Abstract
Aim
To investigate the potential cardioprotective effects of QiShenYiQi Pill® (QSYQ) on myocardial ischemia/reperfusion (I/R) injury through antioxidative stress and mitochondrial protection.
Methods and results
Sprague Dawley rats were pretreated with QSYQ or saline for 7 days and subjected to ischemia (30 minutes occlusion of the left anterior descending coronary artery) and reperfusion (120 minutes). Cardiac functions were evaluated by echocardiogram and hemodynamics. Myocardial mitochondria were obtained to evaluate changes in mitochondrial structure and function, immediately after 120 minutes reperfusion. Pretreatment with QSYQ protected against I/R-induced myocardial structural injury and improved cardiac hemodynamics, as demonstrated by normalized serum creatine kinase and suppressed oxidative stress. Moreover, the impaired myocardial mitochondrial structure and function decreased level of ATP (accompanied by reduction of ATP5D and increase in the expression of cytochrome C). Myocardial fiber rupture, interstitial edema, and infiltrated leukocytes were all significantly ameliorated by pretreatment with QSYQ.
Conclusion
Pretreatment of QSYQ in Sprague Dawley rats improves ventricular function and energy metabolism and reduces oxidative stress via ameliorating multiple mitochondrial dysfunctions during I/R injury.
Keywords: QSYQ, ischemia/reperfusion injury, energy metabolism, mitochondria
Introduction
Ischemic cardiomyopathy remains a public health concern with high morbidity and mortality throughout the world.1 In coronary artery disease, the most effective therapy to reduce ischemic myocardial injury and infarct size is efficient myocardial reperfusion and early sustained restoration of blood flow (reperfusion) through the occluded coronary artery. However, the myocardial reperfusion process can also induce further myocardial cell death, a phenomenon known as myocardial ischemia/reperfusion (I/R) injury.2,3 Thus, reperfusion can lead to worsening of ischemic myocardial injury.4 Reperfusion causes cell damage and cell death mostly by initiating a localized oxidative burst and regional inflammatory response.5 I/R injury includes distinct phases of cellular injury, including ATP depletion, lactate accumulation and acidosis observed during ischemia, and the production of reactive oxygen and nitrogen species during reperfusion.6 The ATP depletion caused by ischemic hypoxia leads to production of the reactive oxygen species (ROS) during reperfusion, which exaggerates myocardium and endothelial cell injury.7 Animal studies have suggested that reperfusion injury is responsible for up to 50% of the final infarct size.8 Thus, myocardial protection against I/R injury becomes a primary goal of therapeutic intervention.
Because diverse pathological factors, such as inflammation, oxidative stress, and apoptosis, contribute to the pathological mechanism underlying myocardial I/R (MI/R) injury,9–13 most research efforts have been focused on interference with production of peroxide,14 expression of adhesion molecules,15 release of inflammatory factors,16,17 and apoptosis of cardiac myocytes.18 A number of strategies and pharmacological agents are shown to ameliorate reperfusion injury in animal models.19 However, translation of these single-target therapeutic strategies to a clinical setting has been disappointing.19 Thus, multi-target therapeutic strategies for MI/R injury are urgently required.
In recent years, there has been a growing interest in the treatment of MI/R-induced cardiac dysfunction with plant-based therapy, including Traditional Chinese Medicine (TCM). Generally, multiple active ingredients in the formula of TCM aim at multiple targets, exerting a systemic effect.20 An excellent example of such a formula is QiShenYiQi Pills® (QSYQ), which is a compound of Chinese medicine approved by China State Food and Drug Administration in 2003 for treatment of coronary heart disease, angina pectoris, and cardiac dysfunction.21 QSYQ has been commonly used in the clinic for the integrative treatment of patients with ischemic heart disease who are also diagnosed with Qi deficiency and blood stasis syndrome.22 The active components of QSYQ are extracted from Radix astragali, Salvia miltiorrhiza, Panax notoginseng, and rosewood, which have the effects of nourishing Qi, promoting blood circulation, and relieving pain.23 Our previous studies have shown that QSYQ could inhibit platelet aggregation, prevent enlargement of end-diastolic diameter, improve left ventricular function, slow down ventricular remodeling, and attenuate pressure overload and I/R-induced cardiac hypertrophy and myocardial fibrosis.24–27 The present study aims to define mechanisms by which QSYQ exerts cardioprotective effect during MI/R.
Materials and methods
Animals
All animals were handled according to the guidelines of Tianjin University of TCM Animal Research Committee (TCM-LAEC2014005). Male Sprague Dawley rats, weighing 250±10 g, were purchased from Beijing Hua Fu Kang Bio-Technology Co., Ltd. Beijing, People’s Republic of China, (Certificate number SCXK [Jing] 2009-0017). The rats were housed in cages at a temperature of 22°C±2°C and humidity 40%±5%, under a 12-hour light/dark cycle, and received standard diet and water ad libitum. The animals were fasted for 12 hours before experiment, but allowed free access to water. The experimental procedures followed the European Union (EU) adopted Directive 2010/63/EU, and all animals were handled according to the guidelines of Tianjin University of TCM Animal Research Committee (TCM-LAEC2014005).
Drug and reagents
QSYQ (batch number: 20091005) was obtained from Tasly Pharmaceutical Group Co. Ltd. (Tianjin, People’s Republic of China), which was prepared from water–ethanol extracts of R. astragali, S. miltiorrhiza, P. notoginseng, and rosewood according to the guidelines of Good Manufacturing Practice and Good Laboratory Practice, and the content of its major components was determined by the Chinese Government agency. QSYQ was dissolved in saline to make a solution at concentrations of 20 mg/mL and 10 mg/mL for experiments.
Chloral hydrate (batch number: Q/12HB 4218-2009) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, People’s Republic of China), freshly prepared to 5% solution with saline before experiment. The interleukin-1β (IL-1β) assay kit and tumor necrosis factor-alpha (TNF-α) assay kit were from Uscn Life Science Inc. (Wuhan, People’s Republic of China). Mitochondria extraction kit (Solarbio, Beijing Solarbio Science & Technology Co., Ltd. Beijing), mitochondrial membrane potential detection kit (JC-1), and ATP detection kit were purchased from Pik-day Institute of Biotechnology. Fluo-4 fluorescent probe, DCFH-DA fluorescent probe, protein test kit, total-superoxide dismutase (T-SOD) detection kit, and malondialdehyde (MDA) detection kit were purchased from Nanjing Jiancheng Bioengineering Institute. The antibodies against cytochrome C and ATP5D were from Abcam Inc. (Cambridge, UK).
MI/R model and drug administration
Rats were randomly divided into four groups: Sham, I/R, QSYQ 10 mg/mL +I/R, and QSYQ 20 mg/mL +I/R groups. Seven days before I/R surgery, the rats in treatment groups were administered with QSYQ via gavage in saline at the dose of 20 mg/mL and 10 mg/mL once daily for 7 days. The animals in Sham-operated and I/R groups received saline at 10 mL/kg at the same time. The care and administration procedure in this current study was in accordance with the clinical guidelines.28
For MI/R model preparation, rats were anesthetized with 5% chloral hydrate (300 mg/kg) by peritoneal injection and placed in a supine position. After left thoracotomy was performed to expose the heart, the proximal left anterior descending coronary artery was ligated with a 5/0 silk. The suture silk was released after 30 minutes, allowing reperfusion to occur. Then the thorax was closed, and as soon as spontaneous respiration was sufficient, the rats were released and allowed to recover on an electric blanket. The animals in the Sham group underwent the same procedure but without ligation of suture silk.29 The overall mortality of rats that underwent induction of I/R during the entire experimental period was 30%–35%. The majority of death occurred on, or after, the I/R surgery, probably because of acute pump failure or lethal arrhythmias.
Adequacy of anesthesia was controlled by monitoring corneal reflex and the lack of response to toe-pinching. Hearts were excised from rats after terminal anesthesia by intraperitoneal (ip) injection of 5% chloral hydrate at dose 300 mg/kg−1 and heparin (100 U). Euthanasia was performed by excessive inhalation of isoflurane. Death was monitored by the cardiac activity and respiration.
