Abstract
This objective of this study was to determine the effects of a rotating bioreactor in temporomandibular joint (TMJ) disc tissue engineering. Porcine TMJ disc cells were seeded at a density of 20 million cells/mL onto nonwoven poly(glycolic acid) (PGA) scaffolds in spinner flasks for 1 week and then cultured either under static conditions or in a rotating bioreactor for a period of 6 weeks. A series of analyses was performed, including mechanical testing, measurement of cellularity, quantification of matrix biosynthesis with a hydroxyproline assay and enzyme-linked immunosorbent assays, and observation of matrix distribution with immunohistochemistry. Between the bioreactor and static cultures, there were marked differences in gross appearance, histological structure, and distribution of collagen types I and II. Engineered constructs from the bioreactor contracted earlier and to a greater extent, resulting in a denser matrix and cell composition. In addition, immunostaining intensity was generally uniform in static constructs, in contrast to higher intensity around the periphery of bioreactor constructs. Moreover, bioreactor constructs had higher amounts of collagen II than did static constructs. However, differences in total matrix content and compressive stiffness were generally not significant. On the basis of the results of this study there is no clear benefit from use of the rotating bioreactor, although a sequence of static culture followed by rotating bioreactor culture may prove in the future to be more beneficial than either alone.
INTRODUCTION
Tissue engineering has made great strides toward regenerating cartilaginous tissues such as hyaline cartilage and even the knee meniscus. In comparison, the temporomandibular joint (TMJ) disc has received far less attention from the tissue-engineering community, with under 10 such studies available in the current literature. However, there is a vital need for a tissue-engineered disc,1 and there are several groups that have started working toward this goal. Fortunately, precedent has now been set for attempting to tissue engineer the TMJ disc. Early studies used rabbit TMJ disc cells in collagen meshes,2 bovine shoulder cartilage cells in disk-shaped poly(lactic acid)–poly(glycolic acid) (PLA–PGA) scaffolds,3 and monkey condylar cartilage cells in collagen–fibrinogen scaffolds.4 More recent attempts have investigated scaffold choice,5,6 seeding method,6 and growth factor effects with TMJ disc cells.7,8 PGA was chosen over alginate for TMJ disc tissue engineering, and a spinner flask was chosen as the preferred seeding method for these PGA scaffolds over an orbital shaker and a novel centrifugal pelleting technique.6 Using PGA scaffolds with spinner flask seeding, insulin-like growth factor (IGF) at 10 ng/mL was determined to be more beneficial than transforming growth factor β1 (TGF-β) or basic fibroblast growth factor (bFGF) at their examined concentrations.8 The timing is now appropriate for investigating the effects of bioreactors in TMJ disc tissue engineering.
Some of the best overall results to date for hyaline cartilage tissue engineering have come from using the rotating wall bioreactor.9 Constructs cultured in the rotating bioreactor remain suspended in the medium by a balance of gravitational and drag forces, simulating a continuous free fall. A rotating bioreactor has been shown to produce higher fractions of glycosaminoglycan (GAG) and/or collagen compared with mixed flasks or static culture.10–15 Morphologically, the constructs show a more continuous and homogeneous matrix compared with high shear bioreactors,11,15,16 and improved mechanical integrity compared with static and high shear environments.15 These results may be due in large part to improvement in the mass transfer characteristics of the rotating bioreactor. With a continuous flow of medium over the construct, there is no stagnant localized region of nutrient depletion or buildup of waste around the constructs, keeping concentration gradients and thus passive diffusion driving force at a maximum. Moreover, for a highly permeable scaffold such as nonwoven PGA, medium flows directly through the construct during the initial phase before the scaffold begins to be replaced by cells and matrix and permeability decreases.
A porcine animal model has been recommended for TMJ disc tissue-engineering experiments.1 Fortunately, characterization studies of the native porcine TMJ disc that are pertinent to tissue engineering are available in the literature.17–22 With regard to cell content, the cells of the porcine TMJ are 70% fibroblasts and 30% fibrochondrocytes, heterogeneously distributed, and distinct from chondrocytes of hyaline cartilage.19 The TMJ disc is 71 ± 2% water,20 and collagen makes up the majority of the dry weight.22 The collagen is primarily type I, although type II collagen is also present.20 Glycosaminoglycans and their corresponding proteoglycans are also an important part of the extracellular matrix. For instance, chondroitin-6-sulfate, chondroitin-4-sulfate, and dermatan sulfate proteoglycan (decorin) account for 2.19 ± 0.71, 1.71 ± 0.51, and 0.87 ± 0.71% of the dry weight, respectively. Regarding mechanical integrity, the aggregate modulus of the porcine TMJ disc under indentation varies by region, and ranges from 16 to 29 kPa.17
The objective of the current study was to combine and capitalize on tissue-engineering studies and native disc characterization studies, while moving forward by utilizing bioreactor technology for TMJ disc tissue engineering for the first time. The original hypothesis was that constructs cultured in the rotating bioreactor would exhibit higher levels of biosynthesis and improved mechanical integrity compared with static culture. Drawing from previous tissue-engineering studies, TMJ disc cells were seeded to PGA scaffolds with spinner flasks, and cultured in the presence of IGF. Drawing from characterization studies, the mechanical integrity was evaluated, and biochemical contents of specific extracellular matrix constituents were quantified and their distribution examined.
