Abstract
Background
Pancreaticoduodenectomies (PD) still have a substantial mortality rate. Recently, different scores have been published to predict the mortality risk pre-operatively after PD. This retrospective study was designed to perform an external assessment of an Early Mortality Risk Score (EMRS).
Methods
From 2000 to 2012, all PD cases performed at our institution were documented. Only patients treated for pancreatic head adenocarcinomas were included. Survival time and EMRS (based on age, tumour size, tumour differentiation and comorbidities) were calculated for every patient. Relative risks (RR) of early death 9 and 12 months after PD were then calculated.
Results
Of 270 PD for various aetiologies, 120 PD for adenocarcinomas were included. The median follow-up was 37 months, and the overall median survival was 19 months. EMRS of 4 showed a mortality RR of 5.1 at 9 months (P = 0.048) and of 4.5 at 12 months (P = 0.020).
Conclusions
EMRS of 4 is a predictor of tumour-related mortality at 9 and 12 months after PD for adenocarcinoma. The EMRS was externally assessed in our patient cohort and can be implemented in clinical practice. Clinical implications of this score still need to be studied.
Introduction
Pancreatic cancer is the fourth cause of cancer-related mortality in the United States and is predicted to become the second leading cause of cancer death by 2020.1,2
Technically, pancreas surgery has made important progress during the two recent decades and has become a safe procedure with mortality rates below 5% in experienced centres with high patient volumes.3–5 Nevertheless, it has to be emphasized that post-operative complication rates remain high, ranging from 20% to 60%, and even more important, long-term survival is still poor with a reported 5-year survival <20% for operated patients with curative intent.6–9 One-year mortality of patients undergoing a pancreaticoduodenectomy (PD) for adenocarcinoma can be as high as 30%.10
The development of pancreas cancer takes several years from first mutations of tumour suppressor genes to clones of tumour cells with metastatic capacity, and finally macroscopically established cancer.11–13 Unfortunately, the majority of the carcinogenesis steps cannot be detected by current diagnostic tools; subsequently, clinical diagnosis is established late, and patients often present locally non-resectable or even metastatic disease.14 Until earlier tumour detection will be feasible, careful patient selection remains crucial to identify potential candidates who could benefit from pancreas resection. If surgery is considered too risky for an individual patient, alternative treatment options such as chemotherapy, eventually combined with local tumour destruction or radiotherapy could fit better. Patients with good chances for a prolonged long-term survival should undergo surgery, and surgery-related morbidity is acceptable, whereas in patients with predicted limited survival, i.e. <12 months, preservation of a reasonable quality of life is more important.10,15,16
To this end, different pre- and post-operative scores using various risk factors have been developed and may be used to ease decision-making and to tailor treatment plans for individual patients.7,9,17–22 One of these, the Early Mortality Risk Score (EMRS) created by Hsu et al. from Johns Hopkins University is a simple four-item score. With a goal of identifying patients at high risk of early mortality after pancreas surgery, the EMRS is a predictive risk score of early death (9 and 12 months) after pancreatic head resection for adenocarcinoma.18 It includes patient age, tumour size, tumour differentiation and comorbidities.18 This score was selected because it can be obtained pre-operatively and it is easy to use and calculate.
This present study aimed to assess the EMRS with a different cohort and in different settings to test its clinical applicability.18
Methods
Early Mortality Risk Score developed by the group from Johns Hopkins Hospital
Providing pre-operative data, this score enables prediction of the 9- and 12-month mortality risk for patients undergoing PD for pancreatic adenocarcinoma.18 The four parameters (0 or 1 point for each score parameter) that compose this score are the age (1 point if <75 years), the tumour size measured on a CT scan (1 point if ≥3 cm), the tumour differentiation (1 point if poor differentiation) and the comorbidities (1 point if presence of any one of these: hypertension, diabetes mellitus, cardiac disease, or chronic obstructive pulmonary disease). A score ≥2 is significantly associated with the 9- and 12-month mortality risk. In case of absence of pre-operative histology, Hsu et al. developed the modified EMRS (mEMRS) allowing to take simply into consideration age, tumour size and comorbidities without changing the prognostic validity of the score.
