and cells can be slowed or prevented. Strategies to produce collagen at a faster rate include varying cell seeding density and use of other scaffold materials. A larger number of cells may be expected to produce a larger total amount of collagen, which is currently being investigated in our laboratory. Other scaffold materials, such as collagen or PLLA, or even different preparations of PGA, may be more beneficial to cell proliferation and biosynthesis. However, very little has been done to examine the effects of scaffold selection on TMJ disc cell behavior, and certainly more work is warranted.
In confluent monolayer culture, bFGF was selected as the most beneficial growth factor for its effects on proliferation and biosynthesis compared to IGF and PDGF, and IGF was selected over PDGF due to its efficacy in promoting collagen synthesis.5 In the current study, differences in proliferation and biosynthesis were not as pronounced as in monolayer culture. In three dimensions, other effects such as scaffold degradation, cell attachment, and cell migration may have contributed to the outcome of the study, and perhaps served to lessen the differences among growth factor treatments. However, as in monolayer culture, IGF was superior to other growth factors in terms of collagen biosynthesis. In monolayer culture, a high IGF concentration increased collagen production more than any other group.5 In contrast, in the current study, IGF was more effective at its lower concentration. It should be emphasized that the IGF concentration has potentially significant effects on 3D PGA scaffolds. At 100 ng/ml, IGF produced the largest drop in cell number and GAG content from week 3 to 6, and was the only group that did not produce significantly more collagen than the control. Therefore, it is possible that a concentration of IGF slightly higher or lower than 10 ng/ml would be more effective.
The results of the present study have demonstrated that IGF at 10 ng/ml was beneficial for collagen biosynthesis in comparison to other growth factors. This is especially important because other measured responses—GAG biosynthesis, cell number, mechanical integrity, and histology— were similar among the growth factor groups, making the differences in collagen synthesis more significant. TGF-β at 5 ng/ml was the only group that was not statistically outperformed by IGF at 10 ng/ml in terms of collagen synthesis. However, in light of the drop-off in collagen synthesis by TGF-β groups over time, and in light of the economic advantage of IGF over TGF-β, the conclusion of this investigation is that the most beneficial growth factor for TMJ disc cells on PGA scaffolds is IGF at a concentration of 10 ng/ml. In addition, future work may be warranted toward examining a range of IGF concentrations around 10 ng/ml.
Acknowledgments
We gratefully acknowledge funding from the National Institute of Dental and Craniofacial Research, grant number R01 DE015038-01A2. In addition, we gratefully acknowledge funding from the Whitaker Foundation and from the Nettie S. Autrey Fellowship at Rice University.
References
- 1.Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2005 doi: 10.1016/j.jbiomech.2004.11.012. in press. [DOI] [PubMed] [Google Scholar]
- 2.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for the tissue engineering of the temporomandibular joint disc. Tissue Eng. 2004;10:1787–1795. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 3.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical content and distribution in the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2005 doi: 10.1016/j.bjoms.2005.05.002. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 4.Darling EM, Athanasiou KA. Biomechanical strategies for articular cartilage regeneration. Ann Biomed Eng. 2003;31:1114–1124. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- 5.Detamore MS, Athanasiou KA. Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol. 2004;49:577–583. doi: 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Detamore MS, Athanasiou KA. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;9:1065–1087. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 7.Detamore MS, Athanasiou KA. Structure and function of the temporomandibular joint disc: Implications for tissue engineering. J Oral Maxillofac Surg. 2003;61:494–506. doi: 10.1053/joms.2003.50096. [DOI] [PubMed] [Google Scholar]
- 8.Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 2003;125:558–565. doi: 10.1115/1.1589778. [DOI] [PubMed] [Google Scholar]
- 9.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, Athanasiou KA. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2005 doi: 10.1016/j.joms.2005.10.009. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005 doi: 10.1016/j.matbio.2004.11.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrar WB, McCarty WL., Jr The TMJ dilemma. J Ala Dent Assoc. 1979;63:19–26. [PubMed] [Google Scholar]
- 12.Girdler NM. In vitro synthesis and characterization of a cartilaginous meniscus grown from isolated temporomandibular chondroprogenitor cells. Scand J Rheumatol. 1998;27:446–53. doi: 10.1080/030097498442280. [DOI] [PubMed] [Google Scholar]
- 13.Kim KW, Wong ME, Helfrick JF, Thomas JB, Athanasiou KA. Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann Biomed Eng. 2003;31:924–930. doi: 10.1114/1.1591190. [DOI] [PubMed] [Google Scholar]
- 14.Korvick D, Athanasiou KA. Variations in the mechanical properties of cartilage from the canine scapulohumeral joint. Am J Vet Res. 1997;58:949–953. [PubMed] [Google Scholar]
- 15.Landesberg R, Takeuchi E, Puzas JE. Cellular, biochemical and molecular characterization of the bovine temporomandibular joint disc. Arch Oral Biol. 1996;41:761–767. doi: 10.1016/s0003-9969(96)00068-4. [DOI] [PubMed] [Google Scholar]
- 16.Landesberg R, Takeuchi E, Puzas JE. Differential activation by cytokines of mitogen-activated protein kinases in bovine temporomandibular-joint disc cells. Arch Oral Biol. 1999;44:41–48. doi: 10.1016/s0003-9969(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 17.Mak AF, Lai WM, Mow VC. Biphasic indentation of articular cartilage: I. Theoretical analysis. J Biomech. 1987;20:703–714. doi: 10.1016/0021-9290(87)90036-4. [DOI] [PubMed] [Google Scholar]
- 18.Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage: II. A numerical algorithm and an experimental study. J Biomech. 1989;22:853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 19.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes in monolayer cultures. Tissue Eng. 2005;11 doi: 10.1089/ten.2005.11.1141. in press. [DOI] [PubMed] [Google Scholar]
- 20.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes in three dimensional cultures. J Orthop Res. 2005 doi: 10.1089/ten.2005.11.1141. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 21.Puelacher WC, Wisser J, Vacanti CA, Ferraro NF, Jaramillo D, Vacanti JP. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. Oral Maxillofac Surg. 1994;52:1172–1177. doi: 10.1016/0278-2391(94)90538-x. discussion, 1177–1178. [DOI] [PubMed] [Google Scholar]
- 22.Springer IN, Fleiner B, Jepsen S, Acil Y. Culture of cells gained from temporomandibular joint cartilage on non absorbable scaffolds. Biomaterials. 2001;22:2569–2577. doi: 10.1016/s0142-9612(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 23.Thomas M, Grande D, Haug RH. Development of an in vitro temporomandibular joint cartilage analog. J Oral Maxillofac Surg. 1991;49:854–856. doi: 10.1016/0278-2391(91)90015-e. discussion, 857. [DOI] [PubMed] [Google Scholar]