Abstract
Brain network activity associated with altered motor control in individuals with chronic pain is not well understood. Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) is a debilitating condition in which previous studies have revealed altered resting pelvic floor muscle activity in men with CP/CPPS compared to healthy controls. We hypothesized that the brain networks controlling pelvic floor muscles would also show altered resting state function in men with CP/CPPS. Here we describe the results of the first test of this hypothesis focusing on the motor cortical regions, termed pelvic-motor, that can directly activate pelvic floor muscles. A group of men with CP/CPPS (N = 28), as well as group of age-matched healthy male controls (N = 27), had resting state functional magnetic resonance imaging scans as part of the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network study. Brain maps of the functional connectivity of pelvic-motor were compared between groups. A significant group difference was observed in the functional connectivity between pelvic-motor and the right posterior insula. The effect size of this group difference was among the largest effect sizes in functional connectivity between all pairs of 165 anatomically-defined subregions of the brain. Interestingly, many of the atlas region pairs with large effect sizes also involved other subregions of the insular cortices. We conclude that functional connectivity between motor cortex and the posterior insula may be among the most important markers of altered brain function in men with CP/CPPS, and may represent changes in the integration of viscerosensory and motor processing.
Highlights
-
•
We studied men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
-
•
First resting state neuroimaging comparison CP/CPPS and healthy controls (HC)
-
•
Motor cortex connectivity to insula distinguishes CP/CPPS from HC.
-
•
Motor cortex connectivity to insula is among largest changes in CP/CPPS resting brain.
-
•
Results provide additional evidence of motor network changes in chronic pain.
1. Introduction
A large body of literature suggests that pain affects muscle activity. Altered muscle activity in regions affected by chronic pain have been reported for patients with a wide range of chronic pain conditions, including low back pain (Arendt-Nielsen et al., 1996; Leinonen et al., 2001), temporomandibular joint disorder (Castroflorio et al., 2012), and chronic pelvic pain (Hetrick et al., 2006; Jantos, 2008). Recent evidence suggests that altered muscle activity in chronic pain may be related to changes in motor cortical structure and function (Tsao et al., 2008; Jacobs et al., 2010; Seminowicz et al., 2011; Wand et al., 2011; Baliki et al., 2012). However, large-scale brain networks contributing to altered motor cortical function in individuals with chronic pain remain poorly understood.
A number of previous studies have identified abnormalities in pelvic floor muscle activity in men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS), even during supine resting (Hellstrom et al., 1987; Anderson et al., 2005; Cornel et al., 2005; Hetrick et al., 2006; FitzGerald et al., 2009; Davis et al., 2011). CP/CPPS is a debilitating condition affecting men in which sufferers report persistent pain associated with many fundamental activities of daily living — including bladder function, sitting, and sexual activity — and report lower quality-of-life compared to other prevalent chronic conditions (Allsop et al., 2011). A number of studies have indicated that CP/CPPS has a worldwide prevalence of 2–10% (Collins et al., 1998; Krieger et al., 2002; Bartoletti et al., 2007; Marszalek et al., 2009), indicating that it is a major healthcare problem with significant economic and social cost. CP/CPPS is currently a symptom-based diagnosis, and the etiology of the disorder remains poorly understood. To further study the benign urologic conditions of CP/CPPS and interstitial cystitis/bladder pain syndrome (IC/BPS), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the U.S. National Institutes of Health (NIH) initiated the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network in 2008 (Clemens et al., 2014; Landis et al., 2014). The MAPP Research Network collected clinical, biomarker, and neuroimaging data from a large cohort of individuals with chronic pelvic pain — results of an analysis of neuroimaging data from the MAPP Network are described in this manuscript.
We hypothesized that men with CP/CPPS may have altered brain network function related to pelvic floor control. Based on previous studies relating pelvic floor muscle activity to CP/CPPS pain intensity (Cornel et al., 2005; Davis et al., 2011), we further hypothesized that function in the brain network of pelvic floor control might further change with CP/CPPS pain intensity. Functional connectivity among brain regions, as derived from functional magnetic resonance imaging (fMRI) data of participants at rest, has emerged as an important tool to quantify the interaction among different brain regions in health and disease, including chronic urogenital pain conditions (Kilpatrick et al., 2014). We first addressed our hypothesis in this manuscript by comparing the functional connectivity of a motor cortical region — that directly controls the pelvic floor — between men with CP/CPPS and healthy controls (HC). We then sought to define the importance of motor cortical changes by determining if group differences of effect sizes larger than motor cortical functional connectivity could be found by a global screen of functional connectivity between pairs of 165 anatomically-defined brain regions. The results to be described below show that chronic pain can affect the interaction between motor cortical areas controlling painful body regions and distant non-motor cortical brain regions.
2. Methods
2.1. Participants
fMRI and questionnaire data from 69 men were analyzed in this study. A cohort of HC (n = 14), recruited outside of the MAPP Network at the University of Southern California (the USC cohort), performed a task-based neuroimaging procedure to localize nodes in the normal brain network of pelvic floor control. Resting state neuroimaging procedures (and no task-based procedures) were performed in men with CP/CPPS (n = 28) and HC (n = 27) as part of the MAPP Research Network study (the MAPP cohort). Two MAPP recruiting sites, Northwestern University (NU) and the University of California, Los Angeles (UCLA), recruited and performed neuroimaging on a significant number of men with CP/CPPS and were included in the analysis for this manuscript. For eligibility, CP/CPPS patients had to report an unpleasant sensation of pain, pressure, or discomfort perceived to be related to the bladder and/or pelvic region for most of the time during the most recent 3 months. HC were recruited by community advertisements and reported an absence of current pain problems and no history of chronic pain in the pelvic or bladder region. At each site, the Institutional Review Board approved the study. All participants provided informed consent.
2.2. Questionnaire data
All participants in the MAPP cohort completed the validated Genitourinary Pain Index (GUPI), which measures the intensity of CP/CPPS symptoms in domains of urinary function, pain, and quality-of-life (Clemens et al., 2009). To assess pain localization, MAPP cohort participants also completed the Brief Pain Inventory (BPI) pain map indicating yes or no to the presence of pain in 45 pre-defined body regions (Cleeland and Ryan, 1994). To assess recent history of urologic and non-urologic pain in MAPP cohort participants, each participant rated the severity of their urologic or pelvic pain symptoms over the past 2 weeks on a 0–10 scale as well as the severity of non-urologic or pelvic pain symptoms (e.g. back pain, headache) over the past 2 weeks on a 0–10 scale.
2.3. Overview of rationale
BPI pain map data from the MAPP cohort indicated that men with CP/CPPS report pain focused in the pelvic floor region, and not in the extremities such as the hand (see Results section and Fig. 1). The rationale for our analysis of MAPP cohort neuroimaging data were therefore to compare the whole-brain functional connectivity of two regions-of-interest (ROI): motor cortex more associated with a painful body region (pelvic floor) and motor cortex more associated with a non-painful body region (e.g. hand). However, to our knowledge, there is no existing data set in which the same set of men on the same scanner performed pelvic floor and hand muscle contractions. We obtained the needed ROI by re-analyzing recently published fMRI data from our laboratory on pelvic floor muscle control in healthy men (Asavasopon et al., 2014), with the methods of our re-analysis described below.