Database and collected information
The Department of Visceral Surgery of the University Hospital of Lausanne (CHUV), Switzerland, maintains a prospective database of all pancreas resections since 2000. It encompasses more than 150 items of pre-, intra- and post-operative data.23 For this current analysis, only patients who underwent PD with curative intent for adenocarcinoma of the pancreatic head from 2000 to 2012 were included, whereas other tumour entities were not considered. Patients who died during the 60 days after the operation or during their hospital stay after the index operation were excluded as done in the original EMRS article.
Technical aspects of the operation and discharge criteria
Most performed PD (n = 105, 87.5%) were classic pancreatic head resections, and 15 patients had a pylorus-preserving PD (12.5%). The pancreatic head was resected en-bloc together with the duodenum, the distal common bile duct, as well as the distal stomach. Moreover, the resection also included the first jejunal loop, the gallbladder and the loco-regional lymph nodes. An omega-type jejunal loop reconstruction was the standard procedure consisting of a pancreatico-enteric anastomosis, a bilio-enteric anastomosis and lastly a gastro-enteric anastomosis. In a few cases, a pancreaticogastric anastomosis was performed by one surgeon who preferred this technique in case of soft pancreas texture. Two drains were routinely left in place near the pancreatico-enteric and bilio-enteric anastomoses. They were removed on post-operative day 3 and 5, if there was no suspicion of leakage, i.e. amylase content in the drain fluid not higher than three times the serum amylase level and no bilirubin detected. Single-shot prophylactic antibiotics were given before the incision.
Technical aspects of the operation did not vary during the study period. Patients were discharged when the pain was controlled by oral medication, the patient was autonomous (ambulation, shower, eating and getting out of bed) and an oral diet was well tolerated.
Score parameters
The time point for the age was the operation date. In our study, tumour size was measured on pre-operative CT as recommended by the original article by Hsu et al. Tumour differentiation was based on post-operative pathology as a pre-operative tumour biopsy was not routinely undertaken at our institution. Poor differentiation was defined as G3 or G4 according to the American Joint Committee on Cancer.24 Of note, in their article Hsu et al. also used the post-operative pathology results and not the pre-operative biopsy to define and validate the EMRS.18 Hypertension, diabetes mellitus, cardiac disease or chronic obstructive pulmonary disease were assessed pre-operatively.
Post-operative complications and resection margins
Post-operative complications were assessed according to the Dindo–Clavien classification.25 Definitions of complications were based according to a previous article from Johns Hopkins.26 The definition of a clearance margin was based on the 7th Edition of the TNM classification by the American Joint Committee on Cancer (AJCC).27 Microscopically, a positive resection margin (R1) was defined as the presence of tumour cells at the resection margin (‘0 mm rule’).27–29 A R2 resection was defined as a macroscopic positive margin and R0 as the absence of microscopic tumour cells at the resection margin.27–29
Outcomes and statistical analysis
Survival time and EMRS were calculated for every single patient. Based on these two results, relative risks (RR) of 9- and 12-month mortality were calculated (EMRS of 0 was defined as the reference, corresponding to a RR of 1). The found RR were compared to the RR of the original EMRS article. Comparisons were performed with Mann–Whitney U-tests for non-Gaussian continuous variables and with Fisher's exact tests for discrete variables. Univariate analysis was performed to identify parameters predicting early death 9 and 12 months after PD. Survival curves were calculated using the Kaplan–Meier technique. The overall median follow-up was calculated by inverting the status indicators of the Kaplan–Meier survival curve as described by Schemper and Smith.30 Comparisons of the survival curves were done by a log-rank (Mantel–Cox) test. A P-value <0.05 was considered to be statistically significant. The RR at which the P-value was <0.05 defined our cut-off value for the EMRS. GraphPad Prism 5 for Mac OS X (GraphPad Software Inc.) was used for calculation and analysis.
The study was approved by the local Ethics Committee (protocol number: 34/13).