Echocardiographic assessment of left ventricular function
Using a Vevo 2100 ultra-high resolution small animal ultrasound imaging system in real time (VisualSonics Vevo 2100, Canada) with a MS-250 ultrasound scanning transducer (model C5) to evaluate left ventricular function.30 Briefly, animals were anesthetized with 1.5%–2.0% isoflurane by mask, and the chest was shaved and placed in the supine position on a 37° pad. Electrocardiogram limb electrodes were placed, at 2 hours after reperfusion, evenly spread layer of ultrasound coupling agent on the rat thoracic, and the console downward sloping 30°–45°. Two-dimensional cine loops and guided M-mode frames were recorded from the parasternal long axis.
The following parameters were measured as indicators of function and remodeling: left ventricular internal diameter diastole (LVIDd), left ventricular posterior wall diastole (LVPWd), left ventricular internal diameter systole (LVIDs), left ventricle ejection fraction percentage (EF%), and left ventricle fractional shortening percentage (FS%). All data were analyzed off-line at the end of the study with software resident on the ultrasound system.26
Hemodynamic evaluation of cardiac function
Two hours after reperfusion, the cannulation was done to the left ventricle through right carotid artery, which was connected to a biofunction experiment system MP100-CE (BIOPAC Systems, Inc., Santa Barbara, CA, USA) after induction of anesthesia with 5% chloral hydrate (300 mg/kg) by peritoneal injection. Heart rate (HR), left ventricular systolic pressure, left ventricular development pressure, left ventricular end diastolic pressure, left ventricular maximum upstroke velocity (+dp/dtmax), and left ventricular maximum descent velocity (−dp/dtmax) were measured at baseline, immediately at 2 hours after reperfusion with MP100-CE equipment.31
Histopathological examination of myocardial tissues
Two hours after reperfusion, thoracotomy was performed on rats after induction of anesthesia with 5% chloral hydrate (300 mg/kg) by peritoneal injection. The heart was removed, fixed in 4% paraformaldehyde solution for more than 48 hours, and further prepared for paraffin sectioning. Serial sections (5 μm) were cut and stained with hematoxylin–eosin. The sections were analyzed under light microscope. The images were captured by a digital camera connected to an optical microscope (Digital Sight Leica DM3000, Leica Microsystems Wetzlar, Germany) and processed with the Leica Application Suite (Leica Application Suite, Leica Microsystems Wetzlar, Germany).
At the end of the experiments, blood was collected and centrifuged at 1,000 rpm for 10 minutes. The plasma was used to detect SOD, MDA, IL1-β, and TNF-α using a enzyme linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions. The content of creatine kinase (CK), creatine kinase-MB (CK-MB), and lactate dehydrogenase (LDH) of serum was also assessed with ELISA by automatic biochemical detector (Multiskan MK3; Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instruction.
Quantitative real-time PCR
Total RNA was extracted using TRIzol lysis buffer. Total RNA concentrations were measured with ND-1000 (NanoDrop Technologies), and equal amounts of total RNA were reverse transcribed to cDNA using high-capacity cDNA reverse transcription kits (Applied Biosystems, F. Hoffmann-La Roche Ltd, Switzerland), following the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) in a step one real-time PCR system (Applied Biosystems) was used to determine the mRNA expression of SOD, GSH, CAT, and NOX. Standard curves were made by serial dilution of cDNA of Sham-operated groups. cDNA was run in duplicates. To account for differences in cDNA preparation and cDNA amplification efficiency, the mRNA expression was normalized by glyceraldehyde-3-phosphate dehydrogenase. The sequences of the sense and antisense primers used for amplification are listed in Table 1.
Table 1.
RT-PCR primers
| Gene | Sequence | |
|---|---|---|
| SOD | Sense | 5′AGATGACTTGGGCAAAGGTG3′ |
| Antisense | 5′CAATCCCAATCACACCACAA3′ | |
| GSR | Sense | 5′GGAAGTCAACGGGAAGAAGTTCACTG3′ |
| Antisense | 5′CAATGTAACCGGCACCCACAATAAC3′ | |
| CAT | Sense | 5′ACATGGTCTGGGACTTCTGG3′ |
| Antisense | 5′CCATTCGCATTAACCAGCTT3′ | |
| NOX | Sense | 5′ATCTGGGTCTGCAGAGACAT3′ |
| Antisense | 5′CTGAGGTACAGCTGGATGTT3′ | |
| GAPDH | Sense | 5′CCATCACTGCCACTCAGAAGAC3′ |
| Antisense | 5′TCATACTTGGCAGGTTTCTCCA3′ |
Abbreviations: RT-PCR, reverse transcription polymerase chain reaction; SOD, superoxide dismutase; GAPDH, glyceraldehyde-3-phosphatedehydrogenase; CAT, Catalase; NOX, triphosphopyridine nucleotide oxidative enzymes; GSR, glutathione reductase.
Preparation and purification of mitochondria
Two hours after reperfusion, rats were sacrificed under anesthesia and the hearts were removed. An aliquot of the hearts (~150 mg) from left ventricle (~2 mm under ligature) was used for mitochondria isolation using mitochondrial extraction kit (Solarbio), and the remainder samples were quickly frozen in liquid nitrogen. The sample was stored at −80°C for a maximum of 1 week before use. Protein content of the mitochondria was quantified with Coomassie Brilliant Blue (Jianchen, Nanjing, People’s Republic of China), according to the manufacturer’s instructions.
Measuring changes in mitochondrial structure and function
Determination of myocardial mitochondrial Ca2+
To measure myocardial mitochondrial Ca2+, the Fluo-4 AM (2 μM)-loaded purified mitochondria were incubated for 30 minutes at 37°C and then washed for further 30 minutes. The Fluo-4 AM-loaded mitochondria were excited at 494 nm, and the fluorescence emission was collected at 516 nm and then incubated for 30 minutes.
Determination of myocardial mitochondrial ROS
The intramitochondrial ROS level was measured using 2′,7′-dichlorofluorescin diacetate (DCFH-DA) fluorescent probes detection kit. Briefly, purified mitochondria were incubated with DCFH-DA (1 μM) for 30 minutes at 37°C and then washed with phosphate-buffered saline for three times. The DCFH-DA-loaded mitochondria were excited at 488 nm, and the fluorescence emission was collected at 525 nm and then incubated for 30 minutes.
Determination of mitochondrial inner membrane potential (ΔΨm)
Myocardial mitochondrial inner membrane potential was monitored by applying the fluorescence dye JC-1.32 For this purpose, myocardial mitochondria were loaded with JC-1 working solution (JC-1 200×: ultrapure water 5× buffer solution: buffer solution = 1:160:40:800 [V:V]). The loaded mitochondria were excited at 490 nm, and the emitted fluorescence was collected at 590 nm.
Determination of mitochondrial permeability transition pore opening
Mitochondrial permeability transition pore (MPTP) opening was determined by analyzing the mitochondrial calcein leak as previously described. For this purpose, cardiac mitochondria were incubated for 5 minutes with buffer (320 mmol/L sucrose, 10 mmol/L Tris-HCl, 10 mmol/L−1 Tris, pH 7.4) and then added in the swelling solution (120 mmol/L KCl, 20 mmol/L MOPS, 10 mmol/L Tris-HCl, 5 mmol/L KH2PO4, pH 7.4) for further 20 minutes. The optical density at a wavelength of 540 nm was measured. One minute after the measurement, CaCl2 (200 μmol/L) was added and the optical density at a wavelength of 540 nm was monitored continuously for 10 minutes.
Determination of ATP
The content of ATP of myocardial mitochondrial was assessed with ELISA by ATP assay kit (Jianchen), according to the manufacturer’s instruction.
Western blot analysis
Rats were sacrificed under anesthesia at 2 hours after reperfusion, and a piece of approximately 50 mg of myocardial tissue was cut from the surrounding of infarct area of left ventricle and stored at −80°C (n=3). The whole protein was extracted and Radio Immunoprecipitation Assay (RIPA) lysis buffer (Sangon Biotech Co., Ltd., Shanghai, People’s Republic of China) was used. The concentration of whole protein was determined with a Bicinchoninic acid (BCA) protein assay kit (Tiangen Biotech (Beijing) Co., Ltd., Bejing, People’s Republic of China) according to the instructions, and the mean values of concentration were computed. For each sample, the assessment was taken twice, and the values were averaged. Then the samples were preserved at −80°C.