Fig. 1.
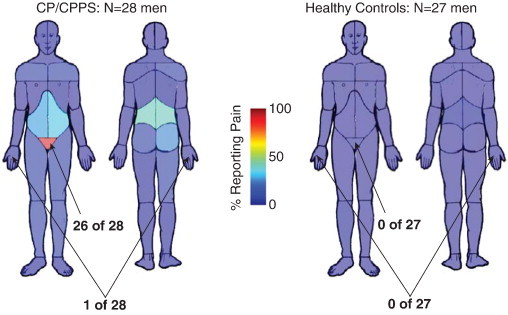
Spatial distribution of CP/CPPS pain in the MAPP cohort. Body maps of pain for the MAPP patients with CP/CPPS and MAPP healthy controls (HC) used in the neuroimaging analysis. Each participant filled out a body map indicating yes or no to pain in the 45 body regions define by the image above. Color maps indicate the percentage of participants indicating pain in each region in each cohort. 26 of 28 men with CP/CPPS indicated pain in the pubic/perineal region, but only 1 of 28 men with CP/CPPS indicated pain in the right hand. By contrast, none of 27 HC indicated either pubic/perineal pain or pain in the right hand.
2.4. Pelvic floor motor cortical representation localization task procedure
The task-based fMRI procedure to localize brain regions associated with pelvic floor muscle and right hand muscle contractions have been described previously (Asavasopon et al., 2014). Briefly, using a video projection screen, we cued participants to voluntarily contract the pelvic floor and the first dorsal interosseous (FDI) muscle of the hand (both to approximately 20% effort) in separate runs consisting of six 30 s blocks of 10 repeated contractions interspersed with 30 s blocks of rest.
2.5. MAPP resting state procedures
MAPP resting state neuroimaging procedures have been described previously (Kilpatrick et al., 2014). Briefly, before entering the scanner, subjects were asked to empty their bladder. During the 10 minute resting state scan, participants in the MAPP cohort were asked to rest with eyes closed without going to sleep.
2.6. fMRI acquisition and preprocessing
The USC cohort was imaged using a 3 Tesla scanner (GE Signa Excite) with an 8-channel head coil (Asavasopon et al., 2014). We positioned participants supine viewing a fixation crosshair, and placed foam pads to limit head motion. As in previous fMRI studies of pelvic floor muscle contraction (Schrum et al., 2011), we collected T2-weighted echo planar image volumes with blood oxygen level dependent (BOLD) contrast (echo time 34.5 ms, flip angle 90°, field of view 220 mm, pixel size 3.43 mm) continually every 2.5 s during 3 imaging runs. Each volume consisted of 37 axial slices (3 mm slice thickness, 0.5 mm interslice gaps) that covered the brain from vertex to cerebellum. We additionally acquired a T1-weighted anatomical image from each participant.
The MAPP cohort was imaged using a 3 Tesla scanners (Siemens Trio) at NU and UCLA according to the following procedures. A high resolution structural image was acquired from each subject with a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence, with repetition time (TR) = 2200 ms, echo time (TE) = 3.26 ms, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 13 mm voxel size. Resting state scans were acquired while subjects rested with eyes closed for 10 min in 40-slice whole brain volumes, with slice thickness = 4 mm, TR = 2000 ms, TE = 28 ms, and flip angle = 77°. MAPP neuroimaging data were collected, quality controlled (independently of the authors) and archived according to multi-site imaging procedures (PAINrepository.org).
USC cohort and MAPP cohort fMRI data were preprocessed using the FMRIB Expert Analysis Tool (FEAT, http://www.fmrib.ox.ac.uk) (Jenkinson et al., 2012), which included skull extraction using the brain extraction tool (BET) in FSL, slice timing correction, motion correction, spatial smoothing using a Gaussian kernel with full-width half-maximum of 5 mm and nonlinear high-pass temporal filtering (100 s).
2.7. Task-based analysis to determine regions of interest
We used a general linear model (GLM) to examine the changes in BOLD signal associated with muscle contraction for the pelvic floor and the FDI (right hand). We performed participant-level whole-brain GLM analyses of individual runs in each participant to determine the regression coefficients during the muscle contraction blocks compared to the rest blocks for both the pelvic floor and FDI. We then performed a group-level mixed-effect (FLAME 1 in FSL) analysis to identify voxels in standard Montreal Neurological Institute (MNI) coordinates with significant differences in regression coefficients between the two tasks: voxels that were more associated with pelvic floor contraction (pelvic floor > right hand) and voxels that were more associated with right FDI contraction (right hand > pelvic floor). We thresholded group-level images with cluster-based correction for multiple comparisons with Z > 2.3 and p < 0.05. To extract motor cortical ROI from the activation maps we first extracted the probabilistic maps of precentral and postcentral gyri from Harvard–Oxford Cortical Structural Atlas within FSL, and then defined motor cortical voxels as those for which the probability of belonging to precentral gyrus exceeded the probability of belonging to postcentral gyrus. ROI were then derived within motor cortex as the centroid of the cluster where pelvic floor > right hand was significant (termed pelvic-motor) and the centroid of the cluster where right hand > pelvic floor (termed hand-motor).
2.8. Functional connectivity analysis of resting state data
We performed functional connectivity analyses on MAPP resting state data as a multi-level GLM using FSL according to established pre-processing methods used previously (Roy et al., 2009; Baliki et al., 2012). Briefly, resting state data from each participant in the MAPP cohort was preprocessed according to the following procedures: slice time correction, motion correction, spatial smoothing using a Gaussian kernel of full-width half-maximum of 5 mm and nonlinear high-pass temporal filtering (150 s). At the individual-participant level, we performed a motor connectivity analysis by contrasting the whole-brain connectivity of signals derived from 10 mm-spheres centered at the pelvic-motor and hand-motor ROI. We controlled for several sources of noise by including the following covariates in the participant-level functional connectivity analysis: six parameters obtained by rigid body correction of head motion, the whole-brain signal averaged over all voxels of the brain, a signal from a ventricular ROI, and a signal from a white matter ROI. We performed group-level analyses using FLAME 1, contrasting the participant-level contrasts between HC and men with CP/CPPS. We assessed significance with cluster-based corrections for multiple comparisons using Gaussian random field theory using typical thresholds (Z > 2.3; cluster significance: p < 0.05, corrected). All participants in the MAPP cohort, from both scanning sites, were included in the group-level analysis.
Regression coefficients were averaged across significant clusters to define a single outcome variable for each participant for each significant cluster.
2.9. Statistical analysis of behavioral variables
A multiple linear regression model was used to determine, in the CP/CPPS cohort, the effect of symptom scores in different domains (pain symptoms, urinary symptoms, and quality-of-life) from the GUPI questionnaire on functional connectivity outcome variable described in the previous section. Symptom scores in each domain were converted into standard scores within the CP/CPPS cohort prior to regression.