MATERIALS AND METHODS
Cell harvest
Cells were obtained from porcine TMJ discs. Both TMJs of a PIC Genetic Breed hog (Fisher Ham & Meat, Spring, TX) were removed en bloc with capsule intact, and then placed in ethanol for 10 to 15 min. In a laminar flow hood, the TMJs were then air dried and scrubbed with sterile iodine pads before aseptically removing the discs. The left and right discs were subsequently minced with a scalpel and digested for 24 h in 40 mL of collagenase at 2.5 mg/mL (type II, 315 U/mg; Worthington Biochemical, Lakewood, NJ). Approximately 37 million cells were obtained from each disc. The TMJ disc cells were passaged twice in culture medium consisting of Dulbecco’s modified Eagle’s medium (Cambrex, East Rutherford, NJ), 10% fetal bovine serum (Cambrex), ascorbic acid (50 μg/mL; Sigma, St. Louis, MO), 1% nonessential amino acids (Invitrogen Life Technologies, Carlsbad, CA), and 1% penicillin–streptomycin–amphotericin B (Fungizone) (Cambrex). Cells were fed four times (every 2 or 3 days) over an 11-day expansion period.
Spinner flask seeding
A scalpel and an elliptically-shaped leather punch were used to cut 5 × 7 mm scaffolds from a sheet of a nonwoven mesh of poly(glycolic acid) (PGA) with a thickness of 2.0 mm (Albany International Research, Albany, NY). According to the manufacturer, the PGA was 45–55% crystalline and 95% porous. Cells were seeded onto these scaffolds using spinner flasks, as previously described.6,8 Fifty scaffolds were used in this study, with 25 scaffolds in each of 2 spinner flasks. The spinner flasks (including stir bars, 3.8 cm long × 0.8 cm thick) were autoclaved and then scaffolds were skewered onto the thin wire of each spinner flask. Scaffolds were then sterilized together with the spinner flasks in ethylene oxide and aired for 1 day. Scaffolds were subsequently wetted by a sequence of sterile-filtered ethanol, two washes of sterile phosphate-buffered saline (PBS), and then left overnight with 225 mL of cell culture medium at 37°C.
Approximately 94 million cells were added to each flask, and stir bars were set to rotate at 90 rpm. Stirring was stopped after 2 days, and cells were given an additional 5 days in the spinner flask to firmly adhere to the scaffolds. An additional 225 mL of penicillin–streptomycin–Fungizone was added 4 days after cells were initially added to the flasks. The medium was not changed and no growth factors were included during the seeding period.
Culture of engineered constructs
Scaffolds from the spinner flask were transferred to either a rotating bioreactor or static culture. The cell culture medium for both the rotating bioreactor and the static culture was supplemented with recombinant human insulin-like growth factor I (IGF) at 10 ng/mL (Peprotech, Rocky Hill, NJ).
Static culture consisted of individual wells in 24-well untreated plates. The wells were precoated with 600 μL of 2% agarose to further prevent cell migration from the scaffolds. A total of 1 mL of cell culture medium with growth factor was added to each well. Scaffolds were then added to the wells, 1 scaffold per well for a total of 19 scaffolds. Half of the medium was changed every other day for a period of 6 weeks. To prevent and counteract the effects of evaporation over a 6-week period, wells around the periphery were filled with sterile PBS, and complete medium changes were performed at 2 and 4 weeks.
The rotating bioreactor setup was composed of two 50-mL γ-sterilized cylindrical vessels with an inner diameter of 8.5 cm and length of 1.0 cm (Synthecon, Houston, TX). Nine scaffolds were cultured in one vessel for testing at 2 and 4 weeks, and 10 scaffolds were cultured in the other vessel for testing at 6 weeks. As with the static groups, half of the medium was changed every other day for a period of 6 weeks. The vessels were rotated at an angular velocity such that constructs were in a state of continuous free fall (stationary with respect to an observer, not to the moving vessel walls). A rotation speed of 11 rpm was used to achieve this desired effect.
The purpose of this study was to compare two techniques—scaffold culture and rotating bioreactor culture. Inherent differences, such as medium volume, were included intentionally. The objective was not to make a simplistic comparison between stationary and flowing medium, but rather to evaluate two culture methods, including their respective innate differences.
Analysis of engineered constructs
Constructs were assessed for quantitative biochemical content, matrix distribution, and mechanical integrity. Biochemical content assays were performed 0, 2, 4, and 6 weeks after seeding. These time points refer to the time spent in bioreactor or static culture, and do not include the immediately preceding 1-week seeding period in the spinner flasks. Matrix distribution and mechanical integrity were evaluated 0 and 6 weeks after seeding.
