Abstract
Microsphere-based polymeric tissue-engineered scaffolds offer the advantage of shape-specific constructs with excellent spatiotemporal control and interconnected porous structures. The use of these highly versatile scaffolds requires a method to sinter the discrete microspheres together into a cohesive network, typically with the use of heat or organic solvents. We previously introduced subcritical CO2 as a sintering method for microsphere-based scaffolds; here we further explored the effect of processing parameters. Gaseous or subcritical CO2 was used for making the scaffolds, and various pressures, ratios of lactic acid to glycolic acid in poly(lactic acid-co-glycolic acid), and amounts of NaCl particles were explored. By changing these parameters, scaffolds with different mechanical properties and morphologies were prepared. The preferred range of applied subcritical CO2 was 15–25 bar. Scaffolds prepared at 25 bar with lower lactic acid ratios and without NaCl particles had a higher stiffness, while the constructs made at 15 bar, lower glycolic acid content, and with salt granules had lower elastic moduli. Human umbilical cord mesenchymal stromal cells (hUCMSCs) seeded on the scaffolds demonstrated that cells penetrate the scaffolds and remain viable. Overall, the study demonstrated the dependence of the optimal CO2 sintering parameters on the polymer and conditions, and identified desirable CO2 processing parameters to employ in the sintering of microsphere-based scaffolds as a more benign alternative to heat-sintering or solvent-based sintering methods.
Keywords: microsphere-based scaffolds, subcritical CO2, PLGA, human umbilical cord mesenchymal stromal cells
INTRODUCTION
Microspheres have long been an ingredient in scaffold fabrication strategies, but more recently have incited interest as the sole building block of scaffolds given the versatility they afford the scaffold in terms of 3D shape, high-resolution spatiotemporal control over mechanical integrity and growth factor release, and pore interconnectivity.1–4 Microsphere-based scaffolds have many proven merits, such as ease of fabrication, the aforementioned control over varying spatial composition and spatiotemporal release of bioactive signals, and physicochemical characteristics.5,6 Furthermore, the microsphere-based scaffold properties can be modulated by modifying the microsphere composition, size, and fabrication method. The most crucial process that microsphere-based scaffolds have in common is the need to sinter the microspheres into a single, cohesive structure. Heat7–11 or ethanol5,12,13 (or 5% acetone in ethanol for some nanocomposites) 14,15 have traditionally been used to join microspheres together, and approaches to compare various solvents such as acetone and tetrahydrofuran (together with hexane as a non-solvent) have been investigated as well for sintering microspheres.16 However, CO2 may be an attractive alternative for sintering these scaffolds.
Supercritical and subcritical CO2 have been widely used in the processing of polymeric materials. The critical point of CO2 is 31.1°C and 73.8 bar. The degree of plasticization and swelling of polymers, and consequently their free volume, can be controlled simply by changing the pressure (leading to increased CO2 solubility), exposure duration, and release rate of the CO2. The relatively low temperature of the process and the stability of CO2 also allow for minimal damage or denaturing to processed compounds. An advantage of using the CO2 as a plasticizer is that it is easily removed from the processed polymers (i.e., lyophilization may not be required, unlike with ethanol sintering). Furthermore, CO2 is seen as a promising green solvent because it has low toxicity and low environmental impact when used from non-sequestered sources.17–19
The traditional method using CO2 for making tissue-engineered scaffolds is a gas-foaming technique. However, a significant disadvantage of gas-foaming techniques to make a porous scaffold is that constructs made by this method may suffer from a closed pore structure. Sometimes very high pressures are used to create pore interconnectivity by sheer virtue of extremely high porosities, but this may lead to drawbacks in mechanical integrity as well as inappropriate pore sizes for cell adhesion and growth. In this study, we plasticized the surfaces of microspheres and sintered them into interconnective porous scaffolds by reducing the applied CO2 pressure and exposure time. In an attempt to increase the porosity with this sintering approach, NaCl was included with the microspheres during the sintering step.11 The objective of this research was to investigate the mechanical integrity and cell compatibility of poly(lactic-co-glycolic acid) (PLGA) microsphere-based porous scaffold systems using gaseous or subcritical CO2 for tissue engineering.
MATERIALS AND METHODS
Materials
PLGA (50:50, 75:25, and 85:15 lactic acid:glycolic acid; intrinsic viscosity 0.34–0.35 dL/g, MW 30,000–40,000 Da) was purchased from Lakeshore Biomaterials (Birmingham, AL, USA). Poly(vinyl alcohol) (PVA; 88% hydrolyzed, 25,000 Da) was obtained from Polysciences (Warrington, PA, USA). Methylene chloride (MC; HPLC grade) was obtained from Fisher Scientific (Pittsburgh, PA, USA).
