Abstract
Background
Robotic distal pancreatectomy (RDP) is performed increasingly, but knowledge of the number of cases required to attain procedural proficiency is lacking. The aim of this study was to identify the learning curve associated with RDP at a high-volume pancreatic centre.
Methods
Metrics of perioperative safety and efficiency for all consecutive RDPs were evaluated. Outcomes were followed to 90 days. Cumulative sum (CUSUM) analysis was used to identify inflexion points corresponding to the learning curve.
Results
Between 2008 and 2013, 100 patients underwent RDP. There was no 90-day mortality. In two patients (2.0%), surgery was converted to laparotomy. Thirty procedures were performed for pancreatic adenocarcinoma. Precipitous operative time reductions from an initial operative time of 331 min were observed after the first 20 and 40 cases to 266 min and 210 min, respectively (P < 0.0001). The likelihood of readmission was significantly lower after the first 40 cases (P = 0.04), and non-significant reductions were observed in incidences of major (Clavien–Dindo Grade II or higher) morbidity and Grade B and C leaks, and length of stay.
Conclusions
In this experience, RDP outcomes were optimized after 40 cases. Familiarity with the platform and dedicated training are likely to contribute to significantly shorter learning curves in future adopters.
Introduction
Laparoscopic distal pancreatectomy (LDP) is associated with reductions in blood loss, analgesic requirements, hospital stay and morbidity compared with the open approach.1–7 Similarly, robotic distal pancreatectomy (RDP) has been demonstrated to be equally safe and effective, and to confer benefits similar to those associated with laparoscopic surgery.8–11 However, RDP may also allow for substantial reductions in blood loss (< 500 ml) and rates of conversion to open surgery, particularly when it is performed for cancer.8,11 Additionally, the costs associated with RDP may become comparable with those of the open or laparoscopic approaches when reductions in conversions translate into reduced hospital lengths of stay (LoS).9
The benefits of minimally invasive distal pancreatectomy (DP),7,12 coupled with the advantageous ergonomics of the robotic platform, have led more surgeons to attempt RDP for the treatment of benign and malignant disease.13,14 As for other procedures, a learning curve may exist for RDP, which, if identified, may allow new adopters the insight and benefit of prior experiences. For RDP, this learning curve entails the mastery of important facets unique to the use of robotic technology, including optimal port placement, the development of close coordination between the console surgeon and bedside assistant, and the overcoming of the loss of tactile feedback.
The present paper reports the outcomes of the first 100 consecutive RDPs to be performed at one centre with the aim of identifying major inflexion points and milestones in the optimization of perioperative outcomes.
Materials and methods
Design and study population
A retrospective (institutional review board-approved) review of a prospectively maintained database of all RDP procedures carried out at the University of Pittsburgh from August 2008, when the gastrointestinal robotic programme at the University of Pittsburgh Medical Center (UPMC) was implemented, to July 2013 was performed. The cohort consisted of the first 100 consecutive cases to be operated using the Da Vinci S or Si Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA). Three surgeons (HJZ, AJM and AHZ) performed 86.0% of all RDPs in this cohort. These three surgeons had extensive prior experience with LDP, but no substantial prior robotic experience. Contraindications to RDP at the study institution evolved significantly over the study period, but from 2011 were limited to: (i) pancreatic body tumours involving the splenoportal confluence for which the resection and reconstruction of the superior mesenteric vein and/or portal vein were anticipated, and (ii) tumours for which a negative margin resection was anticipated to require large multivisceral resections. Importantly, patients with neoadjuvant therapy, anticipated ‘side-bite’ resections of the splenoportal confluence or concomitant ‘minor’ resections of adjacent viscera (adrenalectomy, partial colectomy, duodenectomy, gastrectomy) were not excluded from the robotic approach.