Biochemical content
Engineered constructs were evaluated quantitatively for total collagen content, chondroitin-4-sulfate (C4S) content, chondroitin-6-sulfate (C6S) content, decorin content, and cell number. Scaffolds designated for analysis of biochemical content were lyophilized for 2 days and then digested overnight in 1.5 mL of papain at 60°C. The total collagen content was measured with a modified hydroxyproline assay23 as previously described.7 Briefly, collagen standards (Accurate Chemical & Scientific, Westbury, NY) and samples were hydrolyzed with sodium hydroxide at 121°C and neutralized with hydrochloric acid, and then combined with p-dimethylaminobenzaldehyde in perchloric acid and read at 550 nm. Cell numbers were measured via DNA content, determined by a reaction between DNA and PicoGreen reagent (fluorescence assay kit; Molecular Probes, Eugene, OR). DNA standards were provided with the kit, and a conversion factor of 7.7 pg of DNA per cell for TMJ disc cells was used.7
C4S, C6S, and decorin were measured with enzyme-linked immunosorbent assays (ELISAs) according to a modified protocol used for the native TMJ disc.20 All samples were tested at dilutions of 1:5, 1:10, 1:25, and 1:50. The primary antibodies for ELISAs were all mouse monoclonal IgG. The anti-C6S and anti-C4S primary antibodies24 and the goat anti-mouse IgG secondary antibody were obtained from Chemicon International (Temecula, CA). The anti-dermatan sulfate proteoglycan primary antibody for decorin was obtained from Calbiochem (San Diego, CA). Standards were mixed with papain and diluted with PBS such that sample calculations would be consistent at different dilutions. The appropriate procedures as determined in preliminary experiments were as follows. Decorin standards were prepared by diluting a 2-mg/mL stock solution to 15 μg/mL with 20% papain–80% PBS, which was further diluted to a range of 0.05–1 μg/mL with PBS. Standard solutions for C4S and C6S were prepared by diluting 300-μg/mL stock solutions to 20 μg/mL with papain, and then to 0.05–1 μg/mL with PBS.
An indirect ELISA was used to quantify decorin. Briefly, high-protein-binding well plates were first incubated overnight with bovine decorin (Sigma-Aldrich, St. Louis, MO) and samples. The next day, plates were blocked with 5% bovine serum albumin, incubated with the primary antibody at a 1:1200 dilution, exposed to the secondary antibody, visualized with 3,3′,5,5′-tetramethylbenzidine (TMB), and read at 450 nm. Inhibition ELISAs were used to quantify C4S and C6S. Plates were coated overnight with bovine aggrecan (25 ng/mL; Sigma-Aldrich). The next day, chondroitinase ABC was added to the plates and followed by a block with 5% bovine serum albumin. Meanwhile, samples and standards were incubated with chondroitinase ABC and incubated with the primary antibody (diluted 1:1200 for C4S, 1:5000 for C6S). The sample–antibody and standard–antibody solutions were then added to the plates, followed by the secondary antibody and TMB.
Extracellular matrix distribution
The distributions of collagen I, collagen II, dermatan sulfate (DS), C4S, and C6S were evaluated by immunohistochemistry as previously described.20 The same primary antibodies used for C4S and C6S with ELISAs were used for immunohistochemistry. The anti-collagen I and anti-collagen II antibodies were obtained from Accurate Chemical & Scientific and Chondrex, LLC (Redmond, WA), respectively. The PG-4 antibody against DS was a generous gift from the group of A. Caplan.25 Briefly, a cryotome was used to prepare frozen 12-μm sections of samples, which were placed on slides and fixed in chilled acetone. Staining was performed using a BioGenex i6000 autostainer (BioGenex, San Ramon, CA). Using the autostainer, slides were first exposed to 1% hydrogen peroxide in methanol, blocked with secondary antibody–host serum, and incubated with a primary antibody. Then, a secondary antibody and avidin–biotin complex (Vector Laboratories, Burlingame, CA) were added and samples were visualized with 3,3′-diaminobenzidine (DAB). Slides were removed from the autostainer, counterstained with hematoxylin, dehydrated in graded ethanol, and mounted. An additional digestion step was required for DS (chondroitinase ACII) and for C4S and C6S (chondroitinase ABC) between the block and primary antibody steps. Negative controls were run by omitting the primary antibody from the protocol.
Mechanical integrity
Mechanical integrity was evaluated as previously described for the native TMJ disc.17 Briefly, a constant feedback-controlled compressive force of 0.5 g was applied to hydrated constructs until equilibrium was reached (maximum time, 45 min). This creep indentation was performed with a porous indenter tip with a diameter of 1.66 mm. A tare load of 0.2 g was applied to determine contact. The linear biphasic theory with a numerical solver was used to calculate the aggregate modulus, shear modulus, Poisson’s ratio, and permeability of the engineered constructs.17,26–28
Statistical analysis
Analyses of data for biochemical content, cell content, and mechanical properties were performed by analysis of variance (ANOVA), followed by a Fisher’s protected least significant difference post hoc test when significance was detected by ANOVA (p < 0.05). A single-factor ANOVA with a sample size of n = 4 was used for all quantitative analyses. One sample was tested from each group for immunohistochemistry. ELISAs were performed in duplicate, and cell content and collagen content assays were performed in triplicate.
