Abstract
Background
There is no consensus regarding the optimal adjuvant treatment after resection of non-pancreatic periampullary adenocarcinoma (NPPC; distal common bile duct, ampulla, duodenum).
Objectives
The present study was conducted to evaluate the impacts on longterm survival and recurrence of adjuvant intra-arterial chemotherapy (IAC) and concomitant radiotherapy (RT) in patients submitted to resection for NPPC or pancreatic ductal adenocarcinoma (PDAC) in a randomized controlled trial.
Methods
A total of 120 patients with PDAC (n = 62) or NPPC (n = 58) were prestratified at a ratio of 1:1 for tumour origin and randomized. Half of these patients were treated with adjuvant IAC/RT and the other half were treated with surgery alone. Follow-up was completed for all patients up to 5 years after resection or until death.
Results
There was no survival benefit in either the whole group (primary endpoint) or the PDAC group after IAC/RT. In the NPPC group, longterm survival was observed in 10 patients in the IAC/RT group and five patients in the control group: median survival was 37 months and 28 months, respectively. The occurrence of liver metastases was reduced by IAC/RT from 57% to 29% (P = 0.038). Cox regression analysis revealed a substantial effect of IAC/RT on survival (hazard ratio: 0.44, 95% confidence interval 0.23–0.83; P = 0.011).
Conclusions
This longterm analysis shows that median and longterm survival were improved after IAC/RT in patients with NPPC, probably because of the effective and sustained reduction of liver metastases. The present results illustrate that NPPC requires an adjuvant approach distinct from that in pancreatic cancer and indicate that further investigation of this issue is warranted.
Introduction
The treatment of adenocarcinoma of the pancreas and periampullary region remains challenging. Even in the few patients with disease suitable for resection with curative intent, overall survival remains poor. Most tumours arise in the pancreatic head near the ampulla of Vater. The majority of these tumours represent pancreatic ductal adenocarcinoma (PDAC). Less frequently, tumours in the pancreatic head region arise from the distal common bile duct, ampulla of Vater and duodenum, and are collectively known as periampullary cancers.1 Although histologically very similar, these tumours bear a more favourable prognosis. The common assumption is that these tumours are diagnosed at an earlier stage because they lead to jaundice early as a result of their anatomical location. For these reasons non-pancreatic periampullary adenocarcinoma (NPPC) may be amenable to surgical resection more frequently than its pancreatic counterpart. However, evidence that NPPC represents a separate family of tumours with different biological behaviour is increasing.
Currently, evidence supports the administration of adjuvant chemotherapy after resection for PDAC.2 There is no clear evidence to recommend adjuvant therapy after resection for NPPC.3,4 The present paper reports a single-centre randomized trial in which outcomes after adjuvant treatment with intra-arterial chemotherapy (IAC) and concomitant radiotherapy (RT) were compared with those after surgery alone. The choice of IAC was supported by several small Phase I and II trials showing promising results for a 5-fluorouracil (5-FU), mitoxantrone and cisplatinum-based IAC regimen in advanced and resected pancreatic cancer.5–7 Radiotherapy was added to prevent local recurrence.
In a protocol similar to that of the well-known European Organization for Research and Treatment of Cancer (EORTC) trial3,8 and – at the start of the trial – in the absence of any available evidence-based standard adjuvant treatment for either group, patients with NPPC and PDAC were included in this trial on an equal basis. Patients were prestratified for either NPPC or PDAC after surgical resection. In 2008, shortly after the inclusion of the last patient, the first results were published.9 These showed the study's inability to demonstrate a survival benefit in PDAC. However, adjuvant IAC/RT reduced the number of patients with NPPC who developed liver metastases. Subsequent to this publication, discussion ensued as to whether this was just a temporary effect or whether such treatment might actually lead to better longterm survival. The current paper presents longterm data from this randomized controlled trial, in which all patients were followed for at least 5 years or until death, with special focus on NPPC.