Total proteins were mixed with 3 μL 5× SDS sample buffer. After separation on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (12% gel), the proteins were transferred to polyvinylidene difluoride membrane. After 2-hour blocking with 5% nonfat dry milk or 5% bovine serum albumin (BSA), washing with Tris-HCl buffered saline (TBS)-Tween for three times, 5 minutes each, the membrane with target proteins was cut and incubated overnight at 4°C with primary antibody against cytochrome C (1:1,000) and ATP5D (1:1,000). Afterward, the membranes were rinsed three times, 5 minutes each, and incubated with secondary antibody for 2 hours at room temperature, following rinsing with TBS-Tween five times, 5 minutes for each time. The membranes were developed with ECL reagent, exposed in a dark box, and the protein signal was quantified by scanning densitometry in the X-film using a multifunctional imaging analysis system (VersaDoc MP 5000; Bio-Rad Laboratories Inc., Hercules, CA, USA). The result of each group was expressed as a relative optical density compared with that from the control group.
Statistical analysis
All data were expressed as mean ± SD. Statistical analysis was performed using SPSS 17.0 statistical software. One-way analysis of variance followed by Tukey test for multiple comparisons was used between groups. A value of P<0.05 was considered as statistically significant.
Results
Echocardiography
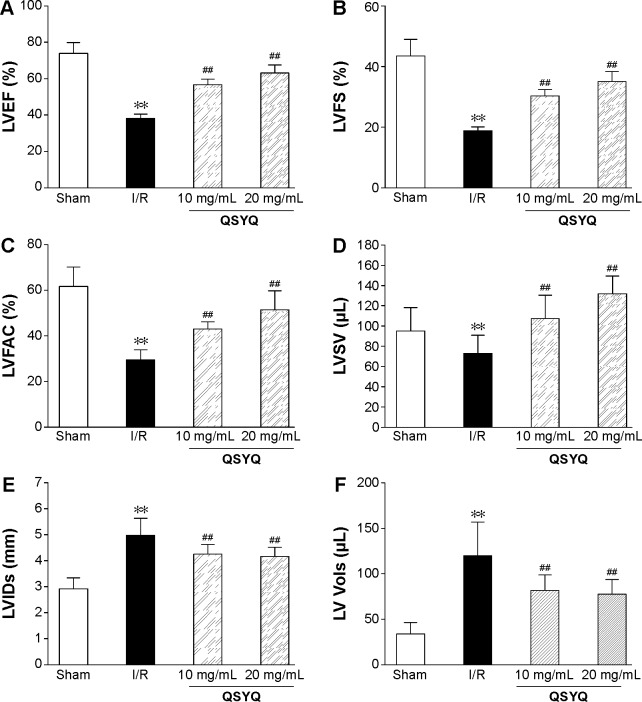
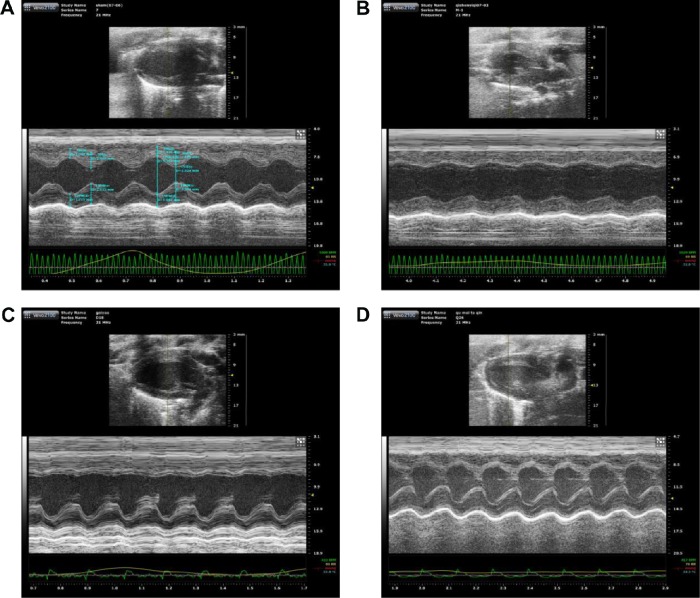
The beneficial effect of QSYQ pretreatment on cardiac insults induced by I/R was confirmed by quantitative analysis of echocardiograms. Results of echocardiography analysis in various groups are summarized in Figure 1. Compared with the Sham group, the I/R group had a significant imaging increase in color Doppler flow LVIDs and systolic volume (Vols). The decrease in FAC%, EF%, FS%, and left ventricular stroke volume (LVSV) in the I/R group was attenuated by QSYQ pretreatment for 1 week. The representative echocardiograms in different groups are presented in Figure 2.
Figure 1.
Echocardiographic characterization of cardiac systolic function in rats subjected to I/R injury.
Notes: Quantitative assessment of dilation and systolic function based on LVEF (A), LVFS (B), LVFAC (C), LVSV (D), LVIDs (E), and LV systolic volume (LV Vols) (F). Data are expressed as mean ± SD from eight animals. **P<0.01 compared with the Sham group; ##P<0.01 compared with the I/R group.
Abbreviations: LVEF, left ventricle ejection fraction; LVFS, left ventricle fractional shortening; LVFAC, left ventricle fraction area change; LVSV; Left ventricular stroke volume; LVID, left ventricular internal diameter; LV, left ventricle; SD, standard deviation; I/R, ischemia/reperfusion; QSYQ, QiShenYiQi Pill®; LVIDs, LV end-systolic inner dimension.
Figure 2.
Representative echocardiographic images (M-mode) in different groups.
Notes: (A) Sham group, (B) I/R group, (C) QSYQ 10 mg/mL +I/R group, and (D) QSYQ 20 mg/mL +I/R group.
Abbreviations: I/R, ischemia/reperfusion; QSYQ, QiShenYiQi Pill®.
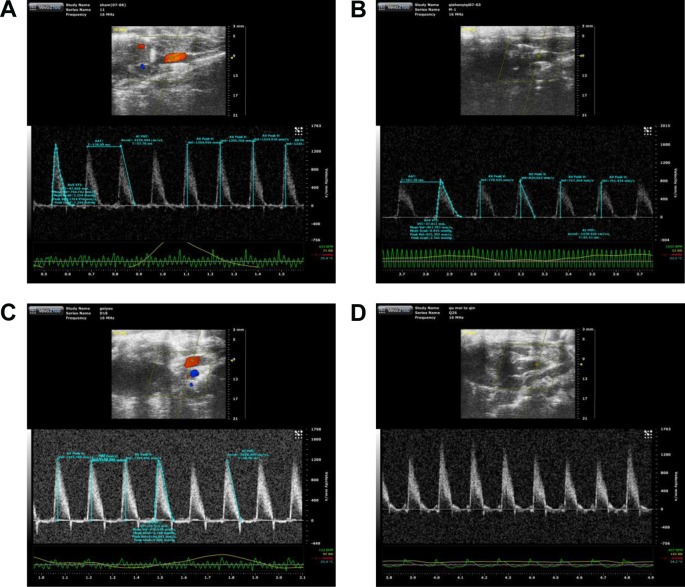
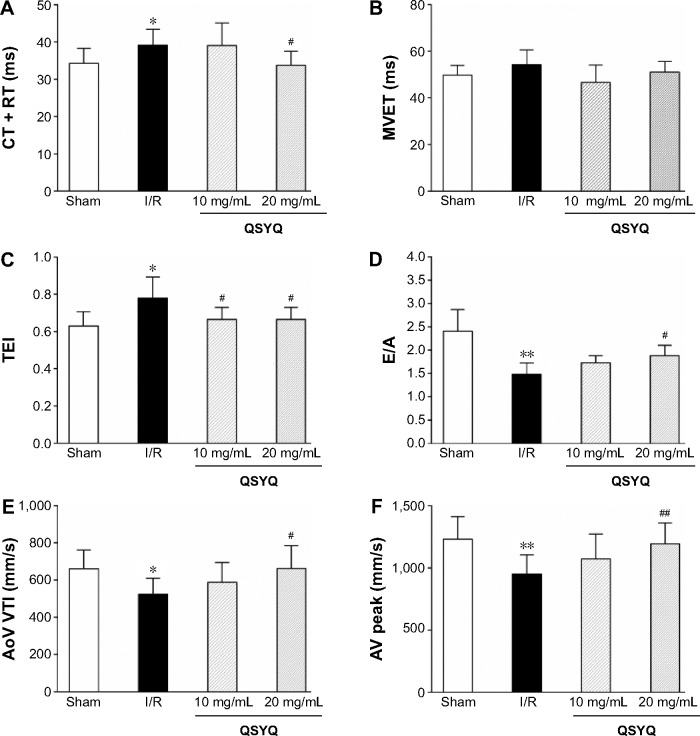
The color images acquired by color Doppler ultrasonography at 2 hours after reperfusion are shown in Figure 3, where the white shadow represents the aortic flow velocity. As expected, the I/R group of maximum blood flow velocity and the velocity time integral was significantly lower than that in the Sham group. However, this situation was improved markedly in the QSYQ (20 mg/mL) group. This result was verified by quantitative evaluation of coronary blood flow as shown in Figure 4. In the I/R group, the ratio of E-wave to A-wave (E/A) index evaluation of left ventricular diastolic function gave rise to an apparent decrease compared to that in the Sham group, and the TEI value, which reflects clinical heart function, was increased significantly compared with the I/R group and Sham group, which was attenuated apparently by pretreatment with QSYQ (Figure 4).