Operative technique
The technique for RDP used at this institution has been published previously.2,11 Initial laparoscopic mobilization is limited to: (i) mobilization of the greater curvature of the stomach up to the angle of His cranially and the right gastroepiploic pedicle inferiorly, and (ii) lowering of the splenic flexure. The remainder of the operation is performed robotically. Splenic preservation was performed according to surgeon preference. When performed for pancreatic ductal adenocarcinoma (PDA), en bloc resection of the retroperitoneal fascia according to the method described by Strasberg and Fields15 was used. Intraoperative frozen-section margins of the pancreatic neck were routinely performed. A closed suction drain was used in all cases.
Definitions and statistical analysis
Procedure duration was calculated as the length of time between skin incision and closure, including the time required to dock the robot. Postoperative outcomes were followed to 90 days. Postoperative pancreatic fistula (POPF) was defined according to International Study Group on Pancreatic Fistula (ISGPF) criteria16 and complications were graded according to the Clavien–Dindo system of classification.17,18 Analysis was performed on an intent-to-treat basis. Student's t-test and analysis of variance (anova) were used to compare normally distributed variables between groups. The Wilcoxon rank-sum test and Kruskal–Wallis test were used for non-normally distributed variables. Fisher's exact test was used to compare categorical variables between groups. A P-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using stata Version 10 (StataCorp LP, College Station, TX, USA).
Cumulative sum analysis of operative time
Cumulative sum (CUSUM) analysis was used to define the learning curve. CUSUM is the accumulated total difference between each data point and the mean of all data points for a particular metric. It is well suited to and widely employed in the assessment of new technical skills.19–21 Starting with the earliest surgical date, cases were ordered chronologically; the difference between the operative time (OT) of each of the 100 cases and the mean OT of all cases (μOT) was then obtained. The CUSUMOT was obtained by adding up the calculated difference from the overall mean, starting with the first case to the next cumulatively. If the OT for a case is more than μOT, the addition to the running value of CUSUMORT is a positive number (upwards slope on the graph). Conversely, it is a negative number if the OT for a case is less than μOT (downwards slope). This cumulative process is sustained until CUSUMORT for the last case is calculated as zero. This allows for a graphical representation of the learning curve and simultaneously outlines deviations from the OT norm.
Results
Perioperative outcomes for the entire cohort
Table1 demonstrates preoperative parameters for the entire cohort (n = 100). The mean age of the patients was 60 years. A total of 58.0% were female and 66.0% of patients had undergone prior abdominal surgery. Indications for RDP included neuroendocrine tumour (n = 35), PDA (n = 30), chronic pancreatitis or benign or premalignant cystic disease (n = 30), and metastatic lesions (n = 5). Table2 displays operative and postoperative outcomes. The average OT was 246 min and the median estimated blood loss (EBL) was 150 ml. Two patients (2.0%) required conversion to laparotomy for failure to progress in a large 11-cm mucinous cystic neoplasm (MCN) (case 41), and significant adhesions (case 47). No 90-day mortalities were recorded. Clavien–Dindo Grade III and IV complications occurred in 14.0% of patients and POPF occurred in 42.0% (Grade A, 24.0%; Grade B, 13.0%; Grade C, 5.0%).
Table 1.
Demographics and preoperative characteristics for 100 robotic distal pancreatectomies (RDPs) and the first 40 versus the last 60 RDPs
| Parameter | Total | Early experience | Late experience | P-value |
|---|---|---|---|---|
| n = 100 | n = 40 | n = 60 | ||
| Age, years, mean ± SD | 60.13 ± 12.7 | 60.1 ± 14.1 | 60.2 ± 11.8 | 0.985 |
| Female, n (%) | 58 (58.0%) | 25 (62.5%) | 33 (55.0%) | 0.537 |
| Preoperative BMI, kg/m2, mean ± SD | 29.9 ± 7.2 | 29.3 ± 7.1 | 30.3 ± 7.2 | 0.506 |
| Prior surgery, n (%) | 66 (66.0%) | 28 (70.0%) | 38 (63.3%) | 0.525 |
| ASA class, n (%) | ||||
| 2 | 27 (27.0%) | 12 (30.0%) | 15 (25.0%) | 0.565 |
| 3 | 71 (71.0%) | 28 (70.0%) | 43 (71.7%) | |
| 4 | 2 (2.0%) | 0 | 2 (3.3%) | |
| CCI, mean ± SD | 3.7 ± 2.9 | 3.7 ± 3.2 | 3.7 ± 2.8 | 0.890 |
| Preoperative albumin, mean ± SD | 3.8 ± 0.50 | 3.8 ± 0.54 | 3.9 ± 0.48 | 0.403 |
| CT size, cm, mean ± SD | 3.1 ± 2.4 | 3.2 ± 2.3 | 3.1 ± 2.5 | 0.974 |
| EUS size, cm, mean ± SD | 3.4 ± 2.6 | 3.8 ± 2.8 | 3.1 ± 2.4 | 0.198 |
| PDA, n (%) | 30 (30.0%) | 16 (40.0%) | 14 (23.3%) | 0.118 |
ASA, American Society of Anesthesiologists; BMI, body mass index; CCI, charlson comorbidity index; CT, computerized tomography scan; EUS, endoscopic ultrasound; PDA, pancreatic ductal adenocarcinoma; SD, standard deviation.