PLGA microsphere preparation
Microspheres composed of 50:50, 75:25, and 85:15 PLGA were made using Precision Particle Fabrication technology from our previous reports.5,20 In brief, 2 g of PLGA dissolved in 10 mL of MC (20% w/v) was injected through a small-gauge needle of diameter 1.54 mm (BD Precision Glide needle, 16G1-1/2, BD Biosciences, San Jose, CA, USA). Uniform polymer droplets were produced by an ultrasonic transducer (Branson Ultrasonics, Danbury, CT, USA) and a waveform generator (model 33220A; Agilent Technologies, Santa Clara, CA, USA). A carrier stream (0.5% PVA w/v in D.I. water) surrounded and separated the droplets individually. The polymer/carrier stream was then collected into a beaker containing approximately 0.5% w/v PVA. Polymer droplets were agitated for 1 day to evaporate residual MC. The hardened microspheres were rinsed with distilled water and filtered. Finally, the microspheres were lyophilized for 2 days (Freezone, Labconco benchtop model, Kansas City, MO, USA). Although some degree of polydispersity existed in the diameter range of the microspheres, the target diameter was approximately 200 microns, to be consistent with our previous studies.5,21
Scaffold fabrication
Cylindrical-shaped molds were loaded with 80 mg of microspheres for scaffolds without NaCl and 60 mg of microspheres for NaCl mixed scaffolds and exposed to dense-phase CO2 at various subcritical levels (10, 14, 15, 20, 25, 30, and 50 bar) for 1 h. The CO2 pressurization rate was 3–5 bar/min, and the depressurization rate (Back pressure regulator (ABPR), Waters Technologies Corp, Milford, MA, USA) was 0.5 bar/min. CO2 sintering was performed using a custom-made stainless steel high-pressure reaction chamber. The custom-made CO2 chamber (Figure 1) had a pressure safety rating of 60 bar and consisted of cylindrical-shaped Teflon molds of the dimensions 9.5 ± 0.3 mm height and 3.7 mm diameter. Nearly 34 scaffolds can be prepared using this chamber in a single run. After depressurization, scaffolds remained in the reaction chamber for 24 h to allow any residual CO2 to escape from the PLGA microspheres. Scaffolds were then lyophilized for 2 days as a precaution and stored at room temperature prior to analyses.
FIGURE 1.

Custom-made stainless-steel CO2 chamber. Maximum pressure rating: 60 bar. The chamber contains Teflon molds inside. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To increase the porosity of the scaffolds, 12.5, 25, and 50 wt % of NaCl particles (200–250 μm) were mixed with PLGA microspheres, then both mixtures were placed into a cylindrical mold prior to sintering. 15 bar (scanning electron microscopy [SEM] and porosity) and 25 bar (mechanical characterization) were used to sinter the scaffolds with the different PLGA ratios and weight fractions of NaCl. Scaffolds with incorporated NaCl particles were submerged in deionized water (Milli-Q, Millipore, Billerica, MA, USA) for 3 days to leach out NaCl particles, with the deionized water being replaced every 12 h.
Mechanical characterization
Compression tests (37°C, phosphate buffered saline) were performed using a uniaxial testing apparatus (Instron Model 5848, Canton, MA, USA) with a custom-built compressive platen-bath assembly as previously described.5 Cylindrical scaffolds of the dimensions 9.4 ± 0.3 mm height and 3.7 ± 0.1 mm diameter were compressed at a strain rate of 1 mm/min. Elastic moduli were obtained from the initial linear regions of the stress-strain plots.
Porosity measurement
Porosities of the scaffolds were calculated using the density (ρ) of the bulk PLGA and the apparent densities (ρapp) of the scaffolds (n = 5). Apparent density and porosity were calculated as:
| (1) |
| (2) |
where m, d, and h are the mass, diameter, and height of the scaffolds respectively. Our previous reports have indicated a close match between the calculated porosity [Eq. (2)] and porosities measured by microCT.5 In addition, the current calculated porosities were validated by comparing values of selected groups (n = 5) with measurements taken via mercury intrusion and extrusion methods using AUTOPORE IV 9500 (Micromeritics, Norcross, GA, USA) with an equilibration time of 10 s (differences between values from each group were found to be smaller than 5% of the mean calculated value for each group).