Materials and methods
The study design was described in detail in the primary analysis.9 The trial was approved by the local medical ethics committee. Patients were randomized after resection into two groups according to whether they were to be treated with IAC/RT or not. All specimens were reviewed by one specialized pathologist (HvD), and graded and staged according to the 2002 guidelines of the Union for International Cancer Control (UICC).10 Tumour origin was determined according to the micro- and macroscopic evaluation of the resected specimen. Duodenal, cystic and neuroendocrine tumours were excluded. Other exclusion criteria were: age <75 years; a Karnofsky index of ≤50; uncontrolled infection; previous chemo- or radiotherapy, and an aberrant vascular supply to the liver. Enrolled patients were prestratified for tumour origin (PDAC or NPPC). After recovery from surgery, patients were randomized during their first visit to the outpatient clinic, according to a computer-generated randomization list provided by the trial statistician. Treatment started within 6–12 weeks after surgery. Patients with complications resulting in a prolonged hospital stay were not randomized. During or before the first IAC administration and before radiotherapy, all patients were restaged by computed tomography (CT). Follow-up consisted of clinical and laboratory examinations every 3 months. During the first 2 years, CT was performed every 3 months and subsequently every 6 months. Clinical signs of recurrence were indications for additional imaging. All patients were monitored for 5 years or until death. All survival data were cross-checked with the national population registry.
Adjuvant treatment
The treatment schedule has been described in full detail previously.9 Chemotherapy was administered through a catheter placed in the coeliac trunk and left in place during the five treatment days of each cycle. Heparin was infused to prevent thrombosis. Cycles consisted of mitoxantrone on day 1, followed by 5-FU/folinic acid on days 2–4 and cisplatinum on day 5. Toxicity was monitored and the dose was reduced by 20% in the event of toxicity of greater than World Health Organization (WHO) Grade II toxicity. After 2 weeks radiotherapy was started. A total cumulative dose of 54 Gy was delivered in single doses of 1.8 Gy on 5 days per week. Intra-arterial chemotherapy was continued for up to a total of six cycles with intervals of 4 weeks between cycles. Therapy was discontinued in the event of serious toxicity (WHO Grades III and IV) (Table1).
Table 1.
Cycles of intra-arterial chemotherapy and concomitant radiotherapy (IAC/RT) administered in 28 patients resected for non-pancreatic periampullary adenocarcinoma (NPPC) and reasons for cessation of therapy. Over half (n = 15) of all patients received all six cycles
| Cycles of IAC/RT | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | Total | |
| Patients, n (%) | 28 (100%) | 25 (89%) | 23 (82%) | 18 (64%) | 17 (61%) | 16 (57%) | 15 (54%) | 28 |
| Dropouts, n (%) | 3 (11%) | 2 (7%) | 5 (18%) | 1 (4%) | 1 (4%) | 1 (4%) | 0 | 13 (46%) |
| Progression | 1 (4%) | 2 (7%) | 3 (11%) | |||||
| Angio-related | 1 (4%) | 1 (4%) | ||||||
| Toxicity | 1 (4%) | 2 (7%) | 1 (4%) | 1 (4%) | 1 (4%) | 6 (21%) | ||
| Patient factors | 2 (7%) | 1 (4%) | 3 (11%) | |||||
Statistics
The primary outcome was overall survival. Secondary endpoints were toxicity and disease-free survival. Using an α-value of 0.05 (two-sided) and a β-value of 0.10, and prestratification by tumour origin, 120 patients were required to be enrolled in each trial arm assuming 2-year survival rates of 30% in the control groups and 50% in the experimental groups. Inclusion was stopped after 120 patients because gemcitabine-based adjuvant therapy had come to represent the standard of care for PDAC and an observation-only group for PDAC was therefore considered unethical. Separate continuation of the trial for NPPC was not part of the protocol and therefore not considered.
Analyses were performed on an intention-to-treat basis. IBM spss Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA) was used for the current analyses. The chi-squared test was used in analyses of categorical variables. Student's t-test was used in analyses of continuous variables. A P-value <0.05 (two-sided) was considered to indicate statistical significance. All P-values were rounded to three decimals. Survival was estimated using the Kaplan–Meier method. Significance was calculated using the log-rank test.