Results
There were 270 patients who underwent a PD for various aetiologies during the study period. PD for adenocarcinoma of the pancreatic head was performed in 130 patients. Three patients were excluded as a result of missing data and another seven patients owing to early post-operative death (grade V complication), leaving 120 patients for analysis. The seven post-operative deaths were due to haemorrhage (3x), multiple organ failure (1x), a massive pulmonary embolism (1x), a gastric fistula (1x) and colon ischaemia (1x). None of these seven deaths were related to a tumour progression. Among the 10 excluded patients, three patients had an EMRS of 1, three patients an EMRS of 2, three patients an EMRS of 3 and one patient an EMRS of 4. Calculations of the RR with these excluded patients did not change the study findings (RR for EMRS of 4 = 5.7).
Table 1 resumes the patients' characteristics, pre-operative data, operative results and post-operative outcomes classified regarding the 9- and 12-month mortality, respectively. No patient received neoadjuvant treatment. Age <75 years, tumour size ≥3 cm, low differentiation grade (G3, G4), the presence of comorbidities (i.e. diabetes mellitus, hypertension, cardiovascular diseases, or chronic obstructive pulmonary disease), R1 or R2 resection, and vascular or perineural invasion were associated with a significantly decreased survival <9 months after PD. A tumour size ≥3 cm, low differentiation grade (G3, G4) and R1 or R2 resection were statistically significant risk factors for survival <12 months. The four EMRS parameters were thus significant risk factors for 9-month mortality. Pre-operative available statistically significant risk factors for early tumour-related death (9-month mortality) were increased patient age, tumour size ≥3 cm and comorbidities.
Table 1.
Risk factors for early mortality 9 (a) and 12 months (b) after a pancreaticoduodenectomy (PD) for adenocarcinoma
| (a) | |||
|---|---|---|---|
| Survival ≥ 9 months N = 87 | Survival < 9 months N = 33 | P-value | |
| Age <75 years | 21 | 15 | 0.028 |
| BMI <25 kg/m2 | 29 | 8 | 0.383 |
| Women | 42 | 15 | 0.840 |
| Tumour size ≥ 3 cm | 33 | 27 | <0.001 |
| Differentiation (G1/2/3) | 16/51/20 | 2/18/13 | 0.029 |
| Comorbidities | 44 | 24 | 0.039 |
| Postoperative CHT | 51 | 17 | 0.539 |
| Pylorus preservation | 9 | 6 | 0.352 |
| Portal vein resection | 31 | 10 | 0.669 |
| R0/R1/R2 | 59/25/3 | 15/14/4 | 0.047 |
| Positive nodes | 71 | 30 | 0.270 |
| T stage (1/2/3/4) | 3/16/65/3 | 0/4/27/2 | 0.522 |
| Major post-operative complications (III-IV) | 25 | 11 | 0.659 |
| Vascular invasiona | 34 | 21 | 0.023 |
| Perineural invasion | 38 | 28 | <0.001 |
| (b) | |||
|---|---|---|---|
| Survival ≥ 12 months N = 75 | Survival < 12 months N = 45 | P-value | |
| Age < 75 years | 21 | 18 | 0.227 |
| BMI < 25 kg/m2 | 23 | 12 | 0.683 |
| Women | 37 | 20 | 0.706 |
| Tumour size ≥ 3 cm | 26 | 34 | <0.001 |
| Differentiation (G1/2/3) | 15/45/15 | 4/23/18 | 0.036 |
| Comorbidities | 39 | 29 | 0.254 |
| Post-operative CHT | 44 | 24 | 0.575 |
| Pylorus preservation | 8 | 7 | 0.570 |
| Portal vein resection | 28 | 13 | 0.428 |
| R0/R1/R2 | 53/20/2 | 21/19/5 | 0.017 |
| Positive nodes | 60 | 41 | 0.127 |
| T stage (1/2/3/4) | 3/14/56/2 | 0/6/36/3 | 0.325 |
| Major postoperative complications (III–IV) | 22 | 14 | 0.839 |
| Vascular invasiona | 31 | 24 | 0.257 |
| Perineural invasion | 37 | 29 | 0.131 |
BMI, body mass index; CHT, chemotherapy.
Microscopic invasion of the small vessels.
Significant P-values appear in bold type.