Table 2.
Operative and postoperative outcomes in 100 robotic distal pancreatectomies (RDPs) and in the first 40 and the last 60 RDPs
| Totala | Early experienceb | Late experiencec | P-value | |
|---|---|---|---|---|
| n = 100 | n = 40 | n = 60 | ||
| OR time, min, mean ± SD | 245.7 ± 89.5 | 298.6 ± 92.9 | 210.4 ± 67.5 | <0.0001 |
| Pure RDP time, min, mean ± SD | 236 ± 79 | 283 ± 87 | 205 ± 56 | <0.0001 |
| RDP with additional resections, min, mean ± SD | 291 ± 119 | 361 ± 97 | 236 ± 109 | <0.05 |
| EBL, ml, median (IQR) | 150 (100–300) | 175 (100–300) | 150 (100–300) | 0.670 |
| Conversion, n (%) | 2 (20.0%) | 0 | 2 (3.3%) | 0.515 |
| Postoperative transfusion, n (%) | 4 (4.0%) | 3 (7.5%) | 1 (6.7%) | 0.299 |
| Mortality, n | 0 | 0 | 0 | 1 |
| Any complication, n (%) | 72 (72.0%) | 29 (72.5%) | 43 (71.7%) | 1 |
| Complications, Clavien Grade <II, n (%) | 14 (14.0%) | 8 (20.0%) | 6 (10.0%) | 0.239 |
| Reoperation, n (%) | 1 (1.0%) | 0 | 1 (1.7%) | 1.00 |
| All pancreatic leaks, n (%) | 42 (42.0%) | 18 (45.0%) | 24 (40.0%) | 0.682 |
| POPF Grades B and C, n (%) | 18 (18.0%) | 11 (27.5%) | 7 (11.7%) | 0.062 |
| Readmission, n (%) | 28 (28.0%) | 16 (40.0%)d | 12 (20.0%)e | 0.041 |
| Length of stay, days, median (IQR) | 6 (5–8) | 6 (5–6) | 5 (4.5–6) | 0.672 |
| Lymph node count (malignant cases), median (IQR) | 12.5 (10–22) | 16 (8–20.5) | 15.5 (10–24) | 0.475 |
| Positive margin,f n (%) | 3 (4.3%) | 1 (3.1%) | 2 (5.3%) | 1.00 |
The 100 RDPs included 82 pure RDPs and 18 RDPs with additional procedures (cholecystectomy, n = 6; duodenal/gastric resection, n = 5; liver radiofrequency ablation, n = 3; adrenalectomy, n = 2; colectomy, n = 1; inferior vena cava filter placement, n = 1).
Early experience (n = 40) included 32 pure RDPs and eight RDPs with concomitant resection.
Late experience (n = 60) included 50 pure RDPs and 10 RDPs with concomitant resection.
Reasons for the 16 (early experience) readmissions were: fluid collections (n = 8; all had Grade B or C leaks);fever (n = 2; both had Grade B leaks but no collection); nausea/pain (n = 2; both had Grade A leaks), postoperative haematoma (self-limiting) (n = 2), seizure activity (no leak) (n = 1), and superficial wound infection (n = 1).