In addition, near-significant factors (P < 0.100) from the univariate analysis were entered in a multivariate Cox proportional hazards model, in accordance with the criteria for proportional hazards.11 Hazard ratios (HRs) are shown with 95% confidence intervals (95% CIs). Grade variables were considered of ordinal level and therefore coded as dummy variables.
Overall survival and disease-free survival
Primary analysis (whole group, n = 120)
The primary analysis was based on 82 deaths. Longer follow-up led to the registration of a further 18 events. Therefore, this survival analysis is based on 100 deaths. Longterm survival (defined as survival for <60 months) was observed in 19 patients. Median overall survival in patients with PDAC (20 months) was lower than that in patients with NPPC (32 months), regardless of therapy (P < 0.001). In the combined groups (whole study group), IAC/RT did not improve survival. Disease-free survival was longer in patients treated with IAC/RT in the whole (PDAC and NPPC) study group (13 months versus 8 months; P = 0.031). Results in each of the prestratified groups are very different.
Subgroup PDAC (n = 62)
Concomitant radiotherapy did not influence survival in PDAC (20 months versus 21 months; P = 0.929). There was no significant effect of IAC/RT on time to progression in PDAC alone (12 months versus 8 months; P = 0.214).
Subgroup NPPC (n = 58)
In the NPPC group, the number of patients who achieved longterm survival after IAC/RT (n = 10) was twice that of longterm survivors after surgery alone (n = 5). Although the survival curves (Fig.1) clearly diverge, log-rank analysis did not indicate statistical significance (median actual survival of 37 months versus 28 months; P = 0.077). However, Cox regression revealed a substantial effect of IAC/RT on survival (HR 0.44, 95% CI 0.23–0.83; P = 0.011).
Figure 1.
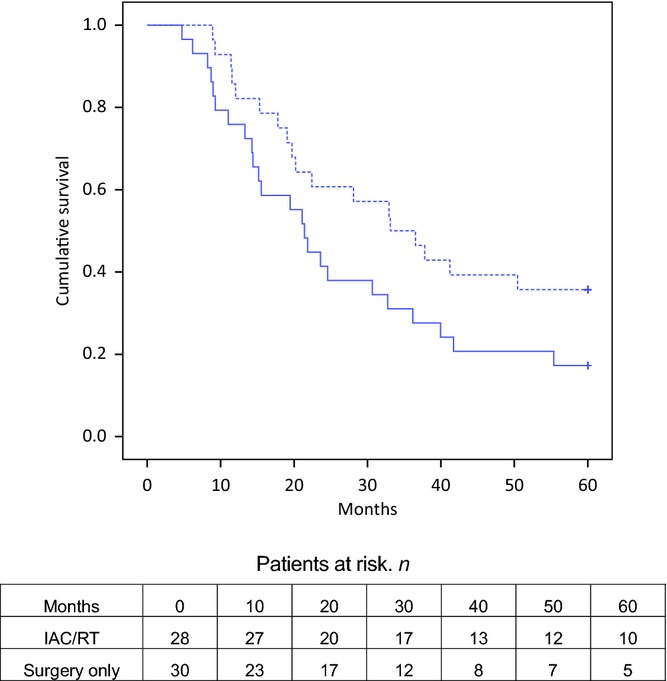
Kaplan–Meier curves for overall survival in patients submitted to resection of non-pancreatic periampullary adenocarcinoma with (dotted line) and without (black line) intra-arterial chemotherapy and concomitant radiotherapy (IAC/RT) (log-rank test, P = 0.077; hazard ratio 0.44, 95% confidence interval 0.23–0.83; P = 0.011)
The other independent factor was differentiation grade. Regardless of therapy, well-differentiated NPPC bore a more favourable prognosis than moderately and poorly differentiated NPPC (Fig.2). A detailed description of univariate and multivariate analyses for overall survival is shown in Table2. Factors were tested for their independent contributions in the model.
Figure 2.
