Abstract
Background
Hepatic arterial anomalies (HAAs) are not infrequently encountered during pancreatic resections. In view of the current emergence of the robotic platform as a safe alternative to open surgery in experienced centres, this study sought to determine the implications of HAAs on the safety and oncologic outcomes of robotic pancreaticoduodenectomy (RPD).
Methods
A prospectively maintained database of patients with HAAs who underwent RPD (RPD + HAA) at a single institution between 2008 and 2013 was retrospectively reviewed. Demographic information and perioperative outcomes of RPD were compared for patients with and without HAAs.
Results
A total of 142 patients underwent RPD; 112 (78.9%) did not have and 30 (21.1%) did have HAAs. The majority (90.0%) of RPDs in patients with HAAs were performed for malignant indications and all aberrant vessels were preserved without conversion to laparotomy. There were no statistically significant differences between RPD patients with and without HAAs with respect to preoperative demographics, tumour characteristics, operative metrics (operative time, estimated blood loss, conversion) and postoperative outcomes, including complications, length of stay and readmissions. Negative margin (R0) rates were similar in both groups.
Conclusions
Robot-assisted pancreaticoduodenectomy is safe and feasible in patients with HAAs and has outcomes similar to those in patients with normal arterial anatomy.
Introduction
Hepatic arterial anomalies (HAAs) are not unusual and are encountered in 20–45% of pancreaticoduodenectomies (PDs), adding to the difficulty of an already technically challenging operation.1–7 The variations in hepatic arterial blood supply were classically delineated by Michels in 1966 and updated by Hiatt et al. in 1994 (Table1).3,5 The most common anomaly according to the Hiatt et al. system of classification is a type III variant: a replaced or accessory right hepatic artery (RHA) that arises from the superior mesenteric artery (SMA).4 This variant is of great concern during PD because the anomalous vessel can course near or through the pancreatic head and posterior to the common bile duct.6,8 Similarly, the less common type V variant, in which the common hepatic artery (CHA) arises from the SMA, can also impede dissection of the pancreatic head, common bile duct and gastroduodenal artery (GDA) during PD.2 Injury to the hepatic arteries can lead to liver ischaemia and also affect bilioenteric anastomosis because the RHA provides the chief blood supply to the common bile duct.1,9,10
Table 1.
| Michels | Anatomy | Hiatt et al. |
|---|---|---|
| I | Normal (RHA and LHA arise from the proper hepatic artery) | I |
| II | Replaced LHA from the LGA | II |
| III | Replaced RHA from the SMA | III |
| IV | Replaced LHA from LGA and replaced RHA from SMA | IV (Combination of accessory and/or replaced LHA and RHA) |
| V | Accessory LHA from LGA | II |
| VI | Accessory RHA from the SMA | III |
| VII | Accessory LHA and accessory RHA | IV |
| VIII | Replaced RHA and accessory LHA or Replaced LHA and accessory RHA | IV |
| IX | Replaced CHA from SMA | V |
| X | Replaced CHA from the LGA | |
| Replaced CHA from the aorta | VI |
LGA, left gastric artery; LHA, left hepatic artery; RHA, right hepatic artery; SMA, superior mesenteric artery.
Several series from experienced centres have demonstrated that the emerging use of the robotic platform for PD can be a safe alternative to the open surgery approach.11–14 The technical advantages of the robotic platform (three-dimensional visualization, magnification and dexterity) may be useful for the meticulous dissection required in PD in the presence of HAAs.15–17 However, the method is disadvantaged by the lack of haptic feedback, which can potentially cause vascular injury and compromise margins.15,18 Although several reports have established outcomes equivalent to those of open PDs in patients with normal versus aberrant hepatic arterial anatomy (particularly Hiatt et al. type III variants), the safety and outcomes of robotic PD (RPD) in the presence of anomalous hepatic arterial anatomy remain unknown.8,19–21
Materials and methods
Following University of Pittsburgh Institutional Review Board approval, a retrospective review of a prospectively collected database of patients submitted to RPD between 2008 and 2013 was performed. Patients who underwent RPD with HAAs (RPD + HAA group) were identified based on operative reports and electronic medical records. Outcomes in this group were compared with those in RPD patients without HAAs (RPD − HAA group). All outcomes were followed to 90 days. Pancreatic fistulae were graded according to International Study Group of Pancreatic Fistula (ISGPF) criteria.22 Postoperative complications were graded based on the Clavien–Dindo system of classification.23 The pancreatic and bile duct margins were the only margins routinely assessed intraoperatively.