Cell harvest
Human umbilical cord collection and cell harvests were approved by the University of Kansas Human Subjects Committee (KU-Lawrence IRB approval no. 15402 and KU Medical Center IRB approval no. 10951). Human umbilical cord mesenchymal stromal cells (hUCMSCs) were harvested from the Wharton’s jelly as we have described previously.22 Briefly, one cord was used for this study (male, 10-cm long), which was first cut into 3–5 cm pieces, then the vessels were removed, and the segments were minced and incubated in 0.75 mg/mL type II collagenase (Worthington Biochemical, Lakewood, NJ) at 37°C for 6 h. Cells were plated and fed every 2 to 3 days and passaged to P4.
Cell seeding on scaffolds and viability
Scaffolds were sterilized by ethylene-oxide prior to cell seeding after which they were air dried in a fume hood for 24 h and placed in a 24-well plate. Cells were seeded drop-wise, in a volume equal to about 50% of the scaffold volume, directly on top of the scaffolds at a density of 2 × 106 cells/mL of scaffold, and allowed to penetrate into the scaffold via capillary action. They were then allowed to attach for 3 h, after which 1.5 mL of the medium was added and cultured for 7 days. The culture medium consisted of Dulbecco’s modified Eagle medium (DMEM-LG; Invitrogen, Carlsbad, CA, USA), 1% penicillin–streptomycin (Invitrogen) and 10% fetal bovine serum ((FBS; Gemini, West Sacramento, CA, USA) and was replaced at Day 1 and 5. After 7 days of culture, the constructs were stained with LIVE/DEAD assay reagent (Molecular Probes, Carlsbad, CA, USA) and incubated for 30 min, then viewed via fluorescence microscopy (Olympus/Intelligent Innovations Spinning Disk Confocal Microscope). Another group of constructs after 7 days of culture was treated with glutaraldehyde and dehydrated by critical point drying, then inspected with a Leo 1550 field emission scanning electron microscope at an accelerating voltage of 5 kV under a high vacuum.
Statistical analysis
Data are presented as mean ± standard deviation of six measurements. All data were compared among groups by analysis of variance (ANOVA). Post-hoc comparisons were made using the Tukey–Kramer comparison test when the p-value was significant (p < 0.05).
RESULTS
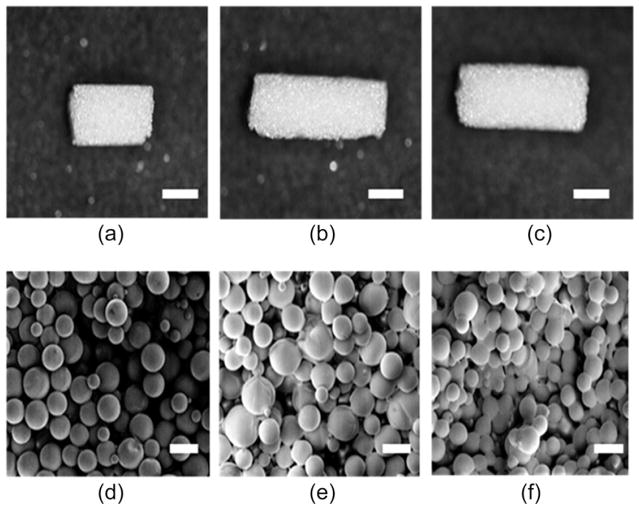
PLGA microspheres were prepared using the Precision Particle Fabrication method.5,20 Cylindrical scaffolds were produced using subcritical CO2 sintering as described previously. The appearance and microstructure of scaffolds after exposure to various CO2 pressures was visualized using SEM (Figure 2). SEM imaging revealed that the scaffolds exposed to 25 bar for 1 h and a depressurization rate of 0.5 bar/min demonstrated a higher degree of sintering and more bridges between the adjoining microspheres when compared to the other groups sintered at different CO2 pressures. The spherical shape of the microspheres was retained in all of the groups.
FIGURE 2.
SEM images of scaffolds. The appearance and microstructure of scaffolds obtained with various CO2 pressures are represented: 15 bar (a, d), 20 bar (b, e), and 25 bar (c, f). Higher sintering effect was observed in scaffolds sintered at 25 bar than that sintered at 15 and 20 bar. Scale bars = 3 mm for a–c and 200 μm for d–f. CO2 exposure: 1 h, 50:50 PLGA, intrinsic viscosity: 0.34–0.35 dL/g.
The scaffolds made at 10 and 14 bar showed insufficient mechanical integrity to maintain their shape in an aqueous environment (data not shown). Other groups prepared at 30 and 50 bar showed a foamy and glassy state on the scaffold on visual inspection (data not shown), indicating over-sintering. Therefore, the pressure range from 15 to 25 bar and 1 h period of CO2 exposure were selected to sinter all of the microspheres.
