Abstract
The mosquito Aedes japonicus (Diptera: Culicidae) has spread rapidly through North America since its introduction in the 1990s. The mechanisms underlying its establishment in container communities occupied by competitors Aedes triseriatus and Aedes albopictus are unclear. Possibilities include (A) temporal separation of A. japonicus from other Aedes, (B) oviposition avoidance by A. japonicus of sites containing heterospecific Aedes larvae, and (C) non-additive competitive effects in assemblages of multiple Aedes. Containers sampled throughout the summer in an oak-hickory forest near Eureka, MO showed peak abundance for A. japonicus occurring significantly earlier in the season than either of the other Aedes species. Despite this, A. japonicus co-occurred with one other Aedes species in 53 % of samples when present, and co-occurred with both other Aedes in 18 % of samples. In a field oviposition experiment, A. japonicus laid significantly more eggs in forest edge containers than in forest interior containers, but did not avoid containers with low or high densities of larvae of A. triseriatus, A. albopictus, or both, compared to containers without larvae. Interspecific competitive effects (measured as decrease in the index of performance, λ′) of A. triseriatus or A. albopictus alone on A. japonicus larvae were not evident at the densities used, but the effect of both Aedes combined was significantly negative and super-additive of effects of individual interspecific competitors. Thus, neither oviposition avoidance of competitors nor non-additive competitive effects contribute to the invasion success of A. japonicus in North America. Distinct seasonal phenology may reduce competitive interactions with resident Aedes.
Keywords: Container community, Multispecies competition, Seasonal phenology, Oviposition behavior
Introduction
Successful invasive species have diverse traits that contribute to successful establishment and spread in a novel environment (reviewed by Sakai et al. 2001). Common contributing traits include superior resource competitive ability (Sakai et al. 2001), superior colonization ability (Moyle 1986), chemical (Callaway and Ridenour 2004) or physical (e.g., intraguild predation, Snyder et al. 2004) reduction of competitors, release from pathogens or natural enemies in the introduced range (Mitchell and Power 2003), or exploitation of an unfilled niche (Moles et al. 2008; Zaiko et al. 2007). The variety of traits that can affect invasive species success increases the difficulty of determining the mechanisms by which any given species becomes established and impacts residents, and therefore limits the ability to control the spread of that invader.
Mosquito species, particularly those whose larvae have adapted to inhabit artificial containers (e.g., tires, buckets), are frequently introduced to new countries via international transport (reviewed by Lounibos 2002), and mechanisms contributing to their success and dynamics are of great interest as these invaders are vectors of multiple important human disease-causing pathogens (Juliano and Lounibos 2005; Lounibos 2002). Invasion success of these mosquitoes appears to be related to oviposition behavior and competitive ability (Leisnham et al. 2014; Vonesh and Blaustein 2010; Reiskind and Wilson 2008; Reiskind and Lounibos 2013) and larval resource competitive ability (reviewed by Juliano 2009; Juliano and Lounibos 2005). Aedes albopictus, in particular, appears to succeed as an invader due to superior larval resource competitive ability relative to resident mosquitoes (Juliano 1998, 2009, 2010; Juliano et al. 2010; Juliano and Lounibos 2005). In contrast, Culex adults, including invasive Culex pipiens (L), are highly sensitive to conditions within aquatic environments, often avoiding oviposition sites with predators (Vonesh and Blaustein 2010). The worldwide invaders Aedes aegypti (L.) and A. albopictus (Skuse) exhibit less discriminating oviposition behavior toward aquatic predators (Juliano et al. 2010; Vonesh and Blaustein 2010; Albeny-Simões et al. 2014), but density of intra-or inter-specific competitors can affect oviposition choice by these invasive Aedes (Zahiri and Rau 1998; Allan and Kline 1998; Yoshioka et al. 2012). These effects are context dependent, ranging from preference to avoidance of potential competitors, and may depend primarily on microbial abundances and only indirectly on density of larvae (Fader and Juliano 2014). Among other mosquitoes, some Anopheles species also avoid ovipositing in containers with heterospecifics (Wachira et al. 2010; Sumba et al. 2004).
The invasive mosquito Aedes japonicus (Theobold) does not appear to fit the patterns of “superior competitor” or “choosy colonizer”. First documented in North America in 1998 (Peyton et al. 1999), this species has since spread from New York and New Jersey to nearly all states east of the Mississippi and two Canadian provinces, and has also been established in western United States and Europe (Kaufman and Fonseca 2014). Despite its rapid range expansion and high abundance, and some evidence that its invasion is associated with declines in abundances of other species (Andreadis and Wolfe 2010; Rochlin et al. 2013), the causes of its success as an invader are unclear. A. japonicus is competitively inferior to A. albopictus (Kesavaraju et al. 2010; Armistead et al. 2008a, b), and merely competitively equivalent to the North American tree hole mosquito Aedes triseriatus (Say) (Hardstone and Andreadis 2012; Alto 2011), two container-dwelling Aedes species that are often sympatric and syntopic with A. japonicus in Eastern North America. A. japonicus also does not appear to be less vulnerable than other Aedes species to the larval predator Toxorhynchites rutilus (Coquillett) (Murrell and Juliano 2013).
Compared to other container Aedes, A. japonicus larvae compose a larger proportion of the relative abundance of container mosquitoes earlier in the year (Fonseca et al. 2013; Kaufman et al. 2012; Burger and Davis 2008; Andreadis et al. 2001), are associated with colder temperature containers (Bartlett-Healy et al. 2012), and are found in larger containers (Kaufman and Fonseca 2014). These patterns suggest two hypotheses for traits of A. japonicus that contribute to its success as a North American invader. The first hypothesis is that A. japonicus minimizes overlap with other Aedes species by ovipositing in containers earlier than other Aedes, resulting in temporal separation that alleviates effects of interspecific competition. The second hypothesis is that A. japonicus minimizes overlap with other Aedes species by avoiding oviposition in containers already occupied by Aedes heterospecifics, thus minimizing resource competition.
A third possibility is that indirect effects of multispecies competition (i.e., among A. japonicus, A. triseriatus, and A. albopictus) may reduce inter-specific competitive effects on A. japonicus, and enhance the likelihood of this species becoming established in containers with more than one interspecific competitor. In community ecology, indirect effects can arise when chains of interactions or interaction modification among community members affect population dynamics of a particular species (Billick and Case 1994; Werner and Peacor 2003). This means that a given species’ population dynamics depends on not only its direct interactions with predators and competitors, but also on the presence of and interactions among those other species. Such non-additive effects have been demonstrated in animal and plant communities, particularly when strong predator–prey interactions are present (e.g., Peacor and Werner 1997; Preisser et al. 2005). The result is a community of co-occurring species that have effects on a focal species that go beyond the sum of effects of each pairwise interaction involving the focal species (Billick and Case 1994; Vonesh and Osenberg 2003). Although theory suggests that such indirect effects can facilitate coexistence among competing species (e.g., Frean and Abraham 2001; Werner and Peacor 2003; Dormann and Roxburgh 2005), experimental demonstrations have been few, with most involving plant communities (Dormann and Roxburgh 2005; Fortner and Weltzin 2007; Weigelt et al. 2007; Engel and Weltzin 2008). Though this phenomenon has not been documented in mosquito communities, different mosquito species have variable context-dependent feeding strategies (Merritt et al. 1992; Yee et al. 2004; Skiff and Yee 2014). If feeding strategies among Aedes species are modified by the presence of one or more competitors, one outcome may be that the effects of both A. albopictus and A. triseriatus on A. japonicus in a multispecies assemblage might be either super- or sub-additive (i.e., more or less, respectively, than the summed effects of each competitor alone on A. japonicus). The possibility of sub-additivity leads to our third hypothesis: Non-additive effects of competing A. albopictus and A. triseriatus reduce the impact of interspecific resource competition on A. japonicus and contribute to its invasion and spread in communities where both of these competitors are present.
