Abstract
Parkinson’s disease (PD) results from the loss of dopaminergic neurons in the substantia nigra portion of the midbrain, and represents the second most common neurodegenerative disease in the world. Although the etiology of PD is currently unclear, oxidative stress and redox dysfunction are generally understood to play key roles in PD pathogenesis and progression. Aging and environmental factors predispose cells to adverse effects of redox changes. In addition to these factors, genetic mutations linked to PD have been observed to disrupt the redox balance. Mutations in leucine-rich repeat kinase 2 (LRRK2) are associated with autosomal dominant PD, and several of these mutations have also been shown to increase the levels of reactive oxygen species in cells. Anti-oxidant proteins are necessary to restore the redox balance and maintain cell viability. Over the past decade studies have started to demonstrate the critical importance for redox proteins mediating neuronal protection in models of PD. This commentary briefly describes some of the factors hypothesized to contribute to PD, specifically regarding the redox changes that occur in PD. Dysregulation of redox proteins in PD is highlighted by some of the work detailing the roles of peroxiredoxin-3 and thioredoxin-1 in models of PD. In an attempt to generate novel therapies for PD, several potent inhibitors of LRRK2 have been developed. The use of these compounds, both as tools to understand the biology of LRRK2 and as potential therapeutic strategies is also discussed. This mini-review then provides a historical prospective on the discovery and characterization of glutaredoxin (Grx1), and briefly describes current understanding of the role of Grx1 in PD. The review concludes by highlighting our recent publication describing the novel role for Grx1 in mediating dopaminergic neuronal protection both in vitro and in vivo.
Keywords: Glutaredoxin, Parkinson’s disease, LRRK2, C. elegans, redox
Overview of Parkinson’s disease and the role of oxidative stress
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the world, resulting from loss of the dopaminergic neurons in the substantia nigra of the midbrain [1, 2]. Clinically, PD patients present with resting tremor, postural instability, bradykinesia, and short shuffling steps, usually accompanied by progressive cognitive losses [3]. PD is a debilitating disease that is classified as “sporadic” or “familial,” based on whether a specific cause for the disease is unknown or it is attributed to a heritable trait, respectively. Although the underlying mechanisms for cases of sporadic PD are largely unresolved, aging-dependent changes in the neurons and environmental factors are believed to play major roles. In particular, increased oxidative stress-mediated damage and diminished anti-oxidant defenses have been implicated importantly as likely contributors to sporadic PD [2, 4, 5]. Oxidative damage and loss of anti-oxidant defenses are likely due to a combination of age related changes and exposure to xenobiotics. Within the past two decades genetic mutations in specific proteins have been linked to PD in humans, including those in SNCA (α-synuclein) [6], PARK7 (DJ-1) [7], PINK1 (Pink1) [8], PARK2 (Parkin) [9], and LRRK2 (LRRK2) [10]. Genetic mutations account for approximately 10% of all PD cases, among which LRRK2 represents the most prevalent cause of familial PD.
Although genetic mutations can be attributed only to a fraction of PD cases, posttranslational modifications of these same PD proteins may result in functional changes that promote sporadic PD in a manner similar to what occurs in familial PD [2, 11]. For example, Parkin has cysteine residues that are sensitive to oxidative modification, and it has been reported that treatment with H2O2 resulted in decreased Parkin activity [12]. A prevalent form of oxidative post-translational modification of cysteine residues on proteins is mixed disulfide formation with glutathione; i.e., S-glutathionylation (protein-SSG formation). Glutathionylation can result in changes in protein activity, sub-cellular localization, and steady-state protein content [13, 14]. Hence, homeostatic regulation of this modification is required to maintain cell viability under oxidative stress conditions. For example, oligomerization of α-synuclein is accelerated by oxidized glutathione [11], suggesting that formation of α-synculein-SSG promotes its aggregation, which is associated with neuronal cell death in PD [11]. Regardless of the classification of the disease, increased oxidative stress is thought to play a key role in degeneration of dopaminergic neurons in PD. Thus, consideration of oxidative stress represents a point of convergence for understanding development of both sporadic and familial PD. Accordingly, re-establishment of redox homeostasis represents a novel therapeutic strategy for slowing dopaminergic degeneration and the progression of PD.
