Abstract
Objectives
Adjuvant gemcitabine with or without chemoradiation is a standard therapeutic option for patients with resected pancreatic cancer. The feasibility and toxicity of gemcitabine with docetaxel before and after 5-fluorouracil (5FU)-based chemoradiation in the adjuvant pancreatic and biliary cancer setting were investigated.
Methods
After a curative-intent resection, eligible patients with pancreaticobiliary cancers were treated with two cycles of gemcitabine and docetaxel followed by 5FU-based chemoradiation. Four weeks after completing chemoradiation, two cycles of gemcitabine and docetaxel were administered. The primary endpoint was the incidence of severe toxicities. Secondary endpoints included disease-free survival (DFS) and overall survival (OS).
Results
Fifty patients with pancreaticobiliary cancers were enrolled. Twenty-nine patients had pancreatic cancer whereas 21 patients had biliary tract or ampullary cancers. There was one death as a result of pneumonia, and 15% of patients experienced grade 3 or greater non-haematological toxicities. The median DFS and OS for patients with pancreatic cancer were 9.6 and 17 months, respectively, and for those with resected biliary tract cancer were 12 and 23 months, respectively.
Conclusions
This combination of gemcitabine and docetaxel with chemoradiation is feasible and tolerable in the adjuvant setting. Future studies utilizing a different gemcitabine/taxane combination and schedule may be appropriate in the adjuvant treatment of both pancreatic cancer and biliary tumours.
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States.1 Surgical resection is curative, however, only 20% of patients present with resectable pancreatic cancer. The 5-year overall survival (OS) rate for patients with resected pancreatic cancer is low at 20–25%.2 Cancers of the biliary tract, ampulla of Vater and gallbladder are uncommon cancers. Similar to pancreatic cancer, most patients with cholangiocarcinoma or gallbladder carcinoma present with locally advanced or metastatic disease. The prognosis is poor with these tumours with a 5-year OS of < 5% reported in several retrospective studies.3–8
Adjuvant therapy for resected pancreatic cancer has been established based on multiple positive randomized trials.9–13 A multivariate analysis of the ESPAC-3 study demonstrated a significant survival benefit for adjuvant chemotherapy for patients with periampullary cancers.14 Only one randomized study demonstrated the benefit of adjuvant chemotherapy for gallbladder cancer.15 A meta-analysis supported the use of adjuvant therapy for resected biliary tract cancers especially for patients with lymph node involvement and R1 resection.16
Adjuvant gemcitabine improved the 5-year OS compared with observation [20.7% versus 10.4%, hazard ratio (HR) 0.76, P = 0.01] for patients with resected pancreatic cancer.9 Compared with 5-fluorouracil (5FU), adjuvant gemcitabine conferred comparable median survival times (23 versus 23.6 months, P = 0.39) but was associated with a better safety profile.10 Gemcitabine administered pre- and post-chemoradiation also resulted in a 5-year OS of 22% (versus 18% with 5FU, P = 0.08).12
Building on this gemcitabine-based chemoradiation platform, the addition of a taxane, docetaxel, to gemcitabine in the adjuvant setting for pancreaticobiliary cancers prior to, and after, 5FU-based chemoradiation for patients with resected pancreatic and biliary tract cancers was investigated. A phase II study by the European Organisation for Research and Treatment of Cancer (EORTC) showed better survival and safety with the combination of gemcitabine plus docetaxel over cisplatin plus docetaxel in mestastatic pancreatic cancer.17 The GTX regimen with the docetaxel, gemcitabine, capecitabine regimen showed a median survival of 11.3 months in metastatic disease and 25 months with locally advanced disease.18 The aim of the present study was to establish the feasibility and safety of gemcitabine plus docetaxel with 5FU chemoradiation for patients with resected pancreaticobiliary cancers.