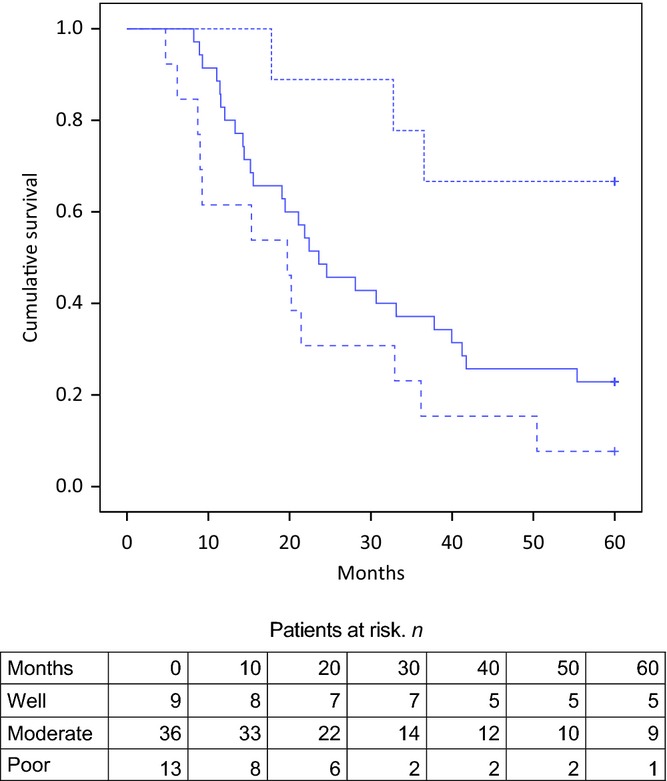
Kaplan–Meier curves for overall survival according to tumour differentiation in patients submitted to resection of well (dotted line), moderate (black line) and poorly (dashed line) differentiated non-pancreatic periampullary adenocarcinoma. Well versus poorly differentiated, P < 0.01; well versus moderately differentiated, P = 0.022; moderately versus poorly differentiated, P = 0.122
Table 2.
Summary of univariate and multivariate analyses in patients submitted to resection of non-pancreatic periampullary adenocarcinoma (NPPC)
| Univariate and multivariate analyses: overall survival in NPPC | ||||||||
|---|---|---|---|---|---|---|---|---|
| IAC/RT group, n | Surgery only group, n | Univariate (log-rank) | Multivariate (Cox) | |||||
| Median survival, months | 95% CI | P-value | HR | 95% CI | P-value | |||
| All patients | 28 | 30 | ||||||
| Male | 11 | 17 | 21 | 17–25 | ||||
| Female | 17 | 13 | 33 | 16–50 | 0.285 | |||
| PPPD | 22 | 23 | 28 | 13–43 | ||||
| Whipple | 6 | 7 | 19 | 7–31 | 0.303 | |||
| Pathology | ||||||||
| T2 | 9 | 8 | 33 | 11–55 | Reference | |||
| T3 | 13 | 16 | 28 | 10–47 | 0.435 | |||
| T4 | 6 | 6 | 18 | 8–28 | 0.025 | |||
| N0 | 12 | 12 | 36 | 23–49 | ||||
| N1 | 16 | 18 | 21 | 17–25 | 0.037 | |||
| R0 | 23 | 29 | 25 | 13–36 | ||||
| R1 | 5 | 1 | 20 | 0–51 | 0.081 | |||
| Differentiation | ||||||||
| Good | 4 | 5 | 60 | – | Reference | |||
| Moderate | 17 | 19 | 24 | 7–36 | 0.022 | |||
| Poor | 7 | 6 | 20 | 7–33 | 0.003 | 2.55 | 1.53–4.25 | 0.000 |
| Surgery only | 30 | 21 | 17–26 | |||||
| Treatment IAC/RT | 28 | 33 | 20–46 | 0.077 | 0.44 | 0.23–0.83 | 0.011 | |
95% CI, 95% confidence interval; HR, hazard ratio; IAC/RT, intra-arterial chemotherapy and concomitant radiotherapy; N0, node-negative disease; N1, node-positive disease; PPPD, pylorus-preserving pancreaticoduodenectomy; R0, negative margin; R1, positive margin; T1–3, tumour stage.