Epidemiological evidence suggests that environmental agents play a role in development of sporadic PD [15], and the effects of these agents have been linked to oxidative stress in most cases. Exposure to the pesticides rotenone, paraquat and dieldrin, as well as heavy metals like manganese, increases the risk of developing PD [15, 16, 17]. Experimentally, rats injected with rotenone showed a decreased level of dopamine in the posterior striatum and prefrontal cortex along with diminished tyrosine hydroxylase indicative of dopaminergic cell loss [18]. Additionally, mice injected with paraquat displayed a significant loss of tyrosine hydroxylase-positive neurons in the midbrain compared to control animals [19]. Finally, treatment of human model dopaminergic neurons (SH-SY5Y cells) with dieldrin resulted in caspase 3 cleavage and cell death [20]. Direct evidence for toxin-based dopaminergic degeneration was reported when induction of Parkinson’s disease-like symptoms in humans was attributed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a chemical byproduct of illicit drug synthesis. This evidence was confirmed when monkeys were injected directly with MPTP and developed PD-like symptoms, which were dampened by administration of the PD drug L-DOPA [21]. Notably, all of the toxins described above have been found to promote increased oxidative stress; and/or to exert decreased toxicity when anti-oxidants were co-administered. For example, rotenone and MPTP are inhibitors of mitochondrial complex I, leading to increased production of superoxide and peroxide. Little is certain about the direct causes of sporadic PD. However, it is especially noteworthy that critical proteins whose mutations are responsible for familial PD are also sensitive to functional changes by oxidative modification. This insight provides an opportunity for discovering potential causes of the sporadic disease.
In 2002 a genome-wide association study revealed a novel locus linked to autosomal dominant PD, and in 2004 LRRK2 was described as the gene within this domain responsible for causing PD in humans [22, 23]. A year later the glycine to serine (G2019S) mutation, located in the kinase domain of LRRK2, was found to segregate with autosomal dominant PD [24]. This mutation is now known to represent the most common mutation in familial PD, found in 1–3% of all cases of PD. The precise molecular mechanism(s) for the neuronal toxicity induced by mutant LRRK2 remains unclear, although over-expression of pathogenic LRRK2 has been shown to drive increased levels of reactive oxygen species (ROS) [25]. Furthermore, LRRK2 contains a MAPKKK domain analogous to ASK1, which is known to undergo oxidative stress-induced alteration in functionality. This fact suggests that the LRRK2 kinase domain may also be redox sensitive. Notably, the G2019S mutation is widely accepted as a gain of function mutation that enhances LRRK2 kinase activity. Because of the prevalence of the G2019S mutation, several inhibitors targeted to the kinase domain have been characterized in attempts to combat the LRRK2 toxicity. “LRRK2-IN-1” was the first available highly potent inhibitor of LRRK2, and it was demonstrated to alleviate LRRK2 toxicity in cell culture [26]. Studies have also shown that LRRK2-IN-1 and other LRRK2 inhibitors mediate protection against LRRK2 toxicity in vivo. Specifically, both LRRK2-IN-1 and TTT-3002, another small molecule LRRK2-kinase inhibitor, were shown to protect dopaminergic neurons in C. elegans models of PD involving mutant LRRK2 expression [27]. This protection demonstrated the in vivo efficacy of small molecule kinase inhibitors against LRRK2 toxicity. Unfortunately, the highly potent LRRK2-IN-1 does not cross the blood brain barrier and cannot be developed for therapeutic use. Recently, two other molecules GNE-0877 and GNE-9605 were shown to inhibit LRRK2; and, in contrast to LRRK2-IN-1, they can penetrate the blood brain barrier [28]. These and other brain permeable drugs need to be tested further for efficacy in clinical trials, with the hope that inhibiting LRRK2 kinase activity may be a viable solution at least for PD patients with LRRK2 mutations. Such targeted therapeutic intervention for patients with LRRK2 mutations represents a new frontier in PD treatment. While only small numbers of PD patients harbor LRRK2 mutations, it is possible that post-translational oxidative modifications alter non-mutant LRRK2 proteins (analogous to ASK1 modification) and result in dysregulated function, similar to what occurs with the mutant LRRK2 proteins. If this is the case, then targeting LRRK2 may be a viable therapeutic strategy for both sporadic and familial PD. Extending this logic, identification of proteins dysregulated in both familial and sporadic PD is needed in order to develop agents with broader therapeutic impact.