Patients and methods
Eligibility
Patients 18 years or older with biopsy-proven, curatively resected cholangiocarcinoma, gallbladder, pancreatic or ampullary adenocarcinoma were eligible. Other eligibility criteria included the following: Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0–2, no prior chemotherapy or radiation therapy and at least 3 weeks had elapsed since any surgery requiring general anaesthesia. Required laboratory values included absolute neutrophil count (ANC) ≥ 1500/mm3, platelet count ≥ 150 000/mm3, haemoglobin ≥ 9 g/dl, serum creatinine ≤ 2 mg/dl, bilirubin ≤ 3.0 mg/dl and serum transaminases ≤ five-fold the institutional upper limits. Exclusion criteria included prior malignancies except basal or squamous skin cancers, cervical carcinoma in situ and co-existing severe medical illnesses, such as unstable angina, uncontrolled diabetes mellitus, uncontrolled arrhythmia or an uncontrolled infection. The study was approved by our Institutional Review Board (IRB) and informed consent was obtained from all participants prior to enrolment.
Study design and treatment plan
This was an open-label, single-arm phase II study. The primary aim was to evaluate the feasibility and toxicities associated with this regimen. Stopping rules according to the sequential probability rate method was used to ensure accrual was stopped should excessive severe toxicities occur (defined as < 30% grade 3–5 non-haematologic adverse events). Institutional Data and Safety Monitoring (DSM) was performed. Chemotherapy began within 8–12 weeks after surgery. Gemcitabine was given at a dose of 1000 mg/m2 as a 30-min intravenous (i.v.) infusion on days 1 and 8 with docetaxel at 35 mg/m2 i.v. on days 1 and 8 of a 21-day cycle for two cycles prior to radiation therapy. 5FU was given at 225 mg/m2 per day as a continuous infusion throughout radiation starting 3 weeks after the second cycle of gemcitabine. Radiation was given with computed tomography (CT) based three-dimensional (3D) treatment planning. Patients received a daily dose of 1.8 Gy 5 days per week to a planning target volume 1 (ptv1) which included celiac trunk and nodes, porta hepatis after the portal vein from the hilum of the liver to the confluence with the superior mesenteric vein, paraaortic and paracaval nodes at the level of the tumour bed and pancreatico-duodenal nodes. After 45 Gy, portals were reduced to encompass planning target volume 2 (ptv2) that included the pre-operative tumour bed and gross tumour volume. The boost dose to ptv2 would be determined by the tolerance of the extraduodenal small bowel. Three to 4 weeks after completing chemoradiation, two more cycles of gemcitabine and docetaxel were given.
Assessment of toxicity and efficacy
At study entry, a full history and a physical examination were obtained, including vital signs, height and weight, ECOG performance status and toxicity assessment. Prior to enrolment, a complete blood count, comprehensive metabolic profile-human chorionic gonadotropin in females, lactic dehydrogenase as well as tumour markers including carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA)and a baseline CT scan of the chest, abdomen and pelvis were obtained. At the start of each cycle, as well as at the end of the treatment, a history and physical examination were performed, as well as laboratory studies above.
Responses were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.0). Toxicity was graded according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0. For patients who developed treatment-related toxicities, the doses of gemcitabine, docetaxel and 5FU were adjusted according to protocol-defined parameters. Treatments were delayed if the ANC was < 1500 cells/ml and platelet counts were <75 000 cells/ml, or for any Grade III non-haematological toxicities or any hypersensitivity reaction. Upon recovery, gemcitabine, docetaxel or 5FU doses were reduced by 25% when therapy was resumed. For patients who developed Grade III or IV toxicity, both radiation and chemotherapy were held until the toxicity resolved to Grade 2 or less. Grade II or less toxicities were treated symptomatically with supportive care. After the completion of study treatments, patients were followed with clinic visits and tumour markers every 3 months for survival and scans were obtained every 6 months for 2 years and then annually for an additional 3 years.
Study endpoints and statistical analysis
The primary endpoint of this study was the incidence of severe toxicities. Secondary endpoints included tumour response, disease-free survival (DFS) and OS. OS was defined as the time from the initiation of treatment to death from any cause or last follow-up, whereas DFS was defined as the time from the initiation of treatment to relapse or death, whichever occurred first. The data analysis was descriptive in nature. Patient demographic and clinical characteristics were summarized using mean (standard deviation) or counts (frequency) as appropriate. The median OS and DFS, 1- and 2-year OS and DFS, as well as their 95% confidence intervals, were estimated using the Kaplan–Meier product-limit method. All analyses were performed using statistical packages SAS 9.3 (SAS Institutes, Cary, NC, USA).