Thirty-three patients (28%) died during the first 9 months after the operation and 45 patients (38%) during the first post-operative year. Sixty-eight patients (57%) received post-operative chemotherapy, whereby most of them (94%) received gemcitabine, and the remaining patients received oxaliplatin or FOLFIRI (leucovorin, fluorouracil and irinotecan). The R0 resection rate was 62% (74 patients), the R1 resection rate 33% (39 patients) and the R2 resection rate 5% (7 patients). The predominant T stage was T3 in 92 patients (77%). The T1 stage was observed in three patients, T2 stage in 20 patients and T4 stage in 5 patients. The median tumour size based on pathology reports was 3 cm (interquartile range: 2.3–4 cm).
Overall complications appeared in 64% (77/120) of the patients. Major complications (IIIa–IVb) appeared in 30% (36/120) of patients, and minor complications (I–II) appeared in 34% (41/120) of patients. Patients with an EMRS of 0 or 1 had a complication rate of 69% vs. 61% for the patients with an EMRS <1 (P = 0.438). Among patients with EMRS of 0 or 1 the most predictive factor of complications was the presence of comorbidities (P = 0.001).
EMRS and mEMRS were calculated for every patient. Seventeen patients had an EMRS of 0, 27 patients an EMRS of 1, 45 patients an EMRS of 2, 25 patients an EMRS of 3, and 6 patients an EMRS of 4. For the modified version, 21 patients had a mEMRS of 0, 32 patients a mEMRS of 1, 52 patients a mEMRS of 2, and 15 patients a mEMRS of 3. RR of 9- and 12-month death after PD for the diverse scores are summarized in Tables2 and 3. A patient with an EMRS of 4 had 5.1 times and 4.5 times more risks of mortality at 9 and 12 months, respectively. In the original article, EMRS of 4 was associated with a mortality RR of 10.7 at 9 months and 5.3 at 12 months. A patient with a mEMRS of 3 had 3.7 times and 3.2 times more risks of mortality at 9 and 12 months, respectively. In the original article, mEMRS of ≥2 was associated with a mortality RR of 2.5 at 9 months and 2.2 at 12 months.
Table 2.
Early Mortality Risk Score (EMRS) associations with early mortality 9 and 12 months after a pancreaticoduodenectomy (PD) for adenocarcinoma
| EMRS | Mortality at 9 months | Mortality at 12 months | ||
|---|---|---|---|---|
| Relative risk (95% CI) | P-value | Relative risk (95% CI) | P-value | |
| Score 0 | Reference = 1 | Reference = 1 | ||
| Score 1 | 1.8 (0.4–8.0) | 0.690 | 1.8 (0.6–5.8) | 0.488 |
| Score 2 | 2.5 (0.6–9.8) | 0.200 | 2.0 (0.7–6.1) | 0.225 |
| Score 3 | 3.1 (0.8–12.5) | 0.151 | 2.9 (1.0–8.8) | 0.054 |
| Score 4 | 5.1 (1.2–22.5) | 0.048 | 4.5 (1.5–13.9) | 0.020 |
CI, confidence interval.
Table 3.
Modified Early Mortality Risk Score (mEMRS) associations with early mortality 9 and 12 months after a pancreaticoduodenectomy (PD) for adenocarcinoma
| mEMRS | Mortality at 9 months | Mortality at 12 months | ||
|---|---|---|---|---|
| Relative risk (95% CI) | P-value | Relative risk (95% CI) | P-value | |
| Score 0 | Reference = 1 | Reference = 1 | ||
| Score 1 | 1.8 (0.5–5.9) | 0.493 | 2.0 (0.7–5.3) | 0.223 |
| Score 2 | 2.6 (0.8–7.7) | 0.090 | 2.3 (0.9–5.9) | 0.061 |
| Score 3 | 3.7 (1.2–11.8) | 0.025 | 3.2 (1.2–8.3) | 0.017 |
CI, confidence interval.
The median follow-up time was 37 months (interquartile range: 20–61 months). Kaplan–Meier survival curves are shown in Figure 1. The overall median survival time according to Kaplan–Meier analysis was 19 months (interquartile range 9–40).
Figure 1.
