Abstract
Despite the proven efficacy of statins, they often fail to achieve low-density lipoprotein (LDL) cholesterol goals, especially in high-risk patients. Moreover, a large number of subjects cannot tolerate statins or full doses of these drugs, in particular patients with familial hypercholesterolemia. Thus, there is a need for additional effective LDL cholesterol-reducing agents. Evolocumab (AMG145) is a monoclonal antibody inhibiting proprotein convertase subtilisin/kexin type 9 that binds to the liver LDL receptor and prevents it from normal recycling by targeting it for degradation. Phase I, II, and III trials revealed that, on subcutaneous injection, either alone or in combination with statins, evolocumab is able to reduce high LDL cholesterol levels from 54% to 80%, apolipoprotein B100 from 31% to 61%, and lipoprotein(a) from 12% to 36%, in a dose-dependent manner. The incidence of side effects seems to be low and mainly limited to nasopharyngitis, injection site pain, arthralgia, and back pain. Evolocumab is an innovative powerful lipid-lowering drug, additive to statins and/or ezetimibe, with a large therapeutic range associated with a low rate of mild adverse events. If the available data are confirmed in long-term trials with strong outcome measures, evolocumab will become an essential tool in the treatment of a large number of high-risk patients, such as those affected by familial hypercholesterolemia, those who are unable to tolerate an efficacious statin dosage, and those at very high cardiovascular risk and unable to achieve their target LDL cholesterol levels with currently available lipid-lowering therapies.
Keywords: hypercholesterolemia, lipid-lowering drugs, proprotein convertase subtilisin/kexin type 9, AMG145
Introduction
Serum low-density lipoprotein (LDL) cholesterol levels seem to be the only reversible risk factor for cardiovascular disease, pharmacological reduction of which is not associated with a limit beyond which increased risk to health is found.1 The paradigm of “the lower, the better” acting simultaneously on different pharmacological targets, has recently been confirmed by the results of the IMPROVE-IT trial, where simultaneous use of statins (inhibitors of liver cholesterol synthesis) and ezetimibe (inhibitor of cholesterol absorption from the bowel) led to a dramatic decrease in LDL cholesterol and a parallel decrease in the risk of cardiovascular disease.2 However, despite this evidence, a large number of patients are not able to reach the desired LDL cholesterol target, mainly because of intolerance to standard treatment or very high baseline values related to genetic diseases of lipid metabolism.3 In this context, the search for new drugs with different mechanisms of action could improve control of LDL cholesterol in patients at increased risk of developing cardiovascular disease.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a regulatory serine protease that binds to the epidermal growth factor-like repeat A domain of the LDL receptor, inducing its degradation (Figure 1).4 PCSK9 is synthesized primarily in the liver, then enters the circulation and binds to the hepatic LDL receptor, thus reducing the ability of the liver to remove LDL cholesterol. Therefore, increased activity of PCSK9 results in high LDL cholesterol levels, as shown in patients with gain-of-function mutations of this protease,5 whereas loss-of-function mutations are associated with low LDL cholesterol levels and a reduced risk of coronary heart disease.6 Low intracellular cholesterol levels (eg, in response to statin treatment) lead to activation of sterol regulatory element-binding protein-2, which causes coexpression of the LDL receptor and PCSK9; it is thought (albeit not clinically demonstrated as yet) that this coexpression could reduce the therapeutic effect of statins.7,8 On the basis of this evidence, specific human monoclonal antibodies directed against PCSK9 have now been developed, ie, 1D05-IgG2, evolocumab (AMG145), and alirocumab (REGN727/SAR236553). Phase II studies showed evolocumab and alirocumab to be effective in reducing LDL cholesterol from 40% to 80%.9 Recent data from the first Phase III studies of PCSK9 inhibitors show LDL cholesterol reduction ranging from 54% to 80%.10 This review summarizes the available clinical evidence on the efficacy and safety of evolocumab (AMG145), for which a relatively large amount of short-term and middle-term clinical data are currently available.
Figure 1.
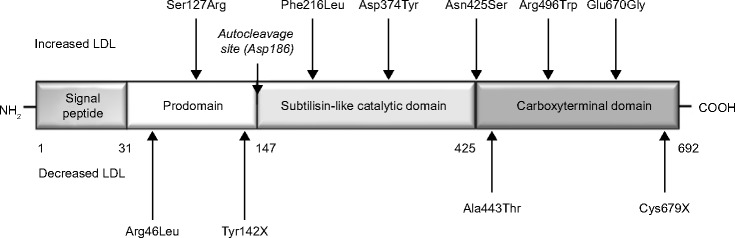
PCSK9 protein and its variants.
Abbreviations: PCSK9, proprotein convertase subtilisin/kexin type 9; LDL, low-density lipoprotein.