Results
Patient characteristics
From February 2003 to June 2010, 50 patients were enrolled: 29 patients (58%) had pancreatic cancer, nine patients (18%) had ampullary cancer and 12 patients (24%) had biliary tract cancers. Out of all the patients, 29 of those were male and 21 were female. The majority of the patients were Caucasian (44) and only six patients were African American. Twenty-four patients had an ECOG score 0 and 26 patients had ECOG 1. The mean follow-up was 24 months (range 3.2–97). The distribution of various baseline patient characteristics is shown in Table1. The median age at the time of study entry was 59 years (range, 41–76).
Table 1.
Pathological characteristics
| Pancreatic cancer | Other cancer | |
|---|---|---|
| Overall stage | ||
| I | 2 | 1 |
| II | 27 | 19 |
| III | 0 | 1 |
| IV | 0 | 0 |
| T-stage | 1 | |
| 1 | 1 | 1 |
| 2 | 4 | 7 |
| 3 | 24 | 11 |
| 4 | 0 | 2 |
| N-stage | ||
| 0 | 8 | 2 |
| 1 | 21 | 15 |
| X | 0 | 4 |
| Differentiation | ||
| Well | 1 | 3 |
| Moderate | 8 | 9 |
| Poor | 20 | 8 |
| NR | 0 | 1 |
| Lymphovascular involvement | ||
| Y | 19 | 17 |
| N | 8 | 1 |
| NR | 2 | 3 |
| CA 19-9 | ||
| < 40 | 20 | 14 |
| 40–100 | 6 | 5 |
| < 100 | 3 | 2 |
NR, not reported; Y, yes; N, No.
Treatment
Of the 50 patients enrolled, two withdrew consent prior to any study therapy as a result of insurance and transportation issues. Out of 48 patients who received at least one cycle of chemotherapy, 30 patients (62.5%) completed all aspects (pre- and post RT chemotherapy and 5FU-based CRT) of the study treatment. Thirty-seven patients (77%) received pre-RT chemotherapy with radiation. Eight patients (16%) did not complete study treatment owing to early disease progression during therapy. Ten patients (21%) did not complete all planned study treatment owing to adverse events or physician decision.
Out of the 30 patients who completed all study treatments, 14 patients had pancreatic cancer, and 16 had biliary and ampullary cancers. Seventeen patients (57%) required a 25–50% dose reduction of gemcitabine and taxotere. Only eight patients (26%) required a 25% reduction of 5FU during chemoradiation. All patients tolerated full-dose radiation.
Adverse events
All 48 patients who received any study treatment were evaluable for toxicity assessments.
There was one death during the study as a result of clostridium difficile complicated with ventilator-acquired pneumonia. Seven patients (14.5%) discontinued study therapy owing to adverse events or physician decision. Two to fifteen percentage experienced grade 3–5 non-haematological toxicities. The early stopping rules for excessive toxicities were not met, thus the planned accrual was completed. The most common non-haematological toxicities and haematological toxicities are listed in Table2. The most common graded 3–4 non-haematological toxicities included diarrhoea (15%), infection (15%), fatigue (8%), dehydration (4%) and liver enzyme abnormalities (4%). The most common grade 3–4 haematological toxicity included neutropenia (23%), thrombocytopenia (6%) and anaemia (4%).
Table 2.
Toxicities
| N = 48 | Grade (1–2) (%) | Grade (3–4) (%) |
|---|---|---|
| Mucositis | 31 | 2 |
| Nausea | 48 | 4 |
| Vomiting | 23 | 4 |
| Diarrhoea | 31 | 15 |
| Dehydration | 17 | 4 |
| Weight loss | 27 | 0 |
| Fatigue | 67 | 8 |
| Renal toxicity | 13 | 0 |
| Hepatotoxicity | 69 | 4 |
| Infection | 13 | 15 |
| Neutropenia | 46 | 23 |
| Anaemia | 65 | 4 |
| Thrombocytopenia | 46 | 6 |
Outcomes
At the time of analysis, 41 (85%) patients had already died. The median, 1- and 2-year OS for the 29 patients with pancreatic cancer was 17.6 months, 60.7% [95% confidence interval (CI): 0.450–0.818] and 32.1% (95% CI: 0.188–0.551), respectively. The median, 1- and 2-year OS for the 21 patients with ampullary and biliary cancers was 23.8 months, 75% (95% CI: 0.58–0.966) and 49.1% (95% CI: 0.312–0.773), respectively (Fig.1). Excluding all ampullary cancer patients, the 12 patients with biliary tract malignancies had a median OS of 27.6 months (95%CI: 9.5–57.1).