Reasons for the 12 (late experience) readmissions: fluid collection (n = 4; Grade B, n = 3; Grade A, n = 1), fever (n = 3; Grade B leak but no collections), postoperative pain/constipation (no leak or collections) (n = 2), poorly controlled new postoperative diabetes (n = 2), Clostridium difficile (n = 1), and flu-like symptoms (n = 1).
70 total malignancy cases; 32 in early experience, and 38 in late experience.
P-values in bold are significant at <0.05.
EBL, estimated blood loss; IQR, interquartile range; OR, operating room; POPF, postoperative pancreatic fistula; SD, standard deviation.
Identification of learning curve based on CUSUM OT
An analysis of all perioperative outcomes was performed by grouping patients chronologically into 10 groups (data not shown). This analysis revealed OT as the only variable to exhibit improvement across the 10 groups. A significant reduction in OT was observed after the first 40 cases (from 298 min to 210 min; P < 0.0001) (Fig.1). Further analysis revealed two distinct inflexion points within these 40 cases; reductions in OT were observed between the first two cohorts (cases 1–20, 330 min) and the second two cohorts (cases 21–40, 266 min) (P < 0.0001) and between the third and fourth cohorts (266 min) and the latter six groups (210 min) (P < 0.002). CUSUM analysis is illustrated in Fig.2(a). This confirmed the two distinct phases of the learning curve in the first 40 cases, followed by a predominantly downward slope indicating that OTs beyond the learning curve continued to gradually improve. Figure2(b–d) shows the learning curve phases individually. Moreover, when plotted on a C-chart (Fig.3), cases performed beyond the learning curve exhibited minimal variations from the mean, indicating that RDP was being performed with reliable consistency and efficiency once the learning curve had been attained.
Figure 1.
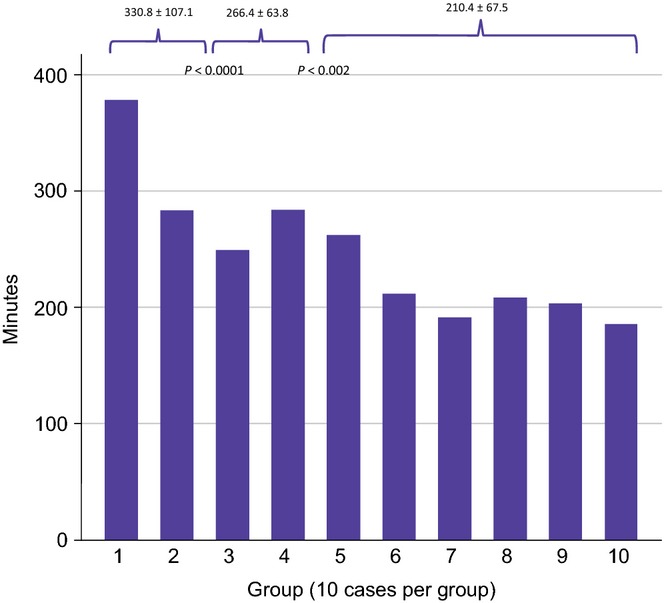
Operative times in 100 robotic distal pancreatectomies by consecutive groups of 10 patients per group
Figure 2.