At the study institution, all RPD patients undergo a preoperative triphasic computed tomography (CT) scan. Hepatic arterial anomalies considered relevant to a PD were a replaced or accessory RHA or CHA, and arteries that arose in a classic (non-aberrant) fashion but had an anomalous course similar to that of a replaced RHA or CHA (Fig.1).
Figure 1.
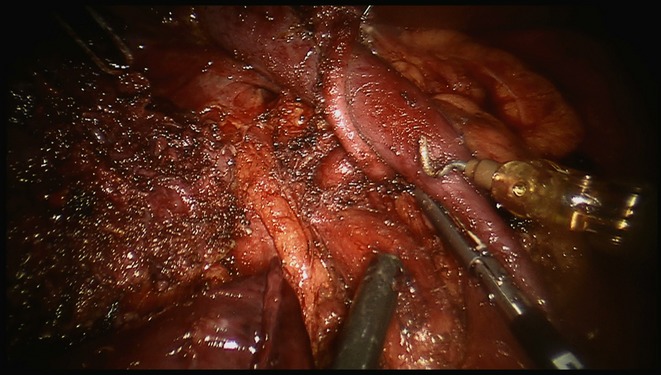
Intraoperative view of a resection bed in a robotic pancreaticoduodenectomy in a 42-year-old patient with pancreatic head adenocarcinoma. Note the anomalous common hepatic artery arising from the coeliac trunk and coursing posterior to the portal vein. The tip of the suction lies on the superior mesenteric artery, which has been skeletonized in 180 ° fashion in order to maximize the R0 outcome. The resected specimen is shown on the far left aspect of the field.
Statistical analysis was performed using stata Version 12.0 (StataCorp LP, College Station, TX, USA). The distribution of continuous variables was checked for normality. The two-tailed Student's t-test was used to compare normally distributed variables between the normal and anomalous arterial anatomy groups. The Wilcoxon rank-sum test was used for continuous variables that were not normally distributed. The two-tailed Fisher's exact test was used to compare categorical variables. Values are presented as the mean ± standard deviation (SD) or median with interquartile range (IQR) as appropriate. P-values of <0.05 were considered to indicate statistical significance.
Results
Prevalence of anomalous hepatic arterial anatomy
Robotic PD was performed in 142 patients, of whom 30 (21.1%) harboured HAAs (RPD + HAA group). The most common vascular anomaly encountered was a replaced RHA (n = 15, 50.0%) followed by a replaced CHA (n = 9, 30.0%) (Table2). All of the replaced RHAs and CHAs arose from the SMA except in one case, in which the replaced CHA arose directly from the aorta. There was a single case of an accessory RHA coming off the GDA. Another patient had a GDA arising from an aberrant RHA deep in the neck of the gland. In both cases, the GDA was transected while the accessory and aberrant RHAs were preserved. Additionally, in four patients either the RHA (n = 3) or CHA (n = 1) took an anomalous path, coursing posterior and lateral to the portal vein.
Table 2.