In this study we assessed these three hypotheses for processes contributing to invasion success of A. japonicus. We used sampling of artificial containers over the active season at a field site at which all three species occur to test the first hypothesis, that A. japonicus temporally avoids overlap with A. triseriatus and A. albopictus because of earlier seasonal activity. We used containers with or without populations of developing competitors to test the second hypothesis, that A. japonicus avoids interspecific competition with other Aedes by ovipositing less in field containers with developing A. triseriatus or A. albopictus larvae, compared to containers without larvae. Finally, we used a laboratory experiment of a design described by Billick and Case (1994) to test the third hypothesis, that sub-additive multispecies indirect effects alleviate competitive effects on A. japonicus, and assessed larval competitive responses of all three Aedes species in intraspecific, 2-species interspecific, and 3-species interspecific situations.
Methods
Our field studies were conducted at Tyson Research Center near Eureka, MO. This site produced the first record of A. japonicus for Missouri in 2005 (Gallitano et al. 2005), so that our field work occurred at a relatively early point in the invasion of this site. Other container mosquitoes at this site include A. triseriatus, A. albopictus, A. hendersoni, Culex restuans, Culex territans, C. pipiens/quinquefasciatus, Culex salinarius, Orthopodomyia signifera, Anopheles barberi, and T. rutilus (Gallitano et al. 2005; Murrell et al. 2014).
Aedes colonization time assessment
This field study occured from May 22 to July 31, 2009. Black plastic buckets ranging in size from 3.79 (“small,”) to 7.57 L (“medium,”) were established along a road in an oak-hickory forest. Ten small and ten medium buckets were placed 10 m from the center of the road and were spaced at least 20 m from adjacent containers. Each small container was filled with 3 L of water and each medium container received 6 L of water. All containers then received 2 g/L dried, senescent white oak leaves (Quercus alba), and 10 mL hay infusion (50 g hay per L water), as an initial microbial inoculum. Rainfall was sufficient to keep containers filled throughout the summer. Two small buckets and one medium bucket were spilled early in the experiment and were excluded from the final analysis (final count: small n = 8, medium n = 9).
Each container was sampled weekly for larval composition by placing a 90-lb pull magnet in the bottom of each container, waiting 3 min for detritus and organisms to recover from this disturbance, then quickly plunging a 6.4 cm-diameter steel tube into the container and onto the magnet. The magnet adhered tightly to the end of the tube, isolating a water column sample containing surface and subsurface species (Murrell et al. 2014). This water sample was transported back to the laboratory at Illinois State University, where mosquito larvae were sorted, identified to species, and counted.
We used survival analyses with the individual container as a random effect (PROC NLMIXED, SAS 9.3) to test differences in (1) time to first colonization in each container, (2) time to greatest abundance in each container, with container size (small vs. medium), species (A. japonicus, A. triseriatus, and A. albopictus), and the interaction of container size and species as fixed effects. In cases where species was significant, we ran follow-up survival analyses for each pairwise combination of species (A. japonicus vs. A. triseriatus, A. triseriatus vs. A. albopictus, A. japonicus vs. A. albopictus).
Aedes oviposition response experiment
This field study was conducted during two periods: 3 July–24 July and 24 August–13 September, 2008. Fourteen 1 L black plastic cups were placed at each of four transects along gravel roads at Tyson. Each container was filled with 500 mL of water, 1 g dried oak leaf detritus, and 10 mL hay infusion. Holes were drilled into each container to prevent the container from overfilling with rainwater and hatching eggs during the field portion of the study. At each site, containers were affixed to trees with cable ties approximately 1–2 m above the ground. Seven containers were placed within 2 m of the road-forest edge and spaced 50 m apart, and seven containers were placed 50 m into the forest, and also spaced 50 m apart.
Within each row of seven containers, one container was stocked with first instar larvae in the each of the following treatments: no larvae; 20 A. albopictus; 40 A. albopictus; 20 A. triseriatus; 40 A. triseriatus; 10 A. albopictus + 10 A. triseriatus; or 20 A. albopictus + 20 A. triseriatus. The A. albopictus and A. triseriatus larvae were obtained from laboratory colonies originating at Tyson Research Center. After 1 week we collected all surviving larvae, and replaced them with a similar set of newly-hatched larvae for the appropriate treatment, and water was topped up to its original level. After 3 weeks, cups were collected and taken to Illinois State University and any eggs on the container walls were hatched in nutrient broth (0.5 g/ L) for 24–48 h at 24 °C. All resulting Aedes larvae were reared to a size at which they could easily be identified (2nd or 3rd instar), identified to species, and counted.
As the data were not normally distributed and data transformations were ineffective, we used a nonparametric mixed-model analysis (PROC MIXED, SAS 9.3) on ranked oviposition data (PROC RANKS, SAS 9.3) to test whether the number of A. japonicus hatched from eggs was significantly affected by sampling date (July, September), container location (edge vs. forest interior), Aedes larval treatment (see “Methods” section), and all possible interactions, with transect included as a random effect.
Multispecies larval competition experiment
This experiment was conducted in environmental chambers at Illinois State University at 24.8 °C (+0.5 °C), 80–90 % RH, and a 14:10 L:D photoperiod in April–May 2009. The A. albopictus and A. triseriatus larvae used were F2 larvae hatched from laboratory colonies originating at Tyson Research Center. A. japonicus larvae were hatched from eggs obtained from a colony maintained since 2005 (generation unknown) at the Headlee Research Laboratory Mosquito Research and Control Unit at Rutgers University in New Brunswick, NJ (Armistead et al. 2008a, b).
Inter- and intra-specific larval competition among the three species of Aedes was investigated using an experimental design based on that described by Billick and Case (1994). Treatment levels were: a target species at a baseline density of 20 larvae; interspecific competition with each of the target species’ competitors individually at the baseline density for a total density of two times baseline density (40 larvae total); interspecific competition with both competitors, each at the baseline density for a total density of three times baseline density (60 larvae total); and intraspecific competition at two times and three times baseline density (Table 1; see Billick and Case 1994 for the logic of this design). The goal was to determine if summing the effects on the target species of adding 20 of each competitor species alone predicts accurately the combined effect on the target species of adding 20 each of both competitor species (Billick and Case 1994). Two parallel experiments with A. albopictus and A. triseriatus, respectively, as the target species used the same design (Table 1). Each combination was replicated five or six times (Table 1), yielding 69 containers. Containers with two or three species each contributed data to two or three, respectively, analyses, once for effects on each of the species present in the container.