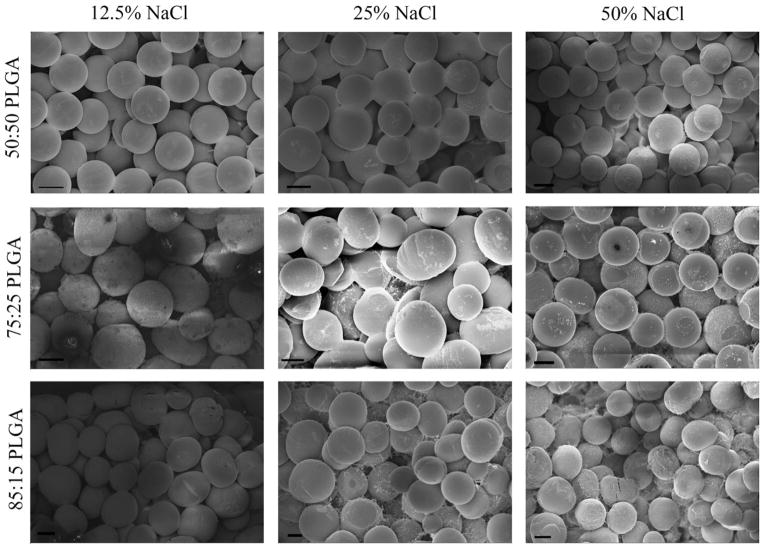
Figure 3 shows the appearance and visualization of the scaffolds synthesized using various salt concentrations (0%, 12.5%, 25%, and 50% NaCl) and different lactic and glycolic acid ratios. It was difficult to discern any significant difference in the degrees of sintering among the different PLGA ratios at a given salt concentration. However, larger void regions were apparent in the groups prepared with higher salt contents.
FIGURE 3.
SEM images of scaffolds with different salt concentrations (0, 12.5, 25, and 50% NaCl) and different lactic/glycolic acid ratios (50:50, 72:25, and 85:15 PLGA). Although differences in degree of sintering were difficult to discern among the PLGA ratios at a given salt concentration, it was readily apparent that there were more void spaces present with the higher salt concentration groups. It is noteworthy that the sizes of the salt particles and the microspheres were very similar. Scale bars = 100 μm. CO2 exposure: 1 h, applied pressure: 15 bar, intrinsic viscosity: 0.34–0.35 dL/g.
The elastic moduli of the scaffolds were found to be from 0.24 to 1.1 MPa (Figure 4). Greater mechanical integrity was observed for scaffolds sintered at 25 bar when compared to 15 and 20 bar pressure. The scaffolds fabricated at 25 bar exhibited 78.8% and 50% higher elastic moduli than at 15 bar and 20 bar, respectively (p < 0.05). In contrast, for scaffolds sintered at 15 bar, the elastic modulus was lower than other groups (p < 0.05).
FIGURE 4.
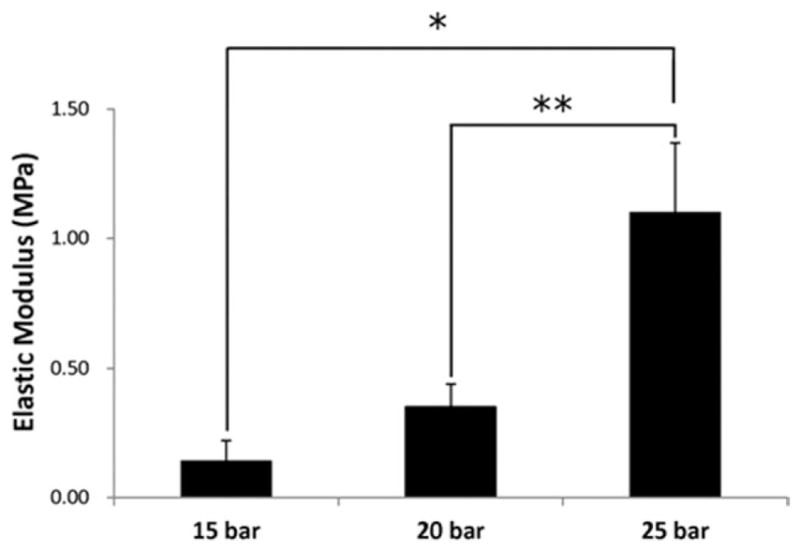
Compressive elastic moduli of scaffolds prepared under different pressures (15, 20, and 25 bar); CO2 exposure time: 1 h, 50:50 PLGA, intrinsic viscosity: 0.34–0.35 dL/g. Greater moduli were observed for scaffolds sintered at 25 bar. Values are reported as mean ± standard deviation, n = 6. Statistically significant difference between groups *p < 0.01 and **p < 0.05.