Distribution of relevant hepatic arterial anomalies encountered in 30 of 142 patients undergoing robotic pancreatoduodenectomy
| Nature of aberrant/anomalous vessel | n (%) |
|---|---|
| Replaced RHA | 15 (50.0%) |
| Off SMA | 14 |
| Off SMA and replaced LHA from LGA | 1 |
| Replaced CHA | 9 (30.0%) |
| Off SMA | 8 |
| Off aorta (Hiatt type VI) | 1 |
| Anomalous RHA course | 3 (10.0%) |
| Anomalous CHA course | 1 (3.3%) |
| Accessory RHA off GDA | 1 (3.3%) |
| Replaced GDA off RHAa | 1 (3.3%) |
Although this is not a classic hepatic arterial anomaly, it was included because the RHA left the GDA and coursed lateral to the common bile duct.
CHA, common hepatic artery; LGA, left gastric artery; LHA, left hepatic artery; RHA, right hepatic artery; SMA, superior mesenteric artery.
Preoperative characteristics
Preoperative demographics for patients submitted to RPD with and without anomalous hepatic arterial anatomy are summarized in Table3. No differences were observed between the groups in demographics or final histologic diagnoses.
Table 3.
Preoperative variables in patients undergoing robotic pancreatoduodenectomy (RPD) with (RPD + HAA) and without (RPD − HAA) hepatic arterial anomalies
| RPD + HAA (n = 30) | RPD − HAA (n = 112) | P-value | |
|---|---|---|---|
| Patient demographics | |||
| Age, years, mean ± SD | 65.9 ± 12.7 | 67.7 ± 12.6 | 0.476 |
| Female, n (%) | 10 (33.3%) | 56 (50.0%) | 0.149 |
| BMI, kg/m2, mean ± SD | 27.3 ± 6.3 | 27.2 ± 5.4 | 0.945 |
| CCI age-adjusted, mean ± SD | 4.0 ± 2.3 | 4.1 ± 2.7 | 0.882 |
| CCI age-unadjusted, mean ± SD | 2.23 ± 1.4 | 2.02 ± 1.8 | 0.547 |
| Pathology, n (%) | |||
| Malignancy | 27 (90.0%) | 88 (78.6%) | 0.196 |
| Histology, n (%) | |||
| PDAC | 14 (46.7%) | 43 (38.4%) | 0.832 |
| Ampullary adenocarcinoma | 7 (23.3%) | 20 (17.9%) | |
| Cholangiocarcinoma | 4 (13.3%) | 5 (4.5%) | |
| IPMN | 3 (10.0%) | 16 (14.3%) | |
| Neuroendocrine tumour | 1 (3.3%) | 11 (9.8%) | |
| DCA | 0 | 4 (3.6%) | |
| GIST | 0 | 2 (1.8%) | |
| Others | 1a (3.3%) | 11 (9.8%) | |
Renal cancer metastasis.
BMI, body mass index; CCI, Charlson Comorbidity Index; DCA, duodenal adenocarcinoma; GIST, gastrointestinal stromal tumour; IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma; SD, standard deviation.
Operative parameters
In the RPD + HAA group, mean operative time was 501 min and median estimated blood loss (EBL) was 250 ml. In six patients (20.0%) EBL exceeded 500 ml. None of the HAA RPDs required conversion to laparotomy and all aberrant or anomalous vessels were preserved without the need for resection or reconstruction. Operating time, EBL (including EBL of <500 ml), number of patients requiring blood transfusions, and rates of conversion to open surgery did not differ statistically between RPDs performed with and without standard hepatic arterial anatomy (Table4).
Table 4.