Table 1.
Treatment level abbreviations, sample size (N), and species compositions for the laboratory competition experiments testing for nonadditive effects of multispecies competition
| Treatment | Abbreviation | N | Numbers of larvae present | Used for analysis of target species
|
||
|---|---|---|---|---|---|---|
| Aedes japonicus | Aedes albopictus | Aedes triseriatus | ||||
| Single species | J | 6 | 20 A. japonicus | X | ||
| JJ | 5 | 40 A. japonicus | X | |||
| JJJ | 5 | 60 A. japonicus | X | |||
| A | 5 | 20 A. albopictus | X | |||
| AA | 5 | 40 A. albopictus | X | |||
| AAA | 5 | 60 A. albopictus | X | |||
| T | 5 | 20 A. triseriatus | X | |||
| TT | 5 | 40 A. triseriatus | X | |||
| TTT | 5 | 60 A. triseriatus | X | |||
| Two species | JT | 6 | 20 A. japonicus + 20 A. triseriatus | X | X | |
| JA | 6 | 20 A. japonicus + 20 A. albopictus | X | X | ||
| AT | 5 | 20 A. albopictus + 20 A. triseriatus | X | X | ||
| Three species | JAT | 6 | 20 A. japonicus + 20 A. albopictus + 20 A. triseriatus | X | X | X |
| 69 | ||||||
Each target species’ response to treatment levels was analyzed separately using data from larvae that species in the indicated treatments (X). N specifies the number of containers; two- and three-species containers yield data for two and three of the analyses, respectively, one for the response variable (survivorship, λ′) for each species in the container
Three days prior to adding larvae (30 March, 2009), 250 mL tri-cornered plastic beakers were filled with 0.15 g of ground rabbit chow (Kaytee Supreme Daily Blend Rabbit Pellets®) and 200 mL of Nanopure™ water and incubated in the conditions described above. After 3 days, synchronously hatched larvae (Yee et al. 2004) were added to containers as 1st instars in the combinations described above. All beakers were housed in a single environmental chamber and randomly rotated daily to limit location effects of temperature, relative humidity, and light exposure. Cups were checked daily beginning at day 6 and all pupae were isolated in cotton stoppered 1.8 mL shell vials. After emergence, the sex, species, and days to eclosion were recorded for each adult. Each female was assigned a unique number and oven dried at 50 °C. The experiment ended on 23 May, 2009 (day 51). Dried females were weighed individually on a Cahn C-31 microbalance, and female wing length was recorded using a dissecting microscope and Scion® image analysis system.
Billick and Case (1994) noted that tests for non-additive interspecific interactions can be highly dependent on the response variable chosen. We thus tested non-additive effects on both female proportion survivorship to eclosion (assuming initial larval populations were 50 % female), and population dynamics. The latter was estimated as finite rate of increase (λ′) via Juliano’s (1998) modification of Livdahl and Sugihara’s (1984) index of performance:
The numerator of this estimates the natural log of net reproductive rate of the cohort, whereas the denominator estimates the mean cohort generation time (Livdahl and Sugihara 1984; Chmielewski et al. 2010). Livdahl and Sugihara’s (1984) estimate has been shown to yield accurate estimates of per capita rate of increase in mosquitoes (Chmielewski et al. 2010), and it provides a more comprehensive understanding of how environmental conditions may affect population dynamics than separate analyses of survival, development time, and fecundity. N0 is the initial number of females (assumed to be 50 % of the larvae), Ad is the number of females eclosing on day d, D is the estimated number of days between eclosion and oviposition, and f (wd) was a published wing length-fecundity regression relationship (Armistead et al. 2008a, b; Livdahl and Sugihara 1984; Lounibos 2002) with wd = mean wing length of females eclosing on day d. Using the index λ′ has the advantage of yielding estimates even when there are no survivors (λ′ = 0) (Juliano 1998).
For each species female proportion survivorship and λ′ were analyzed for fixed effects of intraspecific and interspecific treatment levels using 1-way ANOVA (PROC GLM, SAS 9.3). Significant results for female survivorship were then further analyzed using pairwise comparisons with a Tukey adjustment. For both variables we tested for nonadditivity using a contrast testing for interaction of effects of the two competing species (Billick and Case 1994). Because λ′ for all species did not meet the assumptions of normality and homogeneity of variances, randomization ANOVAs (Cassell 2002) were also run. Significant effects in the randomization tests matched those of the parametric tests, so we report only the results of the parametric tests.
Results
Aedes colonization time assessment
Ten Diptera taxa were recorded in the small and medium containers: A. albopictus, A. japonicus, A. triseriatus, Aedes hendersoni (Cockerell), A. barberi (Coquillett), Chironomus sp., C. restuans (Theobald), Culicoides sp., Megaselia imitatrix (Borgmeier), and T. rutilus. The three Aedes species that were the focus of this study composed 75 % of all individuals, 82 % of all mosquitoes, and 99 % of all Aedes. Larval abundances were relatively low, averaging 16.70 ± 1.96 Aedes larvae/L. This was likely due to the fact that the containers were new rather than extant.
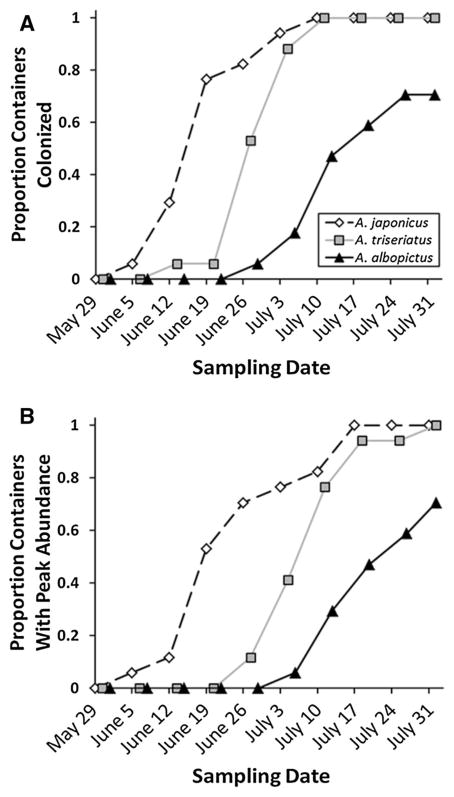
There was no significant effect of container size on either time to first colonization (t16 = 0.44, p = 0.6661) or time to peak abundance of Aedes (t16 = −0.76, p = 0.4559). However, there was a significant effect of species on both time to first colonization (t16 = −5.01, p = 0.0001) and time to peak abundance (t16 = −4.30, p = 0.0005). Interaction effects of container size and species were not significant for initial colonization time (t16 = −2.00, p = 0.0633) or for time to peak abundance (t16 = −0.20, p = 0.8468). Follow-up tests on the significant species effects showed that A. japonicus first colonized significantly sooner than A. albopictus, but not A. triseriatus, and A. triseriatus also colonized significantly sooner than A. albopictus (Fig. 1a). Peak abundance significantly differed among all three Aedes species, with A. japonicus reaching peak abundance in more containers earlier than A. triseriatus and A. albopictus (Fig. 1b). Mean abundances of each species over time, standardized for sample volume, reflect this pattern (Fig. 2), with greatest mean abundance of A. japonicus across containers occurring before notable colonization of A. triseriatus and A. albopictus.