Figure 1.
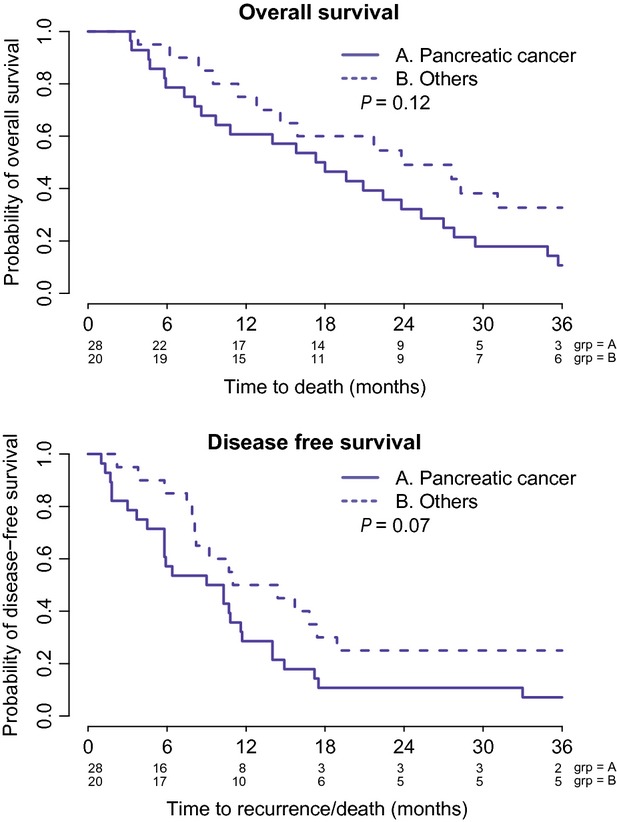
Overall survival and disease-free survival of pancreatic cancer patients and others (ampullary and biliary tract cancer) patients who underwent the study
Disease recurrence was noted in 40 of 48 patients (83%). The majority of recurrence occurred in the liver (37.5%), peritoneum (20%) and lung (17.5%). The median recurrence-free survivals for pancreatic cancer and ampullary/biliary cancers were 9.6 and 12.7 months, respectively (Fig.1). For the 12 patients with biliary tract cancers (excluding ampullary cancer), the median PFS was 16.25 months (95% CI: 5.8 – 57.1).
For the 30 patients who completed all components of study therapy, the median and 1-year OS for the 14 pancreatic cancer patients was 24.5 months and 78.6% (95% CI: 0.59–1). The median DFS was 12.8 months. For the 16 patients with biliary tract/ampullary cancers who completed all study treatment, the median and 1 year OS was 23.8 months and 81.2% (95% CI: 0.642–1), respectively. The median DFS was 15.0 months.
Discussion
This single-institution Phase II study demonstrated the feasibility of administering adjuvant 5FU-based chemoradiation with pre- and post-RT gemcitabine with docetaxel. The current regimen was evaluated to build on the RTOG 9704 platform that initially showed an improvement in 3-year OS with gemcitabine-based chemoradiation. An updated report on the results of RTOG 9704 no longer demonstrated an overall survival improvement at 5 years.12 The diminution of OS benefit associated with the gemcitabine arm was attributed to subsequent salvage therapies, disproportionately higher T3/T4 disease in this arm and possibly the prolonged interruption of systemic chemotherapy necessitated by chemoradiation. This last issue is pertinent to the present study. Although two cycles of gemcitabine with docetaxel were administered prior to chemoradiation, an average of 12–14 weeks occurred during the last systemic therapy to the resumption of gemcitabine/docetaxel. Moreover, systemic chemotherapy was administered for a longer period (6 months) for both CONKO-001 and ESPAC-3 studies whereas systemic therapy in this study was interrupted and only given for a total of four cycles (3 months). Lastly, the benefit of radiation therapy in the adjuvant setting, and the optimal timing of RT delivery, if given, has yet to be confirmed. In the present study, fluoropyrimidine-based radiation was given, and RT was sandwiched between systemic therapy. Current clinical practice utilized systemic chemotherapy alone or chemotherapy plus consolidative chemoRT in the adjuvant treatment of pancreatic cancer. The ongoing RTOG 0848 is evaluating six cycles of adjuvant gemcitabine with or without consolidative fluoropyrimidine-based chemoradiotherapy after systemic therapy (Table3).