2.10. Functional connectivity analyses beyond pelvic-motor
To address the importance and robustness of functional connectivity changes with the pelvic-motor seed, we performed two additional analyses. First, to screen for non-motor regions of potentially important functional connectivity changes in men with CP/CPPS, and compare them to altered pelvic-motor connectivity, we performed a whole-brain analysis by extracting signals of interest from 165 anatomically-defined ROI within the Destrieux structural atlas (Destrieux et al., 2010; Irimia et al., 2012). This atlas has been used previously to extract functional signals from anatomical ROI (Han et al., 2014; Hong et al., 2014). Resting state preprocessing in the atlas-based functional connectivity analysis was identical to that described above. Each of the 165 regions, defined in standard MNI coordinates, were mapped into participant-specific functional space by the same linear transformation used to map the pelvic-motor and hand-motor ROI in the seed-based functional connectivity analysis described above. Signals from all functional voxels within each of the 165 regions were averaged to create a temporal activity profile for each region. A GLM was then constructed — using the same confounds of motion parameters, global signal, white matter signal, and ventricular signal described above — to assess the strength of functional connectivity in the temporal activity profile among pairs of regions. For all pairs of 165 regions, the effect size (and 95% confidence interval) was calculated for the group difference in regression coefficients relating the activity in the pair of regions. Effect size was quantified using Hedges' g formula, implemented in the Measures of Effect Size Toolbox in MATLAB (Hentschke and Stüttgen, 2011). Connectivity between regional pairs was visualized using the Circos software package (Krzywinski et al., 2009).
Second, to assess stability of altered functional connectivity of motor cortex in CP/CPPS, we perturbed the pelvic-motor ROI in CP/CPPS patients (not in HC) by 10 mm in the anterior, posterior, left, and right directions. 10 mm shifts were chosen because a previous study has found approximately 1 cm changes in cortical representation of trunk muscles in patients with chronic low back pain (Tsao et al., 2008).
3. Results
3.1. Participant characteristics
Table 1 lists the characteristics for the participants in the MAPP Network Study. Men with CP/CPPS in the MAPP cohort expressed heightened indications of pain in specific regions on a body map (Fig. 1). Men with CP/CPPS were very likely to indicate pain in the pubic/perineal region (26/28, 93%), with a lower likelihood of indicating abdominal (9/28, 32%) or low back (12/28, 43%) pain. By contrast, men with CP/CPPS were unlikely to indicate more widespread pain across the rest of the body, with a maximum of 4/28 (14%) reporting pain in a region outside of the pelvic/low back/abdominal/gluteal group of regions — these non-pelvic regions included the upper thigh and shoulder area. For the purposes of our fMRI analysis to be described next, we noticed that only 1/28 (3%) of men with CP/CPPS indicated right hand pain. None of the men in the healthy control group of the MAPP cohort reported either pubic/perineal pain or right hand pain. Most men in the HC group did not report any non-urologic pain symptoms in the 2 weeks prior to scan (22/27), and importantly no men in the HC group reported non-urologic symptoms as intense as the average intensity of urologic symptoms in the CP/CPPS cohort (3.85/10). Men in the USC cohort did not report any history of pain in any body region, but were younger on average (mean age ± SE of 32.7 ± 1.5) than the HC in the MAPP cohort. We therefore tested for age-dependence within the pelvic floor motor cortical representation localization results: we found that regression coefficients for the contrast (pelvic floor > rest) within the pelvic-motor region did not depend on age (p = 0.21), and the regression coefficients for the contrast (right hand > rest) within the hand-motor region similarly did not depend on age (p = 0.68).
Table 1.
MAPP male participant characteristics.
| Group | No. pts | Mean ± SE age (years) | Mean ± SE duration (years) | Mean ± SE GUPI |
|
|---|---|---|---|---|---|
| Total | Pain | ||||
| Overall | |||||
| Control | 27 | 43.4 ± 2.7 | Not applicable | 2.1 ± 0.8 | 0.3 ± 0.2 |
| CP/CPPS | 28 | 42.0 ± 2.9 | 8.9 ± 2.1 | 23.5 ± 1.4 | 11.6 ± 0.8 |
| UCLA: | |||||
| Control | 16 | 42.6 ± 3.2 | Not applicable | 2.2 ± 0.9 | 0.4 ± 0.3 |
| CP/CPPS | 16 | 37.3 ± 3.5 | 9.1 ± 2.9 | 23.6 ± 2.1 | 11.2 ± 1.0 |
| NWU | |||||
| Control | 11 | 44.4 ± 5.2 | Not applicable | 2.0 ± 1.4 | 0.1 ± 0.1 |
| CP/CPPS | 12 | 48.1 ± 4.3 | 8.6 ± 3.2 | 23.4 ± 1.9 | 12.4 ± 1.2 |
3.2. Distinct regions-of-interest for pelvic floor muscle and hand muscle contractions
Following the pain localization results described above, we next localized regions of motor cortex (precentral gyrus) associated with pelvic floor muscle contractions and hand muscle contractions in the USC cohort of healthy men. Brain activity during right hand muscle contractions (compared to pelvic floor contractions) was localized in the left motor cortex (Fig. 2A). Brain activity during pelvic floor muscle contractions (compared to right hand muscle contractions) were localized in more medial aspects of motor cortex (Fig. 2B). Coordinates corresponding to the centroid of activation within motor cortex for the hand muscles and pelvic floor muscles were derived from motor cortex: pelvic-motor (4, −26, 66 mm), hand-motor (−34, −20, 62 mm) (Fig. 2C). The pelvic-motor representation was found to be slightly right of midline; previous literature has noted lateralization in the motor representation of the pelvic floor (Turnbull et al., 1999).
Fig. 2.
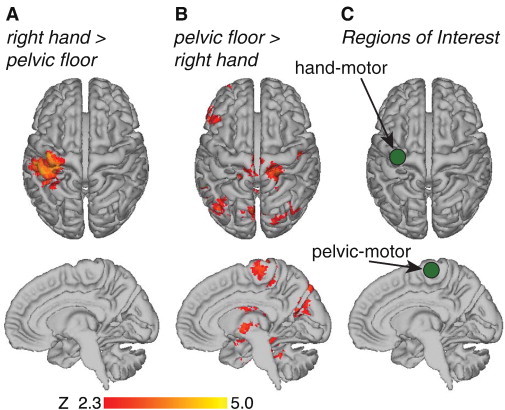
Selection of ROI (regions-of-interest) for analysis of MAPP resting state fMRI data 14 healthy men (USC cohort) were recruited to localize brain regions preferentially associated with voluntary hand muscle contraction, and preferentially associated with voluntary pelvic floor muscle contraction. A. Right hand > pelvic floor shows brain regions where there was significantly more activity during right index finger muscle contraction compared to pelvic floor muscle contraction. B. Pelvic floor > right hand shows brain regions where there was significantly more activity during pelvic floor muscle contraction compared to right index finger muscle contraction. C. 10-mm radius spherical ROI, represented as green circles, were derived within motor cortex (precentral gyrus) to form the pelvic-motor ROI and the hand-motor ROI.