RESULTS
Construct morphology
Immediately after seeding, cells were distributed in a uniform manner. The gross morphology of the constructs changed over time. The constructs in static control noticeably began to contract at about 4 weeks (beyond the 1-week seeding period). However, the bioreactor constructs contracted much earlier, showing a noticeable change in size within 10 days. At 2 weeks, the diameters of the constructs in static and bioreactor cultures were approximately 5 and 3 mm, respectively (approximate due to elliptical shape). Whereas fibers were easily recognizable with the static constructs under a microscope, bioreactor constructs showed no readily observable signs of PGA fibers and were already opaque. By 4 weeks, both groups were opaque under a microscope, with no observable signs of PGA fibers. In addition, the constructs had contracted to approximate diameters of 3 and 1.5 mm for static and bioreactor culture, respectively. By 6 weeks, the constructs in static culture had contracted to a diameter of just under 2 mm. By this time, the constructs in the rotating bioreactor had contracted to a diameter of approximately 0.6 mm, and had to be finely separated from each other with a scalpel. At 6 weeks, the thicknesses of static and bioreactor constructs that were measured before mechanical testing were 0.77 ± 0.11 and 0.47 ± 0.15 mm, respectively.
Construct cellularity
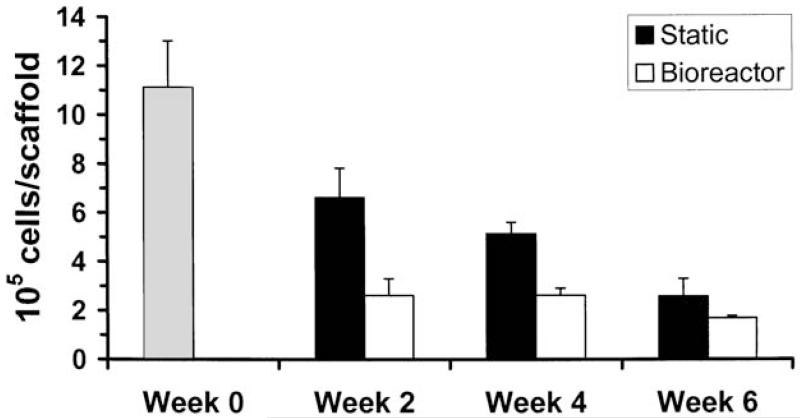
Constructs showed a steady decline in cell number over time (Fig. 1), which has been observed in previous studies.6,8 However, cell density did not decline at the same rate because of the decrease in construct volume over time. At week 0 (immediately after the 1-week seeding period), there were 1.11 ± 0.19 million cells per scaffold, corresponding to a seeding density of 20 million cells per milliliter of scaffold volume. Constructs at week 0 had higher cell numbers than any other group (p 0.0001). The static groups had 59.6, 46.2, and 23.3% of the original week 0 cell number at 2, 4, and 6 weeks, respectively. The bioreactor groups generally had fewer cells, with 23.5, 23.6, and 15.2% of the original week 0 cell number at 2, 4, and 6 weeks, respectively. At 2 weeks, constructs from the static culture group had more cells than those from the bioreactor culture group (p 0.0001). The static group at 2 weeks also had more cells than both groups at 4 and 6 weeks (p < 0.0001, except p < 0.05 for static at week 4). The static group after 4 weeks still had more cells than the bioreactor group had at any time point (p < 0.002 for 2 and 4 weeks, p < 0.0001 at 6 weeks). In addition, the static group had more cells at 4 weeks than at 6 weeks (p < 0.001).
FIG. 1.
Cell number per construct; means ± standard deviation. Time points refer to time in bioreactor or static culture, that is, the time beyond the 1-week seeding period. The cell number at week 0 corresponded to a seeding density of 20 million cells/mL.
Mechanical integrity of engineered constructs
Large differences among groups were observed for construct permeability (Fig. 2), as the constructs at week 0 were 5.9 and 28.7 times more permeable than bioreactor and static constructs at 6 weeks, respectively (p < 0.0001) (Fig. 2). There were no statistically significant differences among groups for construct stiffnesses—the aggregate moduli fell in the narrow range of 5.4 to 6.5 kPa, and the shear moduli fell in the range of 2.3 to 3.1 kPa (Table 1). These values represent 25 to 35% of the aggregate and shear moduli reported for the center of the native porcine TMJ disc. Two representative creep curves are displayed in Fig. 3.
FIG. 2.