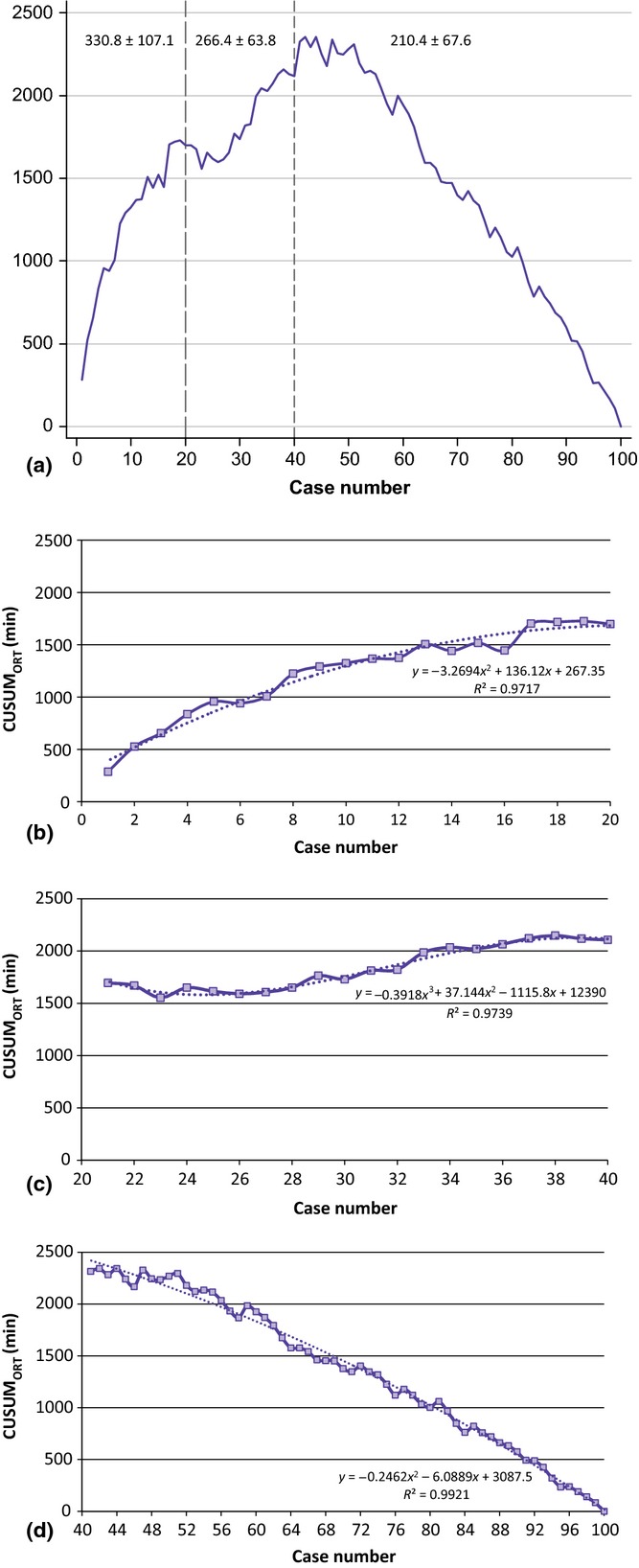
(a) CUSUM analysis of operative time showing three distinct phases of the learning curve for robotic distal pancreatectomy (RDP). CUSUMORT is plotted on the vertical axis against the respective case number. (b) Phase 1 indicates a rise in CUSUMORT with relative stabilization at case 20. (c) Phase 2 demonstrates a reduction in CUSUMORT after case 20. This is followed by a very slow increase in CUSUMORT with stabilization near case 40. (d) Phase 3 demonstrates a significant reduction in CUSUMORT after case 40
Figure 3.
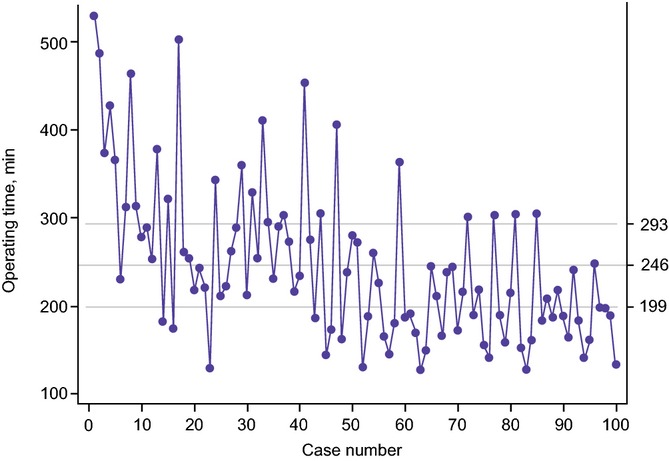
C-chart demonstrating decreasing variance from the mean operative time with increasing experience in robotic distal pancreatectomy
Perioperative outcomes in relation to the learning curve
Based on the identification of the OT learning curve of 40 cases, an analysis of perioperative outcomes comparing those in the learning curve cohort (first 40 cases) with those in the later experience (post-learning curve cohort: cases 41–100) was performed (Tables1 and 2). Both groups were homogeneous with respect to all preoperative demographics (Table1). A significant reduction in the rate of 90-day readmission was observed after the learning curve had been surpassed (P = 0.041). Additionally, marked reductions in the incidence of clinically significant Grade B or C fistulae (27.5% versus 11.7%; P = 0.062), and Clavien–Dindo Grade III or IV complications (20.0% versus 10.0%; P = 0.239) were observed after 40 cases (Table2).