Figure 3.
Representative transmitral valvular flow profiles and the color images acquired by color Doppler ultrasonography at 2 hours after reperfusion, where the white shadow represents the aortic flow velocity.
Notes: (A) Sham group, (B) I/R group, (C) QSYQ 10 mg/mL +I/R group, and (D) QSYQ 20 mg/mL +I/R group.
Abbreviations: I/R, ischemia/reperfusion; QSYQ, QiShenYiQi Pill®.
Figure 4.
Echocardiographic characterization of cardiac diastolic function and coronary blood flow.
Notes: Quantitative assessment of cardiac diastolic function based on isovolumic contraction time plus isovolumic relaxation time (IVCT + IVRT) (A), mitral valve ejection time (MVET) (B), TEI = (IVCT + ICRT)/MVET) (C), and ratio of E-wave to A-wave (E/A) (D); quantitative assessment of coronary blood flow based on aorta velocity time integral mean velocity (AoV VTI) (E) and aortic valve peak velocity (AV peak) (F). Data are expressed as mean ± SD from eight animals. *P<0.05, **P<0.01 compared with the Sham group; #P<0.05, ##P<0.01 compared with the I/R group.
Abbreviations: SD, standard deviation; I/R, ischemia/reperfusion; QSYQ, QiShenYiQi Pill®.
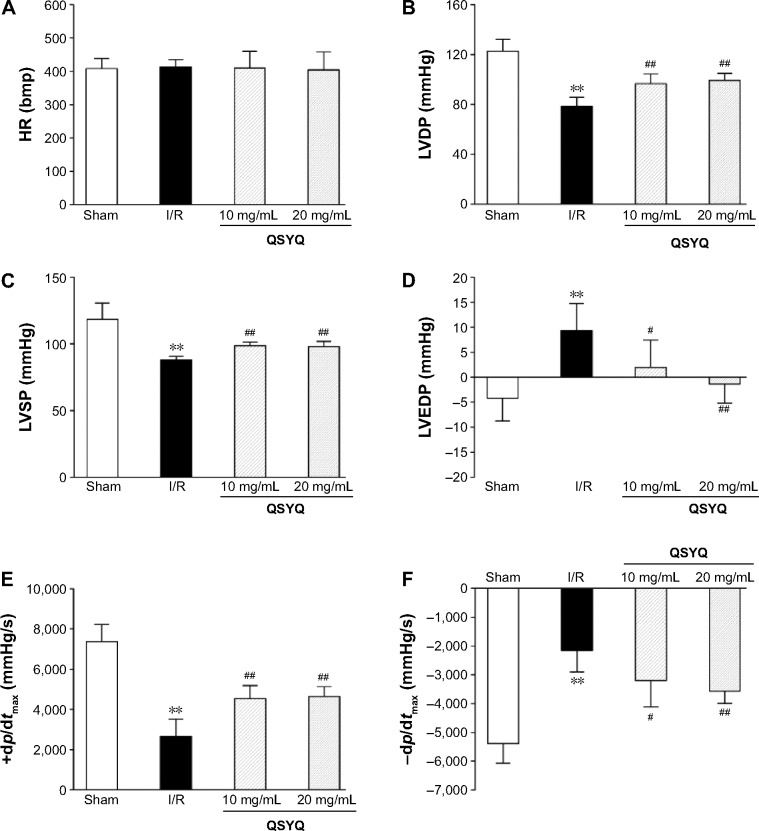
Hemodynamics
Heart function assessment was done in four groups to evaluate the role of QSYQ in preventing heart from I/R injury. As shown in Figure 5, in comparison with the Sham group, I/R caused a significant decline in left ventricular systolic pressure and left ventricular end diastolic pressure and there was an decline in left ventricular development pressure and ±dp/dtmax, indicating an impairment on heart function. Evidently, these impairments were prevented by pretreatment with QSYQ. Meanwhile, no significant difference in HR between the groups was observed.
Figure 5.
Hemodynamic assessment was done to the left ventricle through right carotid artery to evaluate the role of QSYQ pretreatment in preventing heart from I/R injury.
Notes: Quantitative assessment of hemodynamic on cardiac function based on heart rate (HR) (A), LVDP (B), LVSP (C), left ventricular end diastolic pressure (LVEDP) (D), left ventricular maximum upstroke velocity (+dp/dtmax) (E), and left ventricular maximum descent velocity (−dp/dtmax) (F). Data are expressed as mean ± SD from six animals. **P<0.01 compared with the Sham group; #P<0.05, ##P<0.01 compared with the I/R group.
Abbreviations: QSYQ, QiShenYiQi Pill®; I/R, ischemia/reperfusion; HR, heart rate; LVDP, left ventricular development pressure; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end diastolic pressure; SD, standard deviation.
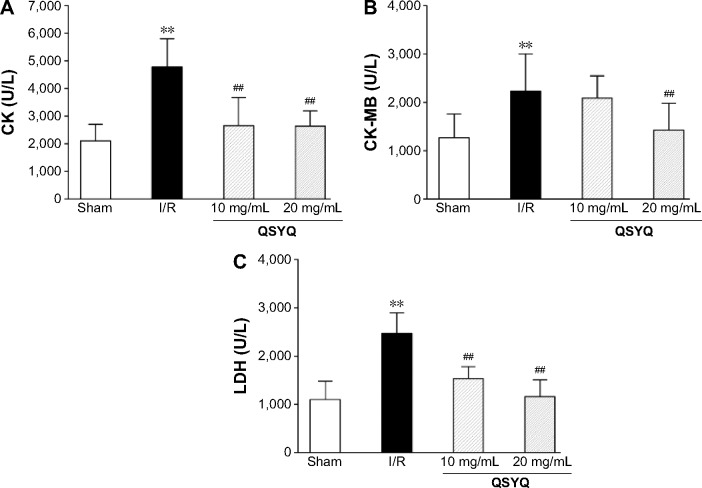
Effects of QSYQ on CK, CK-MB, and LDH
To determine the effect of QSYQ pretreatment on cardiac enzyme activities induced by I/R, CK, CK-MB, and LDH levels were determined by microplate reader (Figure 6). The results showed that as compared to the Sham group, the model group had significantly higher levels of CK, CK-MB, and LDH. Compared to the I/R group, QSYQ 20 mg/mL +I/R group and QSYQ 10 mg/mL +I/R group showed significantly decreased CK and LDH levels, while QSYQ 20 mg/mL +I/R group also showed significantly reduced content of CK-MB but the QSYQ 10 mg/mL +I/R group had markedly no effect on the CK-MB level.
Figure 6.
The effect of QSYQ on the content of CK, CK-MB, and LDH of rat serum.
Notes: Statistical results from ELISA for CK (A), CK-MB (B), and LDH (C). Pretreatment groups were treated with QSYQ for 7 days before operation, 30 minutes before ischemia, and 120 minutes before reperfusion, respectively. Data are expressed as mean ± SD (each group, n=8). **P<0.01 compared with the Sham group; ##P<0.01 compared with the I/R group.