Chemistry and pharmacodynamics
Evolocumab (C6242H9648N1668O1996S56) is a 141.8 kDa, fully human monoclonal immunoglobulin G2-lambda, with a gamma 2 heavy chain (1–441) [human VH (Homo sapiens IGHV1-18*01 (93.9%)-(IGHD)-IGHJ6*01) [8.8.8] (1–115)-Homo sapiens IGHG2*01 (116–441)] (129–214′)-disulfide and lambda light chain (1′–215′) [human V-LAMBDA (Homo sapiens IGLV2-14*01 (95.9%)-IGLJ2*01 [9.3.9] (1′–109′)-IGLC2*01 (110′–215′)]. It binds to the LDL receptor binding domain of PCSK9 with high affinity (kDa <100 pM), strongly inhibiting its activity.11 As a consequence, administration of evolocumab is associated with a rapid (within 14 days) and impressive dose-dependent reduction of LDL cholesterol, with a parallel reduction in apolipoprotein B100 (ApoB) levels.12,13
Pharmacokinetics
Evolocumab is administered subcutaneously. The drug exhibits nonlinear pharmacokinetics at a dose of 21–420 mg, meaning that the drug concentration in plasma does not increase strictly proportionally to the administered dose. Clearance can be described by parallel linear and nonlinear clearance pathways, ie, the pK for evolocumab reaches linearity at single doses >210 mg injected subcutaneously, with peak concentrations reached at 72 hours. Repeated doses above 140 mg exhibited linear kinetics. Again, in Phase II and Phase III studies, the kinetics of evolocumab follow an approximately linear profile at doses >140 mg every 2 weeks, when administered alone or with statins.14
Clinical efficacy
In a Phase Ia study, 56 healthy subjects were randomized to receive evolocumab (n=42) or placebo (n=14),15 and a mean LDL cholesterol decrease of up to 64% was found when compared with placebo (P<0.0001). In a Phase Ib study, 42 hypercholesterolemic patients receiving statin treatment and 14 patients on placebo were enrolled, with the finding of a mean LDL cholesterol decrease of up to 81% versus placebo. Both studies showed that doses >20 mg start to have an effect on LDL cholesterol and that doses >420 mg/every 4 weeks lead to an LDL cholesterol reduction of over 60%. Lipoprotein(a) [Lp(a)] decreased by up to 38% (P<0.001) and ApoB decreased by up to 59% (P<0.001) in the Phase Ib trial.13 The mechanism by which evolocumab reduces Lp(a) is not known; however, evolocumab probably could induce Lp(a) clearing via the LDL receptor or very low-density lipoprotein receptors.
In the Phase II MENDEL trial, 406 patients not treated with statins who had LDL cholesterol levels of 100–190 mg/dL, triglycerides <400 mg/dL, and Framingham risk scores ≤10% were randomized to: evolocumab 70 mg, 105 mg, or 140 mg, or placebo every 2 weeks; evolocumab 280 mg, 350 mg, or 420 mg, or placebo every 4 weeks; or oral ezetimibe 10 mg/day.16 Evolocumab significantly reduced LDL cholesterol in all groups. Changes from baseline LDL cholesterol level were dose-dependent, ranging from −41% (95% CI−46, −36) to −51% (CI −56, −46) with administration of evolocumab every 2 weeks, and from −39% (CI −44, −34) to −48% (−53, −43) with evolocumab every 4 weeks (P<0.0001 for all doses versus placebo or ezetimibe).
The Phase III MENDEL-2 trial compared the effect of AMG145 (140 mg every 2 weeks or 420 mg monthly) with placebo and ezetimibe in hypercholesterolemic patients.17 A total of 614 patients with fasting LDL cholesterol ≥100 mg/dL and <190 mg/dL and Framingham risk scores ≤10% were randomized. Evolocumab reduced LDL cholesterol from baseline by 55%–57% more than placebo and 38%–40% more than ezetimibe (P<0.001). Approximately 70% of the patients on evolocumab achieved an LDL cholesterol level <70 mg/dL compared with ~1.5% of those on ezetimibe and ~0.5% on placebo. Evolocumab also significantly decreased ApoB, triglycerides, Lp(a), and non–high-density lipoprotein (HDL) cholesterol levels (all P<0.05). HDL cholesterol concentrations were also significantly (P<0.05) increased.
In the Phase II LAPLACE-TIMI 57 study,18 631 hypercholesterolemic patients were randomly assigned to evolocumab 70 mg, 105 mg, or 140 mg, or matching placebo every 2 weeks; or to evolocumab 280 mg, 350 mg, and 420 mg, and matching placebo every 4 weeks. At week 12, the mean LDL cholesterol concentrations were dose-dependently reduced by evolocumab every 2 weeks (ranging from 41.8% to 66.1%; P<0.0001 for each dose versus placebo) and evolocumab every 4 weeks (ranging from 41.8% to 50.3%; P<0.0001).19 This study also confirmed a dose-dependent effect of evolocumab on Lp(a) level, proportional to the baseline level of Lp(a),20 ranging from −18% to −32.3%. This effect was also observed with other PCSK9 antibodies, and is likely to be a class effect.21
The Phase III LAPLACE-2 study evaluated the efficacy and tolerability of evolocumab when used in combination with a moderate-intensity versus high-intensity statin in patients with primary hypercholesterolemia and mixed dyslipidemia.22 This 12-week, randomized, double-blind, placebo-controlled and ezetimibe-controlled trial enrolled 1,899 patients, who were randomized to compare evolocumab (140 mg subcutaneous every 2 weeks or 420 mg subcutaneously monthly) with placebo subcutaneously (every 3 weeks or monthly) or ezetimibe (10 mg or placebo daily only for patients receiving atorvastatin) when added to statin therapies. Evolocumab reduced LDL cholesterol levels by 63%–75% versus placebo at a mean of weeks 10 and 12 in the moderate-intensity and high-intensity statin-treated groups. Evolocumab also reduced non-HDL cholesterol concentrations at a mean of weeks 10 and 12 by 58%–65%, ApoB by 51%–59%, and Lp(a) by 21%–36% (all versus placebo). Triglyceride concentrations were also decreased by 12%–30% in patients receiving evolocumab versus placebo, while HDL cholesterol levels were increased by 4%–10% versus placebo.