Figure 5 shows the elastic moduli of scaffolds having different lactic and glycolic acid ratios with the same sintering treatment. With respect to the PLGA composition, the 50:50 PLGA group was 25% stiffer than the 75:25 group (p < 0.05) and 3.5 times stiffer than the 85:15 group.
FIGURE 5.
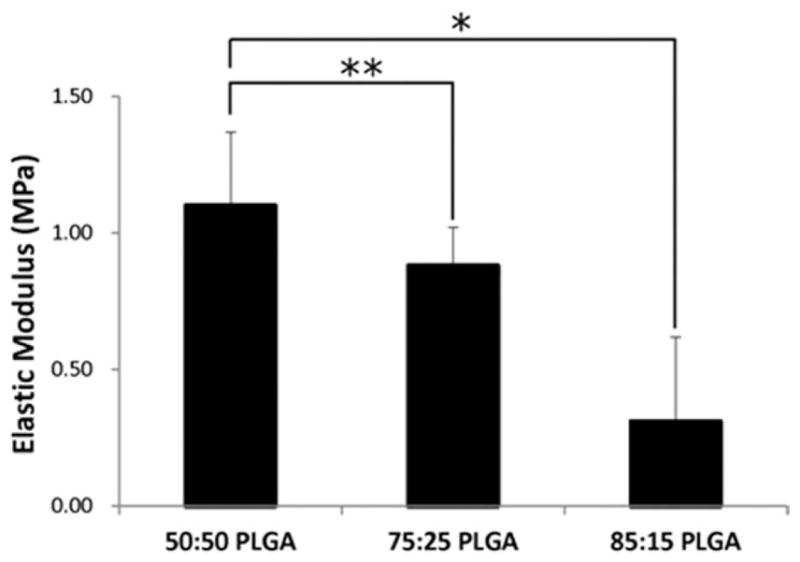
Compressive elasticmoduli of scaffolds prepared using different lactide/glycolide ratios in PLGA (50:50, 75:25, and 85:15); CO2 exposure time: 1 h, intrinsic viscosity: 0.34–0.35 dL/g, applied pressure: 25 bar. Elastic moduli decreased as the ratio of lactide/glycolide content increased. Values are reported as mean ± standard deviation, n = 6. Statistically significant difference between groups, *p < 0.005 and **p< 0.05.
Figure 6 shows the elastic moduli of scaffolds with different weight fractions of NaCl and sintered at 25 bar. It was observed that scaffolds with 12.5, 25, and 50 wt % NaCl particles had significantly lower elastic moduli (90, 72, and 31%, respectively) (p < 0.05) than scaffolds without salt particles.
FIGURE 6.
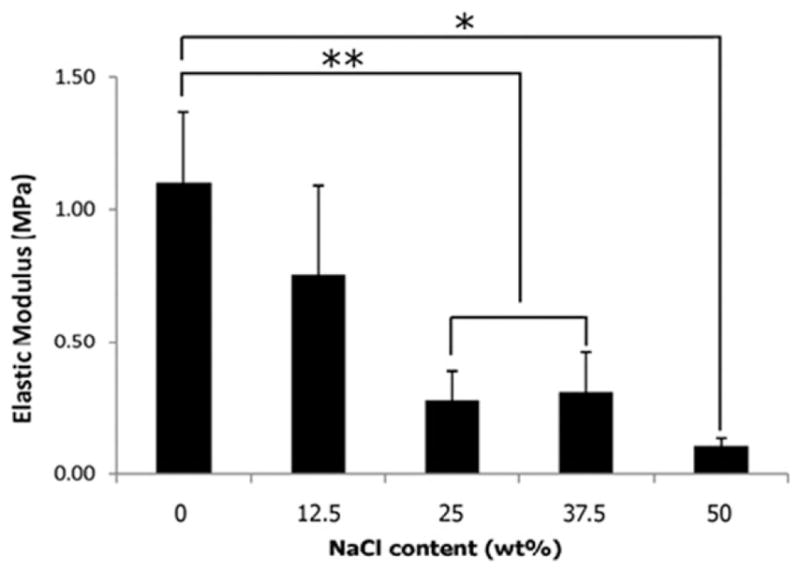
Compressive elastic moduli of scaffolds prepared by blending different amount of NaCl particles (0, 12.5, 25, and 50 wt % of PLGA microspheres); CO2 exposure time: 1 h, 50:50 PLGA, intrinsic viscosity: 0.34–0.35 dL/g, applied pressure: 25 bar. An inverse relationship was observed between elastic moduli and NaCl content. Values are reported as mean ± standard deviation, n = 6. Statistically significant difference between groups, *p < 0.001 and **p < 0.05.