Abbreviations: QSYQ, QiShenYiQi Pill®; CK, creatine kinase; CK-MB, creatine kinase-MB; LDH, lactate dehydrogenase; SD, standard deviation; I/R, ischemia/reperfusion; ELISA, enzyme linked immunosorbent assay.
Effect of QSYQ on I/R-induced changes in energy metabolism
To determine energy metabolism under different conditions, we first measured the content of ATP in heart mitochondria. In comparison to that of the Sham group, the ATP content of rat myocardial mitochondria in I/R group was significantly decreased (Figure 7). The decreased ATP content in the I/R group and Sham group was restored by QSYQ at 20 mg/mL and 10 mg/mL, respectively.
Figure 7.
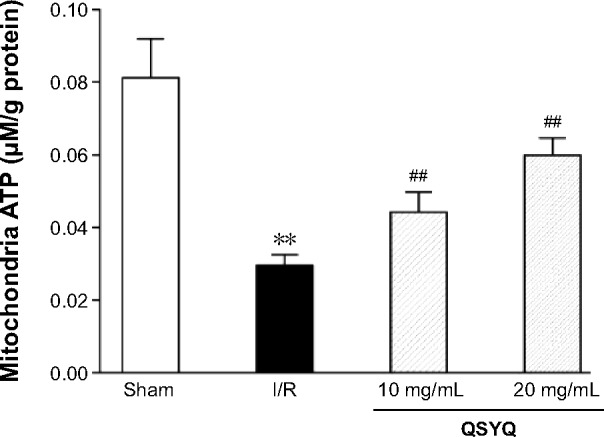
The effect of QSYQ pretreatment on energy metabolism in the myocardium of rats subjected to I/R for 120 minutes.
Notes: Data are expressed as mean ± SD (each group, n=8). **P<0.01 compared with the Sham group; ##P<0.01 compared with the I/R group.
Abbreviations: QSYQ, QiShenYiQi Pill®; I/R, ischemia/reperfusion; SD, standard deviation; ATP, adenosine 5′-triphosphate.
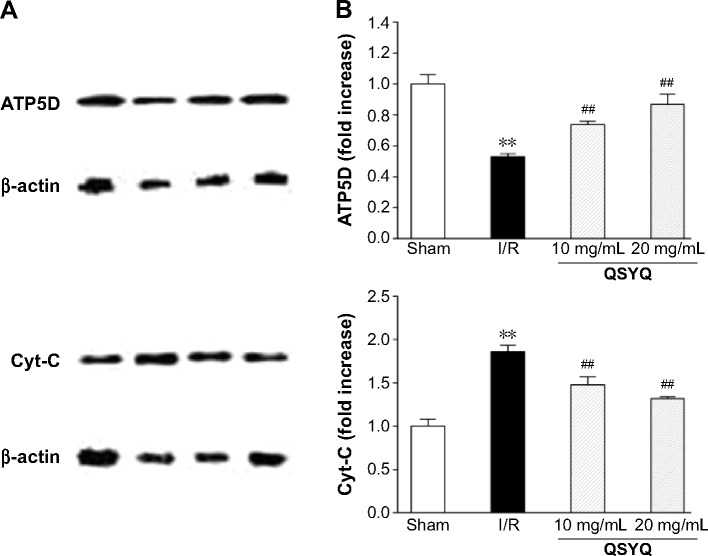
Changes in ATP content correlate to the level of ATP5D expression. Accordingly, ATP5D was reduced significantly in the I/R group, as compared to that in the Sham group, whereas pretreatment with QSYQ for 1 week restored normal ATP5D expression (Figure 8). Likewise, increased cytochrome C levels upon I/R injury were reversed by QSYQ pretreatment (Figure 8).
Figure 8.
Protein expression was measured in adult SD rats pretreated with QSYQ for 7 days and after I/R for120 minutes.
Notes: Representative Western blotting bands (A) and semi-quantitative (B) analysis of ATP5D and Cyt-C in various groups. In (A), each row represents images cropped from different gels probed with different antibodies as shown. Data are expressed as mean ± SD (each group, n=3). **P<0.01 compared with the Sham group; ##P<0.01 compared with the I/R group.
Abbreviations: SD rats, Sprague Dawley rats; QSYQ, QiShenYiQi Pill®; I/R, ischemia/reperfusion; ATP, adenosine 5′-triphosphate; Cyt-C, cytochrome C; SD, standard deviation.
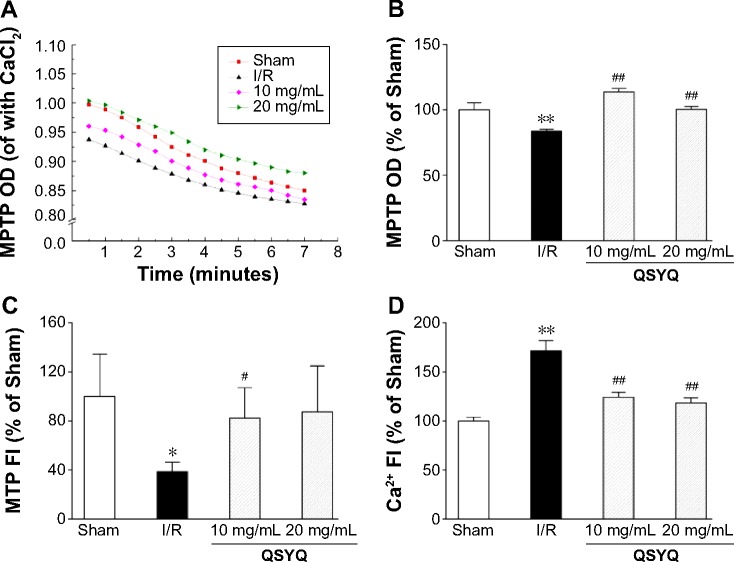
Effect of QSYQ on mitochondrial structure and function
MPTP plays an important role in I/R injury. We examined the extent and speed of MPTP opening. As shown in Figure 9A and B, the extent of MPTP opening in the I/R group was significantly increased as compared to that in the Sham group, either before or after the induction of calcium. Pretreatment with QSYQ significantly attenuated mitochondrial swelling.
Figure 9.
Effect of QSYQ on mitochondrial structure and function in response to I/R-induced injury.
Notes: Myocardial mitochondria was prepared and purified after 120 minutes reperfusion. Quantitative assessment of mitochondrial structure and function was done based on the speed (A) and extent (B) of MPTP opening, MTP (C), and mitochondrial calcium overload (Ca2+) (D). The whole process was completed within 4 hours as described previously (Ref). The linear mixed effect models were analyzed for repeated measurement data, and least squares means were calculated between the groups at different time points. Data are expressed as mean ± SD (each group, n=8). *P<0.05, **P<0.01 compared with the Sham group; #P<0.05, ##P<0.01 compared with the I/R group.
Abbreviations: QSYQ, QiShenYiQi Pill®; I/R, ischemia/reperfusion; MPTP, mitochondrial permeability transition pore; MTP, mitochondrial transmembrane potential; SD, standard deviation; OD, optical density; FI, fluorescence intensity; Ref, reference.
The mitochondrial transmembrane potential (MTP) was examined using the isolated myocardial tissues from each group. In comparison to the Sham group, the MTP in the I/R group was significantly decreased, and the decrease was ablated in the QSYQ 20 mg/mL +I/R group and QSYQ 10 mg/mL +I/R group (Figure 9C). No significant differences in MTP were observed between I/R and QSYQ 20 mg/mL +I/R groups (Figure 9C).
Mitochondrial calcium overload is known to cause MPTP opening. Calcium fluorescence intensity assay showed that I/R challenge dramatically increased calcium fluorescence intensity as compared to Sham treatment. QSYQ pretreatment attenuated calcium fluorescence intensity to the levels of 70.9% (in QSYQ 20 mg/mL +I/R group) and 72.5% (in QSYQ 10 mg/mL +I/R group) in the I/R group (Figure 9D).