Permeability of engineered constructs; means ± standard deviation. Time points do not include the 1-week seeding period. Week 0 constructs were significantly more permeable than bioreactor (B) and static (ST) constructs (p < 0.0001). The permeability of the native disc represents the center of the porcine TMJ disc, obtained in a previous study using the same testing approach.17
Table 1. Compressive Stiffness of Engineered Constructsa.
| Group | HA (kPa) | G (kPa) |
|---|---|---|
| Week 0b | 6.4 ± 1.4 | 3.1 ± 0.6 |
| Static, 6 weeksb | 5.4 ± 1.0 | 2.5 ± 0.5 |
| Bioreactor, 6 weeksb | 6.5 ± 1.1 | 2.3 ± 0.5 |
| Native discc | 18.6 ± 5.2 | 8.8 ± 2.5 |
Abbreviations: HA, aggregate modulus; G, shear modulus.
Reported values represent means ± standard deviations.
Represents time after seeding; does not include 1-week seeding period.
Obtained from center of the porcine TMJ disc in a previous study.17
FIG. 3.
Representative creep curves from a single construct each from static culture and bioreactor culture. Note that the bioreactor sample appears to be stiffer and more permeable, which is reflected in the calculated aggregate modulus (Table 1) and permeability value (Fig. 2) with all constructs included.
Biosynthesis of extracellular matrix
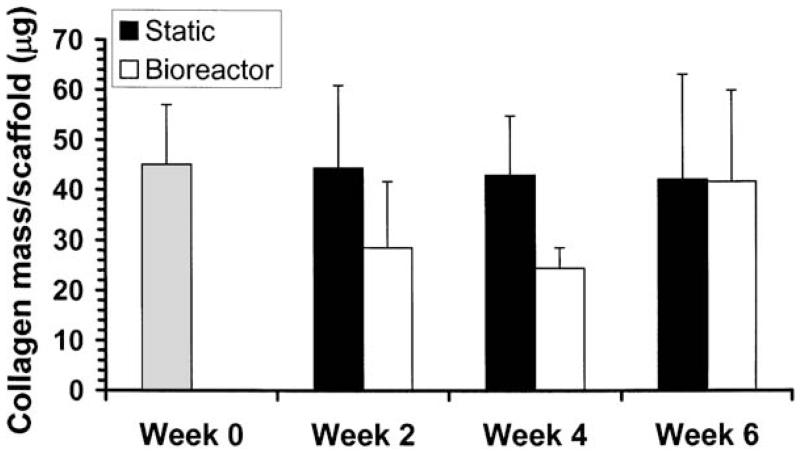
As with construct stiffnesses, there were no statistically significant differences in collagen content among groups (Fig. 4). The constructs contained 45 ± 12 μg of collagen directly out of the seeding period (week 0). Constructs in static culture essentially maintained collagen levels, with at least 94% of this original amount at all time points. In contrast, the bioreactor group had 64% of this original amount at 2 weeks, dropped to 54% of this amount at 4 weeks, and increased to 93% of the original amount by 6 weeks.
FIG. 4.
Collagen content per construct; means ± standard deviation. Time points refer to time in bioreactor or static culture, that is, the time beyond the 1-week seeding period. There were no statistically significant differences among groups.
Unlike collagen content, there were statistically significant differences in glycosaminoglycan content among groups (Fig. 5). Constructs at week 0 had the most chondroitin-4-sulfate (C4S), with 60 to 72% more than both groups at 2 and 6 weeks (p < 0.0001) and 23% more than the static group at 4 weeks (p < 0.05). At 4 weeks, the bioreactor group contained 50 to 60% more C4S than both groups at 2 and 6 weeks (p ≤ 0.0005), and the static group contained 30 to 40% more C4S than both groups at 2 and 6 weeks (p < 0.05). Differences among groups were less pronounced with chondroitin-6-sulfate (C6S) content, although statistically significant differences did exist and trends were different from C4S.
FIG. 5.
GAG/proteoglycan content per construct; means ± standard deviation. ST, static culture; B, bioreactor culture; and following number represents time after seeding in weeks. C4S, chondroitin-4-sulfate; C6S, chondroitin-6-sulfate; DSPG, dermatan sulfate proteoglycan. The highest levels appear at week 0, whereas there is a general gradual increase and peak in chondroitin sulfate content at 4 weeks, and a general increase in DSPG throughout.
C6S content was lowest in static constructs at 2 weeks and bioreactor constructs at 6 weeks. The C6S contents in these two groups were approximately 65% of C6S content in week 0 constructs and bioreactor constructs at 2 weeks (p < 0.0005), approximately 68% of C6S content in bioreactor constructs at 4 weeks (p < 0.002), and about 79% of C6S content in static constructs at 4 weeks (p < 0.05). The third lowest C6S content belonged to the static constructs at 6 weeks, which contained 72 to 75% of the C6S measured in week 0 constructs and in bioreactor constructs at 2 and 4 weeks (p < 0.01). There were no statistically significant differences among groups for dermatan sulfate proteoglycan content, which ranged from 2.4 ± 1.2 μg in static constructs at 2 weeks to 4.6 ± 0.7 μg at week 0.