Fig. 1.
Results of survival analysis of the 2009 colonization study. a Cumulative proportion of containers that have been initially colonized by each Aedes species by each sample week. Both A. japonicus and A. triseriatus colonization curves differed significantly from A. albopictus, but did not differ from each other. b Cumulative proportion of containers that have attained peak abundance of each species by each sample week. All species peak abundance curves significantly differed from one another
Fig. 2.
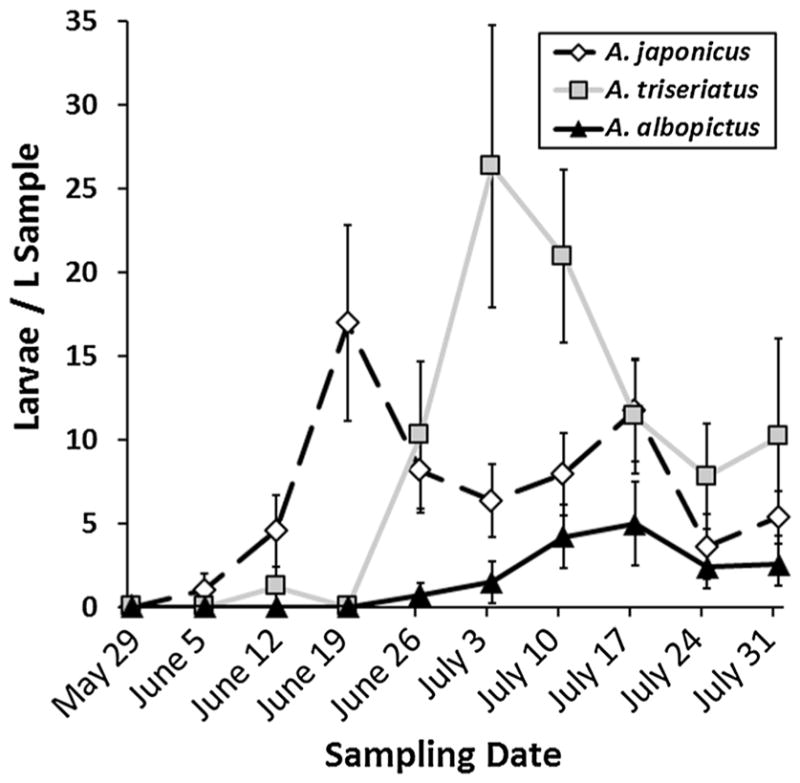
Mean (±SE) abundance of larvae of each Aedes species per liter of sample during the 2009 colonization study
Although A. japonicus colonization and peak abundance occurred earlier in the season, this species was present in containers throughout the 10-week sampling period and frequently co-occurred with other Aedes species (Table 2). From June 26 to July 31, when all three species were present, mean co-occurrence of A. japonicus with only A. triseriatus was 13.68 % (±6.33 % SE) of all samples, and mean co-occurrence with both A. triseriatus and A. albopictus was 26.85 % (±5.06 % SE) of all containers.
Table 2.
Occurrences of A. japonicus, A. triseriatus, and A. albopictus in containers sampled in 2009, as the sole occupants, two species, and three species co-occurrences
| Collection date
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| May 29 | June 5 | June 12 | June 19 | June 26 | July 3 | July 10 | July 17 | July 24 | July 31 | |
| Number of containers sampled | 17 | 17 | 17 | 14 | 17 | 16 | 16 | 16 | 15 | 16 |
| Total containers colonized | 0 | 1 | 6 | 10 | 13 | 13 | 16 | 16 | 12 | 14 |
| A. japonicus only (%) | 0 (0) | 1 (5.88) | 5 (29.41) | 10 (71.43) | 4 (23.53) | 1 (6.25) | 1 (6.25) | 5 (31.25) | 1 (6.67) | 6 (37.50) |
| A. triseriatus only (%) | 0 (0) | 0 (0) | 1 (5.88) | 0 (0) | 2 (11.76) | 3 (18.75) | 4 (25.00) | 1 (6.25) | 5 (33.33) | 2 (12.50) |
| A. albopictus only (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.25) | 2 (13.33) | 0 (0) |
| A. japonicus + A. triseriatus only (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (35.29) | 7 (43.75) | 4 (25.00) | 5 (31.25) | 2 (13.33) | 2 (12.50) |
| A. japonicus + A. albopictus only (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.25) | 0 (0) | 0 (0) |
| A. triseriatus + A. albopictus only (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.88) | 0 (0) | 0 (0) | 2 (12.50) | 0 (0) | 3 (18.75) |
| All 3 Aedes spp. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (12.5) | 7 (43.75) | 1 (6.25) | 2 (13.33) | 1 (6.25) |
The number of samples in which each species or species combination occurs is listed first, with percent of samples occupied displayed in parentheses below
Aedes oviposition response experiment
Aedes japonicus laid significantly more eggs in July than in September (Table 3; Fig. 3a, b). In July there was also a significant effect of container location, with significantly more A. japonicus eggs laid at the forest edge than in the forest interior (Table 3; Fig. 3a). The number of A. japonicus eggs did not significantly differ among the larval treatment levels, nor was there any significant interaction involving treatment (Table 3).
Table 3.
Results of mixed model analyses of Aedes japonicus oviposition in response to collection date (July, September), container location (forest edge vs. forest interior), treatment (numbers of Aedes triseriatus and Aedes albopictus larvae added to container weekly), and all interactions
| Effect | df | F | p |
|---|---|---|---|
| Collection date | 1.81 | 3.79 | 0.0551 |
| Location | 1.81 | 6.20 | 0.0148 |
| Treatment | 6.81 | 0.53 | 0.7868 |
| Collection date × location | 1.81 | 6.08 | 0.0158 |
| Collection date × treatment | 6.81 | 1.69 | 0.1351 |
| Location × treatment | 6.81 | 1.03 | 0.4109 |
| Collection date × location × treatment | 6.81 | 2.08 | 0.0641 |
Significant effects are in bold print
Fig. 3.