Operative and postoperative outcomes in patients undergoing robotic pancreatoduodenectomy (RPD) with (RPD + HAA) and without (RPD – HAA) hepatic arterial anomalies
| RPD + HAA (n = 30) | RPD − HAA (n = 112) | P-value | |
|---|---|---|---|
| Operative information | |||
| Operation time, min, mean ± SD | 500.7 ± 105.2 | 529.5 ± 103.2 | 0.179 |
| Patients transfused, n (%) | 3 (10.0%) | 30 (26.8%) | 0.194 |
| EBL, ml, median (IQR) | 250 (150–400) | 300 (150–550) | 0.742 |
| EBL <500 ml, n (%) | 6 (20.0%) | 34 (30.4%) | 0.361 |
| Duct size, mm, mean ± SD | 5.3 ± 3.8 | 4.9 ± 4.3 | 0.659 |
| Dilated duct (<3 mm), n (%) | 16 (53.3%) | 52 (46.4%) | 0.532 |
| Conversion to open surgery, n (%) | 0 | 11 (9.8%) | 0.120 |
| Perioperative outcomes | |||
| Mortality within 90 days, n (%) | 2 (6.7%) | 3 (2.7%) | 0.285 |
| Complications (Clavien grade), n (%) | |||
| Grades I and II | 15 (50.0%) | 45 (40.2%) | 0.183 |
| Grades III and IV | 4 (13.3%) | 29 (25.8%) | |
| Reoperation, n (%) | 0 | 4 (3.6%) | 0.579 |
| Pseudoaneurysm, n (%) | 2 (6.7%) | 7 (6.3%) | 1.00 |
| Pancreatic leak, n (%) | 8 (26.7%) | 17 (15.2%) | 0.177 |
| Pancreatic leak (ISGPF grade), n (%) | |||
| No leak | 22 (73.3%) | 95 (84.8%) | 0.324 |
| Grade A | 4 (13.3%) | 8 (7.1%) | |
| Grade B | 3 (10.0%) | 5 (4.5%) | |
| Grade C | 1 (3.3%) | 4 (3.6%) | |
| Delayed gastric emptying, n (%) | 3 (10.0%) | 26 (23.2%) | 0.132 |
| Hospital LoS, days, median (range) | 9.5 (7–13) | 10 (8–14) | 0.297 |
| Readmission within 90 days, n (%) | 6 (20.0%) | 33 (29.5%) | 0.363 |
EBL, estimated blood loss; IQR, interquartile range; ISGPF, International Study Group of Pancreatic Fistula; LoS, length of stay; SD, standard deviation.
Postoperative outcomes of RPDs in anomalous hepatic arterial anatomy
Postoperative outcomes are depicted in Table4. In the RPD + HAA group, two deaths (90-day mortality: 6.7%) were recorded. These were caused by biliary sepsis in one patient and a sudden cardiac arrest that occurred outside the hospital in another. Four RPD + HAA patients (13.3%) had a major (Clavien Grades III and IV) complication, two of which were pseudoaneurysms. The first pseudoaneurysm occurred in a patient with a replaced RHA who developed a pseudoaneurysm (in the setting of a pancreatic leak) in a branch of the inferior pancreaticoduodenal arcade, which was treated with coil embolization. The second pseudoaneurysm occurred in a patient with a replaced CHA coming off the SMA, who developed a pseudoaneurysm of the proper hepatic artery which was stented and then coiled. The two other major complications were respiratory failure requiring a tracheostomy and an inferior vena cava filter placement following a pulmonary embolus. Eight RPD + HAA patients (26.7%) had a pancreatic leak; in four (13.3%) patients these leaks were clinically significant ISGPF grade B or C leaks. There were no cases of hepatic ischaemia, hepatic abscess or biliary stricture. The mean length of stay (LoS) was 9.5 days and the 90-day readmission rate was 20.0%. Postoperative outcomes including 90-day mortality, minor and major Clavien complication rates, rates of pancreatic fistula, LoS and readmission rates did not differ significantly between the groups (Table4).
Pathologic data and oncologic outcomes of RPDs in patients with HAAs
Of the 30 patients with HAAs, 27 (90.0%) had malignant pathology [14 pancreatic ductal adenocarcinomas (PDACs), seven ampullary cancers, four cholangiocarcinomas, one neuroendocrine tumour, and one metastatic renal cell carcinoma]. Five of the patients with PDAC had neoadjuvant therapy. Two PDAC resections had R1 positive margins (one pancreatic neck and one common bile duct margin). No positive margins were incurred on any of the anomalous vessels or retroperitoneal margins. The distribution of histologic diagnoses, receipt of neoadjuvant therapy, incidence of positive margins, and lymph node counts were similar between patients undergoing RPD with anomalous and standard hepatic anatomy (Table5).