In patients with NPPC, time to progression appears to be longer after adjuvant IAC/RT (19 months versus 8 months; log-rank test, P = 0.103; HR for recurrent disease 0.48, 95% CI 0.25–0.90; P = 0.022) (Fig.3). The other independent factor in the same regression model was differentiation grade (Table2).
Figure 3.
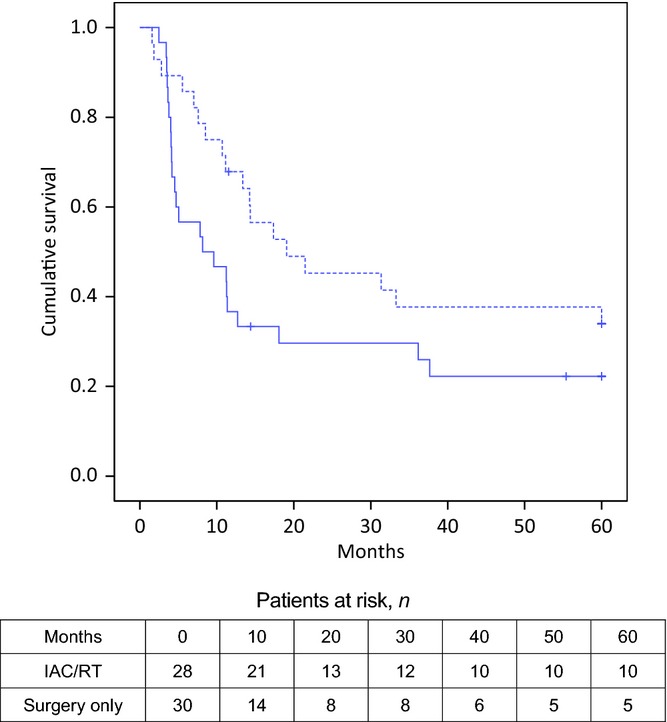
Kaplan–Meier curves for disease-free survival in patients submitted to resection of non-pancreatic periampullary adenocarcinoma (NPPC) with (dotted line) and without (black line) intra-arterial chemotherapy and concomitant radiotherapy (IAC/RT) (19 months versus 8 months; log-rank test, P = 0.103; hazard ratio for recurrent disease 0.48, 95% confidence interval 0.25–0.90; P = 0.022)
Patterns of recurrence are shown in Table3. Interestingly, IAC/RT effectively suppressed the longterm occurrence of liver metastases in patients with NPPC, from 17 to eight cases (HR 3.27, 95% CI 1.10.00–9.80; chi-squared test, P = 0.038). No effect on time to occurrence of liver metastasis was shown.
Table 3.
Patterns of recurrence in patients resected for pancreatic ductal adenocarcinoma (PDAC) or non-pancreatic periampullary cancer (NPPC) with and without adjuvant intra-arterial chemotherapy and concomitant radiotherapy (IAC/RT). Cumulative occurrence of liver metastases is significantly less frequent after IAC/RT than after surgery alone in NPPC (eight versus 17 patients; P = 0.038); this effect was not observed in PDAC. No effect on local recurrence was observed in either NPPC or PDAC
| Recurrence pattern | PDAC | NPPC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IAC/RT | Control | P-value | IAC/RT | Control | P-value | |||||
| Patients, n | 31 | 31 | 28 | 30 | ||||||
| First recurrence, n (%) | ||||||||||
| Local | 14 | (45%) | 19 | (61%) | 0.309 | 11 | (39%) | 9 | (30%) | 0.585 |
| Liver | 14 | (45%) | 11 | (35%) | 0.605 | 7 | (25%) | 13 | (43%) | 0.174 |
| Local and liver | 6 | (19%) | 4 | (13%) | 0.731 | 2 | (7%) | 3 | (10%) | 1.000 |
| Lung | 5 | (16%) | 3 | (10%) | 0.707 | 3 | (11%) | 3 | (10%) | 1.000 |
| Other | 0 | 6 | (19%) | 0.024 | 3 | (11%) | 3 | (10%) | 1.000 | |
| Cumulative recurrence, n (%) | ||||||||||
| Local | 15 | (48%) | 22 | (71%) | 0.120 | 13 | (46%) | 12 | (40%) | 0.791 |
| Liver | 15 | (48%) | 16 | (52%) | 1.000 | 8 | (29%) | 17 | (57%) | 0.038 |
| Local and liver | 7 | (23%) | 11 | (35%) | 0.402 | 4 | (14%) | 7 | (23%) | 0.508 |
| Lung | 5 | (16%) | 3 | (10%) | 1.000 | 3 | (11%) | 5 | (17%) | 0.707 |
| Other | 1 | (3%) | 9 | (29%) | 0.012 | 5 | (18%) | 5 | (17%) | 1.000 |
Discussion
This is a detailed longterm outcome analysis of a randomized clinical trial comparing survival after adjuvant therapy with that after observation alone in patients submitted to resection for PDAC or NPPC. The present findings confirm the results of the original report that IAC/RT does not improve survival in PDAC.9 The effect of IAC/RT in this group is disappointing and confirms that true pancreatic cancer has a dismal prognosis despite the addition of chemotherapy and radiotherapy. However, in patients with NPPC, survival appears to have been better in the IAC/RT group. Intra-arterial chemotherapy and radiotherapy also effectively reduced the occurrence of liver metastases. This effect was sustained throughout the longterm follow-up.