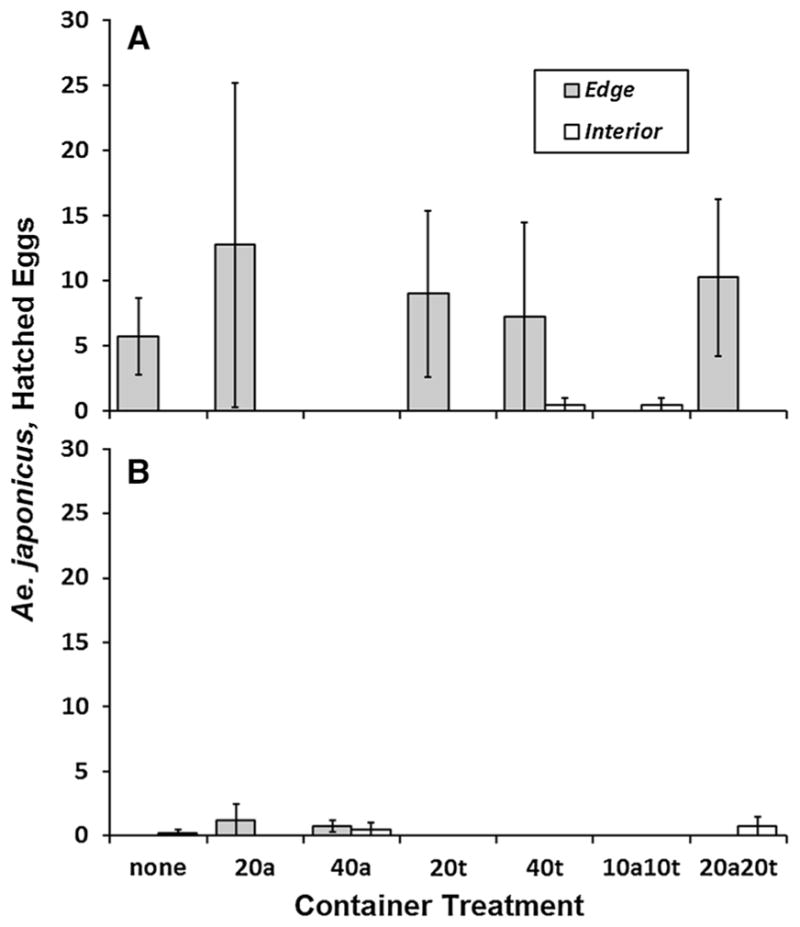
Mean (±SE) number of A. japonicus larvae hatched and reared from eggs collected from the 2008 oviposition study for combinations of container treatment and locations (forest edge vs. interior). a Containers collected July 24. b Containers collected September 13
Multispecies larval competition experiment
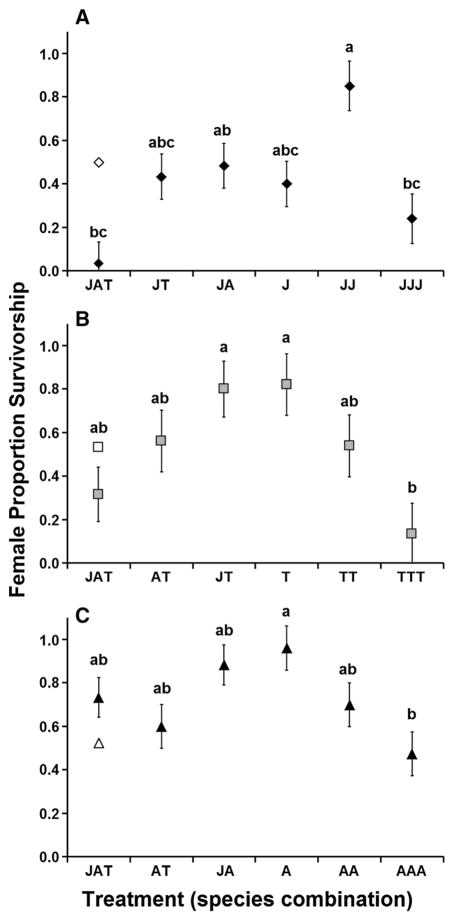
Female proportion survivorship was significantly affected by treatment for all three species (A. albopictus F5,26 = 3.25, p = 0.0207; A. japonicus F5,28 = 6.15, p = 0.0006; A. triseriatus F5,26 = 3.82, p = 0.0100). A. japonicus showed a nonlinear response of survivorship to increasing intraspecific density, with survivorship significantly greater in the double density treatment (JJ) than in the triple density treatment (JJJ) with baseline density (J) intermediate. The JAT treatment level had lowest mean survivorship and was significantly lower than JJ (Fig. 4a). A. japonicus female survival in JAT was significantly lower than that predicted by effects of each competitor alone (JT, JA; F1,28 = 5.40, p = 0.0276; Fig. 4a). A. triseriatus and A. albopictus showed significant declines in survivorship as intraspecific density increased (T vs. TTT and A. vs. AAA, respectively), but no significant differences between baseline density and interspecific treatments (Fig. 4b, c, respectively). Tests for nonadditivity were not significant for A. triseriatus (F1,26 = 0.68, p = 0.4185; Fig. 4b) and A. albopictus (F1,26 = 1.19, p = 0.2862; Fig. 4c).
Fig. 4.
Mean proportion survival of females (+SE) in response to inter- and intraspecific larval densities in multispecies competition experiment, for a A. japonicus, b A. triseriatus, c A. albopictus. Treatment abbreviations correspond to abbreviations given in Table 1. Letters indicate means that do not significantly differ from each other. For each species, filled points represent actual mean survivorship (±SE, =√MSE/n). The open point represents expected survivorship in the multispecies treatments if effects of competing species on target species survivorship were additive and accurately predicted by effects on the target species observed in the 2-species combinations
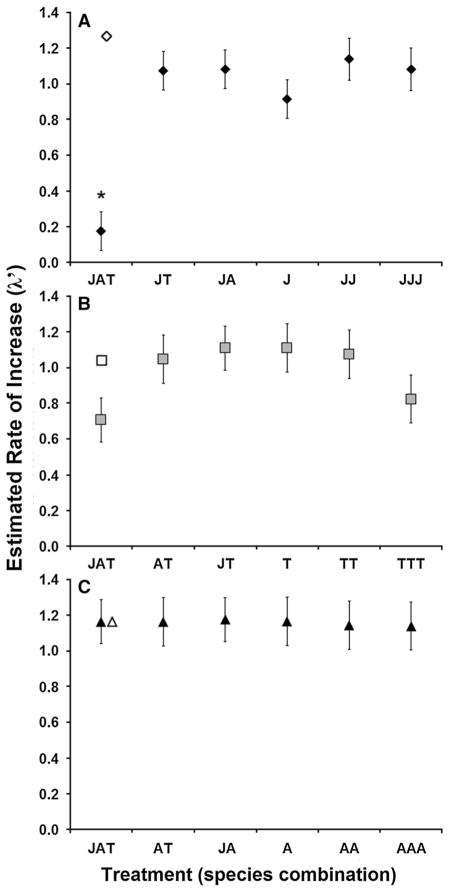
There was a significant effect of treatment on λ′ of A. japonicus (F5,28 = 11.28, p < 0.0001), with estimated finite rate of increase significantly lower in the 3-species treatment level than in any of the intraspecific or 2-species treatments. However, λ′ did not significantly differ between 2-species treatments and intraspecific density treatment levels (Fig. 5a). Observed λ′ when competing with both species (JAT) was significantly lower than the λ′ that would be predicted based on the individual effects of each competitor alone (JA, JT; F1,28 = 24.11, p < 0.0001; Fig. 5a). This low λ′ for the JAT treatment level was not simply a product of higher density, as λ′ for the intraspecific control JJJ at the same density was much greater and comparable to the other treatment levels (Fig. 5a). There were no significant effects of treatment on λ′ for either A. triseriatus (F5,26 = 1.79, p = 0.1493) (Fig. 5b) or A. albopictus (F5,26 = 0.96, p = 0.4623) (Fig. 5c). Tests for nonadditivity were not significant for A. triseriatus (F1,26 = 1.73, p = 0.1996; Fig. 5b) and A. albopictus (F1,26 = 0.13, p = 0.7199; Fig. 5c).