3.3. Altered connectivity between pelvic-motor and right posterior insula
The resting state functional connectivity of pelvic-motor (painful body region) was altered relative to the functional connectivity of hand-motor (non-painful body region) in men with CP/CPPS compared to HC. This alteration in the difference of functional connectivity regression coefficients (β) occurred in a well-confined cluster in the right posterior insular cortex (Fig. 3A). The cluster of altered functional connectivity had 736 voxels and a peak z-score of 4.35 (Table 2). The region of altered functional connectivity also included regions of the superior temporal gyrus (BA 22) and inferior frontal gyrus (BA 44). The difference in regression coefficients, β(pelvic-motor) minus β(hand-motor), were averaged across all 736 voxels in the right posterior insular cortex cluster to derive an outcome measure of pelvic-motor/posterior-insula functional connectivity for each participant. An ANOVA analysis of this outcome measure using patient status and neuroimaging site as factors (Fig. 3B) revealed a significant main effect of patient status (p < 0.0001), no significant main effect of site (p > 0.05), and no significant interaction between patient status and site (p > 0.05). A post-hoc multiple comparison test (with Kramer–Tukey correction for multiple comparisons) revealed that pelvic-motor/posterior-insula functional connectivity was significantly less (p < 0.05) in men with CP/CPPS at both the Northwestern University imaging site and the UCLA imaging site, while there were no significant difference between the sites in either HC or men with CP/CPPS (Fig. 3B). The difference in functional connectivity regression coefficients was positive for HC (p = 0.007, two-sided t-test) and negative for men with CP/CPPS (p = 0.0005, two-sided t-test).
Fig. 3.
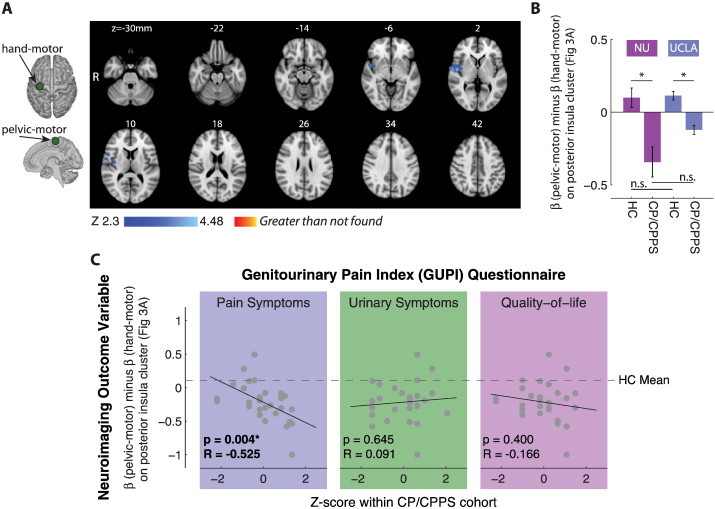
Altered pelvic-motor connectivity in men with CP/CPPS. A. The functional connectivity of pelvic floor motor cortex (pelvic-motor), relative to right hand motor cortex (hand-motor), was significantly different between men with CP/CPPS and healthy controls (HC). Relative to hand-motor, pelvic-motor has less functional connectivity with posterior insular cortex (blue regions) in men with CP/CPPS compared to HC. No regions to which pelvic-motor had increased functional connectivity in men with CP/CPPS compared to HC were identified. B. No significant differences between the two sites (Northwestern University (NU) and UCLA) were identified in the functional connectivity between the motor cortical regions and the posterior insula cluster identified in A. C. Functional connectivity between motor cortex and the posterior insular cortex contributes to differences in pain among men with CP/CPPS. Genitourinary Pain Index (GUPI) score in the domains of pain symptoms, urinary symptoms, and quality-of-life (as standard scores) at time of scan plotted against functional connectivity difference between pelvic-motor and hand-motor with the posterior insular cortex cluster identified to be significantly different between men with CP/CPPS and HC (A). This functional connectivity difference was significantly associated with pain (p = 0.004, R = −0.525) in the CP/CPPS group.
Table 2.
Location, extent, and significance of each local maxima with altered functional connectivity to pelvic floor motor cortex (PFMC) in men with CP/CPPS compared to healthy controls (HC). MNI coordinates in mm.
| Region (hemisphere) |
Peak coordinates |
Cluster size |
Z-score |
GUPI-pain regression |
|---|---|---|---|---|
| Connectivity seed: pelvic-motor (referenced to hand-motor) |
||||
| Less in CP/CPPS vs. HC | ||||
| Posterior insular cortex (right) | 50, −6, 2 | 736 | 4.35 | p = 0.046, R = −0.38 |
| Posterior insular cortex, Brodmann area 13 (right) | 48, 2, −6 | Same cluster | 3.72 | p = 0.074, R = −0.34 |
| Superior temporal gyrus, Brodmann area 22 (right) | 66, −2, 6 | Same cluster | 3.66 | p = 0.224, R = −0.24 |
| Superior temporal gyrus, Brodmann area 22 (right) | 52,2,−2 | Same cluster | 3.59 | p = 0.037, R = −0.40 |
| Posterior insular cortex (right) | 42, −4, 4 | Same cluster | 3.36 | p = 0.016, R = −0.45 |
| Inferior frontal gyrus, Brodmann area 44 (right) | 56, 14, 8 | Same cluster | 2.98 | p = 0.204, R = −0.25 |
We analyzed the functional connectivity regression coefficients for pelvic-motor and hand-motor to the right posterior insula cluster identified to explore the contribution of the separate ROI to the difference between HC and men with CP/CPPS. Within the right posterior insula cluster, we performed an ANOVA of the functional connectivity regression coefficient using ROI (pelvic-motor or hand-motor) and cohort (CP/CPPS or HC) as factors. There was no significant main effect of either ROI or cohort, but there was a significant interaction of ROI and cohort (p = 0.0001). A post-hoc test, with Kramer–Tukey correction for multiple comparisons, revealed that there were significant differences (p < 0.05) in right posterior insula functional connectivity regression coefficient for the pelvic-motor ROI (average of 0.04 for HC and −0.03 for men with CP/CPPS) and for the hand-motor ROI (average of −0.004 for HC and 0.08 for men with CP/CPPS).
3.4. Pelvic-motor connectivity to the right posterior insula is related to pain intensity in CP/CPPS
We found that pelvic-motor/posterior-insula functional connectivity described in the previous section, while identified simply by comparing HC and men with CP/CPPS, also made a significant contribution to variance in subjective pain across the cohort of men with CP/CPPS (Fig. 4). Multiple linear regression indicated that only pain symptoms, and not urinary symptoms or quality-of-life, had a significant (p < 0.05) impact on the altered functional connectivity between motor and insular cortices in the CP/CPPS cohort, such this connectivity deviated more from the healthy control average in men with more intense pain symptoms (p = 0.004, R = −0.525). Pelvic-motor/posterior-insula functional connectivity was also analyzed at local maxima within the 736 voxel cluster for evidence of pain-dependence, and all local maxima suggested a decrease in functional connectivity with increasing score on the GUPI pain subscale (Table 2).
