Abstract
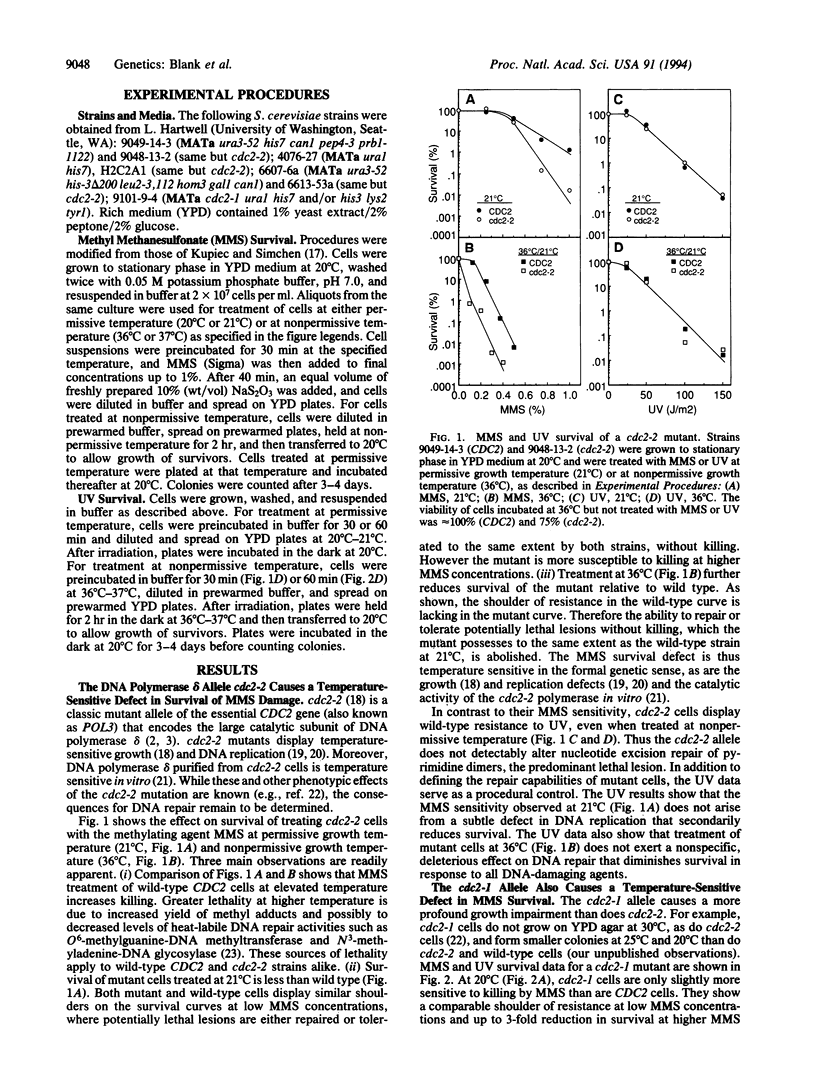
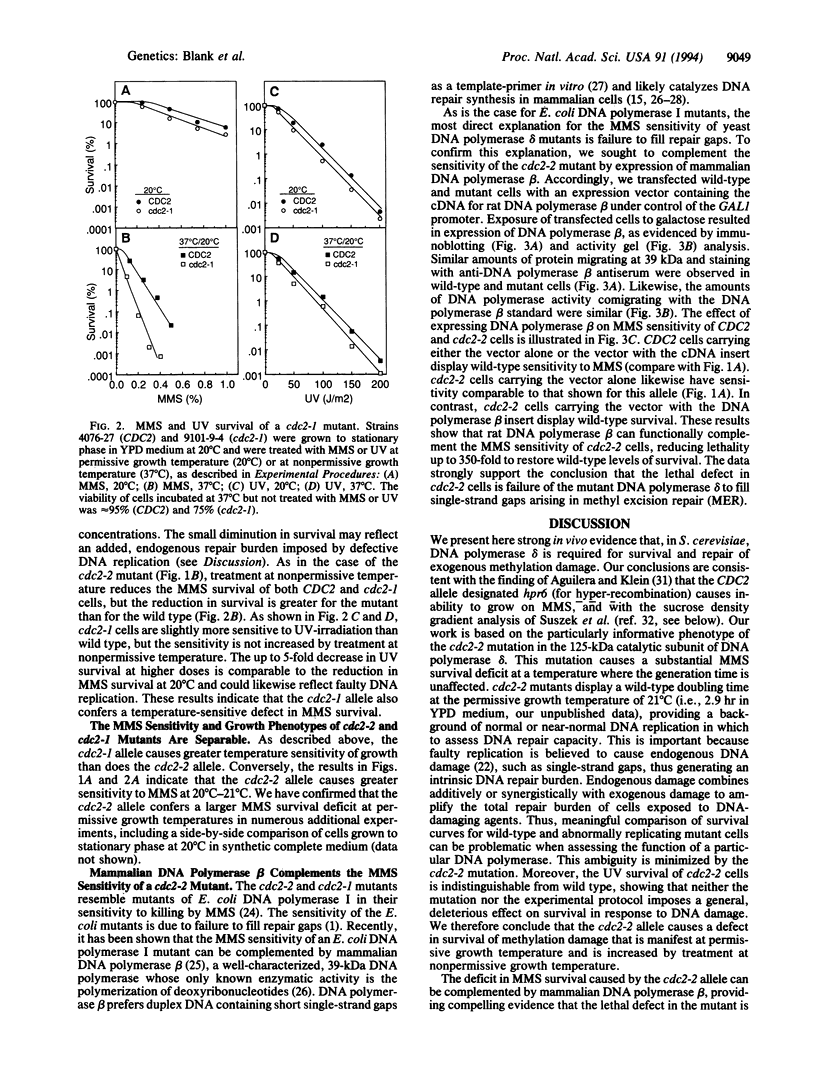
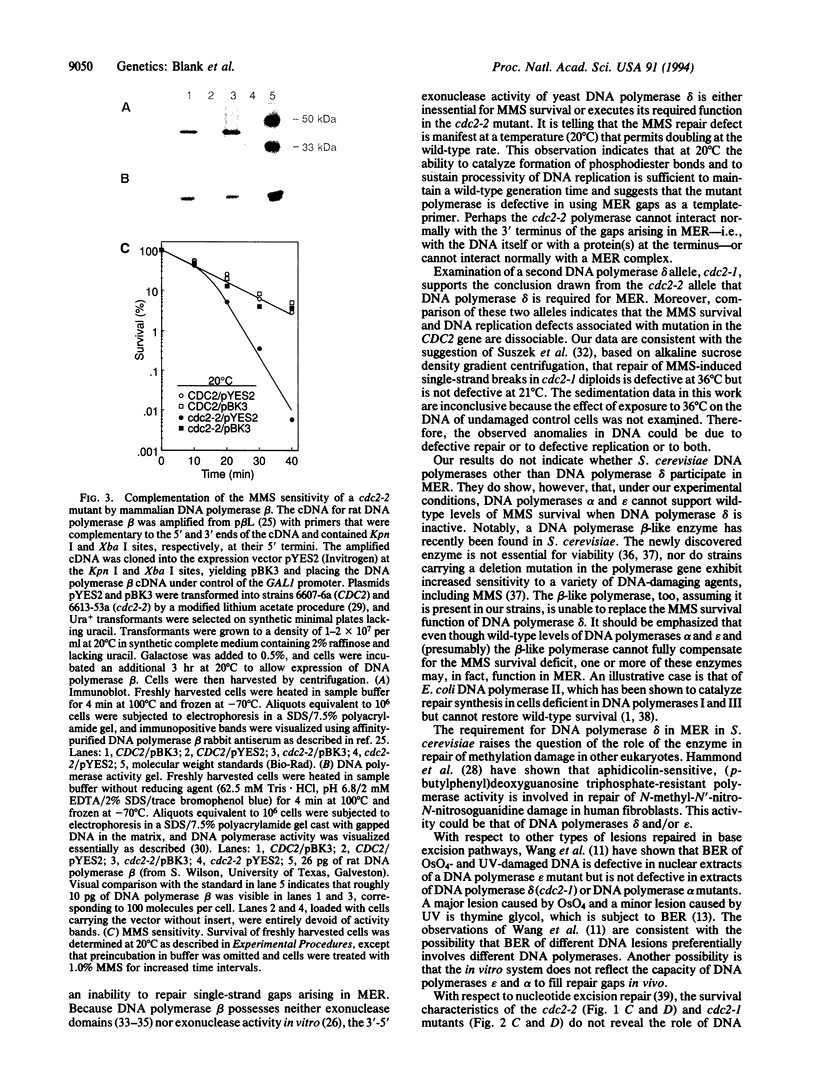
We present evidence that DNA polymerase delta of Saccharomyces cerevisiae, an enzyme that is essential for viability and chromosomal replication, is also required for base excision repair of exogenous DNA methylation damage. The large catalytic subunit of DNA polymerase delta is encoded by the CDC2(POL3) gene. We find that the mutant allele cdc2-2 confers sensitivity to killing by methyl methanesulfonate (MMS) but allows wild-type levels of UV survival. MMS survival of haploid cdc2-2 strains is lower than wild type at the permissive growth temperature of 20 degrees C. Survival is further decreased relative to wild type by treatment with MMS at 36 degrees C, a nonpermissive temperature for growth of mutant cells. A second DNA polymerase delta allele, cdc2-1, also confers a temperature-sensitive defect in MMS survival while allowing nearly wild-type levels of UV survival. These observations provide an in vivo genetic demonstration that a specific eukaryotic DNA polymerase is required for survival of exogenous methylation damage. MMS sensitivity of a cdc2-2 mutant at 20 degrees C is complemented by expression of mammalian DNA polymerase beta, an enzyme that fills single-strand gaps in duplex DNA in vitro and whose only known catalytic activity is polymerization of deoxyribonucleotides. We conclude, therefore, that the MMS survival deficit in cdc2-2 cells is caused by failure of mutant DNA polymerase delta to fill single-strand gaps arising in base excision repair of methylation damage. We discuss our results in light of current concepts of the physiologic roles of DNA polymerases delta and epsilon in DNA replication and repair.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbotts J., SenGupta D. N., Zmudzka B., Widen S. G., Notario V., Wilson S. H. Expression of human DNA polymerase beta in Escherichia coli and characterization of the recombinant enzyme. Biochemistry. 1988 Feb 9;27(3):901–909. doi: 10.1021/bi00403a010. [DOI] [PubMed] [Google Scholar]