Table 5.
Pathology and oncologic outcomes in patients undergoing robotic pancreatoduodenectomy (RPD) with (RPD + HAA) and without (RPD − HAA) hepatic arterial anomalies
| RPD + HAA (n = 27) | RPD − HAA (n = 88) | P-value | |
|---|---|---|---|
| Neoadjuvant therapy, n (%) | 5 (18.5%) | 17 (19.3%) | 0.783 |
| Tumour size, cm, mean ± SD | 2.5 ± 1.4 | 2.8 ± 1.5 | 0.742 |
| Lymph node yield, mean ± SD | 22.3 ± 7.16 | 17.5 ± 7.8 | 0.315 |
| Perineural invasion, n (%) | 20 (74.1%) | 54 (61.4%) | 0.259 |
| Vascular invasion, n (%) | 21 (77.8%) | 63 (71.5%) | 0.803 |
| Margin positive (R1) a, n (%) | 2 (7.4%) | 11 (12.5%) | 0.732 |
No R2 resections.
SD, standard deviation.
Discussion
Variants of the hepatic arterial vasculature are not uncommon and represent an important consideration in PD. There is a potential risk for vascular injury, as well as oncologic concerns for the achievement of negative margins in malignant disease. The presence of an aberrant RHA has been shown in many studies not to impact outcomes in open PD.8,19–21,24 Descriptions of the impacts of other less common arterial anomalies on PD are mostly limited to review articles and small case series or reports.6,25–29 This is the first study to examine the impact of HAAs on the outcomes of minimally invasive PD. Despite the lack of tactile feedback, the robotic platform was found to be safe in this challenging setting at the present study centre.
Preoperative identification of aberrant RHA can be under-recognized, as reported by Stauffer et al.9 Similarly, an artery with an anomalous course is likely to be more difficult to distinguish on preoperative imaging, but carries a similar risk for injury. The incidence of HAA in this study is in line with that in prior reports. At the present centre, a triphasic CT (with a dedicated arterial phase) is employed in the preoperative workup of all patients undergoing PD when possible. Consequently, borderline resectable cancers that are anticipated to undergo major venous or arterial resection (<90 ° abutment) with primary end-to-end anastomosis or interposition vein grafts are assigned to an open surgery protocol. It is likely that the judicious use of triphasic CT and the stereotactic magnification of the robotic platform facilitated the preservation of all 30 aberrant/anomalous hepatic arteries despite a very high percentage of malignant indications (90.0%). This is in line with the low rates of sacrifice of aberrant arteries attributed to malignant involvement reported in the literature on open PD.8,9,19,21,24,26
The technical challenge of performing a PD in the presence of HAA may lead to greater intraoperative blood loss and postoperative complications. In two large, well-matched comparisons of outcomes in this context with those of PDs with standard anatomy, Kim et al.8 and Eshuis et al.19, respectively, reported on 37 and 143 open PDs with aberrant RHAs. Both groups reported an aberrant vessel preservation rate of <90%. Median EBL in the cohorts with an aberrant RHA was 950 ml and 1100 ml, respectively, and did not differ significantly from that in the standard PD cohorts. Additionally, incidences of major postoperative complications were similar. The present results corroborate these findings, indicating that blood loss, transfusion rates and complications were not compromised by the robotic approach. Operative times for RPD + HAA were long but comparable with those published by Kim et al.8 The present group has previously shown precipitous reductions in RPD operative time beyond a learning curve of 80 cases. When data for RPDs performed before the learning curve (11 cases) are eliminated, median operative time for the RPD + HAA cohort is 451 min. Importantly, no patients required conversion.