In the Phase II RUTHERFORD study, evolocumab was administered to statin-treated patients affected by heterozygous familial hypercholesterolemia, mainly because of loss-of-function mutations in the LDL receptor alleles (98%) and less commonly in relation to a defect in ApoB or gain-of-function mutation in PCSK9.23,24 Evolocumab was tested at doses of 350 mg and 420 mg every 4 weeks in 168 patients, whereas other 56 patients were treated with placebo plus statin.25 Mean reduction in LD cholesterol was 43% in the 350 mg group and 55% in the 420 mg group, compared with a 1% increase in the placebo group (P<0.001). At week 12, evolocumab 350 mg every 4 weeks resulted in LDL cholesterol <100 mg/dL in 70% of patients and evolocumab 420 mg every 4 weeks reached this target value in 89% of patients. An LDL cholesterol target <70 mg/dL was obtained in 44% and 65% of patients, respectively. LDL cholesterol changes were higher with evolocumab 420 mg than with alirocumab monthly, with similar statin doses.26 A statistically significant dose-dependent reduction in triglycerides was also observed (15% and 20%, respectively), associated with an HDL cholesterol increase of approximately 7% and a dose-dependent Lp(a) reduction ranging from 23% to 32%.
The Phase III RUTHERFORD-2 trial investigated the relationship between the response of LDL cholesterol to evolocumab and genotype in subjects with heterozygous familial hypercholesterolemia identified on the basis of clinical criteria, on stable lipid-lowering therapy for ≥4 weeks, and with fasting LDL cholesterol ≥100 mg/dL.27 The patients were randomized 2:2:1:1 to receive evolocumab 140 mg every 2 weeks, evolocumab 420 mg monthly, placebo every 2 weeks, or placebo monthly for 12 weeks. Of the 331 randomized patients, 264 agreed to the genetic analysis, and of those, 80% had mutations known to cause familial hypercholesterolemia. Patients found to have an LDL receptor mutation were grouped by LDL receptor functional class as defective or negative.28,29 Evolocumab administered every 2 weeks, or monthly resulted in mean LDL cholesterol reductions at week 12 of 59% and 61%, respectively, versus placebo (P<0.001): LDL cholesterol reductions with every 2 weeks or monthly dosing were 61% and 55% in those with an LDL receptor mutation associated with no function, 49% and 66% in those with defective function, and 62% and 63% where the LDL receptor status was unclassified. At week 12, LDL cholesterol <70 mg/dL was achieved by 68% and 63% of patients in the evolocumab 140 mg every 2 weeks and 420 monthly groups, respectively, compared with 2% of patients in the placebo groups. Compared with placebo, mean reductions in Lp(a) were 32% and 28% at week 12 (both doses P<0.001). Treatment with evolocumab 140 mg every 2 weeks and 420 mg monthly compared with placebo resulted in mean triglycerides decreases of 20% (P<0.001) and 12% (P<0.05), respectively, at week 12. HDL cholesterol was increased by 9% in both evolocumab groups.
In the Phase II GAUSS study, evolocumab was tested in statin-intolerant patients, comparing the effect of evolocumab at doses of 280 mg, 350 mg, and 420 mg with that of placebo/ezetimibe.30 Statin intolerance was defined as inability to tolerate effective doses of at least two different statins because of myalgia, myopathy, myositis, or rhabdomyolysis that resolved after statin discontinuation. Maximal reduction in LDL cholesterol was observed within 2 weeks of treatment, with or without ezetimibe. At week 12, LDL cholesterol changes were dose-related, ranging from −67 to −91 mg/dL, increasing to −110 mg/dL in the 420 mg + ezetimibe group. Lp(a) levels were reduced by 20%–29% in the evolocumab-treated groups (all P<0.001). The increase in HDL cholesterol ranged from 6% to 12%, compared with the placebo/ezetimibe group, with similar increases seen in ApoA1.
In the Phase III GAUSS-2 trial, researchers evaluated the efficacy and safety of evolocumab compared with oral ezetimibe in patients with hypercholesterolemia unable to tolerate effective statin doses (~10%–20% of patients were receiving statins).31,32 The GAUSS-2 trial was a 12-week, double-blind study, which enrolled 307 patients (mean age: 62 ± 10 years, mean baseline LDL cholesterol: 193 ± 59 mg/dL) on no statin or on a low-dose statin (weekly dose seven times the smallest available tablet strength). Mean percent reductions from baseline at a mean of weeks 10 and 12 were 56.1% with 140 mg every 2 weeks and 55.3% with 420 mg once monthly, corresponding to treatment differences versus ezetimibe of 36.9% and 38.7%, respectively (P<0.001), with similar mean percent reductions at week 12 (P<0.001). Evolocumab-treated patients were more likely to achieve LDL cholesterol target levels than ezetimibe-treated patients (~76.5% versus ~5.5%, respectively). Compared with ezetimibe, evolocumab resulted in significant reductions in ApoB, Lp(a), non-HDL cholesterol concentrations, and ApoB/ApoA-I and total cholesterol/HDL cholesterol ratios (P<0.001).