Outcomes in patients with and without PDA
A subgroup analysis of patients with PDA (30.0%) was also performed (data not shown). Although PDA patients were older (66.3 years) than their non-PDA counterparts (57.5 years) (P < 0.001), all other baseline demographics were similar across both groups and there were no significant differences in operative (mean OT: 244 min versus 246 min, respectively; median EBL: 150 ml in both groups; number of conversions: none versus 2.9%, respectively; all non-significant) or postoperative (median LoS: 6 days in both groups; Clavien–Dindo Grade III and IV complications: 13.3% versus 14.3%, respectively; readmissions: 26.7% versus 25.6%, respectively; all non-significant) outcomes. The median number of lymph nodes harvested in PDA patients was 19 and only one patient had a positive margin (3.3%).
Discussion
The cumulative summation technique has been effectively used to evaluate learning curves for surgical procedures22,23 because it displays the variance from the mean on a case-by-case basis. In this analysis, CUSUM yielded a parabolic curve showing three distinct phases from which correlates of the RDP learning curve can be assessed. The mean OTs during the first, second and third phases were 331 min, 266 min and 210 min, respectively (P < 0.0001). The first steep OT drop (phase 1: 20 patients) can be attributed to increased familiarity with the ‘basics’ of the platform, which include optimal port placement, robotic docking and an initial rapid improvement in dissection skills. Phase 2 (cases 20–40) is likely to represent the steep learning curve that reflects the surgeon's development of ability to compensate for reduced haptic feedback with improved visualization and mastery of tissue manipulation. Phase 3 (case 40 onward) represents optimized (post-learning curve) performance metrics for RDP. Importantly, OTs may still gradually decline in phase 3 as a result of refinements in technique and the advent of new instruments or technology.
Although the present data indicate that approximately 40 cases were needed to achieve optimal outcomes, it is likely that the true learning curve may be less steep in other teams wishing to implement this technology today. Robotic surgery has infiltrated most subspecialties and an increasing number of surgeons are already utilizing the platform. Additionally, other reports detailing RDP technique and outcomes have recently emerged and will provide new adopters with further insight and guidance. A short learning curve, however, is predicated on robust prior experience in open and robotic pancreatic surgery; the present surgical team possessed extensive prior experience in open and laparoscopic pancreatic surgery (but minimal robotic experience) prior to attempting RDP.2 Thus, it is likely that phase 1 can be circumvented if all of the above criteria are present in the new adopter. To the present authors’ knowledge, only one prior report has attempted to identify the learning curve for LDP; Braga and colleagues analysed 30 consecutive LDPs (performed in 2009 and 2010) and noted a significant decline in OT after the first 10 cases.24 However, it is unclear if this cohort represented the authors’ first institutional LDPs. Furthermore, a trend in OT reductions was also observed after the 20th case, implying that the true LDP learning curve might have been longer if the sample size had been larger. Additionally, the authors had the benefit of significant laparoscopic experience prior to performing LDP. This is in sharp contrast with the present authors’ RDP learning curve, which was predicated on minimal robotic platform experience.