Fig. 4.
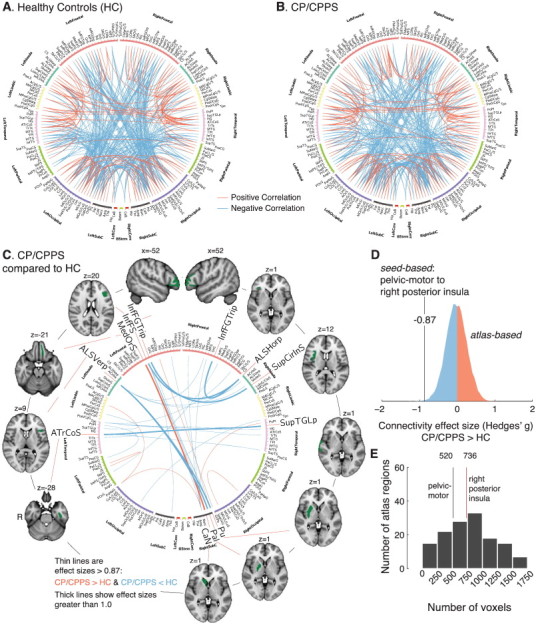
Whole-brain connectivity among all regions in the Destrieux anatomical atlas in men with CP/CPPS and healthy controls (HC). A. Connectivity in the HC group. B. Connectivity in the CP/CPPS group. For clarity, only the strongest 1% of functional connectivity pairs are shown in A and B. C. Connections between atlas region pairs for which the functional connectivity may be affected by group (CP/CPPS vs. HC). Regional pairs with effect size greater than functional connectivity change between pelvic-motor and the right posterior insula (−0.87) in magnitude are shown, with pairs for which effect size was greater than 1 emphasized with stronger line weight. D. The functional connectivity between pelvic-motor and the right posterior insula (Fig. 3A) had an associated effect size greater than the vast majority of atlas-based region pairs. E. Confirmation that the pelvic-motor and right posterior insula cluster had comparable sizes to regions in the structural atlas.
3.5. Altered sensorimotor connectivity compared to whole-brain connectivity analysis screen
We found that HC men (Fig. 4A) and men with CP/CPPS (Fig. 4B) displayed an approximately similar pattern of functional connectivity among pairs of regions defined by the Destrieux anatomical atlas. However, the comparison of HC and CP/CPPS suggested possible group differences (Fig. 4C). The largest effect size magnitudes of all possible pairs of regions was 1.01 (left medial orbital sulcus to right caudate) and −1.2 (left vertical ramus of the anterior segment of the lateral sulcus to right putamen). Of the 7 region pairs with connectivity effect sizes for the difference between CP/CPPS and HC greater than 1 in magnitude, 3 pairs involved frontoinsular connectivity, 3 involved the right basal ganglia connectivity, and 1 involved bilateral temporal cortex connectivity (Fig. 4C). The effect size of the functional connectivity change in CP/CPPS patients between pelvic-motor and the right posterior insula (Hedges' g = −0.87) was among the top 6% of significant (p < 0.05) pair-wise effect sizes in the change in functional connectivity among atlas regions (Fig. 4D). The sizes of pelvic-motor (520 voxels) and the right posterior insula cluster (736 voxels) were not widely different compared to sizes of regions in the atlas, suggesting that the results of the hypothesis-based seed approach and the hypothesis-free atlas are approximately comparable (Fig. 4E).
3.6. Robustness of right posterior insula finding
Moving the pelvic-motor seed in the CP/CPPS group of the MAPP cohort demonstrated that the right posterior insula cortex cluster of altered functional connectivity displayed robustness to perturbations of the seed region (Fig. 5A). We found a nearly identical cluster of altered connectivity in the right posterior insula if the same pelvic-motor region was used in CP/CPPS and HC (Fig. 5B), if the pelvic-motor region was shifted posteriorly in the CP/CPPS group (Fig. 5C), or if the pelvic-motor region was shifted left in the CP/CPPS group (Fig. 5D). The right posterior insula cortex connectivity cluster did not appear when pelvic-motor was shifted anteriorly (Fig. 5E) or right (Fig. 5F) in the CP/CPPS group (see Discussion section).
Fig. 5.
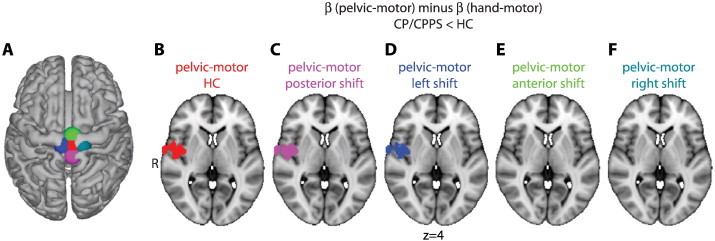
Sensitivity analysis of right posterior insula result. A. The location of pelvic-motor was moved several different locations for the CP/CPPS cohort, and the comparison with healthy controls (HC) of Fig. 3 was repeated. B. Pelvic-motor HC shows the connectivity assuming the same pelvic-motor ROI in both men with CP/CPPS and HC, and is identical to cluster shown in Fig. 3A. Altered connectivity between motor cortex and the right posterior insula cluster persisted when the pelvic-motor was moved 10 mm posterior (C) or 10 mm left (D), but disappeared when pelvic-motor was moved 10 mm anterior (E) or 10 mm right (F).
4. Discussion
To our knowledge, this is the first report of abnormalities in resting state brain activity measures in men with CP/CPPS compared to healthy male controls. Our results reveal disease related alterations in the functional connectivity between the pelvic part of motor cortex (pelvic-motor) and the right posterior insula. Strikingly, not only was the pelvic-motor/posterior-insula functional connectivity different between men with CP/CPPS and HC, it also made a significant contribution to explaining variation in the subjective report of pain intensity in the CP/CPPS cohort. Altered functional connectivity of pelvic-motor to some brain region was hypothesized a priori based on previous literature in CP/CPPS. However, a more hypothesis-free analysis between all possible pairs of regions from a structural brain atlas revealed that the pelvic-motor/posterior-insula connectivity is likely among the largest effects that would be expected in a resting state functional connectivity comparison of men with CP/CPPS and HC. Interestingly, the hypothesis-free analysis independently pointed to other insula subregions, and to frontoinsular connections, as an important ROI in CP/CPPS, consistent with previous task-based fMRI literature (Farmer et al., 2011). Here we discuss interpretations of our findings, possible limitations suggesting future work, and potential clinical implications of our results.