Cells rely on redox enzymes to mediate protection against damage resulting from uncontrolled oxidative stress [2]. Many redox enzymes are necessary for scavenging ROS, maintaining critical intracellular redox balance, and controlling vital signaling events initiated by ROS. These enzymes include thioredoxins (Trx), peroxiredoxins (Prx), and glutaredoxins (Grx) [2]. Studies focusing on models of sporadic PD have shown that shRNA knockdown of Prx3 in SH-SY5Y cells increases susceptibility to apoptosis when the cells are challenged with the active metabolite of MPTP (MPP+) [29]. In contrast, overexpression of Trx1 decreases MPTP toxicity in mice [30]. These same enzymes have been studied also in the context of familial PD. Pathogenic LRRK2-G2019S has been reported to inactivate Prx3 via phosphorylation, thereby disrupting the cells’ ability to control H2O2 [31]. Overexpression of Prx3 was found to protect against LRRK2-G2019S toxicity in SH-SY5Y cells [31]. Moreover, postmortem brain samples from LRRK2-G2019S-positive PD patients showed increased levels of phosphorylated Prx3 compared to non-G2019S PD patients [31], indicating that LRRK2 toxicity may be caused in part by disrupting redox homeostasis.
Considering the immense body of work in the field of redox biology, the studies described above involving Prx3 and Trx1 represent only a sampling of the work describing critical roles for redox enzymes in mediating thiol homeostasis, which is pertinent to models of sporadic and familial PD [32]. These examples serve to set the stage for the primary focus of this article on the glutaredoxin (thioltransferase) enzyme and what we consider to be compelling evidence for its role in protection of dopaminergic neurons, which are the epicenter of PD.
Focus on the neuroprotective role of the glutaredoxin enzyme
Characterization of the first mammalian thioltransferase (glutaredoxin) occurred in 1974 [33], and in 1976 an E. coli enzyme with analogous activity was found and named “glutaredoxin” [34]. Although “thioltransferase” more accurately reflects the enzymatic function of the protein, “glutaredoxin (Grx)” has become the generally used name for the enzyme [35]. In 1991 human Grx1 was isolated from red blood cells [32], and then cloned and intensively biochemically characterized [36]. Grx1 specifically catalyzes the removal of glutathione from cysteine residues [37], restoring the steady-state function to proteins whose activity is changed upon glutathionylation [38]. It is now known that Grx1 is the major deglutathionylating enzyme in the cell, displaying about 5000-fold greater catalytic efficiency for deglutathionylation compared to Trx1 [39], whose primary function is to reduce intramolecular disulfide bonds.