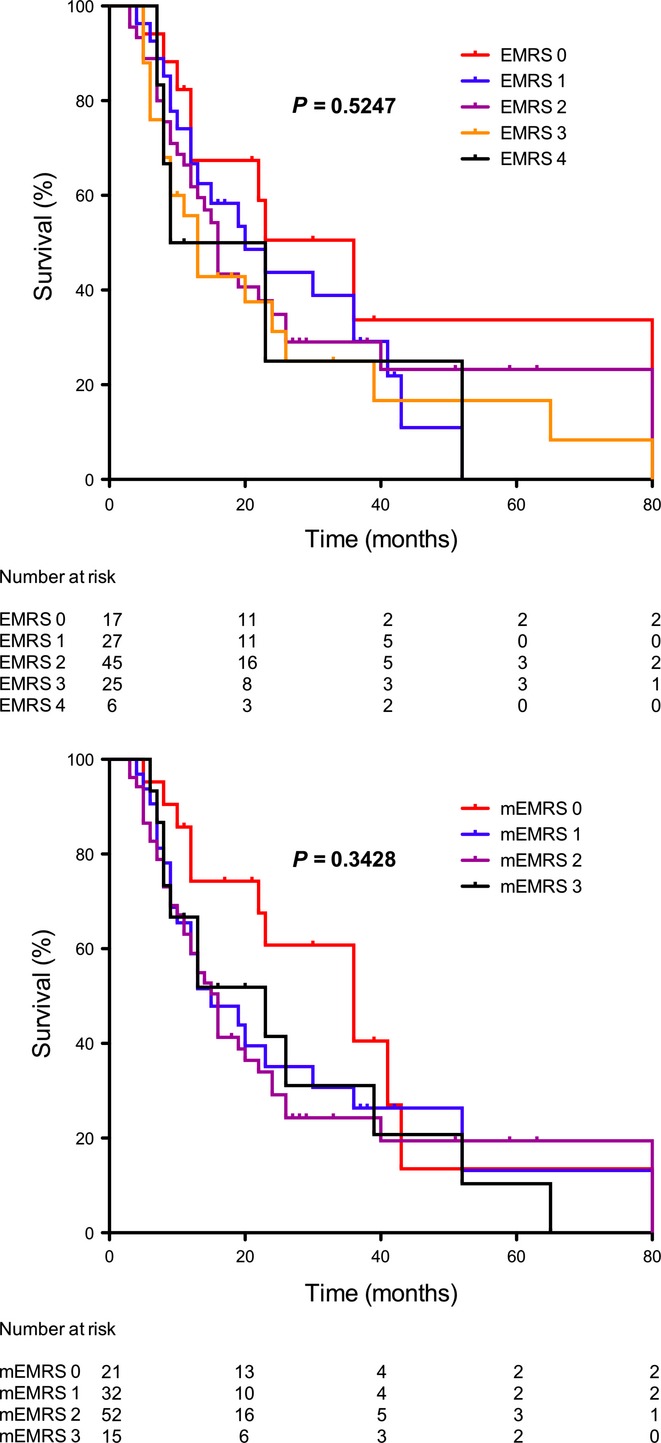
Kaplan–Meier survival curves for Early Mortality Risk Score (EMRS) and modified EMRS (mEMRS)23
The measures of the tumour size on pre-operative CT or based on the pathology report were statistically significantly different (median size on CT: 2.7 cm with interquartile range 2–3, median size on pathology reports: 3 cm with interquartile range 2.3–4, P = 0.001).
Discussion
In our cohort, an EMRS of 4 or a mEMRS of 3 were associated with increased risks of early (9- and 12-month) tumour-related post-operative mortality.
Even when surgery is performed early on for adenocarcinoma of the pancreatic head, around one-third of patients will not survive the first post-operative year.10 Upfront surgery in high-risk patients may not be appropriate, and alternative treatment options may be worthwhile to be considered by avoiding the post-operative morbidity. Simple pre-operative scores like the EMRS could potentially be helpful tools to identify patients at increased risk and to individualize treatment plans.