Although the safety of RPD has been previously established,22 a major concern is the lack of haptic feedback and its implications on arterial dissection, especially when the resection is performed for cancer. Margin distance remains a significant prognostic factor influencing recurrence and survival in patients with pancreatic adenocarcinoma, and a dissection on the peri-adventitial layers of the SMA (or an aberrant hepatic artery) invariably improves the retroperitoneal R0 margin rate but may increase the risk for arterial injury. Rates of post-pancreatectomy haemorrhage (PPH) ranged from 2.7% to 14% in three large series of PD performed with anomalous RHA and do not differ from those in patients with normal hepatic arterial anatomy submitted to PD.8,18,20 The present group observed a rate of PPH of 6.7%, which is within these parameters. When using the robotic platform, arterial injury can be minimized by strict adherence to a no-touch technique when dissecting around these arteries. Adequate tension can be exerted on the surrounding tissue by the third robotic arm or by the laparoscopic bedside assistant to allow for safe dissection. The combination of a low-energy robotic hook and the bipolar grasper ensures the efficient handling of small uncinate vascular branches with minimal heat dissipation to the SMA or aberrant vessels.
From an oncologic perspective, the robotic platform yielded similar R0 margins in the contexts of equivalent tumour size, extent of neoadjuvant therapy administered, and extent of perineural or lymphovascular invasion in both groups. Importantly, none of the aberrant vessels were sacrificed as a result of intraoperative injury, and none of the positive margins were retroperitoneal or involved the aberrant or anomalous hepatic arteries. It is important to note, however, that microscopic assessment of the aberrant or anomalous vessel margin is challenging for the pathologist because this margin is not as clearly defined on the resected specimen as other ‘standardized’ margins may be. Additionally, the definition of ‘no microscopic tumour at the margin’ was used to define R0, regardless of the margin distance. Both of these factors may have influenced the high rate of R0 resection identified in this series. Longer follow-up is needed to ensure that these short-term oncologic surrogate outcomes translate to equivalent survival.
This study has several limitations, the most important of which is its retrospective nature. Despite the similarity between the groups in preoperative and pathologic characteristics, an inherent selection bias may arise in the process of categorizing patients for robotic platform versus open surgery. The present group has not attempted to perform major vascular resections or reconstructions (primary end-to-end and vein interposition) using the robotic platform and thus procedures involving more ‘difficult’ borderline resectable tumours are usually performed in an open fashion at this institution.
In conclusion, this single-institution study demonstrates that RPD can be performed safely in patients with aberrant or anomalous hepatic arterial anatomy with acceptable perioperative and oncologic outcomes.
Conflicts of interest
None declared.
Acknowledgments
TN and BAB are supported by the National Cancer Institute (grant no. T32CA113263). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- Abdullah SS, Mabrut JY, Garbit V, De La Roche E, Olagne E, Rode A, et al. Anatomical variations of the hepatic artery: study of 932 cases in liver transplantation. Surg Radiol Anat. 2006;28:468–473. doi: 10.1007/s00276-006-0121-0. [DOI] [PubMed] [Google Scholar]
- Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50–52. doi: 10.1097/00000658-199407000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G. Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat. 2004;26:239–244. doi: 10.1007/s00276-004-0229-z. [DOI] [PubMed] [Google Scholar]
- Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–347. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 2010;17:186–193. doi: 10.1245/s10434-009-0757-1. [DOI] [PubMed] [Google Scholar]