The Phase III DESCARTES trial evaluated the safety and efficacy of 52 weeks of treatment with evolocumab.33 Patients with an LDL cholesterol level ≥75 mg/dL were randomly assigned in a 2:1 ratio to receive either evolocumab (420 mg) or placebo every 4 weeks for 52 weeks. Nine hundred and one patients were included in the primary analysis. The reduction in LDL cholesterol from baseline in the evolocumab group, taking into account the change in the placebo group, was 57.0% ± 2.1% (P<0.001). LDL cholesterol levels were reduced to below 70 mg/dL in 82.3% of patients in the evolocumab group, compared with 6.4% in the placebo group. Evolocumab also significantly decreased the concentrations of ApoB (44.2%), non-HDL cholesterol (50.3%), Lp(a) (22.4%), and triglycerides (11.5%). HDL cholesterol was significantly increased by 5.4%.
In the Phase III TESLA Part B trial, 50 patients with homozygous familial hypercholesterolemia on stable lipid-lowering therapy and not on lipoprotein apheresis received either subcutaneous evolocumab 420 mg or placebo every 4 weeks for 12 weeks.34 Of the 50 patients randomized, 49 completed the study (16 in the placebo group and 33 in the evolocumab group). Evolocumab significantly reduced LDL cholesterol (as measured by ultracentrifugation) by 30.9% compared with placebo. These data confirm the results of a previous small trial carried out in patients with receptor-negative or receptor-defective homozygous familial hypercholesterolemia, in which eight patients treated with subcutaneous 420 mg evolocumab every 4 weeks for ≥12 weeks, followed by 420 mg evolocumab every 2 weeks for 12 more weeks. At week 12, the mean change from baseline in LDL cholesterol was −16.5% (range 5.2% to −43.6%; P<0.078) and −13.9% (range 39.9% to −43.3%; P<0.148) with 4-week and 2-week dosing, respectively. While no LDL cholesterol reduction was seen in the two receptor-negative patients over the treatment period, the mean LDL cholesterol reduction in the six LDL receptor-defective patients was 19.3%±16% and 26.3%±20% with 4-week and 2-week dosing, respectively (P=0.03 for both values), ranging from 4% to 48% with the 2-week dosing regimen.35
The main characteristics of these studies are summarized in Tables 1–3. The OSLER trial was then started in October 2011 to evaluate whether long-term exposure to evolocumab is safe and well tolerated on a background of standard of care. More than 1,100 hypercholesterolemic participants were enrolled from the Phase II trials, and the study is planned to end in May 2016.36–38
Table 1.
Phase III trials of alirocumab
| Patients (n) | Population | Trial | Dose | Main results |
|---|---|---|---|---|
| 103 | Hypercholesterolemia (with or without statin therapy) | ODYSSEY MONO74 | 75 mg SC, every 2 weeks | LDL-C −47.2%, TC −29.6%, HDL-C +6.0%, Lp(a) −16.7% |
| 251 | Statin intolerance; primary hypercholesterolemia (heterozygous FH or non-FH); and moderate, high, or very high CVD risk (no statin therapy) | ODYSSEY ALTERNATIVE89 | 75–150 mg SC every 2 weeks | LDL-C −52.2%; lower occurrence of muscle symptoms versus ezetimibe |
| 183 | Hypercholesterolemia (heterozygous FH or non-FH) not adequately controlled (atorvastatin with or without other lipid-modifying therapy), and high CVD risk | ODYSSEY OPTIONS I89 | 50–150 mg SC every 2 weeks/200–400 mg every 4 weeks | LDL-C −44%/54% |
| 415 | Hypercholesterolemia not adequately controlled (rosuvastatin ± other lipid-modifying therapy), and high CVD risk | ODYSSEY OPTIONS II89 | 75 mg SC every 2 weeks/150 mg SC every 2 weeks | LDL-C −36%/51% |
| 316 | Hypercholesterolemia not adequately controlled (with maximum dose of a statin with or without other lipid-modifying therapy), and high CVD risk | ODYSSEY COMBO I89 | 75–150 mg SC every 2 weeks | LDL-C −48% |
| 107 | Heterozygous FH not adequately controlled with current lipid-modifying therapy (no specification regarding statin therapy) | ODYSSEY HIGH FH89 | 150 mg SC every 2 weeks | LDL-C −46% |
| 3,109 | Hypercholesterolemia not adequately controlled with current lipid-modifying therapy, and high CVD risk (no specification regarding statin therapy) | ODYSSEY LONG TERM89 | 150 mg SC every 2 weeks | LDL-C −61% |
| 720 | Hypercholesterolemia not adequately controlled (maximum dose of a statin with or without other lipid-modifying therapy), and high CVD risk | ODYSSEY COMBO II54 | 75 mg SC every 2 weeks | LDL-C −50% |
| – | Hypercholesterolemia | ODYSSEY CHOICE 160 | Ongoing | |
| – | Heterozygous FH not adequately controlled with current lipid-modifying therapy (no specification regarding statin therapy) | ODYSSEY FH I55 | Ongoing | |
| – | Heterozygous FH not adequately controlled (with maximally tolerated statin with or without other lipid-modifying therapy) | ODYSSEY FH II55 | Ongoing | |
| – | Recent (in the past 4–16 weeks) acute coronary syndrome event requiring hospitalization | ODYSSEY OUTCOMES56 |
Abbreviations: CVD, cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein(a); SC, subcutaneous; FH, familial hypercholesterolemia.