Immunohistochemical analysis of engineered constructs
A summary of immunohistochemical findings is provided in Table 2. Collagen I was discovered in all three groups (week 0, static at 6 weeks, bioreactor at 6-weeks), and staining was especially intense with 6-week constructs (Fig. 6). Collagen II was not observed at week 0 (data not shown), although it was discovered in both groups at 6 weeks. Only infrequent, isolated islands of collagen II were observed in the static group, whereas larger, more continuous regions of collagen II were observed in the bioreactor group. Collagen staining for both types was more intense around the construct periphery for the bioreactor group, whereas staining was uniform in the static group.
Table 2. Semiquantitative Summary of Immunohistochemical Results.
| Group | Collagen I | Collagen II | C4S | C6S | DS |
|---|---|---|---|---|---|
| Week 0 | + | − | + | +/− | + |
| Static, 6 weeks | +++ | +/− | ++ | +/− | ++ |
| Bioreactor, 6 weeks | +++ | + | +/− | ++ | ++ |
Abbreviations: C4S, chondroitin-4-sulfate; C6S, chondroitin-6-sulfate; DS, dermatan sulfate.
Key: −, not observed; +/−, trace amount; +, slight presence; + +, strong presence; + + +, extreme presence.
FIG. 6.
Immunohistochemical staining for collagen I and II. ST, static culture; B, bioreactor culture. The brown color represents positive staining, the faint blue color is a background stain, and dark blue stains are cell nuclei. Note the intense staining of collagen I throughout both the static and bioreactor constructs at 6 weeks, with higher intensity at the bioreactor construct periphery. Collagen II staining was minimal, with sporadic small islands of staining in static constructs compared with spread-out regions of staining in bioreactor constructs.
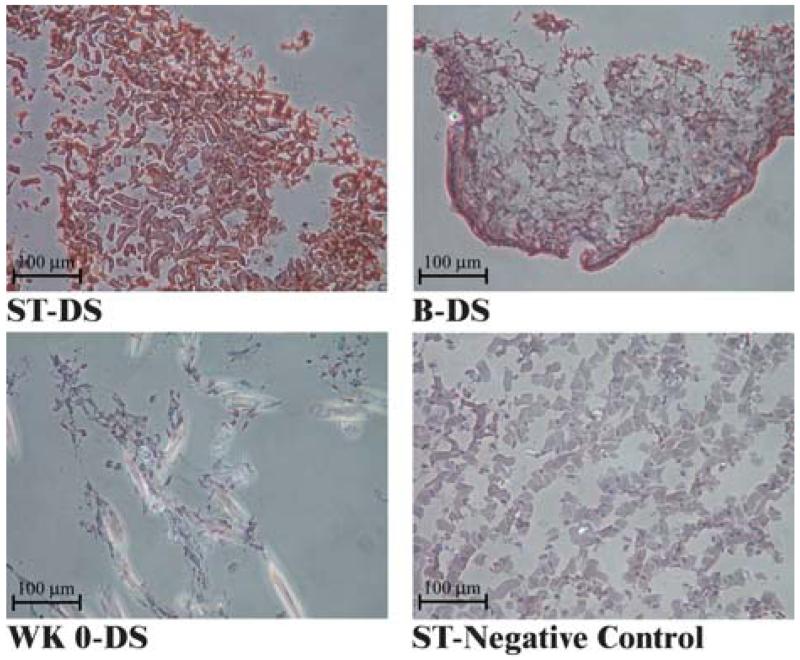
Staining for both C4S and C6S was faint in the week 0 group. However, chondroitin sulfate content was strongly dependent on type at 6 weeks (Fig. 7). C4S staining was intense for the static group, but weak for the bioreactor group. Conversely, C6S staining was weak for the static group, but intense for the bioreactor group. Staining for dermatan sulfate (DS) was intense at 6 weeks, but was faint in comparison at week 0 (Fig. 8). As with collagen staining, CS and DS staining were uniform in the static group and more intense around the periphery in the bioreactor group.
FIG. 7.
Immunohistochemical staining for chondroitin-4-sulfate (C4S) and chondroitin-6-sulfate (C6S). Abbreviations and stain color guide as for Fig. 6. Static constructs stained more intensely for C4S, whereas bioreactor constructs stained more intensely for C6S. Note the additional intensity of the C6S staining around the bioreactor construct periphery.
FIG. 8.
Immunohistochemical staining for dermatan sulfate (DS). Abbreviations and stain color guide as for Fig. 6. Staining for the static construct was not entirely continuous, but homogeneous. In contrast, immunostaining was heterogeneous with the bioreactor construct, preferentially located around the construct periphery.