Oxidative stress
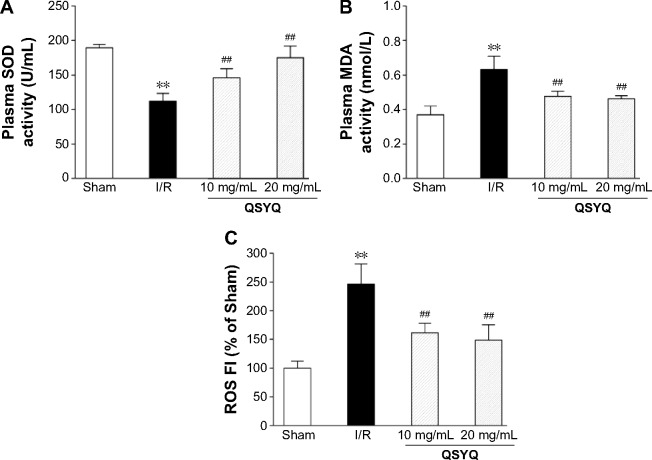
The level of plasma SOD decreased in the I/R group, compared to the Sham group. Pretreatment with QSYQ (20 mg/mL and 10 mg/mL) attenuated I/R-induced decrease in SOD (Figure 10A). The plasma MDA in the I/R group (0.63±0.08) was higher than that in the Sham group (0.37±0.05; P<0.01). In Figure 10B, in the pretreatment with the QSYQ (20 mg/mL and 10 mg/mL) groups, the content of MDA decreased to 0.47±0.04 and 0.48±0.03, respectively (P<0.05). Moreover, fluorescence intensity assay showed that ROS levels in the I/R group were much higher than the Sham group in myocardial mitochondria, but on pretreatment with QSYQ (20 mg/mL and 10 mg/mL), ROS levels significantly decreased compared to the I/R group (Figure 10C).
Figure 10.
Ex vivo characterization of changes in oxidative stress in rats upon I/R-induced injury.
Notes: Quantitative assessment of the antioxidative stress effect of QSYQ was done based on SOD (A), MDA (B) of rat plasma using an ELISA kit following the manufacturer’s instructions (Ref), and the ROS (C) in myocardial mitochondrial using DCFH-DA fluorescent probes detection kit as previously described (Ref). Data are expressed as mean ± SD (each group, n=8). **P<0.01 compared with the Sham group; ##P<0.01 compared with the I/R group.
Abbreviations: I/R, ischemia/reperfusion; QSYQ, QiShenYiQi Pill®; SOD, superoxide dismutase; MDA, malondialdehyde; ROS, reactive oxygen species; DCFH-DA, 2′,7′-dichlorofluorescin diacetate; SD, standard deviation; ELISA, enzyme linked immunosorbent assay; FI, fluorescence intensity.
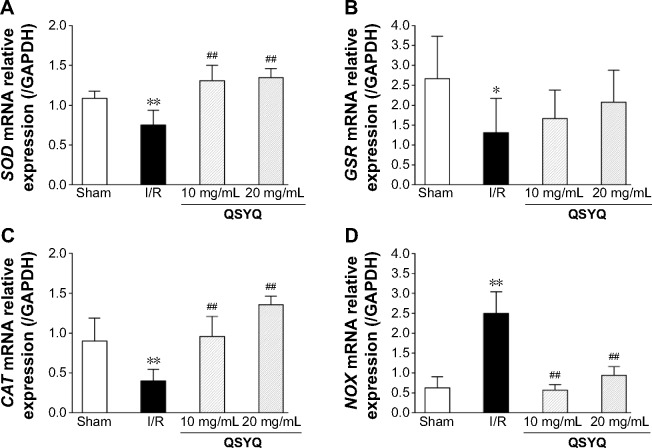
We next quantified SOD, GSH, CAT, and NOX mRNA using reverse transcription polymerase chain reaction (RT-PCR) at the end of QSYQ treatment (Figure 11). The SOD and CAT mRNA levels (Figure 11A and C) were significantly decreased in the I/R group (P<0.01) as compared to the Sham-operated group, while those in QSYQ groups were dramatically higher than the I/R group (P<0.01). There was a significant increase observed in NOX mRNA expression in the I/R group, and the levels were attenuated in QSYQ groups (Figure 11D). Pretreatment of QSYQ upregulated GSR mRNA levels to some extent, but showed no significance when compared to the I/R group (Figure 11B).
Figure 11.
QSYQ regulates expression of SOD, GSR, CAT, and NOX mRNA in rat hearts.
Notes: The relative levels of cardiac SOD (A), GSR (B), CAT (C), and NOX (D) mRNA were assessed by real-time PCR. Results were normalized to GAPDH. Data are expressed as mean ± SD (each group, n=4). *P<0.05, **P<0.01 compared with the Sham group; ##P<0.01 compared with the I/R group.
Abbreviations: QSYQ, QiShenYiQi Pill®; SOD, superoxide dismutase; PCR, polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SD, standard deviation; I/R, ischemia/reperfusion; GSH, glutathione; CAT, Catalase; NOX, triphosphopyridine nucleotide oxidative enzymes; GSR, glutathione reductase.
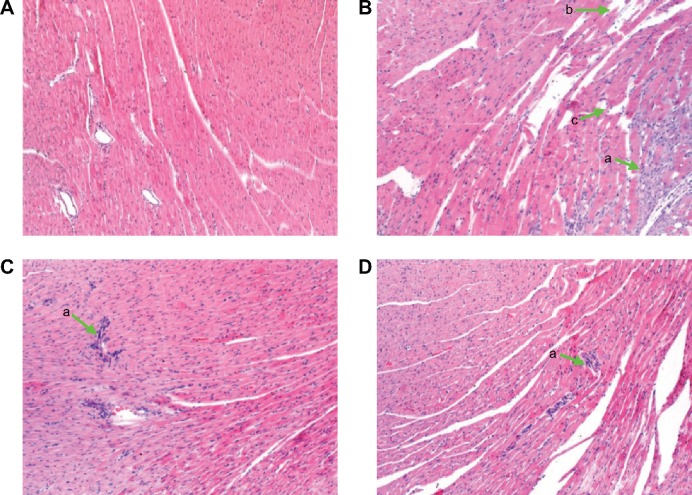
Myocardium histology
In order to directly observe the effect of QSYQ pretreatment on the I/R-induced alteration in myocardium structure, we examined the histology of the left ventricle myocardial tissues (Figure 12). Compared with the Sham group (panel A), distinct alterations occurred in the surrounding infarct areas of the myocardial tissues of the I/R group (panel B), including myocardial interstitial edema, rupture of myocardial fibers, and infiltration of leukocytes. All the I/R-induced injuries were ameliorated by pretreatment with QSYQ (panel C QSYQ 10 mg/mL +I/R group and panel D QSYQ 20 mg/mL +I/R group).
Figure 12.
The effect of QSYQ pretreatment on myocardial histology and myocardial ultrastructure in rats after I/R for 120 minutes.
Notes: Representative photographs of myocardium by HE. (A) Sham group, (B) I/R group, (C) QSYQ 10 mg/mL +I/R group, and (D) QSYQ 20 mg/mL +I/R group. (a) Infiltration of leukocytes, (b) rupture of myocardial fibers, and (c) interstitial edema.
Abbreviations: QSYQ, QiShenYiQi Pill®; I/R, ischemia/reperfusion; HE, hematoxylin–eosin.
Effects of QSYQ on plasma TNF-α and IL-1β
To gain an insight into the effect of QSYQ pretreatment on the I/R-induced inflammation in myocardium tissue, we examined the plasma levels of TNF-α and IL-1β in different groups (Figure 13). Statistically significant difference in TNF-α existed between I/R and the other groups. The respective mean and standard deviation were as follows: Sham group 88.37±20.11, I/R group 177.88±55.49, QSYQ 10 mg/mL +I/R group 87.61±11.28, and QSYQ 20 mg/mL +I/R group 78.47±5.63 (P<0.05). The units of the above means were expressed in picogram per mL of plasma. The ELISA results showed that rats with I/R had plasma TNF-α concentration higher than that in the Sham group (P<0.01); QSYQ 10 mg/mL +I/R group and QSYQ 20 mg/mL +I/R group showed decreased TNF-α levels (P<0.05 and P<0.01, respectively).
Figure 13.