Risk factors of early death after PD identified by Hsu et al. were age <75 years, tumour size ≥3 cm, poor differentiation, the presence of comorbidities, no adjuvant chemotherapy, node positivity, margin resection positivity, vascular invasion (only for 12-month mortality) and post-operative complications.18 As some factors already are pre-operatively available, they can be used for therapeutic decision-making. In our study, age <75 years (only for 9-month mortality), tumour size ≥3 cm, low differentiation grade (G3, G4), the presence of comorbidities (only for 9-month mortality), R1 or R2 resection, and vascular or perineural invasion (only for 9-month mortality) were statistically significant risk factors for early mortality after PD.
EMRS of 4 and modified EMRS of 3 were statistically significant for higher RR of early death after PD for adenocarcinoma. In contrast, long-term survivals based on Kaplan–Meier curves were not different (Figure 1, P = 0.525 for EMRS and P = 0.343 for mEMRS). Of note, Hsu et al. did not calculate the difference in long-term survival in the different scores. The fact that survival curves of the four EMRS are not different but that an EMRS of 4 is a predictor of early mortality is remarkable, but it might be related to the limited sample size of the study. The overall median survival rate was 19 months, and 38% of the patients were dead 12 months after the index operation. This confirms the bad prognosis of pancreatic head adenocarcinomas even when early operated. Moreover, occurrence of severe complications among incomplete resections (R1) affects the long-term survival and predicts a poor outcome.31
Age <75 years and the presence of comorbidities were predictors of survival <9 months but not of survival <12 months. On the contrary, a tumour size ≥3 cm and low differentiation grade were predictors of mortality for both 9 and 12 months. The patient-related factors (i.e. age and comorbidities) reflect the frailty of the patients. Fragile and weak patients are at high risk of dying during the early post-operative period. This could explain the difference in the predictive patient-related factors between the early mortality at 9 months and 12 months (a majority of polymorbid and old patients die before 9 months).
Measurements of the tumour size were based on the pre-operative CT-scanner as recommended by the princeps article by Hsu et al..18 The size of the tumours on CT and measured in pathology were compared and showed a statistically significant difference (P = 0.001). In our study, a pre-operative CT scan underestimated the tumour size as found in other studies.32,33
In our centre, patients diagnosed with adenocarcinoma of the pancreas do not routinely undergo a tumour biopsy before treatment, or only if neoadjuvant chemotherapy is potentially considered. Therefore, tumour differentiation was assessed via pathology reports. In institutions where a pre-operative biopsy is not performed, the modified EMRS is a simple score that can easily be used and calculated in clinical practice. Modified EMRS probably represent a more useful generalizable tool than the standard EMRS comprising the tumour differentiation.
In the article of Hsu et al., 53% of the operated patients received adjuvant chemotherapy compared to 57% in our group.18 There was a difference in terms of chemotherapy type, which can play a role in the differences of the predictive values of the EMRS between this study data and Hsu's. In the EMRS manuscript, 94% of patients with adjuvant chemotherapy received 5-fluorouracil whereas 94% of our patients received gemcitabine.
Complication rates were similar between a low-risk (EMRS of 0 or 1) and a high-risk of mortality (EMRS <1) patients (P = 0.438). This result shows that complications can occur independently of the disease or the patient. EMRS thus represents a good predictor of early mortality but not a good predictor of post-operative complications.
Our study has several limitations that must be acknowledged. First of all, as the scores were calculated in a retrospective way, all possible confounding factors were sometimes not available in the charts. Moreover, 120 patients to validate this score may lack statistical power (in particular for the RR <4). Included patients received either adjuvant chemotherapy or no post-operative treatment. These parameters were not taken into account for the validation process.
Clinical implications of the score still need to be evaluated. Patients with a high EMRS could maybe benefit from neoadjuvant treatment. Moreover, it could be suggested that reduction of malnutrition, smoking cessation, or other interventions to minimize post-operative morbidity would be of interest in high EMRS, but this is not clearly established as these interventions would probably improve the immediate post-operative morbidity and mortality. Finally, post-operative management should be particularly careful in such high-risk patients.
EMRS represents a predictive score of early mortality after PD, but does not correlate with long-term survival. This study shows that the EMRS and the modified EMRS were predictive of short-term mortality in a different patient cohort. EMRS and mEMRS can, therefore, be used in clinical daily practice. Prospective studies to verify our findings and algorithm development based on this score are required to enhance the usefulness of this simple predictive score.