The present results confirm that NPPCs probably represent a separate family of tumours with different biological behaviour. This hypothesis is supported by the superior survival of patients with NPPC, even after adjusting for tumour size, positive lymph nodes and stage.11,12 Evidence is mounting that these tumours may have a different genetic basis and express different proteins, microRNA and growth factor receptors.13–17 It may become possible in the future to use these biomarkers to identify more specific subtypes of NPPC and PDAC and select a more targeted type of adjuvant therapy.
In pursuit of the improvement of survival after surgery for pancreatic cancer, several randomized trials offering adjuvant chemotherapy both with and without concomitant radiotherapy have been conducted. These have led to the consensus that gemcitabine-based adjuvant chemotherapy improves outcomes after surgery for PDAC.18 The role of adjuvant radiotherapy remains doubtful.19
By contrast, the benefit of adjuvant chemotherapy in patients with NPPC remains largely unclear. The first trial was the well-known EORTC trial, which offered a 5-FU-based regimen with concomitant radiotherapy, but which failed to shown an effect on survival.3,8 In the EORTC trial, adjuvant chemotherapy based on 5-FU with concomitant radiotherapy (40 Gy) was compared with surgery alone. Liver metastases occurred in 50% of patients. There were no significant differences in the occurrence of liver metastases between treatment groups. Neoptolemos et al.4 published the only other recent randomized study on adjuvant therapy for NPPC. This trial compared three study groups, in which 5-FU-based chemotherapy, gemcitabine-based chemotherapy and surgery only, respectively, were administered. The authors were unable to demonstrate improved survival after gemcitabine- or 5-FU-based adjuvant chemotherapy in the primary analysis.4 However, in a multivariate analysis, after adjusting for variables of age, bile duct cancer, poor tumour differentiation and positive lymph nodes, the authors observed a modest benefit in association with adjuvant chemotherapy. This study clearly shows that the common adjuvant schedules for PDAC cannot be extrapolated to the treatment of NPPC. In the recent ABC-2 trial,20 the provision of gemcitabine combined with cisplatinum led to a survival benefit in patients with advanced cholangiocarcinomas. The patients included in this study suffered from a range of intrahepatic, extrahepatic and metastastic bile duct cancers. Perhaps the addition of cisplatinum might have evoked a response to IAC in the ampullary and distal bile duct cancers (NPPC) investigated in the present study.