- Aguilera A., Klein H. L. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics. 1988 Aug;119(4):779–790. doi: 10.1093/genetics/119.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Blasco M. A., Salas M. A general structure for DNA-dependent DNA polymerases. Gene. 1991 Apr;100:27–38. doi: 10.1016/0378-1119(91)90346-d. [DOI] [PubMed] [Google Scholar]
- Blank A., Loeb L. A. Isolation of temperature-sensitive DNA polymerase III from Saccharomyces cerevisiae cdc2-2. Biochemistry. 1991 Aug 13;30(32):8092–8096. doi: 10.1021/bi00246a030. [DOI] [PubMed] [Google Scholar]
- Budd M. E., Campbell J. L. DNA polymerases delta and epsilon are required for chromosomal replication in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):496–505. doi: 10.1128/mcb.13.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases delta and epsilon. J Biol Chem. 1991 Nov 25;266(33):22698–22706. [PubMed] [Google Scholar]
- Chen J., Derfler B., Samson L. Saccharomyces cerevisiae 3-methyladenine DNA glycosylase has homology to the AlkA glycosylase of E. coli and is induced in response to DNA alkylation damage. EMBO J. 1990 Dec;9(13):4569–4575. doi: 10.1002/j.1460-2075.1990.tb07910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M. N., Newlon C. S. Saccharomyces cerevisiae cdc2 mutants fail to replicate approximately one-third of their nuclear genome. Mol Cell Biol. 1983 Jun;3(6):1000–1012. doi: 10.1128/mcb.3.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianov G., Price A., Lindahl T. Generation of single-nucleotide repair patches following excision of uracil residues from DNA. Mol Cell Biol. 1992 Apr;12(4):1605–1612. doi: 10.1128/mcb.12.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Kelley W. S. Effects of different alleles of the E. coli K12 pol A gene on the replication of non-transferring plasmids. Mol Gen Genet. 1976 Feb 2;143(3):311–318. doi: 10.1007/BF00269409. [DOI] [PubMed] [Google Scholar]
- Hammond R. A., McClung J. K., Miller M. R. Effect of DNA polymerase inhibitors on DNA repair in intact and permeable human fibroblasts: evidence that DNA polymerases delta and beta are involved in DNA repair synthesis induced by N-methyl-N'-nitro-N-nitrosoguanidine. Biochemistry. 1990 Jan 9;29(1):286–291. doi: 10.1021/bi00453a039. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Mol Biol. 1976 Jul 15;104(4):803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985 Jul;110(3):381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec M., Simchen G. DNA-repair characterization of cdc40-1, a cell-cycle mutant of Saccharomyces cerevisiae. Mutat Res. 1986 Aug;162(1):33–40. doi: 10.1016/0027-5107(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Lifsics M. R., Lancy E. D., Jr, Maurer R. DNA replication defect in Salmonella typhimurium mutants lacking the editing (epsilon) subunit of DNA polymerase III. J Bacteriol. 1992 Nov;174(21):6965–6973. doi: 10.1128/jb.174.21.6965-6973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W., Hanawalt P., Shizuya H. Role of DNA polymerase II in repair replication in Escherichia coli. Nat New Biol. 1973 Aug 22;244(138):242–243. doi: 10.1038/newbio244242a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Bogenhagen D. F. Repair of a synthetic abasic site involves concerted reactions of DNA synthesis followed by excision and ligation. Mol Cell Biol. 1991 Sep;11(9):4441–4447. doi: 10.1128/mcb.11.9.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- Morrison A., Bell J. B., Kunkel T. A., Sugino A. Eukaryotic DNA polymerase amino acid sequence required for 3'----5' exonuclease activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9473–9477. doi: 10.1073/pnas.88.21.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A., Sugino A. DNA polymerase II, the epsilon polymerase of Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol. 1993;46:93–120. doi: 10.1016/s0079-6603(08)61019-3. [DOI] [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Podust V. N., Hübscher U. Lagging strand DNA synthesis by calf thymus DNA polymerases alpha, beta, delta and epsilon in the presence of auxiliary proteins. Nucleic Acids Res. 1993 Feb 25;21(4):841–846. doi: 10.1093/nar/21.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R., Widen S. G., Singhal R. K., Watkins J., Prakash L., Wilson S. H. Yeast open reading frame YCR14C encodes a DNA beta-polymerase-like enzyme. Nucleic Acids Res. 1993 Nov 25;21(23):5301–5307. doi: 10.1093/nar/21.23.