Sources of funding
No funding sources.
Conflicts of interest
Gaëtan-Romain Joliat, David Petermann, Nicolas Demartines, and Markus Schäfer have no conflicts of interest or financial ties to disclose.
References
- Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95:357–362. doi: 10.1002/bjs.5982. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- Pecorelli N, Balzano G, Capretti G, Zerbi A, Di Carlo V, Braga M. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg. 2012;16:518–523. doi: 10.1007/s11605-011-1777-2. [DOI] [PubMed] [Google Scholar]
- Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254:702–707. doi: 10.1097/SLA.0b013e31823598fb. [DOI] [PubMed] [Google Scholar]
- Kennedy EP, Yeo CJ. The case for routine use of adjuvant therapy in pancreatic cancer. J Surg Oncol. 2007;95:597–603. doi: 10.1002/jso.20719. [DOI] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21–23. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JS, Zhou Z, Simons JP, Ng SC, McDade TP, Whalen GF, et al. A simple risk score to predict in-hospital mortality after pancreatic resection for cancer. Ann Surg Oncol. 2010;17:1802–1807. doi: 10.1245/s10434-010-0947-x. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Wolfgang CL, Laheru DA, Pawlik TM, Swartz MJ, Winter JM, et al. Early mortality risk score: identification of poor outcomes following upfront surgery for resectable pancreatic cancer. J Gastrointest Surg. 2012;16:753–761. doi: 10.1007/s11605-011-1811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB. 2010;12:488–97. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragulin-Coyne E, Carroll JE, Smith JK, Witkowski ER, Ng SC, Shah SA, et al. Perioperative mortality after pancreatectomy: a risk score to aid decision-making. Surgery. 2012;152:S120–127. doi: 10.1016/j.surg.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Venkat R, Puhan MA, Schulick RD, Cameron JL, Eckhauser FE, Choti MA, et al. Predicting the risk of perioperative mortality in patients undergoing pancreaticoduodenectomy: a novel scoring system. Arch Surg. 2011;146:1277–1284. doi: 10.1001/archsurg.2011.294. [DOI] [PubMed] [Google Scholar]
- Are C, Afuh C, Ravipati L, Sasson A, Ullrich F, Smith L. Preoperative nomogram to predict risk of perioperative mortality following pancreatic resections for malignancy. J Gastrointest Surg. 2009;13:2152–62. doi: 10.1007/s11605-009-1051-z. [DOI] [PubMed] [Google Scholar]
- Joliat GR, Petermann D, Demartines N, Schäfer M. Prediction of complications after pancreaticoduodenectomy: validation of a postoperative complication score. Pancreas. 2014 doi: 10.1097/MPA.0000000000000399. In press. [DOI] [PubMed] [Google Scholar]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th edn. New York, NY: Springer; 2010. [Google Scholar]
- Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin LH, Gaspodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th edn. New York, NY, USA: Wiley & Sons; 2009. [Google Scholar]
- Hruban RH, Pitman MB, Klimstra D. Tumors of the Pancreas. Washington, DC, USA: Armed Forces Institute of Pathology; 2007. [Google Scholar]
- Schlitter AM, Esposito I. Definition of microscopic tumor clearance (R0) in pancreatic cancer resections. Cancers. 2007;2:2001–2010. doi: 10.3390/cancers2042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- Petermann D, Demartines N, Schäfer M. Severe postoperative complications adversely affect long-term survival after R1 resection for pancreatic head adenocarcinoma. World J Surg. 2013;37:1901–1908. doi: 10.1007/s00268-013-2023-8. [DOI] [PubMed] [Google Scholar]
- Arvold ND, Niemierko A, Mamon HJ, Fernandez-del Castillo C, Hong TS. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys. 2011;80:1383–1390. doi: 10.1016/j.ijrobp.2010.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Wild AT, Wang H, Fishman EK, Hruban RH, Laheru DA, et al. Comparison of conventional and 3-dimensional computed tomography against histopathologic examination in determining pancreatic adenocarcinoma tumor size: implications for radiation therapy planning. Radiother Oncol. 2012;104:167–172. doi: 10.1016/j.radonc.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