Table 3.
Summary of treatment, median overall survival (OS), disease-free survival (DFS) and 5-year OS in previous studies
| Study | No. | Treatment | Median PFS | Median OS | 5 year OS |
|---|---|---|---|---|---|
| GITSG 1985 | 43 | Obs | 9 | 11 months | 8% |
| chemoRT | 11 | 20 months | 19% | ||
| CONKO | 368 | Obs | 6.7 | 12% | |
| Gemcitabine | 13.4 | 23% | |||
| ESPAC-1 | 289 | Obs | No RF S reported | 16.9 | 10.7% |
| 5FU | 21.6 | 29% | |||
| ESPAC -3 (Pancreatic) | 1088 | 5FU | 14.1 | 23.0 | 48.1%(24 months OS) |
| Gemcitabine | 14.3 | 23.6 | 49.1% | ||
| RTOG 9704 | 538 | 5FU-RT | NR | 17.1 | 18% |
| Gem-RT | 20.5 | 22% | |||
| ESPAC-3 (Periampullary) | 434 | Obs | 19.5 | 35.2 | NR |
| 5FU | 23 | 38.9 | |||
| Gem | 29.1 | 45.7 | |||
| Gallbladder (Takada et al.) | 112 | No adjuvant | 11.9 | 16.4 | 14.4 |
| Adj chemo | 12.3 | 14.1 | 26 | ||
| Cho | |||||
| Completed therapy | |||||
| Pancreatic | 30 | 12.8 | 24.5 | NR | |
| Biliary | 15.0 | 23.8 | |||
| All patients | |||||
| Pancreatic | 48 | 9.6 | 17.6 | ||
| Biliary | 12.7 | 23.8 | |||
The addition of a taxane to gemcitabine in the adjuvant setting of pancreaticobiliary neoplasms is of interest especially in light of the positive results of gemcitabine with nab-paclitaxel in metastatic pancreatic cancer.19 Our study utilized docetaxel with gemcitabine based on multiple Phase II data in pancreatic and biliary cancers demonstrating activity against these malignancies.17,20–24 In metastatic pancreatic cancer, gemcitabine with docetaxel is associated with response rates of 12–20%17,20 although the ECOG study reported no superiority among several regimens including gemcitabine/cisplatin or gemcitabine alone.20 In the neoadjuvant setting for locally advanced pancreatic cancer, docetaxel with gemcitabine has been associated with responses as high as 50% and increased resection rates.21,22 To our knowledge, this is the only completed adjuvant trial using the combination of docetaxel and gemcitabine for pancreaticobiliary cancers.
Gemcitabine with docetaxel appears to be tolerable with manageable toxicities when given in the adjuvant setting for pancreaticobiliary cancers. Only 20% developed severe adverse events. These toxicities were likely related more to chemoradiation than to gemcitabine/docetaxel as the majority of toxicities were gastrointestinal adverse events. For the 30 patients who actually completed all aspects of the regimen, the median OS for patients with pancreatic cancer of 24.5 months is comparable with those reported by CONKO-1, RTOG 9704 and ESPAC-3. The median OS for biliary tract cancers reaching 23.8 months is also encouraging. The APACT study currently explores the use of nab-paclitaxel with gemcitabine as adjuvant therapy for patients with resected pancreatic cancer.
There are several limitations of this study. This study is a single institution Phase II trial. Thus clinical bias would be a limitation. One significant issue was the presence of competing studies that limited our enrolment. Compared with the interferon-based study conducted at our institution during the same period,25 patients enrolled in this study had a significant delay in initiating adjuvant therapy from the time of surgery. The median time from surgery date to chemotherapy was 61 days (range: 33–195). Twenty-seven (54%) patients started therapy < 60 days after surgery. Seven (14%) had delayed adjuvant therapy of <90 days post-resection. Fifteen (30%) patients experienced significant post-operative morbidity including infection (8 patients), cardiac (3 patients), gastrointestinal (3), gastrointestinal bleed (1) and metabolic (1). These results are comparable to those experienced in our interferon-based adjuvant therapy.25 This may in part explain our inferior outcomes.