The rationale for using IAC/RT in the present study was two-fold. Treatment was intended to facilitate the reduction of liver metastases (IAC) and improve local control (RT). Indeed, in patients with NPPC, the provision of IAC/RT led to a reduction in the occurrence of liver metastases and had a substantial effect on median survival, disease-free survival and the number of longterm survivors. Interestingly, the present authors were unable to demonstrate an effect on local recurrence. The EORTC study also included radiotherapy, administered at 40 Gy rather than the 54 Gy used in the present study.8 The EORTC trial also failed to show any effect on local recurrence. Therefore, it is more likely that the IAC, rather than the radiotherapy, was responsible for reducing the number of liver metastases and consequently imposing a positive effect on survival. Two Phase II clinical trials and a case study preceded the present trial.5,21,22 Both trials showed a decrease in the occurrence of liver metastases and improved survival after IAC. The underlying principle is that by infusing selectively, a much higher dose can be achieved in the target organ, in this case the liver. The present findings do not preclude the possibility that, in pancreatic cancers, the mitoxantrone, 5-FU and cisplatinum combination used in this study may be inferior to a gemcitabine-based regimen. Furthermore, recent developments of new therapeutic agents and combination therapy have led to more effective systemic therapy in metastatic pancreatic cancer using a 5-FU, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) regimen.23 The present authors speculate that a different agent or combination may be effective as IAC in PDAC and suggest that the IAC concept should not be completely discarded as a possible means of delivering other more effective chemotherapeutics to patients suffering from PDAC.
In the present study, Cox regression analysis showed that both differentiation grade and adjuvant treatment were of independent influence on overall and disease-free survival in NPPC. Differentiation grade was inversely correlated with survival (Fig.2), which is concordant with findings in other studies.4 Survival after resection of well-differentiated cancers is much better compared with that after resection of moderately or poorly differentiated tumours. It is questionable whether well-differentiated tumours should be treated with this adjuvant regimen.
This longterm analysis shows interesting effects of adjuvant IAC/RT on survival in patients with NPPC, which were not revealed in the primary analyses. Although this group was relatively small (n = 58), and despite the premature conclusion of the trial and the fact that a benefit was observed only in this prestratified group, the present study provides some evidence that this IAC/RT protocol may be beneficial in these patients. It must be acknowledged that this study is underpowered. However, a small group size is more likely to lead to a type II error (i.e. no effect of therapy in the analyses although a true effect may have been present) than an overestimation of the effect of IAC/RT. The finding of a positive effect in a prestratified group advocates for the further study of this concept in patients with NPPC, particularly in those with moderately or poorly differentiated tumours. In addition, this is the only study to date to show any substantial beneficial effect of adjuvant therapy in this particular group of patients.
Although the IAC/RT protocol administered in the present study was intense, it did not adversely affect quality of life during the short time that some patients live after ‘curative’ resection.24 Toxicity was relatively mild. Delivering IAC to a large number of patients is logistically challenging and requires the training of medical staff and dedicated nurses. Further developments in minimally invasive isolated perfusion devices may prove to be more practical to use and more effective in delivering chemotherapy to the liver alone, where the effect of the present regimen was most noticeable.
In conclusion, patients with resectable NPPC may benefit from adjuvant IAC as it has a substantial effect on overall and disease-free survival, and effectively and enduringly reduces the occurrence of liver metastases. The value of radiotherapy for local control remains doubtful. The results of this trial warrant further investigation by means of a dedicated trial on adjuvant IAC for NPPC. This trial should enrol patients in three treatment arms: (i) surgery only (control); (ii) systemic gemcitabine plus cisplatinum (based on the ABC trial), and (iii) gemcitabine plus cisplatinum-based IAC.
Conflicts of interest
None declared.