Fig. 5.
Mean finite rate of increase in response to inter- and intraspecific larval densities in multispecies competition experiment, for a A. japonicus, b A. triseriatus, c A. albopictus. Treatment abbreviations correspond to abbreviations given in Table 1. For each species, filled points represent actual mean λ′ (±SE, =√MSE/n); the open point represents expected λ′ in the multispecies treatment if effects of competing species on target species λ′ were additive and accurately predicted by effects on the target species observed in the 2-species combinations. Asterisk a mean for A. japonicus significantly different from means from all other treatments for A. japonicus
Discussion
Consistent with previous results (Fonseca et al. 2013; Kaufman et al. 2012; Burger and Davis 2008; Andreadis et al. 2001), we have shown that A. japonicus in Missouri colonize earlier than A. albopictus and become abundant in containers earlier in the season than either of its common Aedes competitors. Despite this, A. japonicus continues to colonize containers until late July. Our 2009 colonization assessment and 2008 oviposition study show that A. japonicus larvae frequently co-occur in containers with A. triseriatus and A. albopictus, even in containers as small as 1 L, and A. japonicus adults exhibit no avoidance of oviposition in containers with larval Aedes competitors. The multispecies competition experiment clearly shows A. japonicus suffers non-additive, negative competitive effects when encountering two competing Aedes, and that non-additive effects are absent for both A. albopictus and A. triseriatus. These results for A. japonicus in competition clearly refute the hypothesis that multispecies competition facilitates the invasion of A. japonicus; indeed, multispecies competition has the opposite effect on A. japonicus. Taken collectively, our results indicate that the success of A. japonicus invasion in part results from its temporal separation from other container Aedes competitors. A further contributor to its success, not investigated in our experiments, is that A. japonicus tends to colonize larger containers than A. triseriatus and A. albopictus (Kaufman and Fonseca 2014). In the general context of invasion biology, the success of A. japonicus seems best understood as a product of its ability to use resources, defined by seasonal time and water body size, that are not fully exploited by potentially competing residents.
The lack of oviposition avoidance by A. japonicus of heterospecifics is consistent with the lack of strong evidence for interspecific avoidance in other field oviposition studies of Aedes (e.g., Fader and Juliano 2014). Field oviposition patterns can be difficult to interpret as Aedes species can either be positively correlated across containers due to temporal synchrony (Johnson and Sukhdeo 2013) or similar responses to resource levels (Yee et al. 2010), or negatively correlated with each other (Armistead et al. 2012; Sunihara et al. 2002), due to interspecific oviposition avoidance or different habitat preferences (Reiskind and Lounibos 2013). Our study shows that A. japonicus prefers containers at the forest edge over the interior, and lays significantly more eggs earlier in the season, as shown by (Burger and Davis 2008). The lack of avoidance by A. japonicus of oviposition where other Aedes are already present means that encounters within containers with A. albopictus and A. triseriatus are likely where the species overlap, as they do at Tyson. While further tests of A. japonicus oviposition response to other possible competitors (e.g., Culex) would be useful, our results do not support oviposition avoidance of competitors as a mechanism enabling A. japonicus to succeed in invasion of North American containers.
Ours is the first study to test for nonadditive competitive responses of mosquito larvae, and one of the few (see Ho et al. 1989) to test for effects of competition in multispecies assemblages. We observed significant effects of intraspecific density on female survivorship in all three species, indicating that densities were sufficiently high for resource competition to occur. However, the only significant effect on λ′ was the superadditive effect on A. japonicus λ′ by A. albopictus + A. triseriatus relative to the competitive effects of either competitor alone.
Aside from its implications in the invasion biology of A. japonicus, the super-additive effect on A. japonicus λ′ by A. albopictus + Ae triseriatus suggests that there may be complex, nonadditive effects of competition in natural assemblages that may have important impacts on community structure. This has been shown for other terrestrial plant and animal communities (e.g., Werner and Peacor 2003; Dormann and Roxburgh 2005; Fortner and Weltzin 2007; Engel and Weltzin 2008) but to our knowledge has not been previously demonstrated with immature mosquitoes. Such non-additive effects among competitors could arise from interaction modification via context dependent, trait mediated effects (e.g., on foraging) that are expressed differently in response to different species of competitors, or from a variety of other mechanisms (Billick and Case 1994; Werner and Peacor 2003; Dormann and Roxburgh 2005). Our present experiment cannot determine the mechanisms behind this non-additive effect on A. japonicus, but the presence of such an effect suggests that we cannot gain a full understanding of this community by quantifying pairwise interactions (Billick and Case 1994; Dormann and Roxburgh 2005; Weigelt et al. 2007). Though feeding behavior of A. japonicus has never been studied in the presence of competitors, in isolation A. japonicus browses more vigorously on submerged leaves than does A. albopictus (O’Donnell and Armbruster 2007). A. japonicus, like other mosquitoes, also shifts its feeding activity and position in containers in response to cues from predation (Kesavaraju et al. 2011). If such context dependent shifts in feeding strategy and position within the container (by A. japonicus or its competitors) occur in response to different combinations of competitors, the result could be non-additive effects of competitors. Our experiment was not designed to test whether feeding segregation within containers occurs, and we cannot demonstrate whether or not available resources within our containers were being fully utilized. However, investigations of mechanisms of non-additive effects of multispecies competition among Aedes and other mosquitoes could yield interesting insights into mosquito behavior and community structure. More generally, in the context of invasions, such non-additive effects may be one contributor to invasion resistance or susceptibility of resident assemblages (Zarnetske et al. 2013).
The population of A. japonicus we used for the competition experiment was not from Tyson, but rather from a long-established colony at Rutgers University, New Brunswick, NJ. Though this colony has been used for many of the competition studies for A. japonicus (Armistead et al. 2008a, b; Kesavaraju et al. 2010; Alto 2011), it is unknown whether there are population differences in competitive ability of A. japonicus, and specifically if Tyson A. japonicus may exhibit a different competitive response than the Rutgers population. Should better methods for isolating wild populations of A. japonicus or for rearing laboratory colonies of A. japonicus be developed, it would be worthwhile to test whether populations differ in their interspecific competitive ability. At this time, however, our study along with studies have found no compelling evidence that A. japonicus utilizes superior resource competitive ability, in single- or multi-species interspecific competition, as mechanism for invasion.
Our field experiments took place only 3–4 years after A. japonicus was first recorded at this site (Gallitano et al. 2005), and it seems likely that our results reflect a container mosquito assemblage that is in flux, with A. japonicus increasing. Experiments at this site in June-July 2010 indicated that A. japonicus comprised >90 % of the Aedes in 19 L buckets (Murrell and Juliano 2013), whereas experiments in May–August 2011 yielded assemblages in 19 L buckets with A. japonicus and A. triseriatus co-dominant (Murrell et al. 2014). Thus, the relatively low density of A. japonicus in the present field results is not surprising.