Another is the inclusion of multiple gastrointestinal malignancies such as pancreatic cancer, ampullary cancer, gallbladder cancer and intra- and extrahepatic cholangiocarcinoma. Clinical and genomic data demonstrates that these are heterogeneous entities with varying clinical behaviours and outcomes. As feasibility and tolerability of gemcitabine and docetaxel-based chemoradiation was the main endpoint for the study, we included all these groups and described their outcomes. Also, clinically and pathologically, tumours in the periampullary region may be difficult to differentiate from true pancreatic cancer or extrahepatic cholangiocarcinoma.
Currently, evidence for the use of adjuvant therapy for biliary tract cancers is limited. The rarity of each subtype of biliary tract cancers precluded the completion of large enough randomized studies to establish standard treatment recommendations. A meta-analysis did confirm a potential benefit for the use of adjuvant therapy in node positive and margin positive resected biliary tract cancers.16 Patients with gallbladder cancer derived a survival benefit from adjuvant mitomycin C and 5FU resulting in 5-year DFS and OS rates of 20.3% and 26%, respectively, compared with 11.6% and 14.4%.15 All other subgroups, including pancreatic cancer, ampullary cancer and other biliary tract cancer did not benefit from this regimen.
Only recently is there evidence for adjuvant therapy for periampullary cancers. The ESPAC-3 periampullary study randomized 428 patients (297 with ampullary, 96 with bile duct cancer and 35 with other cancers) to 5FU or gemcitabine or observation. The primary analysis did not confirm any significant benefit associated with adjuvant chemotherapy for periampullary cancer (a median survival of 43.1 months compared with 35.2 months for observation, HR 0.86, P = 0.25). After adjusting for prognostic variables using multiple regression analysis, there was a significant, albeit modest, benefit favouring adjuvant chemotherapy compared with observation for these tumours. The median survival for patients with 297 ampullary cancers in the different arms are as follows: 40.6 months in the observation arm; 57.8 months for the 5FU arm and 70.8 months in the gemcitabine arm. The lower survival times were noted in spite of 5FU and gemcitabine treatment for those with bile duct cancer (18.3 month for 5FU and 19.5 months for gemcitabine). The median survival for our patients with biliary tract cancers, including periampullary and gallbladder cancers is 23.8 months. The proportions of patients in our study representing the different subgroups of biliary tract cancers most probably influenced our results. When ampullary cancer patients were excluded, the median DFS and OS for the 12 biliary tract patients were better at 16.25 and 27.6 months, respectively. An extensive cooperative effort would be warranted to assess the benefit of adjuvant therapy for each subtype of biliary tract cancer.
Lastly, subsequent salvage therapy upon recurrence may impact outcomes, including OS. Oxaliplatin-based therapy for pancreaticobiliary cancers was only approved in the United States at the latter portion of our study. Thus, of the 40 patients who experienced recurrence (eight of whom had early recurrence during study therapy), nine patients never had any salvage therapy, nine patients received only capecitabine single-agent therapy, 12 did receive oxaliplatin-based therapy (11 FOLFOX, 1 FOLFIRINOX), eight received further gemcitabine-based therapy and two received experimental therapies.
In summary, we have demonstrated the feasibility and tolerability of a docetaxel/gemcitabine regimen pre- and post 5FU-RT in the adjuvant treatment of pancreaticobiliary cancers. Although feasible, the survival outcomes for pancreatic cancer patients treated with this regimen and schedule appear to be inferior to those reported with other regimens. Future studies utilizing a different taxane (i.e. nab-paclitaxel) with gemcitabine in the adjuvant treatment of both pancreatic cancer and biliary tumours may be appropriate given the significant benefit of the gemcitabine/nab-paclitaxel regimen in metastatic pancreatic cancer.
Acknowledgments
This publication was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclaimers
The manuscript has never been published and is not under consideration for publication elsewhere.