The first evidence that Grx1 may play a role in protection of dopaminergic neurons was found when Grx1 protein and mRNA content were observed to be increased in mouse brain homogenate after MPTP treatment [40], suggesting a homeostatic upregulation in response to the chemical insult. Further work showed that female mice have greater Grx1 content compared to males and are more resistant to MPTP-induced dopaminergic cell death [41]. While these studies are consistent with a role for Grx1 in protection of dopaminergic neurons, additional studies provided more evidence for Grx1 mediating neuronal protection. Treatment of SH-SY5Y cells in culture with the pro-oxidant drug L-DOPA was shown to increase apoptosis. Investigation into the molecular mechanism of this drug-induced cell death revealed that Grx1 was selectively inactivated relative to other redox enzymes by oxidized L-DOPA, which covalently adducted the Grx1 active site (Cys-22) [42]. These findings led to the hypothesis that Grx1 plays a critical role in maintaining neuronal cell viability. To test this concept, Grx1 was knocked down in SH-SY5Y cells. The cells treated with the Grx1-siRNA showed an increased level of apoptosis compared to control cells with non-targeting siRNA [42]. This finding was soon confirmed as knockdown of Grx1 in Neuro-2a cells via shRNA also resulted in cell death [43]. Taken together these data indicated that Grx1 played a direct neuroprotective role in cultured cells. Still little was known about the neuroprotective role of Grx1 in vivo and its implications for PD in humans. These limitations were addressed in our recent study [44] which is highlighted by this commentary.
We investigated the role of Grx1 in mediating protection of dopaminergic neurons in vivo by using several different C. elegans models of PD. C. elegans worms overexpressing LRRK2-G2019S, LRRK2-R1441C, α-synuclein, or tyrosine hydroxylase specifically in the dopaminergic neurons, representing models of both familial and sporadic PD, were crossed into worms lacking the homolog to Grx1, GLRX-10. Each of the hybrid worm lines lacking the Grx1 homolog showed a significantly more severe PD-like phenotype compared to the corresponding control worms with endogenous GLRX-10. Furthermore, re-expression of WT GLRX-10, but not a catalytically dead mutant (C22S), in the dopaminergic neurons of the GLRX-10−/− / LRRK2-R1441C worms, rescued the exacerbated PD-like phenotypes.
In addition to identifying a novel role for Grx1 in protection of dopaminergic neurons in multiple in vivo models of PD, our study also examined Grx1 content in postmortem human brain samples from PD patients and non-PD controls. Western blot analysis of midbrain homogenates indicated an overall diminution of Grx1 protein within the midbrain for the PD patients versus controls. Using immunohistochemistry on midbrain tissue slices, we found that the fraction of dopaminergic (TH-positive) neurons that are Grx1-deficient was higher in PD patients compared to controls. Overall our recent study provides in vivo evidence that Grx1 mediates protection of dopaminergic neurons in models of sporadic and familial PD; and for the first time reports that Grx1 protein content is diminished in PD brain tissue, suggesting that diminution of Grx1 with aging predisposes to PD.
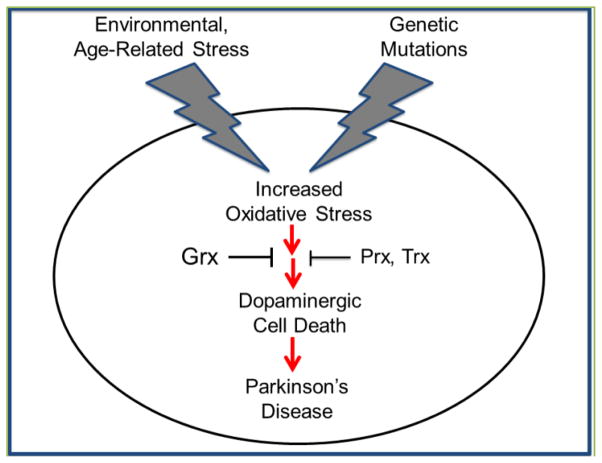
Both sporadic and familial PD develop asymptomatically over decades before they manifest clinically, leading to the idea that PD is a multifactorial disease where pathogenesis likely involves the convergence of multiple insults, including genetic, environmental and aging-dependent factors that impact redox homeostasis (Figure 1). Several anti-oxidant pathways exist within the dopaminergic neurons that mediate protection from toxicity induced not only via ROS-mediated DNA damage, but also from irreversible oxidative damage to proteins critical for cell survival. Prevention of toxicity with exogenous anti-oxidants does not discriminate between toxicity caused by DNA damage, irreversible protein modification, or other effects of increased ROS. Our recent paper revealed that loss of the Grx1 homolog in worms exacerbated LRRK2-mediated dopaminergic neuronal toxicity in vivo. Re-expression of the catalytically active, but not the inactive form of the Grx1 homolog prevented the increased toxicity. In addition, loss of the Grx1 homolog exacerbated PD phenotypes in models of sporadic PD driven by over production of dopamine or α-synuclein. Overall these data clearly implicate reversible protein glutathionylation as the likely mechanistic basis for the catalytic role of Grx1 in mediating dopaminergic neuronal protection against the oxidative stress associated with overexpression of mutant LRRK2 or α-synuclein, or overproduction of dopamine. Thus, removal of the glutathione modification from key regulatory proteins by Grx1 is necessary to restore their function and maintain cellular homeostasis and cell survival. The critical need for Grx1 to combat oxidative stress in dopaminergic neurons identifies manipulation of the activity of this enzyme as a novel therapeutic strategy for PD.