Morbidity after open or minimally invasive DP is not insignificant, even in high-volume institutions.25–30 The primary morbidity after DP is the development of POPF, the incidence of which approaches 40% when POPF is characterized rigorously using ISGPF guidelines.28 The present group observed a decline in the development of clinically significant POPF after the first 40 cases (P = 0.06). This may be attributable to the site of pancreatic transection with the linear cutting stapler; with increasing experience, this group has come to transect all PDA pancreata at the neck in order to maximize negative margin distance and improve lymph node yield. As the parenchyma here is thinnest, clinically significant pancreatic fistulae may have been avoided. Other than the reduction in OT between the early and late phases, the analysis did not reveal any other factors that would have led to a reduction in Grade B and C leaks. Notably, the method of transecting the gland (91.0% stapler, 9.0% electrocautery with robotic oversewing of the cut edge) did not influence the leak rate. However, this reduction in clinically significant POPF is likely to have led to the significant decline in readmissions in the latter part of the experience (from 40.0% to 20.0%; P = 0.041). The association between POPF and readmission is well documented and was confirmed in this analysis: 12 of the 16 readmissions (75.0%) in the early part of the present experience occurred in patients with known leaks (Grade B or C, n = 10; Grade A, n = 2), whereas six of 12 readmissions (50.0%) in the later part of the experience occurred in patients with known leaks (Grade B, n = 4; Grade A, n = 2).
The median EBL of 150 ml and conversion rate of 2.0% identified in the present series compare favourably with equivalent data for most LDP series.3,5,9,22 Unlike in pancreaticoduodenectomy, in which morbidity is much higher as a result of the extent of dissection and multiple anastomoses, the ability to complete RDP without conversion (particularly for PDA) may be the only benefit to distinguish the method from LDP. However, this advantage is of paramount importance because conversions are associated with higher EBL, positive (R1) margins and increased morbidity.11 Thus, even in the PDA cohort, in which average tumour size was 3.6 cm, EBL was maintained at 150 ml, the R1 rate was low, and no conversions were required.
The present analysis has important limitations. Firstly, as three surgeons were involved in the majority of cases (sometimes in a two attending approach in the initial implementation phase), it is particularly difficult to ascertain an individual surgeon's learning curve. Consequently, this learning curve represents that of a ‘group’ of surgeons performing RDP in similar fashion. Secondly, the learning curve may actually be shorter than 40 cases for surgeons already experienced in some facets of the robotic platform; indeed new attending staff (and fellows) with prior robotic experience and training have – anecdotally – been able to climb this learning curve much more rapidly. Thirdly, the present learning curve analysis is based solely on OT because this was the only metric to display constant significant improvements when outcomes were examined in 10 chronologically ordered groups; all other metrics failed to show such a constant significant improvement, probably as a result of small sample sizes. Finally, during this study period, 75 open DPs (ODPs) and 107 LDPs were performed at the study institution (mostly during 2008–2010). Although comparisons among these three modalities (ODP, LDP and RDP) would have been ideal, these were not feasible because the vast majority of DPs performed by the authors from 2011 onward were RDPs. In conclusion, this report identifies the RDP learning curve to be approximately 40 cases in a group of surgeons with minimal prior robotic experience. Familiarity with the platform, mentorship and dedicated training are likely to contribute to the significant shortening of the learning curve in future adopters.
Conflicts of interest
None declared.