The experiments reported the present paper involved a limited range of container sizes, and held constant container composition and detritus resource. We have not directly tested whether A. japonicus exploitation of different sizes or types of containers or different thermal regimes contributes to its invasion success in North America, as proposed by Kaufman and Fonseca (2014). Our results are compatible with the general point made by Kaufman and Fonseca (2014): invasion success of A. japonicus does not depend on superior competitive ability relative to A. triseriatus and A. albopictus. Instead, it may depend on its preference for colder climates, to which A. albopictus is less tolerant (Rochlin et al. 2013), and use of habitats occupied by less competitive species, such as the rock pool mosquito Aedes atropalpus (Coquillett), the native pool and container dweller C. restuans, and invasive generalist C. pipiens (L.). C. restuans and C. pipiens, like A. japonicus, are more commonly found in larger containers (Andreadis et al. 2001), and colonize significantly earlier than other Aedes species (Murrell et al. 2014). Removal of A. japonicus larvae from container communities also significantly increases abundance and development of Culex larvae (Murrell and Juliano 2013), which suggests that A. japonicus is competitively superior to Culex. A. japonicus also frequently overlaps with A. atropalpus in North American rock pools, and in some areas has almost completely displaced this native species (Armistead et al. 2012; Andreadis and Wolfe 2010). Despite this, investigations of A. japonicus competitive effects on these species are rare (but see Hardstone and Andreadis 2012; Armistead et al. 2008a, b). More research into the interaction of A. japonicus with Culex and A. atropalpus in a wider array of container types would likely enhance our understanding of the invasion success of A. japonicus.
Acknowledgments
We thank M. Dunham, B. Kuyken, S. Brandt, and J. Cellini for their assistance with the laboratory experiment, J. Ahlert and C. Jordan for assistance with the colonization study, J. M. Chase and the Tyson Research staff for use of their facilities, D. Fonseca and Headlee Research Laboratory Mosquito Research and Control Unit, Rutgers University, for A. japonicus eggs, P. R. Crump for statistical assistance, and three anonymous reviewers for their helpful comments on the manuscript. This research was funded by NIAID Grant R15 AI075306-01 and AARA supplement 3R15AI075306-01S1 to SAJ. BHN was partially supported during manuscript writing period by the Oklahoma Agricultural Experiment Station (OKL-02909).
References
- Albeny-Simões D, Murrell EG, Elliot SL, Andrade MR, Lima E, Juliano SA, Vilela EF. Attracted to the enemy: Aedes aegypti prefers oviposition sites with predator-killed conspecifics. Oecologia. 2014;175:481–492. doi: 10.1007/s00442-014-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SA, Kline DL. Larval rearing water and preexisting eggs influence oviposition by Aedes aegypti and Ae. albopictus (Diptera: Culicidae) J Med Entomol. 1998;35:943–947. doi: 10.1093/jmedent/35.6.943. [DOI] [PubMed] [Google Scholar]
- Alto BW. Interspecific larval competition between invasive Aedes japonicus and native Aedes triseriatus (Diptera: Culicidae) and adult longevity. J Med Entomol. 2011;48:232–242. doi: 10.1603/me09252. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Wolfe RJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the northeastern United States. J Med Entomol. 2010;47:43–52. doi: 10.1603/033.047.0106. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. J Med Entomol. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Armistead JS, Arias JR, Nishimura N, Lounibos LP. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera: Culicidae) in northern Virginia. J Med Entomol. 2008a;45:629–637. doi: 10.1603/0022-2585(2008)45[629:ilcbaa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead JS, Nishimura N, Escher RL, Lounibos LP. Larval competition between Aedes japonicus and Aedes atropalpus (Diptera: Culicidae) in simulated rock pools. J Vector Ecol. 2008b;33:238–246. doi: 10.3376/1081-1710-33.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead JS, Nishimura N, Arias JR, Lounibos LP. Community ecology of container mosquitoes (Diptera: Culicidae) in Virginia following invasion by Aedes japonicus. J Med Entomol. 2012;49:1318–1327. doi: 10.1603/me11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett-Healy K, Unlu I, Obenauer P, Hughes T, Healy S, Crepeau T, Farajollahi A, Kesavaraju B, Fonseca D, Schoeler G, Gaugler R, Strickman D. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) J Med Entomol. 2012;49:813–824. doi: 10.1603/me11031. [DOI] [PubMed] [Google Scholar]
- Billick I, Case TJ. Higher order interactions in ecological communities: What are they and how can they be detected? Ecology. 1994;75:1529–1543. [Google Scholar]
- Burger JF, Davis H. Discovery of Ochlerotatus japonicus japonicus (Theobald) (Diptera: Culicidae) in southern New Hampshire, USA and its subsequent increase in abundance in used tire casings. Entomol News. 2008;119:439–444. [Google Scholar]
- Callaway RM, Ridenour WM. Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ. 2004;2:436–443. [Google Scholar]
- Cassell DL. A randomization-test wrapper for SAS PROCs. 2002 http://www2.sas.com/proceedings/sugi27/p251-27.pdf.
- Chmielewski MW, Khatchikian C, Livdahl T. Estimating the per capita rate of population change: How well do life-history surrogates perform? Ann Entomol Soc Am. 2010;103:734–741. [Google Scholar]
- Dormann CF, Roxburgh SH. Experimental evidence rejects pairwise modelling approach to coexistence in plant communities. Proc R Soc B Biol. 2005;272:1279–1285. doi: 10.1098/rspb.2005.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel EC, Weltzin JF. Can community composition be predicted from pairwise species interactions? Plant Ecol. 2008;195:77–85. [Google Scholar]
- Fader JE, Juliano SA. Oviposition habitat selection by container-dwelling mosquitoes: responses to cues of larval and detritus abundances in the field. Ecol Entomol. 2014;39:245–252. doi: 10.1111/een.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca DM, Unlu I, Crepeau T, Farajollahi A, Healy SP, Bartlett-Healy K, Strickman D, Gaugler R, Hamilton G, Kline D, Clark GG. Area-wide management of Aedes albopictus. Part 2: gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manag Sci. 2013;69:1351–1361. doi: 10.1002/ps.3511. [DOI] [PubMed] [Google Scholar]
- Fortner AM, Weltzin JF. Competitive hierarchy for four common old-field plant species depends on resource identity and availability 1. J Torrey Bot Soc. 2007;134:166–176. [Google Scholar]
- Frean M, Abraham ER. Rock–scissors–paper and the survival of the weakest. Proc R Soc B Biol. 2001;268:1323–1327. doi: 10.1098/rspb.2001.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallitano S, Blaustein L, Vonesh J. First occurrence of Ochlerotatus japonicus in Missouri. J Vector Ecol. 2005;30:347–348. [PubMed] [Google Scholar]
- Hardstone MC, Andreadis TG. Weak larval competition between the invasive mosquito Aedes japonicus japonicus (Diptera: Culicidae) and three resident container-inhabiting mosquitoes in the laboratory. J Med Entomol. 2012;49:277–285. doi: 10.1603/me11050. [DOI] [PubMed] [Google Scholar]