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sassanfar M., Samson L. Identification and preliminary characterization of an O6-methylguanine DNA repair methyltransferase in the yeast Saccharomyces cerevisiae. J Biol Chem. 1990 Jan 5;265(1):20–25. [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Santocanale C., Ropp P. A., Longhese M. P., Plevani P., Lucchini G., Sugino A. Purification and characterization of a new DNA polymerase from budding yeast Saccharomyces cerevisiae. A probable homolog of mammalian DNA polymerase beta. J Biol Chem. 1993 Dec 25;268(36):27148–27153. [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Singhal R. K., Wilson S. H. Short gap-filling synthesis by DNA polymerase beta is processive. J Biol Chem. 1993 Jul 25;268(21):15906–15911. [PubMed] [Google Scholar]
- Suszek W., Baranowska H., Zuk J., Jachymczyk W. J. DNA polymerase III is required for DNA repair in Saccharomyces cerevisiae. Curr Genet. 1993 Sep;24(3):200–204. doi: 10.1007/BF00351792. [DOI] [PubMed] [Google Scholar]
- Sweasy J. B., Loeb L. A. Detection and characterization of mammalian DNA polymerase beta mutants by functional complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4626–4630. doi: 10.1073/pnas.90.10.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweasy J. B., Loeb L. A. Mammalian DNA polymerase beta can substitute for DNA polymerase I during DNA replication in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1407–1410. [PubMed] [Google Scholar]
- Turchi J. J., Bambara R. A. Completion of mammalian lagging strand DNA replication using purified proteins. J Biol Chem. 1993 Jul 15;268(20):15136–15141. [PubMed] [Google Scholar]
- Waga S., Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994 May 19;369(6477):207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wu X., Friedberg E. C. DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Feb;13(2):1051–1058. doi: 10.1128/mcb.13.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. R., Jiang Y., Zhang S. J., Hao H., Lee M. Y. DNA polymerase delta is involved in the cellular response to UV damage in human cells. J Biol Chem. 1994 May 13;269(19):13748–13751. [PubMed] [Google Scholar]