Table 2.
Phase III trials of evolocumab
| Patients (n) | Population | Trial | Dose | Main results |
|---|---|---|---|---|
| 614 | Framingham risk score ≤10% and LDL-C level ≥100 mg/dL (no specification regarding statin therapy) | MENDEL-268 | 140 mg SC every 2 weeks or 420 mg every 4 weeks | LDL-C −56.9% to 58.8%, HDL-C +3.8% to 3.9%; Lp(a) −18.4% to 19.2% |
| 307 | Statin intolerance; hypercholesterolemia (no statin or low-dose statin) | GAUSS-285 | 140 mg SC every 2 weeks or 420 mg every 4 weeks | LDL-C −53% to 56%, lower occurrence of muscle symptoms versus ezetimibe |
| 901 | LDL-C level ≥85 mg/dL and either at ATP III target with background lipid therapy or taking maximum background lipid therapy (no specification regarding statin therapy) | DESCARTES70 | 420 mg SC every 4 weeks | LDL-C −48.5% to 61.6% |
| 2,067 | Primary hypercholesterolemia or mixed dyslipidemia (taking statin therapy with or without ezetimibe) | LAPLACE-269 | 140 mg SC every 2 weeks or 420 mg every 4 weeks | LDL-C −63% to 75% |
| 331 | Heterozygous FH and LDL-C level ≥100 mg/dL with statin therapy (no specification regarding statin therapy) | RUTHERFORD-271 | 140 mg SC every 2 weeks or 420 mg every 4 weeks | LDL-C −55.7% to 59.2%, HDL-C +5.4% to 8.1%, Lp(a) 21.6% to 22.9% |
| 50 | Homozygous FH and LDL-C level >130 mg/dL with stable lipid therapy (no specification regarding statin therapy) | TESLA72 | 420 mg SC every 4 weeks | LDL-C −23.1%, HDL-C 4.0%, Lp(a) −12.7% |
| – | Hypercholesterolemia or mixed dyslipidemia; completion of previous evolocumab study (no specification regarding statin therapy) | OSLER-257 | Ongoing | |
| – | Coronary heart disease; clinical indication for coronary catheterization; and LDL-C level ≥80 mg/dL or, with additional risk factors, ≥60 mg/dL and <80 mg/dL (no specification regarding statin therapy) | GLAGOV58 | Ongoing | |
| – | Clinical CVD, high risk of recurrent CVD event, and LDL-C level ≥70 mg/dL or non-HDL-C ≥100 mg/dL (no specification regarding statin therapy) | FOURIER59 | Ongoing |
Abbreviations: ATP III, Third Adult Treatment Panel of the US National Cholesterol Education Program; CVD, cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein(a); SC, subcutaneous; FH, familial hypercholesterolemia.
Table 3.
Phase III trials of bococizumab
| Patients (n) | Population | Trial | Main results |
|---|---|---|---|
| – | Heterozygous FH; high or very high CVD risk; LDL-C level >70 mg/dL and TG level ≤400 mg/dL (with statin therapy) | SPIRE-HF | Ongoing |
| – | High or very high CVD risk; LDL-C level >70 mg/dL and TG level ≤400 mg/dL (with statin therapy) | SPIRE-HR | Ongoing |
| – | High or very high CVD risk; LDL-C level ≥70 mg/dL and TG level <400 mg/dL (with statin therapy) | SPIRE-LDL | Ongoing |
| – | High CVD risk; LDL-C level ≥70 mg/dL and <100 mg/dL, or non-HDL-C level ≥100 mg/dL and <130 mg/dL, with lipid-lowering therapy (no specification regarding statin therapy) | SPIRE-1 | Ongoing |
| – | High CVD risk; LDL-C level ≥100 mg/dL or non-HDL-C level ≥130 mg/dL, with lipid-lowering therapy (no specification regarding statin therapy) | SPIRE-2 | Ongoing |
Abbreviations: FH, familial hypercholesterolemia; CVD, cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein(a); SC, subcutaneous; TG, triglycerides.