Previous brain imaging studies of men with CP/CPPS had importantly identified brain structural differences in pain related regions (Farmer et al., 2011; Mordasini et al., 2012), and a neural correlate in the right anterior insula of spontaneous pain in men with CP/CPPS (Farmer et al., 2011). Our study is the first to identify brain activity changes in men with CP/CPPS compared to HC, and points toward an important role for brain motor control in this disorder, consistent with previous findings of altered pelvic floor muscle activity (Hellstrom et al., 1987; Cornel et al., 2005; Hetrick et al., 2006; Davis et al., 2011). Our results are also consistent with several studies demonstrating the importance of primary motor cortex for pain processing. For example, primary motor cortex appears to process nociceptive signals in parallel with primary sensory cortex (Frot et al., 2013), and there is emerging evidence that repetitive transcranial magnetic stimulation over motor cortex has analgesic effects (Lefaucheur et al., 2014), including in patients with chronic pelvic pain (Louppe et al., 2013).
One hypothesis that would explain our findings is that functional connectivity is altered by changes in direct neural communication between right posterior insular cortex and motor cortex. The insula is currently understood to have a posterior-to-mid-to-anterior integration of interoceptive information (Craig, 2011). Primary interoceptive representations of viscerosensory information are believed to be concentrated in the posterior insula, with progressive integration with cognitive and affective aspects and resultant subjective awareness of this interoceptive information developing in the anterior insula. Insular cortex plays a more important role during voluntary pelvic floor muscle contraction compared to other lower limb muscles (Schrum et al., 2011), with posterior insula possibly relaying necessary sensory information to motor cortical structures for control of muscles associated with the viscera (Deen et al., 2011; Cauda et al., 2011; Levinthal & Strick, 2012). The existence of an important functional connection between pelvic-motor cortex and the posterior insula is further supported by our finding that HC participants in the MAPP cohort had significantly greater functional connectivity between pelvic-motor and the posterior insula compared to hand-motor, suggesting that this connection is important in the healthy human male brain. If this hypothesis is correct, the altered functional connectivity between posterior insular cortex and motor cortex controlling the pelvic floor in CP/CPPS patients may relate to a change in the brain connections mediating the sensorimotor communication between these areas.
Screening a large number of functional connections between 165 regions in the Destrieux structural atlas for changes in men with CP/CPPS also indirectly pointed to insular cortex as an important contributor to the difference between the CP/CPPS and HC groups. Specifically, as noted in the results, 3 of 7 pairs with group difference effect size greater than 1.0 involved involved frontoinsular connectivity, all of which were focused on the right insula. Even though the reason for the observed group differences in these frontoinsular connections remains to be determined, it is of interest that the frontoinsular region of the brain, in particular in the right hemisphere, has been shown to be associated with sympathetic autonomic control (Allman et al., 2011). Since changes in autonomic function have been identified in men with CP/CPPS (Anderson et al., 2008; Dimitrakov et al., 2008; Anderson et al., 2009), future research can examine the association between frontoinsular connectivity and measures of altered autonomic function.
Our current study has some limitations. As a single-observation study, we focused our analysis on overall group differences rather than controlling for the effects of other factors, such as treatment, age, and chronicity of symptoms. Another limitation is that the MAPP Research Network study was exploratory in nature and designed to collect general brain anatomical and functional data as well as participant demographic, symptom, and biomarker data — no measures of muscle activity were collected which could have defined a possible peripheral correlate of altered functional connectivity of the motor cortex in men with CP/CPPS. Furthermore, participants in the MAPP study did not perform pelvic floor contractions (or contractions of other muscles) in the scanner, so we were unable to use patient-specific ROI. We compensated for this limitation by carefully localizing the normal cortical representation of pelvic floor and hand contractions in a new sample of healthy men, and using relatively large ROI (2 cm in diameter) to develop a first-order map of neuromotor pathology in men with CP/CPPS. It is surprising that group differences emerged at this level of spatial resolution, and suggest that future studies with better localization of sensorimotor regions in men with CP/CPPS may even further increase the specificity of our findings. We have recently demonstrated, in concert with previous studies, that the pelvic-motor region likely produces corticospinal motor projections as evidenced by very short latency motor evoked potentials (MEP) (Asavasopon et al., 2014). The cortical MEP intensity map of particular muscles can change in individuals with chronic pain (Tsao et al., 2008); thus, future studies of individuals with CP/CPPS must determine if the cortical MEP intensity map of the pelvic floor is changing with respect to HC, and if such changes could contribute to the altered functional connectivity effects observed in this study. As a preliminary analysis of possible changes in motor cortical representation of the pelvic floor in CP/CPPS, we performed a sensitivity analysis by perturbing the pelvic-motor ROI in the CP/CPPS group. Since altered connectivity with the right posterior insula did disappear for some pelvic-motor perturbations in the CP/CPPS group (anterior and right), it is possible that our results might be explained by shifts of the normal motor representation of the pelvic floor in CP/CPPS. Nonetheless, since we identified that motor cortical functional connectivity changes in CP/CPPS have relatively large effect sizes, motor cortex is likely an important region of interest in studying CP/CPPS despite the fact that it remains to be determined if motor representation or fundamental communication with the posterior insula is driving the observed changes.
Several critical lines of future work emerge from this study. First, it is important to determine if effective treatment for CP/CPPS functions to normalize motor cortical/insula connectivity in men with CP/CPPS who improve. Second, it is important to determine if baseline levels of motor cortical/insula connectivity could predict response to treatment, even when controlled for baseline levels of pain intensity. Third, it is important to create a more expansive map of brain functional connectivity alterations in men with CP/CPPS, to determine the relative prominence of motor cortical changes, and to relate this map to changes in gray and white matter structure. Finally, defining the pelvic floor muscle activity correlates of brain functional connectivity changes that accompany CP/CPPS symptoms is important for a more complete understanding of how functional connectivity changes contribute to the experience of pain in men with CP/CPPS.
While the current results are limited to men with CP/CPPS, it is possible that our approach and findings have implications for other chronic pain conditions in which motor control is altered. It has been hypothesized that the threat of pain/injury triggers changes in the motor system that leads to a redistribution of activity within and between muscles (Hodges and Tucker, 2011). However, the location of these neural changes have not yet been localized. Our results suggest that areas that do not generate direct corticospinal projections, such as the posterior insula, could play a critical role in modulating motor cortical activity in individuals with chronic pain. Further research is necessary to determine if the identity of these non-motor cortical regions related to chronic pain are general or are specific to different chronic pain conditions.
Acknowledgements
We thank all of the volunteers who participated in the study. We would like to thank Nina Bradley, Bruce Naliboff, and Kirsten Tillisch for helpful discussions. Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316). This work was also supported, in part, by the USC Division of Biokinesiology and Physical Therapy under award number USCBKN/PT-2013A, the Loma Linda University Physical Therapy Department under award number LLU-647525-2007, and National Center for Medical Rehabilitation Research of the National Institutes of Health under award number T32 HD064578. We declare the following interests: financial interest and/or other relationship with Pfizer, Cerephex, Lilly, Merck, Nuvo, Furest, Tonix, Purdue, Therauance and Johnson & Johnson (DJC), financial interest and/or other relationship with National Institutes of Health and Medtronic (TJN), financial interest and/or other relationship with National Institutes of Health (CM).