Conflict of interest
Authors have no financial interests to declare.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Law LY. Dramatic response to trastuzumab and paclitaxel in a patient with human epidermal growth factor receptor 2-positive metastatic cholangiocarcinoma. J Clin Oncol. 2012;30:e271–e273. doi: 10.1200/JCO.2012.42.3061. [DOI] [PubMed] [Google Scholar]
- Shani M, Hart J, Modan B. Cancer of the biliary system: a study of 445 cases. Br J Surg. 1974;61:98–100. doi: 10.1002/bjs.1800610205. [DOI] [PubMed] [Google Scholar]
- Hamrick RE, Jr, Liner FJ, Hastings PR, Cohn I., Jr Primary carcinoma of the gallbladder. Ann Surg. 1982;195:270–273. doi: 10.1097/00000658-198203000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CJ, Pitt HA, Cameron JL. Cholangiocarcinoma. Surg Clin North Am. 1990;70:1429–1447. doi: 10.1016/s0039-6109(16)45293-x. [DOI] [PubMed] [Google Scholar]
- Bengmark S, Ekberg H, Evander A, Klofver-Stahl B, Tranberg KG. Major liver resection for hilar cholangiocarcinoma. Ann Surg. 1988;207:120–125. doi: 10.1097/00000658-198802000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Listrup DM. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128:871–877. doi: 10.1001/archsurg.1993.01420200045008. discussion 877–9. [DOI] [PubMed] [Google Scholar]
- Kraybill WG, Lee H, Picus J, Ramachandran G, Lopez MJ, Kucik N, et al. Multidisciplinary treatment of biliary tract cancers. J Surg Oncol. 1994;55:239–245. doi: 10.1002/jso.2930550408. [DOI] [PubMed] [Google Scholar]
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- Lutz MP, Van Cutsem E, Wagener T, Van Laethem JL, Vanhoefer U, Wils JA, et al. Docetaxel plus gemcitabine or docetaxel plus cisplatin in advanced pancreatic carcinoma: randomized phase II study 40984 of the European Organisation for Research and Treatment of Cancer Gastrointestinal Group. J Clin Oncol. 2005;23:9250–9256. doi: 10.1200/JCO.2005.02.1980. [DOI] [PubMed] [Google Scholar]
- De Jesus-Acosta A, Oliver GR, Blackford A, Kinsman K, Flores EI, Wilfong LS, et al. A multicenter analysis of GTX chemotherapy in patients with locally advanced and metastatic pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2012;69:415–424. doi: 10.1007/s00280-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke MH, Tempero MA, Niedzwiecki D, Hollis DR, Kindler HL, Cusnir M, et al. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904. J Clin Oncol. 2009;27:5506–5512. doi: 10.1200/JCO.2009.22.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahora K, Kuehrer I, Schindl M, Koelblinger C, Goetzinger P, Gnant M. NeoGemTax: gemcitabine and docetaxel as neoadjuvant treatment for locally advanced nonmetastasized pancreatic cancer. World J Surg. 2011;35:1580–1589. doi: 10.1007/s00268-011-1113-8. [DOI] [PubMed] [Google Scholar]
- Pipas JM, Barth RJ, Jr, Zaki B, Tsapakos MJ, Suriawinata AA, Bettman MA, et al. Docetaxel/Gemcitabine followed by gemcitabine and external beam radiotherapy in patients with pancreatic adenocarcinoma. Ann Surg Oncol. 2005;12:995–1004. doi: 10.1245/ASO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Hribaschek A, Eichelmann K, Rudolph S, Fahike J, Ridwelski K. Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs. 2002;20:351–356. doi: 10.1023/a:1016209901417. [DOI] [PubMed] [Google Scholar]
- Ridwelski K, Fahlke J, Kuhn R, Hribaschek A, Kettner E, Greiner C, et al. Multicenter phase-I/II study using a combination of gemcitabine and docetaxel in metastasized and unresectable, locally advanced pancreatic carcinoma. Eur J Surg Oncol. 2006;32:297–302. doi: 10.1016/j.ejso.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Linehan DC, Tan MC, Strasberg SM, Drebin JA, Hawkins WG, Picus J, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution phase II study. Ann Surg. 2008;248:145–151. doi: 10.1097/SLA.0b013e318181e4e9. [DOI] [PubMed] [Google Scholar]