DISCUSSION
To the best of our knowledge, this is the first report of the use of a bioreactor for TMJ disc tissue engineering. The results of the current study unequivocally demonstrate a difference between constructs cultured in a bioreactor or under static conditions. Gross appearance differs drastically within 2 weeks of seeding. Quantity and organization of synthesized matrix vary between groups, and manifested in observed differences in mechanical properties.
There is an apparent disparity in some cases between immunohistochemical and ELISA findings. For example, C6S and C4S immunostaining is weak in the static and bioreactor constructs, respectively (Fig. 7), despite the similar levels of each detected at 6 weeks by ELISA (Fig. 5). This may be the result of an anomaly in the immunohistochemistry sample, or instead may be attributed to heterogeneous distribution of dense clusters that were not observed due to nonrepresentative 12-μm sections. In addition, high levels of collagen, chondroitin sulfate, and dermatan sulfate were detected in week 0 samples by ELISAs, although immunohistochemical staining was generally more faint in week 0 samples compared with week 6 samples. This is not of concern, as this can simply be attributed to the sheer size of the constructs at week 0 compared with constructs at 6 weeks. If both groups were to have similar total amounts of matrix, a much smaller construct would be more densely packed and result in more intense immunostaining, which is exactly what was observed.
The week 0 constructs were considerably more permeable than both the bioreactor and static constructs at 6 weeks. This is an expected result, considering that freshly seeded cells on a highly porous scaffold should be more permeable than constructs that have contracted, with matrix replacing polymer. It should be mentioned, however, that the assumptions of indentation testing were not valid for constructs that underwent significant shrinkage with culture time. The indenter tip was not changed intentionally to preserve consistent test methodology with the week 0 constructs. As a result, there is likely some degree of error present in the permeability values for the week 6 constructs, whereas the aggregate modulus, which is an equilibrium property, was probably less affected.
Scaffolds in both the static and bioreactor groups appear to undergo an initial loss in construct mass after the seeding period, as revealed by an initial drop in cellularity (Fig. 1) and scaffold contraction. However, this initial loss appears to be more dramatic for constructs cultured in the bioreactor. Our hypothesis is that the initial culture period in the bioreactor, with shear through a highly permeable construct, washes out some cells and matrix, and in addition facilitates removal of degraded PGA and thus slows autocatalytic molecular weight degradation. As a result, collagen production suffers initially (Fig. 4). However, cells around the periphery, perhaps stimulated by appropriate levels of shear, form a protective peripheral layer composed of collagen and proteoglycans, enabling the more tightly packed cells to begin to compensate for the slower start owing to the initial washing out of contents. This contention is supported by the observations that cell numbers and collagen content were maintained for the bioreactor constructs after the initial drop from week 0 to week 2 (Figs. 1 and 4), juxtaposed with the observed high-intensity immunostaining around the periphery of the construct from the bioreactor (Figs. 6–8).
The overall effects of the bioreactor appear to be initial washing out of scaffold degradation products, along with cells and matrix attached to them, and construct contraction, which results in smaller, more densely packed constructs. The original hypothesis of this study was not supported, which is that constructs cultured in the rotating bioreactor would exhibit higher levels of biosynthesis and improved mechanical integrity compared with static culture. Although the histological structure of the bioreactor constructs (Figs. 6–8) is more reminiscent of native TMJ disc appearance,20 it cannot be stated conclusively from the current study that the rotating bioreactor is necessarily beneficial for TMJ disc tissue engineering. Although constructs are more dense, they do not result in significantly elevated levels of total matrix production in the engineered constructs. In addition, collagen type II is produced in greater quantities in the bioreactor group, which is less like the native TMJ disc, which contains primarily type I collagen.20 Moreover, the bioreactor is much more costly to operate because of both the cost of the unit itself and the higher volume of medium required. However, an alternative approach that may be more successful than static or bioreactor culture alone may be to culture under static conditions, after spinner flask seeding long enough for scaffolds to begin to contract (about 3 to 4 weeks), and then to transfer scaffolds to a rotating bioreactor for an additional 2 to 4 weeks. In this manner, enough structural integrity could be generated in static culture to withstand the washing out from the bioreactor shear, such that the benefits of bioreactor culture could be superimposed on a solid foundation built in static culture.
ACKNOWLEDGMENTS
We gratefully acknowledge funding from the National Institute of Dental and Craniofacial Research (grant R01 DE015038-01A2). In addition, we gratefully acknowledge funding from the Whitaker Foundation and from the Nettie S. Autrey Memorial Fellowship at Rice University. We thank Drs. David Carrino and Arnold Caplan for generous contribution of the PG-4 antibody used for ELISAs. We also thank Dr. Alejandro Almarza for expertise in operation of the rotating bioreactor.