Appendix A. Supplementary data
The following is the Supplementary data to this article.

The Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network Study Group.
References
- Allman J.M., Tetreault N.A., Hakeem A.Y., Manaye K.F., Semendeferi K., Erwin J.M., Park S., Goubert V., Hof P.R. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann. New York Acad. Sci. 2011;1225:59–71. doi: 10.1111/j.1749-6632.2011.06011.x. 21534993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop S.A., Erstad D.J., Brook K., Bhai S.F., Cohen J.M., Dimitrakoff J.D. The DABBEC phenotyping system: towards a mechanistic understanding of CP/CPPS. Nat. Rev. Urol. 2011;8(2):107–113. doi: 10.1038/nrurol.2010.227. 21243018 [DOI] [PubMed] [Google Scholar]
- Anderson R.U., Orenberg E.K., Chan C.A., Morey A., Flores V. Psychometric profiles and hypothalamic–pituitary–adrenal axis function in men with chronic prostatitis/chronic pelvic pain syndrome. J. Urol. 2008;179(3):956–960. doi: 10.1016/j.juro.2007.10.084. 18207189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.U., Orenberg E.K., Morey A., Chavez N., Chan C.A. Stress induced hypothalamus–pituitary–adrenal axis responses and disturbances in psychological profiles in men with chronic prostatitis/chronic pelvic pain syndrome. J. Urol. 2009;182(5):2319–2324. doi: 10.1016/j.juro.2009.07.042. 19762053 [DOI] [PubMed] [Google Scholar]
- Anderson R.U., Wise D., Sawyer T., Chan C. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J. Urol. 2005;174(1):155–160. doi: 10.1097/01.ju.0000161609.31185.d5. 15947608 [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L., Graven-Nielsen T., Svarrer H., Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. 1996;64(2):231–240. doi: 10.1016/0304-3959(95)00115-8. 8740599 [DOI] [PubMed] [Google Scholar]
- Asavasopon S., Rana M., Kirages D.J., Yani M.S., Fisher B.E., Hwang D.H., Lohman E.B., Berk L.S., Kutch J.J. Cortical activation associated with muscle synergies of the human male pelvic floor. J. Neurosci. 2014;34(41):13811–13818. doi: 10.1523/JNEUROSCI.2073-14.2014. 25297107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Petre B., Torbey S., Herrmann K.M., Huang L., Schnitzer T.J., Fields H.L., Apkarian A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. 22751038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti R., Cai T., Mondaini N., Dinelli N., Pinzi N., Pavone C., Gontero P., Gavazzi A., Giubilei G., Prezioso D., Mazzoli S., Boddi V., Naber K.G., Italian Prostatitis Study Group Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: results of a multicenter case–control observational study. J. Urol. 2007;178(6):2411–2415. doi: 10.1016/j.juro.2007.08.046. 17937946 [DOI] [PubMed] [Google Scholar]
- Castroflorio T., Falla D., Tartaglia G.M., Sforza C., Deregibus A. Myoelectric manifestations of jaw elevator muscle fatigue and recovery in healthy and TMD subjects. J. Oral Rehabil. 2012;39(9):648–658. doi: 10.1111/j.1365-2842.2012.02309.x. 22490056 [DOI] [PubMed] [Google Scholar]
- Cauda F., D'Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55(1):8–23. doi: 10.1016/j.neuroimage.2010.11.049. 21111053 [DOI] [PubMed] [Google Scholar]
- Cleeland C.S., Ryan K.M. Pain assessment: global use of the Brief Pain Inventory. Ann. Acad. Med. Singapore. 1994;23(2):129–138. 8080219 [PubMed] [Google Scholar]
- Clemens J.Q., Calhoun E.A., Litwin M.S., McNaughton-Collins M., Kusek J.W., Crowley E.M., Landis J.R., Urologic Pelvic Pain Collaborative Research Network Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74(5):983–987. doi: 10.1016/j.urology.2009.06.078. 19800663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J.Q., Mullins C., Kusek J.W., Kirkali Z., Mayer E.A., Rodríguez L.V., Klumpp D.J., Schaeffer A.J., Kreder K.J., Buchwald D., Andriole G.L., Lucia M.S., Landis J.R., Clauw D.J., MAPP Research Network Study Group The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. B.M.C. Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. 25085007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M.M., Stafford R.S., O'Leary M.P., Barry M.J. How common is prostatitis? A national survey of physician visits. J. urol. 1998;159(4):1224–1228. 9507840 [PubMed] [Google Scholar]
- Cornel E.B., van Haarst E.P., Schaarsberg R.W.M., Geels J. The effect of biofeedback physical therapy in men with chronic pelvic pain syndrome type III. Eur. Urol. 2005;47(5):607–611. doi: 10.1016/j.eururo.2004.12.014. 15826751 [DOI] [PubMed] [Google Scholar]
- Craig A.D. Significance of the insula for the evolution of human awareness of feelings from the body. Ann. New York Acad. Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. 21534994 [DOI] [PubMed] [Google Scholar]
- Davis S.N., Morin M., Binik Y.M., Khalife S., Carrier S. Use of pelvic floor ultrasound to assess pelvic floor muscle function in urological chronic pelvic pain syndrome in men. J. Sex. Med. 2011;8(11):3173–3180. doi: 10.1111/j.1743-6109.2011.02452.x. 21883952 [DOI] [PubMed] [Google Scholar]
- Deen B., Pitskel N.B., Pelphrey K.A. Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex. 2011;21(7):1498–1506. doi: 10.1093/cercor/bhq186. 21097516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. 20547229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrakov J., Joffe H.V., Soldin S.J., Bolus R., Buffington C.A., Nickel J.C. Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008;71(2):261–266. doi: 10.1016/j.urology.2007.09.025. 18308097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer M.A., Chanda M.L., Parks E.L., Baliki M.N., Apkarian A.V., Schaeffer A.J. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J. urol. 2011;186(1):117–124. doi: 10.1016/j.juro.2011.03.027. 21571326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald M.P., Anderson R.U., Potts J., Payne C.K., Peters K.M., Clemens J.Q., Kotarinos R., Fraser L., Cosby A., Fortman C., Neville C., Badillo S., Odabachian L., Sanfield A., O'Dougherty B., Halle-Podell R., Cen L., Chuai S., Landis J.R., Mickelberg K., Barrell T., Kusek J.W., Nyberg L.M., Urological Pelvic Pain Collaborative Research Network Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J. urol. 2009;182(2):570–580. doi: 10.1016/j.juro.2009.04.022. 19535099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frot M., Magnin M., Mauguière F., Garcia-Larrea L. Cortical representation of pain in primary sensory-motor areas (S1/M1)—a study using intracortical recordings in humans. Hum. Brain Mapp. 2013;34(10):2655–2668. doi: 10.1002/hbm.22097. 22706963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Mac Donald C.L., Johnson A.M., Barnes Y., Wierzechowski L., Zonies D., Oh J., Flaherty S., Fang R., Raichle M.E., Brody D.L. Disrupted modular organization of resting-state cortical functional connectivity in U.S. military personnel following concussive ‘mild. Neuroimage. 2014;84:76–96. doi: 10.1016/j.neuroimage.2013.08.017. 23968735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom W.J.G., Schmidt R.A., Lue T.F., Tanagho E.A. Neuromuscular dysfunction in nonbacterial prostatitis. Urology. 1987;30(2):183–188. doi: 10.1016/0090-4295(87)90193-2. 3497475 [DOI] [PubMed] [Google Scholar]