Among the Phase III trials aiming to confirm the efficacy and safety of evolocumab in a specific subpopulation of hypercholesterolemic subjects, the main one is PROFICIO, while the FOURIER study, that started in January 2013 and will continue to 2018, is evaluating whether evolocumab decreases cardiovascular events versus placebo in 22,500 patients with cardiovascular disease.39
Safety and tolerability data
Based on the available pharmacological data for evolocumab, we could foresee two main types of possible toxicity. Target-related toxicity includes the effects of antibodies in non-intended tissues. Modality-related toxicity includes acute hypersensitivity reactions. Another restricting point could be the formation of antibodies against murine and chimeric antibodies, ie, an antiantibody response, which is very low for fully humanized antibodies. The most safe/efficacious dosages of ascending doses of evolocumab have been tested in pharmacodynamic and pharmacokinetic studies performed in patients using stable doses of statins.40
No serious adverse events were observed in the Phase I studies of evolocumab, but myositis was reported in three patients, with creatine kinase elevations >2.5–5 times the upper limit of normal, which resolved in less than 4 weeks.13
In the MENDEL study, nonserious adverse events occurred with a similar incidence in evolocumab-treated, placebo-treated, and ezetimibe-treated patients (46%–58%); no serious treatment-related adverse events were reported.16 Also, in the MENDEL-2 trial,17 nonserious adverse events occurred with a similar incidence in evolocumab-treated, placebo-treated, and ezetimibe-treated patients (44%–46%). Serious adverse events occurred in 1.3% of the patients receiving AMG145 versus 0.6% in both the placebo and ezetimibe groups.
The LAPLACE-TIMI 57 study evaluated different doses of evolocumab together with statin ± ezetimibe, but no serious adverse events occurred. Treatment-related adverse events were similar in the evolocumab and placebo groups; none of these events was severe.18,19 In the LAPLACE II trial, nonserious adverse events occurred with a similar incidence in evolocumab-treated, placebo-treated, and ezetimibe-treated patients (36%–40%), with back pain, arthralgia, headache, muscle spasms, and pain in the extremities being the most common adverse events in the evolocumab-treated patients.41
In the RUTHERFORD study,23,24 the most common treatment-related adverse events were nasopharyngitis (12.5% with evolocumab 350 mg, 12.7% with evolocumab 420 mg, and 10.7% with placebo), injection site pain (9.1% with 350 mg, 3.6% with 420 mg, and 1.8% with placebo), and headache (5.5% with 350 mg, 5.4% with 420 mg, and 8.9% with placebo). Nasopharyngitis was thought to be caused by an immune-mediated process, but the exact underlying pathogenetic mechanism has to be fully clarified. Only one patient treated with evolocumab 420 mg experienced creatine kinase increase more than ten times the upper limit of normal without symptoms. An increase in transaminases more than three times (but less than five times) the upper limit of normal was observed in two patients treated with evolocumab. In the RUTHERFORD-2 trial, the incidence of nonserious and serious adverse events was comparable with rates reported in previous studies of evolocumab.42
In the GAUSS study, the most common treatment-related adverse effect was myalgia, which occurred at the highest incidence in 5% of the 280 mg group and 20% in the evolocumab/ezetimibe group.40 Registered serious adverse events were coronary artery disease, acute pancreatitis, hip fracture, and syncope, but they were considered not related to the tested lipid-lowering treatment. Two patients experienced creatine kinase levels higher than ten times the upper limit of normal during the study, but values normalized after treatment cessation.
In the GAUSS II trial, muscle-related adverse effects occurred in 12% of evolocumab-treated patients and 23% of ezetimibe-treated patients. Myalgia occurred in 8% of evolocumab-treated patients and 18% of ezetimibe-treated patients. Patients using low-dose statin therapy were more likely to develop myalgia, both in the ezetimibe group (statin versus no statin, 21% versus 17%) and the evolocumab group (statin versus no statin, 17% versus 6%).43
In the OSLER trial, adverse events occurred in 81.4% of patients treated with evolocumab and in 73.1% of those in the standard treatment group. The five most common adverse events in the evolocumab group compared to the standard group were nasopharyngitis (12.2% versus 9.8%), upper respiratory tract infections (7.7% versus 7.6%), influenza (7.1% versus 5.2%), arthralgia (6.9% versus 4.3%), and back pain (6.5% versus 5.4%). Other reported adverse events included muscle-related events (9.2% versus 9.8%), elevated liver function tests (1.8% versus 1.6%), and elevated creatine kinase (1.0% versus 1.9%). Serious adverse events occurred in a similar percentage in patients treated with evolocumab (7.1%) and in those in the standard care group (6.3%).37,38
In the DESCARTES trial, the overall incidence of adverse events occurring during treatment was similar in the evolocumab group (74.8%) and the placebo group (74.2%). The most common adverse events in the evolocumab group were nasopharyngitis, upper respiratory tract infection, influenza, and back pain.33 In the TESLA Part B trial, adverse events occurred in 63% of the patients in the placebo group and in 36% of the patients in the evolocumab group. No serious adverse events occurred, and no anti-evolocumab antibody development was detected during the study.34
Discussion
In clinical practice, reduction of LDL cholesterol levels is usually achieved by use of reversible inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (statins), also associated with inhibitors of the intestinal absorption of cholesterol (ionic exchange resins, such as cholestyramine or colestimide, colestipol, or ezetimibe).44 This strategy is usually effective and relatively well tolerated; however, subjects at high risk for cardiovascular disease, ie, those requiring more intensive treatment for reduction of hypercholesterolemia, often do not reach the desired LDL cholesterol target, either because of lack of efficacy of the available therapies or because of side effects of the high drug dosages required.45,46