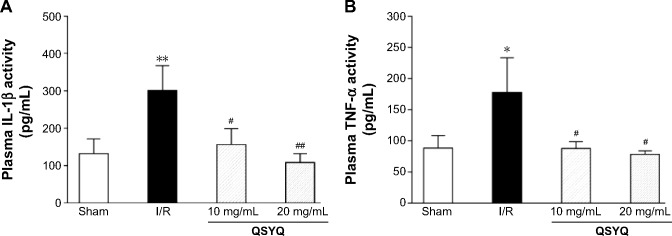
Ex vivo characterization of changes in inflammation in rats upon I/R-induced injury.
Notes: Quantitative assessment of the anti-inflammation effect of QSYQ was done based on IL-1β (A), and TNF-α (B) levels in rat plasma using an ELISA kit following the manufacturer’s instructions (Ref). Data are expressed as mean ± SD (each group, n=8). *P<0.05, **P<0.01 compared with the Sham group; #P<0.05, ##P<0.01 compared with the I/R group.
Abbreviations: I/R, ischemia/reperfusion; QSYQ, QiShenYiQi Pill®; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-alpha; SD, standard deviation; ELISA, enzyme linked immunosorbent assay.
Discussion
Since TCM is composed of multi-active components and possesses multi-target action feature, it can interact with multiple targets and regulate multiple biological pathways at the same time, which has proven to be especially effective in treating complex diseases.23,33 QSYQ has showed its unique advantage in terms of antiplatelet therapy. In this study, we found that pretreatment with QSYQ against MI/R injury via ameliorating multiple mitochondrial dysfunctions, including the reduction of calcium overload, the prevention of the mitochondrial membrane potential collapse, the inhibition of MPTP opening, the decrease in release of cytochrome C, antioxidative stress and improving myocardial energy metabolism.
The therapeutic benefit of QSYQ on various cardiovascular and cerebrovascular diseases begs the questions of underlying mechanisms. The present study analyzed the effect of QSYQ pretreatment on the known factors, including CK, CK-MB, and LDH levels,34 and involved in synergistic anti-inflammatory, antioxidative, and antiapoptotic pathways. The experimental results show that QSYQ pretreatment could effectively improve cardiac hemodynamics, increase coronary blood flow, enhance myocardial contractility, and reduce myocardial reperfusion injury in a rat I/R model.
Oxidative stress, intracellular calcium overload, MPTP opening, and inflammation are important contributing factors in lethal myocardial IR injury.1 Inflammation is closely associated with I/R injury, which is mediated by excessive generation of ROS or oxidative stress.35 Previously, it was reported that posttreatment with QSYQ attenuated myocardial fibrosis in rat cardiac hypertrophy, and suggested that the intervention of inflammatory process played the major role. We measured the levels of pro-inflammatory cytokines and found that I/R resulted in a significant increase in IL-1β and TNF-α levels and QSYQ pretreatment attenuated inflammatory response as well as I/R-induced leukocyte infiltration.
On the other hand, oxidative stress mediators, such as ROS released by inflammatory cells around the infarct areas, also suggested to play a critical role in MI/R injury;36 high concentrations of ROS can inhibit cell proliferation and induce apoptosis.37 A number of antioxidant enzymes are responsible for the removal of excess ROS in the living organism. Among these, SOD is the most crucial enzyme in the cellular antioxidant system.38 Furthermore, as an important product of lipid peroxidation, MDA also indirectly reflects the production of intracellular ROS.39 Results of the present study showed that QSYQ could significantly inhibit I/R-induced oxidative stress and ROS, thus contributing to the attenuation of I/R injury. To further support our findings, expression of oxidative stress-associated genes, such as SOD, GSH, CAT, and NOX, was determined by RT-PCR. In myocardial tissue that underwent I/R, we found that expression of SOD was significantly enhanced and NOX expression was markedly reduced on treatment with QSYQ. Thus, the protective effect of QSYQ pretreatment may be achieved through upregulation of SOD and CAT and reduction of NOX gene expression and the subsequent inhibition of oxidative stress.
Energy metabolism plays a vital role in the pathogenesis of I/R injury. Clearly, ATP generation is the most important role of mitochondria, especially in the heart. Because the heart requires a continuous supply of energy throughout life, cardiomyocytic mitochondria are densely packed to form a complex structure accounting for 35% of cardiac muscle cell volume.40 In the present study, we found that QSYQ not only has effects of antioxidant activity as mentioned earlier but could also improve myocardial energy metabolism and thus prevent I/R injury. Pretreatment with QSYQ can significantly inhibit myocardial intracellular ATP depletion. Many studies have indicated that mitochondrial dynamics may be a fundamental component to maintain normal cellular homeostasis and cardiomyocyte contractility. Some studies have suggested that altered mitochondrial morphology is directly involved in the detriment to cardiac function under stress.41,42 Mitochondria modulate cardiomyocyte contractility by supplying ATP and participating in calcium homeostasis. The outcomes of I/R injury are excessive production of ROS, calcium overload in the mitochondria, matrix dissipating, and the membrane potential collapsing and opening the MPTP, which lead to uncoupling of oxidative phosphorylation and further production of ROS. As a result, ATP will be depleted and mitochondrial rupture is evident.43,44 In our study, QSYQ protecting mitochondrial morphology and function and regulation of the mitochondrial dynamics demonstrate the beneficial effects on cardiac performance after I/R injury.43,44
The evidence from recent studies of Prof JY Han’s group indicates that synthetic barriers in one of the ATP synthase subunits, ATP5D, may participate in depleting ATP during I/R, whereas this disorder is presumably prevented by QSYQ pretreatment. Our findings also support the hypothesis. Indeed, our findings are in agreement with a number of studies that suggest that QSYQ can restrain the decrease of ATP and ATP5D.45 Moreover, our data also show that QSYQ was able to significantly inhibit cardiac mitochondrial calcium overload caused by I/R injury and prohibit the collapse of the membrane potential (ΔΨm) and MPTP opening, thereby reducing the release of cytochrome C, which in turn reduces further injury on cardiac cells, thereby inhibiting generation of ROS. This can promote the generation of ATP and inhibit F0F1-ATPase hydrolysis of ATP. The detailed mechanism of QSYQ protective effect on mitochondria remains to be clarified. Nonetheless, the finding of the present study may open a potentially novel avenue for developing therapy to deal with the cardiac I/R injury.
Mitochondria are typically regarded as energy generators, but the latest data demonstrate other divergent functions such as oxygen free radical production, control of cell ion homeostasis, and regulation of cell apoptosis and necrosis.46 Previously, we have demonstrated that QSYQ can significantly inhibit the generation of ROS. Moreover, some additional benefits of QSYQ were observed in the present study, including diminishing the expression of cytochrome C. Obviously, protection of the cardiomyocytes from apoptosis contributes to the attenuation effect of QSYQ on myocardial infarction via reduction of cytochrome C, while QSYQ may improve myocardial contractility partly through preservation of ATP and calcium homeostasis.
In summary, the current study has demonstrated that pretreatment with QSYQ could protect cardiomyocytes against I/R injury, which may be associated with its potential to protect the mitochondrial function and expression of ATP5D. The results suggest that QSYQ probably by improving cardiac hemodynamics, inhibition of inflammation and myocardial apoptosis, protection of myocardial mitochondrial morphology and function thereby improving myocardial energy metabolism are multi-target and multi-pathway in which QSYQ protect against I/R injury. The fact that QSYQ could protect the heart from injury by I/R needs further clarification, and more studies, particularly using lager animals, are required to verify the feasibility for QSYQ application in clinic.