References
- Merchant NB, Parikh AA, Kooby DA. Should all distal pancreatectomies be performed laparoscopically? Adv Surg. 2009;43:283–300. doi: 10.1016/j.yasu.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Magge D, Gooding W, Choudry H, Steve J, Steel J, Zureikat A, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg. 2013;148:525–531. doi: 10.1001/jamasurg.2013.1673. [DOI] [PubMed] [Google Scholar]
- Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010;210:779–785. doi: 10.1016/j.jamcollsurg.2009.12.033. discussion 786–787. [DOI] [PubMed] [Google Scholar]
- Kim SC, Park KT, Hwang JW, Shin HC, Lee SS, Seo DW, et al. Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution. Surg Endosc. 2008;22:2261–2268. doi: 10.1007/s00464-008-9973-1. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Gonen M, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503–509. doi: 10.1016/j.jamcollsurg.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048–1059. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- Nigri GR, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, et al. Meta-analysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc. 2011;25:1642–1651. doi: 10.1007/s00464-010-1456-5. [DOI] [PubMed] [Google Scholar]
- Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ., 3rd 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258:554–559. doi: 10.1097/SLA.0b013e3182a4e87c. discussion 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JA, Canal DF, Wiebke EA, Dumas RP, Beane JD, Aguilar-Saavedra JR, et al. Robotic distal pancreatectomy: cost effective? Surgery. 2010;148:814–823. doi: 10.1016/j.surg.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Kang CM, Kim DH, Lee WJ, Chi HS. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc. 2011;25:2004–2009. doi: 10.1007/s00464-010-1504-1. [DOI] [PubMed] [Google Scholar]
- Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–132. doi: 10.1097/SLA.0b013e31825fff08. [DOI] [PubMed] [Google Scholar]
- Borja-Cacho D, Al-Refaie WB, Vickers SM, Tuttle TM, Jensen EH. Laparoscopic distal pancreatectomy. J Am Coll Surg. 2009;209:758–765. doi: 10.1016/j.jamcollsurg.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646–1657. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kang CM, Lee WJ, Chi HS. The first experience of robot assisted spleen-preserving laparoscopic distal pancreatectomy in Korea. Yonsei Med J. 2011;52:539–542. doi: 10.3349/ymj.2011.52.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasberg SM, Fields R. Left-sided pancreatic cancer: distal pancreatectomy and its variants: radical antegrade modular pancreatosplenectomy and distal pancreatectomy with celiac axis resection. Cancer J. 2012;18:562–570. doi: 10.1097/PPO.0b013e31827596c5. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. discussion 937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melich G, Hong YK, Kim J, Hur H, Baik SH, Kim NK, et al. Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc. 2015;29:558–568. doi: 10.1007/s00464-014-3698-0. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jolissaint JS, Ramirez A, Gordon R, Yang Z, Sawyer RG. Cumulative sum: a proficiency metric for basic endoscopic training. J Surg Res. 2014;192:61–67. doi: 10.1016/j.jss.2014.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, et al. Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc. 2014;28:2821–2831. doi: 10.1007/s00464-014-3569-8. [DOI] [PubMed] [Google Scholar]
- Chaput de Saintonge DM, Vere DW. Why don't doctors use cusums? Lancet. 1974;1:120–121. doi: 10.1016/s0140-6736(74)92345-9. [DOI] [PubMed] [Google Scholar]
- Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med. 1977;296:1044–1045. doi: 10.1056/NEJM197705052961806. [DOI] [PubMed] [Google Scholar]
- Braga M, Ridolfi C, Balzano G, Castoldi R, Pecorelli N, Di Carlo V. Learning curve for laparoscopic distal pancreatectomy in a high-volume hospital. Updates Surg. 2012;64:179–183. doi: 10.1007/s13304-012-0163-2. [DOI] [PubMed] [Google Scholar]
- Strijker M, van Santvoort HC, Besselink MG, van Hillegersberg R, Borel Rinkes IH, Vriens MR, et al. Robot-assisted pancreatic surgery: a systematic review of the literature. HPB. 2013;1:1–10. doi: 10.1111/j.1477-2574.2012.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JM, Brennan MF, Tang LH, D'Angelica MI, Dematteo RP, Fong Y, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–175. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–698. doi: 10.1097/00000658-199905000-00012. discussion 698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet. 2011;377:1514–1522. doi: 10.1016/S0140-6736(11)60237-7. [DOI] [PubMed] [Google Scholar]
- Nussbaum DP, Penne K, Speicher PJ, Stinnett SS, Perez A, White RR, et al. The role of clinical care pathways: an experience with distal pancreatectomy. J Surg Res. 2014;190:64–71. doi: 10.1016/j.jss.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Reddy DM, Townsend CM, Jr, Kuo YF, Freeman JL, Goodwin JS, Riall TS. Readmission after pancreatectomy for pancreatic cancer in Medicare patients. J Gastrointest Surg. 2009;13:1963–1974. doi: 10.1007/s11605-009-1006-4. discussion 1974–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