- Hentschke H., Stüttgen M.C. Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci. 2011;34(12):1887–1894. doi: 10.1111/j.1460-9568.2011.07902.x. 22082031 [DOI] [PubMed] [Google Scholar]
- Hetrick D.C., Glazer H., Liu Y.W., Turner J.A., Frest M., Berger R.E. Pelvic floor electromyography in men with chronic pelvic pain syndrome: a case–control study. Neurourol. urodyn. 2006;25(1):46–49. doi: 10.1002/nau.20162. 16167354 [DOI] [PubMed] [Google Scholar]
- Hodges P.W., Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152(3 Suppl):S90–S98. doi: 10.1016/j.pain.2010.10.020. 21087823 [DOI] [PubMed] [Google Scholar]
- Hong J.Y., Kilpatrick L.A., Labus J.S., Gupta A., Katibian D., Ashe-McNalley C., Stains J., Heendeniya N., Smith S.R., Tillisch K., Naliboff B., Mayer E.A. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J. Neurosci. 2014;34(43):14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. 25339739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Chambers M.C., Torgerson C.M., Van Horn J.D. Circular representation of human cortical networks for subject and population-level connectomic visualization. Neuroimage. 2012;60(2):1340–1351. doi: 10.1016/j.neuroimage.2012.01.107. 22305988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.V., Henry S.M., Nagle K.J. Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clin. Neurophysiol. 2010;121(3):431–440. doi: 10.1016/j.clinph.2009.11.076. 20071225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantos M. Vulvodynia: a psychophysiological profile based on electromyographic assessment. Appl. Psychophysiol. Biofeedback. 2008;33(1):29–38. doi: 10.1007/s10484-008-9049-y. 18214669 [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. 21979382 [DOI] [PubMed] [Google Scholar]
- Kilpatrick L.A., Kutch J.J., Tillisch K., Naliboff B.D., Labus J.S., Jiang Z., Farmer M.A., Apkarian A.V., Mackey S.C., Martucci K.T., Clauw D.J., Harris R.E., Deutsch G., Ness T.J., Yang C.C., Maravilla K., Mullins C., Mayer E.A. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J. urol. 2014;192(3):947–955. doi: 10.1016/j.juro.2014.03.093. 24681331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J., Ross S., Riley D. Chronic prostatitis: epidemiology and role of infection. Urology. 2002;60(6 Suppl):8–12. doi: 10.1016/s0090-4295(02)02294-x. 12521579 [DOI] [PubMed] [Google Scholar]
- Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. 19541911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J.R., Williams D.A., Lucia M.S., Clauw D.J., Naliboff B.D., Robinson N.A., van Bokhoven A., Sutcliffe S., Schaeffer A.J., Rodriguez L.V., Mayer E.A., Lai H.H., Krieger J.N., Kreder K.J., Afari N., Andriole G.L., Bradley C.S., Griffith J.W., Klumpp D.J., Hong B.A. The MAPP research network: design, patient characterization and operations. B.M.C. Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. 25085119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J.P., André-Obadia N., Antal A., Ayache S.S., Baeken C., Benninger D.H., Cantello R.M., Cincotta M., de Carvalho M., de Ridder D., Devanne H., Di Lazzaro V., Filipović S.R., Hummel F.C., Jääskeläinen S.K., Kimiskidis V.K., Koch G., Langguth B., Nyffeler T., Oliviero A. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin. Neurophysiol. 2014;125(11):2150–2206. doi: 10.1016/j.clinph.2014.05.021. 25034472 [DOI] [PubMed] [Google Scholar]
- Leinonen V., Kankaanpää M., Luukkonen M., Hänninen O., Airaksinen O., Taimela S. Disc herniation-related back pain impairs feed-forward control of paraspinal muscles. Spine (Phila. Pa. 1976) 2001;26(16):E367–E372. doi: 10.1097/00007632-200108150-00014. 11493866 [DOI] [PubMed] [Google Scholar]
- Levinthal D.J., Strick P.L. The motor cortex communicates with the kidney. J. Neurosci. 2012;32(19):6726–6731. doi: 10.1523/JNEUROSCI.0406-12.2012. 22573695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louppe J.-M., Nguyen J.-P., Robert R., Buffenoir K., de Chauvigny E., Riant T., Péréon Y., Labat J.J., Nizard J. Motor cortex stimulation in refractory pelvic and perineal pain: report of two successful cases. Neurourol. Urodyn. 2013;32(1):53–57. doi: 10.1002/nau.22269. 22674567 [DOI] [PubMed] [Google Scholar]
- Marszalek M., Wehrberger C., Temml C., Ponholzer A., Berger I., Madersbacher S. Chronic pelvic pain and lower urinary tract symptoms in both sexes: analysis of 2749 participants of an Urban health screening project. Eur. Urol. 2009;55(2):499–507. doi: 10.1016/j.eururo.2008.03.073. 18395963 [DOI] [PubMed] [Google Scholar]
- Mordasini L., Weisstanner C., Rummel C., Thalmann G.N., Verma R.K., Wiest R., Kessler T.M. Chronic pelvic pain syndrome in men is associated with reduction of relative gray matter volume in the anterior cingulate cortex compared to healthy controls. J. Urol. 2012;188(6):2233–2237. doi: 10.1016/j.juro.2012.08.043. 23083652 [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. 19110061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrum A., Wolff S., van der Horst C., Kuhtz-Buschbeck J.P. Motor cortical representation of the pelvic floor muscles. J. urol. 2011;186(1):185–190. doi: 10.1016/j.juro.2011.03.001. 21575960 [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Wideman T.H., Naso L., Hatami-Khoroushahi Z., Fallatah S., Ware M.A., Jarzem P., Bushnell M.C., Shir Y., Ouellet J.A., Stone L.S. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. 21593339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H., Galea M.P., Hodges P.W. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131(8):2161–2171. doi: 10.1093/brain/awn154. 18669505 [DOI] [PubMed] [Google Scholar]
- Turnbull G.K., Hamdy S., Aziz Q., Singh K.D., Thompson D.G. The cortical topography of human anorectal musculature. Gastroenterology. 1999;117(1):32–39. doi: 10.1016/s0016-5085(99)70547-0. 10381907 [DOI] [PubMed] [Google Scholar]
- Wand B.M., Parkitny L., O'Connell N.E., Luomajoki H., McAuley J.H., Thacker M., Moseley G.L. Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Man. Ther. 2011;16(1):15–20. doi: 10.1016/j.math.2010.06.008. 20655796 [DOI] [PubMed] [Google Scholar]