References
- Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic head cancer. Br J Surg. 2012;99:1036–1049. doi: 10.1002/bjs.8734. [DOI] [PubMed] [Google Scholar]
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246:734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- Beger HG, Gansauge F, Buchler MW, Link KH. Intraarterial adjuvant chemotherapy after pancreaticoduodenectomy for pancreatic cancer: significant reduction in occurrence of liver metastasis. World J Surg. 1999;23:946–949. doi: 10.1007/s002689900604. [DOI] [PubMed] [Google Scholar]
- Link KH, Gansauge F, Rilinger N, Beger HG. Celiac artery adjuvant chemotherapy. Results of a prospective trial. Int J Pancreatol. 1997;21:65–69. doi: 10.1007/BF02785922. [DOI] [PubMed] [Google Scholar]
- Maurer CA, Borner MM, Lauffer J, Friess H, Z'Graggen K, Triller J, et al. Celiac axis infusion chemotherapy in advanced nonresectable pancreatic cancer. Int J Pancreatol. 1998;23:181–186. doi: 10.1007/BF02788395. [DOI] [PubMed] [Google Scholar]
- Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. discussion 782–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morak MJ, van der Gaast A, Incrocci L, van Dekken H, Hermans JJ, Jeekel J, et al. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 2008;248:1031–1041. doi: 10.1097/SLA.0b013e318190c53e. [DOI] [PubMed] [Google Scholar]
- Sobin LH, Wittekind Ch. TNM Classification of Malignant Tumours. New York: Wiley; 2002. International Union Against Cancer (UICC) eds. (. In:, 6th edn. [Google Scholar]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Klempnauer J, Ridder GJ, Pichlmayr R. Prognostic factors after resection of ampullary carcinoma: multivariate survival analysis in comparison with ductal cancer of the pancreatic head. Br J Surg. 1995;82:1686–1691. doi: 10.1002/bjs.1800821233. [DOI] [PubMed] [Google Scholar]
- Smeenk HG, Erdmann J, van Dekken H, van Marion R, Hop WC, Jeekel J, et al. Long-term survival after radical resection for pancreatic head and ampullary cancer: a potential role for the EGF-R. Dig Surg. 2007;24:38–45. doi: 10.1159/000100917. [DOI] [PubMed] [Google Scholar]
- Collins AL, Wojcik S, Liu J, Frankel WL, Alder H, Yu L, et al. A differential microRNA profile distinguishes cholangiocarcinoma from pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21:133–138. doi: 10.1245/s10434-013-3240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee JA, van Eijck CH, Hop WC, van Dekken H, Dicheva BM, Seynhaeve AL, et al. Angiogenesis: a prognostic determinant in pancreatic cancer? Eur J Cancer. 2011;47:2576–2584. doi: 10.1016/j.ejca.2011.08.016. [DOI] [PubMed] [Google Scholar]
- van der Zee JA, ten Hagen TL, Hop WC, van Dekken H, Dicheva BM, Seynhaeve AL, et al. Differential expression and prognostic value of HMGA1 in pancreatic head and periampullary cancer. Eur J Cancer. 2010;46:3393–3399. doi: 10.1016/j.ejca.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Overman MJ, Zhang J, Kopetz S, Davies M, Zhi-Qin J, Stemke-Hale K, et al. Gene expression profiling of ampullary carcinomas classifies ampullary carcinomas into biliary-like and intestinal-like subtypes that are prognostic of outcome. PLoS One. 2013;8:e65144. doi: 10.1371/journal.pone.0065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- Sultana A, Cox T, Ghaneh P, Neoptolemos JP. Adjuvant therapy for pancreatic cancer. Recent Results Cancer Res. 2012;196:65–88. doi: 10.1007/978-3-642-31629-6_5. [DOI] [PubMed] [Google Scholar]
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- Hayashibe A, Kameyama M, Shinbo M, Makimoto S. Clinical results on intra-arterial adjuvant chemotherapy for prevention of liver metastasis following curative resection of pancreatic cancer. Ann Surg Oncol. 2007;14:190–194. doi: 10.1245/s10434-006-9110-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa O, Ohhigashi H, Sasaki Y, Furukawa H, Imaoka S. Extended pancreatectomy and liver perfusion chemotherapy for resectable adenocarcinoma of the pancreas. Digestion. 1999;60(Suppl. 1):135–138. doi: 10.1159/000051470. [DOI] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- Morak MJ, Pek CJ, Kompanje EJ, Hop WC, Kazemier G, van Eijck CH. Quality of life after adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled study. Cancer. 2010;116:830–836. doi: 10.1002/cncr.24809. [DOI] [PubMed] [Google Scholar]