- Yang SH, Yin YH, Jang JY, Lee SE, Chung JW, Suh KS, et al. Assessment of hepatic arterial anatomy in keeping with preservation of the vasculature while performing pancreatoduodenectomy: an opinion. World J Surg. 2007;31:2384–2391. doi: 10.1007/s00268-007-9246-5. [DOI] [PubMed] [Google Scholar]
- Kim PT, Temple S, Atenafu EG, Cleary SP, Moulton CA, McGilvray ID, et al. Aberrant right hepatic artery in pancreaticoduodenectomy for adenocarcinoma: impact on resectability and postoperative outcomes. HPB. 2014;16:204–211. doi: 10.1111/hpb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer JA, Bridges MD, Turan N, Nguyen JH, Martin JK. Aberrant right hepatic arterial anatomy and pancreaticoduodenectomy: recognition, prevalence and management. HPB. 2009;11:161–165. doi: 10.1111/j.1477-2574.2009.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverso LW, Freeny PC. Pancreaticoduodenectomy. The importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg. 1989;55:421–426. [PubMed] [Google Scholar]
- Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg. 2011;35:2739–2746. doi: 10.1007/s00268-011-1276-3. [DOI] [PubMed] [Google Scholar]
- Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;26:2397–2402. doi: 10.1007/s00464-012-2207-6. [DOI] [PubMed] [Google Scholar]
- Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646–1657. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ., III 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258:554–559. doi: 10.1097/SLA.0b013e3182a4e87c. discussion 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly EJ, Talamini MA. Robotic abdominal surgery. Am J Surg. 2004;188(4A Suppl):19S–26S. doi: 10.1016/j.amjsurg.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Zeh HJ, III, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg. 2011;45:323–340. doi: 10.1016/j.yasu.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Zenoni SA, Arnoletti JP, de la Fuente SG. Recent developments in surgery: minimally invasive approaches for patients requiring pancreaticoduodenectomy. JAMA Surg. 2013;148:1154–1157. doi: 10.1001/jamasurg.2013.366. [DOI] [PubMed] [Google Scholar]
- Winer J, Can MF, Bartlett DL, Zeh HJ, Zureikat AH. The current state of robotic-assisted pancreatic surgery. Nat Rev Gastroenterol Hepatol. 2012;9:468–476. doi: 10.1038/nrgastro.2012.120. [DOI] [PubMed] [Google Scholar]
- Eshuis WJ, Olde Loohuis KM, Busch OR, van Gulik TM, Gouma DJ. Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB. 2011;16:1–167. doi: 10.1111/j.1477-2574.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Lee YJ, Kim CW, Moon KM, Kim MW. Clinical implications of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Surg. 2009;33:1727–1732. doi: 10.1007/s00268-009-0063-x. [DOI] [PubMed] [Google Scholar]
- Sulpice L, Rayar M, Paquet C, Bergeat D, Merdrignac A, Cunin D, et al. Does an aberrant right hepatic artery really influence the short- and long-term results of a pancreaticoduodenectomy for malignant disease? A matched case-controlled study. J Surg Res. 2013;185:620–625. doi: 10.1016/j.jss.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. discussion 937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jah A, Jamieson N, Huguet E, Praseedom R. The implications of the presence of an aberrant right hepatic artery in patients undergoing a pancreaticoduodenectomy. Surg Today. 2009;39:669–674. doi: 10.1007/s00595-009-3947-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain RS, El-Sedfy A, Rajkumar D. Aberrant hepatic arterial anatomy and the whipple procedure: lessons learned. Am Surg. 2011;77:517–526. [PubMed] [Google Scholar]
- Rammohan A, Sathyanesan J, Palaniappan R, Govindan M. Transpancreatic hepatomesenteric trunk complicating pancreaticoduodenectomy. JOP. 2013;14:649–652. doi: 10.6092/1590-8577/1641. [DOI] [PubMed] [Google Scholar]
- Woods MS, Traverso LW. Sparing a replaced common hepatic artery during pancreaticoduodenectomy. Am Surg. 1993;59:719–721. [PubMed] [Google Scholar]
- Wood M, Lazo C, Hassid V, Awad ZT. Replaced common hepatic artery from superior mesenteric artery during pancreaticoduodenectomy. Gulf J Oncolog. 2012;11:60–62. [PubMed] [Google Scholar]
- Yang F, Long J, Fu DL, Jin C, Yu XJ, Xu J, et al. Aberrant hepatic artery in patients undergoing pancreaticoduodenectomy. Pancreatology. 2008;8:50–54. doi: 10.1159/000114867. [DOI] [PubMed] [Google Scholar]