Figure 1.
Detrimental effects of environmental/age-related stress and genetic mutations, resulting in death of dopaminergic neurons associated with Parkinson’s disease; and protective effects of redox enzymes.
Most current therapies are designed to manage symptoms of PD mainly by replenishing the levels of dopamine in the brain. There are currently no established treatments that decrease the rate of dopaminergic degeneration and slow the progression of PD. As presented in this feature article, redox proteins have been shown to play an important role in mediating dopaminergic neuronal protection in models of PD. In almost all cases, loss of function/content of redox enzymes sensitizes dopaminergic cells in culture and dopaminergic neurons in whole animals to oxidative stress, resulting in increased cell death. These findings suggest that increasing the activities of redox enzymes may afford dopaminergic neuronal protection and provide a strategy to slow the progression of PD. Thus, therapeutic approaches aimed at enhancing catalytic redox activity represent an exciting and promising new avenue for the treatment of PD.
Acknowledgments
This work was supported in part by the National Institutes of Health (grant NS073170 to A.L.W. and S.G.C.; grant NS085503 to A.L.W., J.J.M. and S.G.C., predoctoral fellowship T32 GM008803 to W.M.J.), Parkinson’s Disease Foundation (summer student fellowship PDF-SFW-1348 to W.M.J.), the Department of Veterans Affairs (merit review grant BX000290 to J.J.M.), the National Science Foundation Advance Institutional Transformation Program (ACES research opportunity grant to S.G.C.)
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WM, Wilson-Delfosse AL, Mieyal JJ. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients. 2012;4:1399–1440. doi: 10.3390/nu4101399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- 4.Zuo L, Motherwell MS. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene. 2013;532:18–23. doi: 10.1016/j.gene.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 5.Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 7.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 8.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 9.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 10.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Paik SR, Lee D, Cho HJ, Lee EN, Chang CS. Oxidized glutathione stimulated the amyloid formation of alpha-synuclein. FEBS Lett. 2003;537:63–67. doi: 10.1016/s0014-5793(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 12.Meng F, Yao D, Shi Y, Kabakoff J, Wu W, Reicher J, et al. Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol Neurodegener. 2011;6:34. doi: 10.1186/1750-1326-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 14.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases--a mechanistic approach. Toxicol Lett. 2014;230:85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med. 2008;44:1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002;136:317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 19.Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, et al. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 20.Cho HS, Kim S, Lee SY, Park JA, Kim SJ, Chun HS. Protective effect of the green tea component, L-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology. 2008;29:656–662. doi: 10.1016/j.neuro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13. 1. Ann Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- 23.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Kachergus J, Mata IF, Hulihan M, Taylor JP, Lincoln S, Aasly J, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse AL, Chen SG, et al. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng X, Dzamko N, Prescott A, Davies P, Liu Q, Yang Q, et al. Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nat Chem Biol. 2011;7:203–205. doi: 10.1038/nchembio.