Acknowledgments
The authors thank Professor Zemin Yao for proofreading this manuscript. This work was supported by grant from the National Key Basic Research Program of China (973 Program) (No 2012CB518404), the National Science Fund for Distinguished Young Scholars (81125024), and the National Natural Science Foundation of China (81273993, 81273891).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Zhong X, Li X, Qian L, et al. Glycine attenuates myocardial ischemia-reperfusion injury by inhibiting myocardial apoptosis in rats. J Biomed Res. 2012;26(5):346–354. doi: 10.7555/JBR.26.20110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piper HM, Garcia-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38(2):291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosio G, Tritto I. Reperfusion injury: experimental evidence and clinical implications. Am Heart J. 1999;138(2 pt 2):S69–S75. doi: 10.1016/s0002-8703(99)70323-6. [DOI] [PubMed] [Google Scholar]
- 5.Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81(5):637–647. doi: 10.1002/bjs.1800810504. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61(3):427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Abd-Elfattah AS, Jessen ME, Hanan SA, Tuchy G, Wechsler AS. Is adenosine 5′-triphosphate derangement or free-radical-mediated injury the major cause of ventricular dysfunction during reperfusion? Role of adenine nucleoside transport in myocardial reperfusion injury. Circulation. 1990;82(5 suppl):IV341–IV350. [PubMed] [Google Scholar]
- 8.Deng C, Sun Z, Tong G, et al. alpha-Lipoic acid reduces infarct size and preserves cardiac function in rat myocardial ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2 pathway. PLoS One. 2013;8(3):e58371. doi: 10.1371/journal.pone.0058371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhang H, Zhang C. Role of inflammation in the regulation of coronary blood flow in ischemia and reperfusion: mechanisms and therapeutic implications. J Mol Cell Cardiol. 2012;52(4):865–872. doi: 10.1016/j.yjmcc.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb RA. Cell death pathways in acute ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther. 2011;16(3–4):233–238. doi: 10.1177/1074248411409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenitzer JR, Freeman BA. Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci. 2010;1203:45–52. doi: 10.1111/j.1749-6632.2010.05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakshmi SV, Padmaja G, Kuppusamy P, Kutala VK. Oxidative stress in cardiovascular disease. Indian J Biochem Biophys. 2009;46(6):421–440. [PubMed] [Google Scholar]
- 13.Liou SF, Ke HJ, Hsu JH, et al. San-Huang-Xie-Xin-Tang prevents rat hearts from ischemia/reperfusion-induced apoptosis through eNOS and MAPK pathways. Evid Based Complement Alternat Med. 2011;2011:915051. doi: 10.1093/ecam/neq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosio G, Becker LC, Hutchins GM, Weisman HF, Weisfeldt ML. Reduction in experimental infarct size by recombinant human superoxide dismutase: insights into the pathophysiology of reperfusion injury. Circulation. 1986;74(6):1424–1433. doi: 10.1161/01.cir.74.6.1424. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima S, Coppen SR, Varela-Carver A, et al. A novel strategy for myocardial protection by combined antibody therapy inhibiting both P-selectin and intercellular adhesion molecule-1 via retrograde intracoronary route. Circulation. 2006;114(1 suppl):I251–I256. doi: 10.1161/CIRCULATIONAHA.105.000794. [DOI] [PubMed] [Google Scholar]
- 16.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia–reperfusion injury. Cardiovasc Res. 1999;43(4):860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 17.Arslan F, Smeets MB, O’Neill LA, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121(1):80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhang X, Ren XP, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122(13):1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolli R, Becker L, Gross G, et al. NHLBI Working Group on the Translation of Therapies for Protecting the Heart from Ischemia Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95(2):125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 20.Boran AD, Iyengar R. Systems approaches to polypharmacology and drug discovery. Curr Opin Drug Discov Devel. 2010;13(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhao X, Gao X, Nie X, Yang Y, Fan X. Development of fluorescence imaging-based assay for screening cardioprotective compounds from medicinal plants. Anal Chim Acta. 2011;702(1):87–94. doi: 10.1016/j.aca.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Hou YZ, Wang S, Zhao ZQ, et al. Clinical assessment of complementary treatment with Qishen Yiqi dripping pills on ischemic heart failure: study protocol for a randomized, double-blind, multicenter, placebo- controlled trial (CACT-IHF) Trials. 2013;14:138. doi: 10.1186/1745-6215-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu J, Yuan G, Zhu Y, Xu X. Computational pharmacological studies on cardiovascular disease by Qishen Yiqi Diwan. Sci China B Chem. 2009;52(11):1871–1878. [Google Scholar]
- 24.Xie DX, Huang XZ, Mao BY. Mechanisms of QiShenYiQi dropping pills on ventricular remodeling after myocardial infarction of rat model. Chin J Exp Tradit Med Formulae. 2011;6(17):180–183. [Google Scholar]
- 25.Song YZ, Guo LP, Shang H, Wang J. Effects of QiShenYiQi dripping pills on lipid metabolism in experimental hypercholesterolemia rabbits. Jilin J Tradit Chin Med. 2011;1(31):71–73. [Google Scholar]
- 26.Li YC, Liu YY, Hu BH, et al. Attenuating effect of post-treatment with QiShen YiQi Pills on myocardial fibrosis in rat cardiac hypertrophy. Clin Hemorheol Microcirc. 2012;51(3):177–191. doi: 10.3233/CH-2011-1523. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Wang Y, Yu L, et al. QI-SHEN-YI-QI accelerates angiogenesis after myocardial infarction in rats. Int J Cardiol. 2010;143(1):105–109. doi: 10.1016/j.ijcard.2008.11.210. [DOI] [PubMed] [Google Scholar]
- 28.Zhang BL, Su Y, Gao XM, Dong ZL, Xu ZP, Wang XH. Clinical and experimental research of compound huangqi danshen granule on treating angina pectoris. TCM Tian Jin. 2002;4(19):12–15. [Google Scholar]
- 29.Zhao N, Liu YY, Wang F, et al. Cardiotonic pills, a compound Chinese medicine, protects ischemia–reperfusion-induced microcirculatory disturbance and myocardial damage in rats. Am J Physiol Heart Circ Physiol. 2010;298(4):H1166–H1176. doi: 10.1152/ajpheart.01186.2009. [DOI] [PubMed] [Google Scholar]
- 30.Ai D, Pang W, Li N, et al. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106(2):564–569. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SQ, Wei XH, Huang P, et al. QiShenYiQi Pills(R) prevent cardiac ischemia–reperfusion injury via energy modulation. Int J Cardiol. 2013;168(2):967–974. doi: 10.1016/j.ijcard.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Di Lisa F, Blank PS, Colonna R, et al. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486(pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Wu L, Liu W, et al. A network pharmacology study of Chinese medicine QiShenYiQi to reveal its underlying multi-compound, multi-target, multi-pathway mode of action. PLoS One. 2014;9(5):e95004. doi: 10.1371/journal.pone.0095004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108(1):150–158. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Yu Y, Ji L, Liang X, Zhang T, Hai CX. Alpha-lipoic acid protects against myocardial ischemia/reperfusion injury via multiple target effects. Food Chem Toxicol. 2011;49(11):2750–2757. doi: 10.1016/j.fct.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 36.Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia–reperfusion injury. Curr Med Chem. 2008;15(1):1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- 37.Stanciu M, Wang Y, Kentor R, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275(16):12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- 38.Bora KS, Sharma A. Evaluation of antioxidant and cerebroprotective effect of Medicago sativa Linn. against ischemia and reperfusion insult. Evid Based Complement Alternat Med. 2011;2011:792167. doi: 10.1093/ecam/neq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin F, Liu YX, Zhao HW, Huang X, Ren P, Zhu ZY. Chinese medicinal formula Guan-Xin-Er-Hao protects the heart against oxidative stress induced by acute ischemic myocardial injury in rats. Phytomedicine. 2009;16(2–3):215–221. doi: 10.1016/j.phymed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Page E, McCallister LP. Quantitative electron microscopic description of heart muscle cells. Application to normal, hypertrophied and thyroxin-stimulated hearts. Am J Cardiol. 1973;31(2):172–181. doi: 10.1016/0002-9149(73)91030-8. [DOI] [PubMed] [Google Scholar]
- 41.Papanicolaou KN, Khairallah RJ, Ngoh GA, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31(6):1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piquereau J, Caffin F, Novotova M, et al. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res. 2012;94(3):408–417. doi: 10.1093/cvr/cvs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol. 2003;35(4):339–341. doi: 10.1016/s0022-2828(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 44.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46(6):850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang DX, Zhao HP, Pan CS, et al. QiShenYiQi pills, a compound Chinese medicine, ameliorates doxorubicin-induced myocardial structure damage and cardiac dysfunction in rats. Evid Based Complement Alternat Med. 2013;2013:480597. doi: 10.1155/2013/480597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95(10):957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]