In this context, an overall well-tolerated new treatment option, enlarging and empowering our ability to manage high-risk patients, especially when statin-intolerant (at least according to the definition used in the above cited trials), could be a great innovation in therapy.47 This is clearly useful when following the indications given by European Society of Cardiology/European Atherosclerosis Society guidelines,48 which focus their attention on baseline cholesterol levels and use a targeted approach, the LDL-C target seems to be less relevant when considering American College of Cardiology/American Heart Association guidelines, which have a statin-centered approach independent of baseline cholesterol levels and a specific LDL cholesterol target to be reached.49 Moreover, evolocumab positively modulates the whole lipid pattern, significantly decreases the Lp(a) level,50 a genetically determined lipid risk factor, and other lipid and lipoprotein fractions such as ApoB, triglycerides, and non-HDL cholesterol, while increasing the HDL cholesterol level. Due to the strong LDL cholesterol-lowering achieved with evolocumab, levels of LDL cholesterol well below 50 mg/dL will be observed in a large number of patients treated with these agents.10
Even if the clinical relevance of the Lp(a)-reducing effect has to be tested in further trials, it could be really useful, especially considering the scarcity of drugs acting on Lp(a).51 What is even more encouraging is that similar efficacy and safety data have been confirmed also with alirocumab, another independently developed human anti-PCKS9 antibody.52,53
In summary, evolocumab is a powerful lipid-lowering drug, with an apparently large dose-dependent therapeutic range associated with a low rate of usually mild adverse events. Its efficacy seems to be evident both when administered alone and when given in association with statins and/or ezetimibe. If the available data are confirmed in long-term trials with strong outcomes, evolocumab will provide an essential tool to efficiently treat high risk patients who need to reach ambitious LDL cholesterol targets.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cholesterol Treatment Trialists’ (CTT) Collaboration Efficacy and safety of LDL-lowering therapy among men and women: analysis of individual data from 174 000 participants in 27 randomized trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 2.Kohno T. Report of the American Heart Association (AHA) Scientific Session 2014, Chicago. Circ J. 2014;79:34–40. doi: 10.1253/circj.CJ-14-1329. [DOI] [PubMed] [Google Scholar]
- 3.Reiner Z, De Bacquer D, Kotseva K, et al. Treatment potential for dyslipidaemia management in patients with coronary heart disease across Europe: findings from the EUROASPIRE III survey. Atherosclerosis. 2013;231:300–307. doi: 10.1016/j.atherosclerosis.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell KN, Breslow JL. Adenoviral mediated expression of PCSK9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. [Google Scholar]
- 6.Abifadel M, Varret M, Rabee JD, et al. Mutations in PCSK9 cause autosomal PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1267. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 7.Urban D, Pöss J, Böhm M, Laufs U. Targetting the proprotein convertase subtisilin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62:1401–1408. doi: 10.1016/j.jacc.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Rashid S, Curtis DE, Garuti R, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking PCSK9. Proc Natl Acad Sci U S A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnier M. PCSK9 inhibitors. Curr Opin Lipidol. 2013;24:251–258. doi: 10.1097/MOL.0b013e3283613a3d. [DOI] [PubMed] [Google Scholar]
- 10.Gouni-Berthold J, Berthold HK. PCSK9 antibodies for the treatment of hypercholesterolemia. Nutrients. 2014;6:5517–5533. doi: 10.3390/nu6125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein EA, Wasserman SM, Dias C, Scott R, Raal F. AMG145. Drugs Fut. 2013;38:451–459. [Google Scholar]
- 12.Chan CY, Piper DE, Cao Q, et al. A proprotein convertase subtilisin/kexin type neutralizing antibody reduces serum cholesterol in mice and non human primates. Proc Natl Acad Sci U S A. 2009;106:9820–9825. doi: 10.1073/pnas.0903849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicero AF, Tartagni E, Ertek S. Efficacy and safety profile of evolocumab (AMG145), an injectable inhibitor of the proprotein convertase subtilisin/kexin type 9: the available clinical evidence. Exp Opin Biol Ther. 2014;14:863–868. doi: 10.1517/14712598.2014.902929. [DOI] [PubMed] [Google Scholar]
- 14.Foltz IN, Karow M, Wasserman SM. Evolution and emergence of therapeutic monoclonal antibodies. What cardologists need to know. Circulation. 2013;127:2222–2230. doi: 10.1161/CIRCULATIONAHA.113.002033. [DOI] [PubMed] [Google Scholar]
- 15.Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a density lipoprotein cholesterol levels. Results from 2 randomized, double-blind, placebo-controlled, ascending dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–1898. doi: 10.1016/j.jacc.2012.08.986. [DOI] [PubMed] [Google Scholar]
- 16.Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG145 on low-monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 17.Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia – the MENDEL-2 randomized, controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Kohli P, Desai NR, Giugliano RP, et al. Design and rationale of the LAPLACE-TIMI 57 trial: a phase II, double-blind, placebo-controlled study of the efficacy and tolerability of a monoclonal antibody inhibitor of PCSK9 in subjects with hypercholesterolemia on background statin therapy. Clin Cardiol. 2012;35:385–391. doi: 10.1002/clc.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128:962–969. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 21.McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 23.Leigh SE, Foster AH, Whittall RA, et al. Uptake and analysis of the University College London low density lipoprotein receptor familial hypercholesterolemia database. Ann Hum Genet. 2008;72:485–498. doi: 10.1111/j.1469-1809.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 24.Innerarity TL, Weisgraber KH, Arnold KS, et al. Familial defective apolipoprotein B-100: low density lipoproteins with abnormal receptor binding. Proc Natl Acad Sci U S A. 1987;84:6919–6923. doi: 10.1073/pnas.84.19.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 26.Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to protein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Raal F, Stein E, Dufour, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2014;385:331–340. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 29.Usifo E, Leigh SE, Whittall RA, et al. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet. 2012;76:387–401. doi: 10.1111/j.1469-1809.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients. The GAUSS Randomized Trial. JAMA. 2012;308:2497–2506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 31.Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 34.Raal FJ, Honarpour N, Blom DJ, et al. for the TESLA Investigators Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2014;385:341–350. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 35.Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–2120. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- 36.Amgen Open label study of long term evaluation against LDL-C trial (OSLER) [Accessed February 1, 2014]. Available from: http://clinicaltrials.gov/show/NCT01439880.