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao C, Johnson WM, Gao Y, Wang W, Zhang J, Deak M, et al. Kinase inhibitors arrest neurodegeneration in cell and C. elegans models of LRRK2 toxicity. Hum Mol Genet. 2013;22:328–344. doi: 10.1093/hmg/dds431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estrada AA, Chan BK, Baker-Glenn C, Beresford A, Burdick DJ, Chambers M, et al. Discovery of highly potent, selective, and brain-penetrant aminopyrazole leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J Med Chem. 2014;57:921–936. doi: 10.1021/jm401654j. [DOI] [PubMed] [Google Scholar]
- 29.De Simoni S, Goemaere J, Knoops B. Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP+ Neurosci Lett. 2008;433:219–224. doi: 10.1016/j.neulet.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 30.Bai J, Nakamura H, Kwon YW, Tanito M, Ueda S, Tanaka T, et al. Does thioredoxin-1 prevent mitochondria- and endoplasmic reticulum-mediated neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine? Antioxid Redox Signal. 2007;9:603–608. doi: 10.1089/ars.2006.1513. [DOI] [PubMed] [Google Scholar]
- 31.Angeles DC, Gan BH, Onstead L, Zhao Y, Lim KL, Dachsel J, Melrose H, et al. Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum Mutat. 2011;32:1390–1397. doi: 10.1002/humu.21582. [DOI] [PubMed] [Google Scholar]
- 32.Gallogly MM, Starke DW, Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal. 2009;11:1059–1081. doi: 10.1089/ars.2008.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Askelof P, Axelsson K, Eriksson S, Mannervik B. Mechanism of action of enzymes catalyzing thiol-disulfide interchange. Thioltransferases rather than transhydrogenases. FEBS Lett. 1974;38:263–267. doi: 10.1016/0014-5793(74)80068-2. [DOI] [PubMed] [Google Scholar]
- 34.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976;73:2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mieyal JJ, Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid Redox Signal. 2012;16:471–475. doi: 10.1089/ars.2011.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mieyal JJ, Starke DW, Gravina SA, Dothey C, Chung JS. Thioltransferase in human red blood cells: purification and properties. Biochemistry. 1991;30:6088–6097. doi: 10.1021/bi00239a002. [DOI] [PubMed] [Google Scholar]
- 37.Gravina SA, Mieyal JJ. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry. 1993;32:3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- 38.Allen EM, Mieyal JJ. Protein-thiol oxidation and cell death: regulatory role of glutaredoxins. Antioxid Redox Signal. 2012;17:1748–1763. doi: 10.1089/ars.2012.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chrestensen CA, Starke DW, Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 40.Kenchappa RS, Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. Faseb J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- 41.Kenchappa RS, Diwakar L, Annepu J, Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. Faseb J. 2004;18:1102–1104. doi: 10.1096/fj.03-1075fje. [DOI] [PubMed] [Google Scholar]
- 42.Sabens EA, Distler AM, Mieyal JJ. Levodopa deactivates enzymes that regulate thiol-disulfide homeostasis and promotes neuronal cell death: implications for therapy of Parkinson’s disease. Biochemistry. 2010;49:2715–2724. doi: 10.1021/bi9018658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saeed U, Ray A, Valli RK, Kumar AM, Ravindranath V. DJ-1 loss by glutaredoxin but not glutathione depletion triggers Daxx translocation and cell death. Antioxid Redox Signal. 2010;13:127–144. doi: 10.1089/ars.2009.2832. [DOI] [PubMed] [Google Scholar]
- 44.Johnson WM, Yao C, Siedlak SL, Wang W, Zhu X, Caldwell GA, et al. Glutaredoxin deficiency exacerbates neurodegeneration in C. elegans models of Parkinson’s disease. Hum Mol Genet. 2014;24:1322–1335. doi: 10.1093/hmg/ddu542. [DOI] [PMC free article] [PubMed] [Google Scholar]