The porosities of scaffolds obtained at different sintering pressures and with various NaCl concentrations are shown in Table I. When the applied pressure was increased from 15 to 20 bar or 25 bar, there were no statistically significant differences in porosity (mean porosity values were 38–41% for all three pressures). There were also no statistically significant changes in porosity with the use of differing PLGA ratios (50:50, 75:25, 85:15) sintered at 15 bar. At a constant applied pressure of 15 bar, blending an additional 12.5 wt % of the porogen resulted in a higher mean porosity value, although it was not statistically significant. However, at salt concentrations of 25% and 50%, the porosities after sintering at 15 bar were 30.3% (p < 0.005) and 58.4% (p < 0.001) larger than they were without salt, respectively.
TABLE I.
Comparison of the Porosities of the Scaffolds
| Applied pressure (bar) | Porogena content (wt %) | PLGA ratio | Porosity (%) |
|---|---|---|---|
| 15 | 0 | 50:50 | 40.2 ± 7.0 |
| 20 | 0 | 50:50 | 38.3 ± 3.4 |
| 25 | 0 | 50:50 | 38 ± 16 |
| 15 | 12.5 | 50:50 | 44.9 ± 4.7* |
| 15 | 25 | 50:50 | 52.4 ± 5.1* |
| 15 | 50 | 50:50 | 63.7 ± 5.2* |
| 15 | 0 | 75:25 | 42.2 ± 7.1** |
| 15 | 0 | 85:15 | 42.2 ± 8.1** |
NaCl (200–250 μm).
0.35 dL/g, CO2 exposure 1 h. At 15 bar, a statistically significant increase in porosity when compared to the 0% NaCl group was observed in the 25% (p < 0.005) and 50% (p < 0.001) groups. n = 6, except for
n = 5 and
n = 10.
hUCMSCs were seeded and cultured on the scaffolds for 1 week to determine the viability of the scaffolds. The majority of the cell population was identified as viable on the surface and inside of the scaffolds after 1 week of culture (Figure 7). Figure 8 shows the hUCMSC morphology on the scaffolds as exhibited by SEM after 1 week of culture. Cells (inside red circles) were seen attached to the scaffold surface.
FIGURE 7.
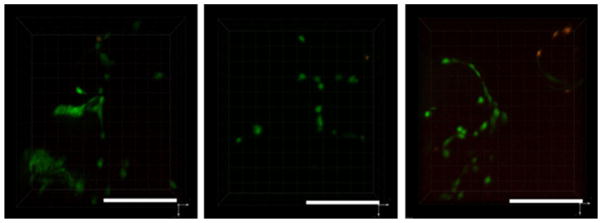
Fluorescent micrographs of live/dead dye-stained hUCMSCs seeded on scaffolds following a 1 week cell-culture period: (a) 15, (b) 20, and (c) 25 bar: live (green) and dead (red) cells. The majority of the cells were found to be viable for all scaffolds. Note the dark circular regions, representing the location of the microspheres, which were surrounded by the cells. 50:50 PLGA intrinsic viscosity: 0.34–0.35 dL/g, CO2 exposure 1 h, applied pressures 25 bar; Scale bars: 500 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 8.

hUCMSC morphology on the scaffolds after 1 week of culture, exhibited by SEM. Cells (inside red circles) attached to scaffolds by extending cell processes to the surface: Gross morphological state of tissues: (a) 20 and (b) 25 bar: 50:50 PLGA intrinsic viscosity: 0.34–0.35 dL/g, CO2 exposure 1 h; Scale bars: 500 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
The present study demonstrated that subcritical CO2 is an effective plasticizer for the sintering process of microsphere-based tissue-engineered scaffolds, and provided general guidelines for the selection of sintering conditions for PLGA microsphere-based scaffolds under various conditions. High pressure CO2 swells and plasticizes glassy polymers.23 During plasticization, the distance between the polymer interchains increases, resulting in an increase in the free volume of the polymer, which consequently enhances the mobility of the polymer segments. This phenomenon is similar to the plasticizing effect achieved during scaffold fabrication using organic solvents.23–25 The traditional gas-foaming method to make porous scaffolds is a saturation of the polymer with CO2 at supercritical pressures with equilibration periods of 2 h, or at subcritical pressures with equilibration periods of more than 24 h. Rapid degassing then leads to the nucleation of the gas and forms pores in the polymer, and the decreased glass transition temperature is restored.26–28 This gas-foaming method is not suitable for the microsphere sintering method because the microspheres would all lose their structural integrity to form a single liquid-like state. The subcritical, or dense-phase CO2 sintering method for manufacturing microsphere-based scaffolds is entirely different from the gas-foaming technique, using significantly lower CO2 pressures and exposure times. When PLGA microspheres are exposed to subcritical CO2 for a shorter period of time, the swollen and plasticized state is limited to the surface of the microspheres. This leads to retention of the entire morphology of the microspheres and sintering of the adjoining microspheres. The scaffolds prepared by this method have an interconnected pore structure, which is essential for the supply of nutrients and circulation of metabolic waste.