- 37.Koren MJ, Giugliano RP, Raal F, et al. Randomized comparison of the safety, tolerability and efficacy of long-term administration of AMG 145 versus standard of care in 1004 patients: 52 week results from the OSLER trial. Circulation. 2014;129:234–243. doi: 10.1161/CIRCULATIONAHA.113.007012. [DOI] [PubMed] [Google Scholar]
- 38.Mearns BM. Dyslipidaemia: 1-year results from OSLER trial of anti-PCSK9 monoclonal antibody evolocumab. Nat Rev Cardiol. 2014;11:63. doi: 10.1038/nrcardio.2013.201. [DOI] [PubMed] [Google Scholar]
- 39.Amgen Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER) [Accessed February 1, 2014]. Available from: http://clinicaltrials.gov/show/NCT01764633.
- 40.Amgen Multiple dose study to evaluate the safety, tolerability, phar-macokinetics and pharmacodynamics of AMG 145 in subjects with hyperlipidemia on stable doses of a statin. [Accessed February 1, 2014]. Available from: http://clinicaltrials.gov/ct2/show/record/NCT01133522.
- 41.Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 42.Raal F, Stein E, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2014;385:331–340. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 43.Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Gotto AM, Jr, Moon JE. Pharmacotherapies for lipid modification: beyond the statins. Nat Rev Cardiol. 2013;10:560–570. doi: 10.1038/nrcardio.2013.117. [DOI] [PubMed] [Google Scholar]
- 45.Catapano AL. Perspectives on low-density lipoprotein cholesterol goal achievement. Curr Med Res Opin. 2009;25:431–447. doi: 10.1185/03007990802631438. [DOI] [PubMed] [Google Scholar]
- 46.Jones P, Nair R, Thakker KM. Prevalence of dyslipidemia and lipid goal attainment in statin-treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc. 2012;1:e001800. doi: 10.1161/JAHA.112.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiner Z. Resistance and intolerance to statins. Nutr Metab Cardiovasc Dis. 2014;24:1057–1066. doi: 10.1016/j.numecd.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Reiner Z, Catapano AL, De Backer G, et al. ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 49.Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Disease Risk in Adults: Synopsis of the 2013 ACC/AHA Cholesterol Guideline. Ann Intern Med. 2014;160:339–343. doi: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 50.Raal FJ, Giugliano RP, Sabatine MS, et al. PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of over 1300 reduction in lipoprotein(a) with the patients in 4 phase 2 trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;23:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tavori H, Melone M, Rashid S. Alirocumab: PCSK9 inhibitor for LDL cholesterol reduction. Exp Rev Cardiovasc Ther. 2014;12:1137–1144. doi: 10.1586/14779072.2014.954551. [DOI] [PubMed] [Google Scholar]
- 53.Cicero AF, Tartagni E, Ertek S. Safety and tolerability of injectable lipid-lowering drugs: a review of available clinical data. Exp Opin Drug Saf. 2014;13:1023–1030. doi: 10.1517/14740338.2014.932348. [DOI] [PubMed] [Google Scholar]
- 54.Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015 Feb 16; doi: 10.1093/eurheartj/ehv028. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kastelein JJ, Robinson JG, Farnier M, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia not adequately controlled with current lipid-lowering therapy: design and rationale of the ODYSSEY FH studies. Cardiovasc Drugs Ther. 2014;28(3):281–289. doi: 10.1007/s10557-014-6523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168(5):682–689. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 57.Amgen Open Label Study of Long Term Evaluation Against LDL-C Trial-2 (OSLER-2) [Accessed May 07, 2015]. Available from: https://clinicaltrials.gov/show/NCT01854918. NLM identifier: NCT01854918.
- 58.Amgen GLobal Assessment of Plaque reGression With a PCSK9 antibOdy as Measured by intraVascular Ultrasound (GLAGOV) [Accessed May 07, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01813422. NLM identifier: NCT01813422.
- 59.Amgen Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) [Accessed May 07, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01764633. NLM identifier: NCT01764633.
- 60.Regeneron Pharmaceuticals Study to Evaluate the Efficacy and Safety of an Every Four Weeks Treatment Regimen of Alirocumab (REGN727/SAR236553) in Patients With Primary Hypercholesterolemia (ODYSSEY CHOICE 1) [Accessed May 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01926782. NLM identifier: NCT01926782.