REFERENCES
- 1.Detamore MS, Athanasiou KA. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;9:1065. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 2.Thomas M, Grande D, Haug RH. Development of an in vitro temporomandibular joint cartilage analog. J. Oral Maxillofac. Surg. 1991;49:854. doi: 10.1016/0278-2391(91)90015-e. [DOI] [PubMed] [Google Scholar]
- 3.Puelacher WC, Wisser J, Vacanti CA, Ferraro NF, Jaramillo D, Vacanti JP. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J. Oral Maxillofac. Surg. 1994;52:1172. doi: 10.1016/0278-2391(94)90538-x. [DOI] [PubMed] [Google Scholar]
- 4.Girdler NM. In vitro synthesis and characterization of a cartilaginous meniscus grown from isolated temporomandibular chondroprogenitor cells. Scand. J. Rheumatol. 1998;27:446. doi: 10.1080/030097498442280. [DOI] [PubMed] [Google Scholar]
- 5.Springer IN, Fleiner B, Jepsen S, Acil Y. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials. 2001;22:2569. doi: 10.1016/s0142-9612(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 6.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for the tissue engineering of the temporomandibular joint disc. Tissue Eng. 2004;10:1787. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 7.Detamore MS, Athanasiou KA. Effects of growth factors on temporomandibular joint disc cells. Arch. Oral Biol. 2004;49:577. doi: 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Detamore MS, Athanasiou KA. Evaluation of three growth factors for TMJ disc tissue engineering. Ann. Biomed. Eng. 2005;33:383. doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darling EM, Athanasiou KA. Articular cartilage bioreactors and bioprocesses. Tissue Eng. 2003;9:9. doi: 10.1089/107632703762687492. [DOI] [PubMed] [Google Scholar]
- 10.Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE, Vunjak-Novakovic G. Modulation of the mechanical properties of tissue engineered cartilage. Biorheology. 2000;37:141. [PubMed] [Google Scholar]
- 11.Freed LE, Hollander AP, Martin I, Barry JR, Langer R, Vunjak-Novakovic G. Chondrogenesis in a cell–polymer–bioreactor system. Exp. Cell Res. 1998;240:58. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 12.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, Freed LE. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem. Biophys. Res. Commun. 2001;286:909. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 13.Saini S, Wick TM. Concentric cylinder bioreactor for production of tissue engineered cartilage: Effect of seeding density and hydrodynamic loading on construct development. Biotechnol. Prog. 2003;19:510. doi: 10.1021/bp0256519. [DOI] [PubMed] [Google Scholar]
- 14.Vunjak-Novakovic G, Obradovic B, Martin I, Freed LE. Bioreactor studies of native and tissue engineered cartilage. Biorheology. 2002;39:259. [PubMed] [Google Scholar]
- 15.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J. Orthop. Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 16.Martin I, Obradovic B, Freed LE, Vunjak-Novakovic G. Method for quantitative analysis of glycosaminoglycan distribution in cultured natural and engineered cartilage. Ann. Biomed. Eng. 1999;27:656. doi: 10.1114/1.205. [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Wong ME, Helfrick JF, Thomas JB, Athanasiou KA. Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann. Biomed. Eng. 2003;31:924. doi: 10.1114/1.1591190. [DOI] [PubMed] [Google Scholar]
- 18.Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J. Biomech. Eng. 2003;125:558. doi: 10.1115/1.1589778. [DOI] [PubMed] [Google Scholar]
- 19.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, Athanasiou KA. Cell type and distribution in the porcine temporomandibular joint disc. J. Oral Maxillofac. Surg. 2005 doi: 10.1016/j.joms.2005.10.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24:45. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J. Biomech. J. Oral Maxillofac. Surg. 2005 doi: 10.1016/j.jbiomech.2004.11.012. in press. [DOI] [PubMed] [Google Scholar]
- 22.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical content and distribution in the porcine temporomandibular joint disc. J. Oral Maxillofac. Surg. 2005 doi: 10.1016/j.bjoms.2005.05.002. in press. [DOI] [PubMed] [Google Scholar]
- 23.Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch. Biochem. Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 24.Poole CA, Glant TT, Schofield JR. Chondrons from articular cartilage. IV. Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J. Histochem. Cytochem. 1991;39:1175. doi: 10.1177/39.9.1717545. [DOI] [PubMed] [Google Scholar]
- 25.Sorrell JM, Carrino DA, Baber MA, Asselineau D, Caplan AI. A monoclonal antibody which recognizes a glycosaminoglycan epitope in both dermatan sulfate and chondroitin sulfate proteoglycans of human skin. Histochem. J. 1999;31:549. doi: 10.1023/a:1003896124595. [DOI] [PubMed] [Google Scholar]
- 26.Korvick D, Athanasiou KA. Variations in the mechanical properties of cartilage from the canine scapulohumeral joint. Am. J. Vet. Res. 1997;58:949. [PubMed] [Google Scholar]
- 27.Mak AF, Lai WM, Mow VC. Biphasic indentation of articular cartilage. I. Theoretical analysis. J. Biomech. 1987;20:703. doi: 10.1016/0021-9290(87)90036-4. [DOI] [PubMed] [Google Scholar]
- 28.Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage. II. A numerical algorithm and an experimental study. J. Biomech. 1989;22:853. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]