The applied CO2 pressure is one of the primary factors for controlling the mechanical properties of the scaffolds. We applied various subcritical CO2 pressures (10, 14, 15, 20, 25, 30, and 50 bar) while fabricating the scaffolds in our preliminary studies, with only the sintering effects of the subcritical CO2 at 15, 20, and 25 bar being adequate for further evaluation. Scaffolds made below 15 bar showed insufficient mechanical strength to maintain their shape in an aqueous environment (data not shown) because lower CO2 pressure and concentration may not have sufficiently increased the mobility of the polymer interchains under the time of CO2 exposure. Other groups prepared above 25 bar showed a foamy and glassy state on visual inspection of the scaffold because high concentrations of CO2 dissolved the microspheres and produced closed and regionally localized foams in the scaffolds (data not shown). In contrast, the scaffolds exposed to 25 bar for 1 h showed a higher degree of sintering between adjoining microspheres (Figure 2) and the highest elastic modulus compared to other groups (Figure 4) because within the efficacious range of 15–25 bar, the 25 bar pressure provided a higher degree of sintering between adjoining microspheres without leading to pore collapse.
Generally, the scaffold groups with higher lactic acid ratios (85:15 and 75:25) in PLGA were less stiff compared to the lower lactic acid ratio (50:50) group. However, these results were for the CO2 parameters selected for the 50:50 group, and perhaps with additional optimization, perhaps with longer sintering times, that the moduli of the higher lactic acid groups could be increased. Overall, the moduli observed in the current study with CO2 sintering were on the same order of magnitude as those we observed previously with ethanol sintering,5 and in particular the previous ethanol sintering for 1 h with 50:50 PLGA (intrinsic viscosity = 0.41 dL/g) and the current CO2 sintering at 20 bar for 1 h with 50:50 PLGA (intrinsic viscosity = 0.35 dL/g) were both approximately 0.3 MPa (both tested under compression in phosphate buffered saline). There were slight variations between the materials (intrinsic viscosity) and compression method (strain rate, height:diameter ratio), but altogether this comparison provides evidence of comparable moduli achieved with the two different sintering methods for PLGA microsphere-based scaffolds.
The data indicated an inverse relationship between scaffold stiffness and salt content (Figure 6). The groups with higher NaCl content in the scaffold provided fewer sintering sites between adjoining microspheres, which resulted in a lack of scaffold stiffness.
In comparison with the higher applied CO2 pressures (20 and 25 bar), 15 bar of applied pressure showed the least degree of sintering (Figure 2) and most of the microspheres retained their spherical morphology. Therefore, the mean porosity (40%) of the scaffold at 15 bar was larger than those of 20 (38.3%) and 25 bar (37.9%). To enhance porosity, a porogen was introduced to generate pores. However, although porosity increased through the inclusion of additional porogen, adding NaCl granules decreased the stiffness of the structures. The porosity and mechanical properties thus exhibited an inverse relationship, as also supported by a very recent related study.11 Overall, it is unknown whether incorporation of NaCl granules in scaffold fabrication with microspheres will ultimately better promote tissue in-growth in vivo, although we do now have a better understanding of how NaCl can be leveraged to shift the balance of mechanical performance and porosity with the use of the current materials and CO2 sintering conditions. Moreover, the use of NaCl as a porogen opens up questions for new research directions, including the effects of porogen size, as well as the use of other porogens, and their incorporation at different steps in the fabrication process.
One limitation of this study was that application of our samples was limited to in vitro evaluation. For some specific preclinical or clinical trials, the shape and size of the scaffold will need to more closely mimic the targeted tissue. Ongoing studies will focus on fabricating shape-specific scaffolds, as we have shown previously, and investigating their performance in vitro and in vivo. In addition, ongoing studies in our group are focusing on controlled release of bioactive signals from microspheres following differing sintering conditions, as well as on longer term degradation studies.
CONCLUSION
Scaffolds were fabricated using a subcritical CO2 sintering method. Scaffolds prepared at 25 bar with a lower lactic acid ratio and without NaCl particles were stiffer, while the constructs made at 15 bar with a lower glycolic acid ratio and salt granules had lower elastic moduli. The optimum range of applying subcritical fluid CO2 was from 15 to 25 bar for the scaffold system investigated, with the ideal pressure depending on the application and polymer system. The future applications of this study are very broad, including tissue engineering and drug delivery. The key advantages of subcritical fluid CO2 sintering are a non-toxic and environmentally friendly approach for scaffold fabrication. A major challenge in tailoring sintering conditions using subcritical CO2 will be the encapsulation and release of growth factors with minimal damage or denaturing.