- Ho BC, Ewert A, Chew L. Interspecific competition among Aedes aegypti, Ae. albopictus, and Ae. triseriatus (Diptera: Culicidae): larval development in mixed cultures. J Med Entomol. 1989;26:615–623. doi: 10.1093/jmedent/26.6.615. [DOI] [PubMed] [Google Scholar]
- Johnson BJ, Sukhdeo MVK. Successional mosquito dynamics in surrogate treehole and ground-container habitats in the northeastern United States: Where does Aedes albopictus fit in? J Vector Ecol. 2013;38:168–174. doi: 10.1111/j.1948-7134.2013.12023.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu Rev Entomol. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, Nishimura N, Greene K. Your worst enemy could be your best friend: predator contributions to invasion resistance and persistence of natives. Oecologia. 2010;162:709–718. doi: 10.1007/s00442-009-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae) Annu Rev Entomol. 2014;59:31–49. doi: 10.1146/annurev-ento-011613-162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Stanuszek WW, Brouhard EA, Knepper RG, Walker ED. Establishment of Aedes japonicus japonicus and its colonization of container habitats in Michigan. J Med Entomol. 2012;49:1307–1317. doi: 10.1603/me12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Alto B, Afify A, Gaugler R. Malathion influences competition between Aedes albopictus and Aedes japonicus. J Med Entomol. 2010;47:1011–1018. doi: 10.1603/me10011. [DOI] [PubMed] [Google Scholar]
- Kesavaraju B, Khan DF, Gaugler R. Behavioral differences of invasive container-dwelling mosquitoes to a native predator. J Med Entomol. 2011;48:526–532. doi: 10.1603/me10200. [DOI] [PubMed] [Google Scholar]
- Leisnham PT, LaDeau SL, Juliano SA. Spatial and temporal habitat segregation of mosquitoes in urban Florida. PLoS One. 2014;9(3):e91655. doi: 10.1371/journal.pone.0091655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. J Anim Ecol. 1984;53:573–580. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–374. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- Moles AT, Gruber MA, Bonser SP. A new framework for predicting invasive plant species. J Ecol. 2008;96:13–17. [Google Scholar]
- Moyle PB. Fish introductions into North America: patterns and ecological impact. In: Mooney HA, Drake JA, editors. Ecology of biol invasions of North America and Hawaii. Springer; New York: 1986. pp. 27–43. [Google Scholar]
- Murrell EG, Juliano SA. Predation resistance does not trade off with competitive ability in early-colonizing mosquitoes. Oecologia. 2013;173:1033–1042. doi: 10.1007/s00442-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell EG, Ives AR, Juliano SA. Intrinsic and extrinsic drivers of succession: effects of habitat age and season on an aquatic insect community. Ecol Entomol. 2014;39:316–324. doi: 10.1111/een.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell DL, Armbruster P. Comparison of larval foraging behavior of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) J Med Entomol. 2007;44:984–989. doi: 10.1603/0022-2585(2007)44[984:colfbo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Peacor SD, Werner EE. Trait-mediated indirect interactions in a simple aquatic food web. Ecology. 1997;78:1146–1156. [Google Scholar]
- Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J Am Mosq Control. 1999;15:238–241. [PubMed] [Google Scholar]
- Preisser EL, Bolnick DI, Benard MF. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- Reiskind MH, Wilson ML. Interspecific competition between larval Culex restuans Theobald and Culex pipiens L. (Diptera: Culicidae) in Michigan. J Med Entomol. 2008;45:20–27. doi: 10.1603/0022-2585(2008)45[20:icblcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Lounibos LP. Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med Vet Entomol. 2013;27:421–429. doi: 10.1111/mve.12000. [DOI] [PubMed] [Google Scholar]
- Rochlin I, Ninivaggi DV, Hutchinson ML, Farajollahi A. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in northeastern USA: implications for public health practitioners. PLoS One. 2013;4:e60874. doi: 10.1371/journal.pone.0060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG. The population biology of invasive species. Annu Rev Ecol Syst. 2001;32:305–332. [Google Scholar]
- Skiff JJ, Yee DA. Behavioral differences among four co-occurring species of container mosquito larvae: effects of depth and resource environments. J Med Entomol. 2014;51:375–381. doi: 10.1603/me13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder WE, Clevenger GM, Eigenbrode SD. Intraguild predation and successful invasion by introduced ladybird beetles. Oecologia. 2004;140:559–565. doi: 10.1007/s00442-004-1612-5. [DOI] [PubMed] [Google Scholar]
- Sumba LA, Okoth K, Deng AL, Githure J, Knols BG, Beier JC, Hassanali A. Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. J Circadian Rhythms. 2004;2:6. doi: 10.1186/1740-3391-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunihara T, Ishizaka K, Mogi M. Habitat size: a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. J Vector Ecol. 2002;27:8–20. [PubMed] [Google Scholar]
- Vonesh JR, Osenberg CW. Multi-predator effects across life-history stages: non-additivity of egg-and larval-stage predation in an African treefrog. Ecol Lett. 2003;6:503–508. [Google Scholar]
- Vonesh J, Blaustein L. Predator-induced shifts in mosquito oviposition site selection: a meta-analysis and implications for vector control. Isr J Ecol Evol. 2010;56:263–279. [Google Scholar]
- Wachira SW, Ndung U, Njagi PGN, Hassanali A. Comparative responses of ovipositing Anopheles gambiae and Culex quinquefasciatus females to the presence of Culex egg rafts and larvae. Med Vet Entomol. 2010;24:369–374. doi: 10.1111/j.1365-2915.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- Weigelt A, Schumacher J, Walther T, Bartelheimer M, Steinlein T, Beyschlag W. Identifying mechanisms of competition in multi-species communities. J Ecol. 2007;95:53–64. [Google Scholar]
- Werner EE, Peacor SD. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84:1083–1100. [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Larval feeding behavior of three co-occurring species of container mosquitoes. J Vector Ecol. 2004;29:315–322. [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kneitel JM, Juliano SA. Environmental correlates of abundances of mosquito species and stages in discarded vehicle tires. J Med Entomol. 2010;47:53–62. doi: 10.1603/033.047.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Couret J, Kim F, McMillan J, Burkot TR, Dotson EM, Kitron U, Vazquez-Prokopec GM. Diet and density dependent competition affect larval performance and oviposition site selection in the mosquito species Aedes albopictus (Diptera: Culicidae) Parasite Vector. 2012;5:225. doi: 10.1186/1756-3305-5-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahiri N, Rau ME. Oviposition attraction and repellency of Aedes aegypti (Diptera: Culicidae) to waters from conspecifics larvae subjected to crowding, confinement, starvation, or infection. J Med Entomol. 1998;35:782–787. doi: 10.1093/jmedent/35.5.782. [DOI] [PubMed] [Google Scholar]
- Zaiko A, Olenin S, Daunys D, Nalepa T. Vulnerability of benthic habitats to the aquatic invasive species. Biol Invasions. 2007;9:703–714. [Google Scholar]
- Zarnetske PL, Gouhier TC, Hacker SD, Seabloom EW, Bokil VA. Indirect effects and facilitation among native and non-native species promote invasion success along an environmental stress gradient. J Ecol. 2013;101:905–915. [Google Scholar]