References
- 1.Borden M, Attawia M, Khan Y, El-Amin SF, Laurencin CT. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J Bone Joint Surg Br. 2004;86:1200–1208. doi: 10.1302/0301-620x.86b8.14267. [DOI] [PubMed] [Google Scholar]
- 2.Chun KW, Yoo HS, Yoon JJ, Park TG. Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: effect of surface modification on cell attachment and function. Biotechnol Prog. 2004;20:1797–1801. doi: 10.1021/bp0496981. [DOI] [PubMed] [Google Scholar]
- 3.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Ruhe PQ, Hedberg-Dirk EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12:789–800. doi: 10.1089/ten.2006.12.789. [DOI] [PubMed] [Google Scholar]
- 5.Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng Part C Methods. 2008;14:299–309. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh M, Sandhu B, Scurto A, Berkland C, Detamore MS. Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO(2) as a sintering agent. Acta Biomater. 2010;6:137–143. doi: 10.1016/j.actbio.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrie Aronin CE, Sadik KW, Lay AL, Rion DB, Tholpady SS, Ogle RC, Botchwey EA. Comparative effects of scaffold pore size, pore volume, and total void volume on cranial bone healing patterns using microsphere-based scaffolds. J Biomed Mater Res A. 2009;89:632–641. doi: 10.1002/jbm.a.32015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29:2869–2877. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrie Aronin CE, Sefcik LS, Tholpady SS, Tholpady A, Sadik KW, Macdonald TL, Peirce SM, Wamhoff BR, Lynch KR, Ogle RC, Botchwey EA. FTY720 promotes local microvascular network formation and regeneration of cranial bone defects. Tissue Eng Part A. 2010;16:1801–1809. doi: 10.1089/ten.tea.2009.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv Q, Nair L, Laurencin CT. Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. J Biomed Mater Res A. 2009;91:679–691. doi: 10.1002/jbm.a.32302. [DOI] [PubMed] [Google Scholar]
- 11.Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng Part A. 2012;18:1376–1388. doi: 10.1089/ten.tea.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A. 2012;100:162–170. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dormer NH, Busaidy K, Berkland CJ, Detamore MS. Osteochondral interface regeneration of rabbit mandibular condyle with bioactive signal gradients. J Oral Maxillofac Surg. 2011;69:e50–e57. doi: 10.1016/j.joms.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dormer NH, Qiu Y, Lydick AM, Allen ND, Mohan N, Berkland CJ, Detamore MS. Osteogenic differentiation of human bone marrow stromal cells in hydroxyapatite-loaded microsphere-based scaffolds. Tissue Eng Part A. 2012;18:757–767. doi: 10.1089/ten.tea.2011.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng Part A. 2011;17:2845–2855. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- 16.Brown JL, Nair LS, Laurencin CT. Solvent/non-solvent sintering: a novel route to create porous microsphere scaffolds for tissue regeneration. J Biomed Mater Res B Appl Biomater. 2008;86:396–406. doi: 10.1002/jbm.b.31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung J, Perrut M. Particle design using supercritical fluids: Literature and patent survey. J Supercritical Fluids. 2001;20:179–219. [Google Scholar]
- 18.Türk M, Upper G, Hils P. Formation of composite drug-polymer particles by co-precipitation during the rapid expansion of supercritical fluids. J Supercritical Fluids. 2006;39:253–263. [Google Scholar]
- 19.Yeo S-D, Kiran E. Formation of polymer particles with supercritical fluids: A review. J Supercritical Fluids. 2005;34:287–308. [Google Scholar]
- 20.Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Release. 2001;73:59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 21.Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng. 2010;38:2167–2182. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Dormer NH, Bonewald LF, Detamore MS. Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng Part A. 2010;16:1937–1948. doi: 10.1089/ten.TEA.2009.0706. [DOI] [PubMed] [Google Scholar]
- 23.Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv Drug Delivery Rev. 2008;60:373–387. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Tsivintzelis I, Pavlidou E, Panayiotou C. Porous scaffolds prepared by phase inversion using supercritical CO2 as antisolvent: I. Poly(l-lactic acid) J Supercritical Fluids. 2007;40:317–322. [Google Scholar]
- 25.Nalawade SP, Picchioni F, Janssen LPBM. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Progr Polymer Sci. 2006;31:19–43. [Google Scholar]
- 26.Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 27.Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396–402. doi: 10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv Drug Deliv Rev. 2008;60:373–387. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]