Abstract
Objectives
Post-hepatectomy liver failure has a major impact on patient outcome. This study aims to explore the impact of the integration of a novel patient-centred evaluation, the LiMAx algorithm, on perioperative patient outcome after hepatectomy.
Methods
Trends in perioperative variables and morbidity and mortality rates in 1170 consecutive patients undergoing elective hepatectomy between January 2006 and December 2011 were analysed retrospectively. Propensity score matching was used to compare the effects on morbidity and mortality of the integration of the LiMAx algorithm into clinical practice.
Results
Over the study period, the proportion of complex hepatectomies increased from 29.1% in 2006 to 37.7% in 2011 (P = 0.034). Similarly, the proportion of patients with liver cirrhosis selected for hepatic surgery rose from 6.9% in 2006 to 11.3% in 2011 (P = 0.039). Despite these increases, rates of post-hepatectomy liver failure fell from 24.7% in 2006 to 9.0% in 2011 (P < 0.001) and liver failure-related postoperative mortality decreased from 4.0% in 2006 to 0.9% in 2011 (P = 0.014). Propensity score matching was associated with reduced rates of post-hepatectomy liver failure [24.7% (n = 77) versus 11.2% (n = 35); P < 0.001] and related mortality [3.8% (n = 12) versus 1.0% (n = 3); P = 0.035].
Conclusions
Postoperative liver failure and postoperative liver failure-related mortality decreased in patients undergoing hepatectomy following the implementation of the LiMAx algorithm.
Introduction
Improvements in rates of operative mortality after hepatic tumour resection have broadened its use in the treatment of patients with benign and malignant hepatobiliary disease.1,2 Extended resection has evolved as a suitable approach to ensure complete tumour clearance in selected patients. Previous large series have reported improved survival rates compared with non-surgical strategies.3–8 As a result, radical approaches in non-cirrhotic livers resulting in smaller remnant volumes have become more commonplace.9 In patients with normal hepatic function, remnant volume of 25% can be sufficient to avoid postoperative hepatic failure.10 However, preoperative liver function and intraoperative variables also have significant influence on patient outcomes and therefore must be considered.11
In patients with impaired hepatic function, there is no consensus on what constitutes a safe residual liver volume following hepatic resection.12,13 Several methods have been proposed to assess remnant liver function.14–18 However, no preoperative approach has been widely accepted and pre-existing hepatic dysfunction remains a major concern when considering patients for hepatic resection. Selection criteria that accurately identify patients in whom a surgical intervention can be safely performed are required.
LiMAx (maximum liver function capacity) has recently been proposed as a novel 13C-liver function breath test for the preoperative assessment of actual liver function before hepatectomy and the prediction of patient outcome after surgery.19 LiMAx has been shown to be unaffected by age, gender or obesity and has been demonstrated to accurately and reliably assess liver function in both healthy subjects and patients with cirrhosis.20–22 Based on these findings, the authors have proposed a patient-centred preoperative evaluation for the risk stratification (LiMAx algorithm) of patients prior to liver surgery (Fig.1).23
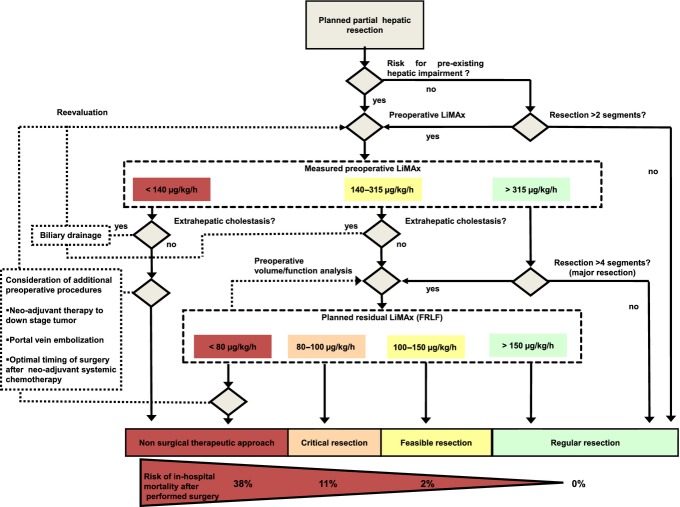
Figure 1.
Clinical decision tree for preoperative evaluation of patients undergoing hepatectomy (modified according to Stockmann et al.23). If pre-existing hepatic injury is unlikely and a small segmental resection (up to two segments) is planned, surgery can be performed safely. However, in cases of suspected hepatic injury or planned larger resections, a preoperative LiMAx test to evaluate actual enzymatic hepatic function is performed. In patients with normal liver function (LiMAx <315 μg/kg/h), resections of up to four segments can be performed, but patients with considerably impaired liver function (LiMAx <140 μg/kg/h) should be refused and alternative management options considered. In patients with intermediate liver function (LiMAx 140–315 μg/kg/h) or in whom major hepatic resection (more than four segments) is planned, clinical decisions should be guided by preoperative volume/function analysis as follows: resections with future remnant liver function (FRLF) of <100 μg/kg/h are feasible and safe; resections with FRLF of 80–100 μg/kg/h represent critical interventions, and resections with an expected FRLF of <80 μg/kg/h should not be considered. In the last category, alternative preoperative options such as portal vein embolization to increase future remnant liver volume,26 stenting in patients with biliary obstruction and application of neoadjuvant chemotherapeutic regimes to reduce tumour volume and facilitate smaller resections (colorectal liver metastases) should be considered.27 Hereafter, close LiMAx monitoring and preoperative repeated volume/function analysis may help to ascertain the optimal timing for partial hepatic resection, even in patients with marginal LiMAx values
Although the prognostic ability of LiMAx has already been shown in prospective cohort studies, the aim of this study was to investigate the effects on patient selection and outcome of the introduction of the LiMAx algorithm.
Materials and methods
A retrospective analysis of all patients undergoing elective hepatectomy at the Department for General, Visceral and Transplantation Surgery, Charité – Universitätsmedizin Berlin between 1 January 2006 and 31 December 2011 was performed. This period was chosen because it centres around the introduction of the LiMAx algorithm in preoperative work-up in 2008 and 2009. Exclusion criteria prevented the inclusion of patients undergoing small wedge resections, additional major extrahepatic procedures, emergency surgery and associated liver partition with portal vein ligation for staged hepatectomy. The institutional ethics committee waived requirements for informed consent because the study was of a retrospective design.
Cases were retrieved from the hospital's medical controlling office. Perioperative and patient variables extracted from the hospital?s information system were evaluated. Effects associated with the integration of the LiMAx algorithm on the clinical management and outcome of patients undergoing hepatectomy were studied. Variables analysed included age, gender, American Society of Anesthesiologists (ASA) score, main diagnoses, frequency of portal vein embolization and preoperative biliary drainage, type of hepatic resection and postoperative variables including Acute Physiology and Chronic Health Evaluation (APACHE) II score after postoperative admission to the intensive care unit (ICU), post-hepatectomy liver failure (PHLF), number of postoperative days in the ICU, postoperative hospital length of stay (LoS) and mortality including cause. Post-hepatectomy liver failure was defined according to the consensus definition of the International Study Group of Liver Surgery (ISGLS) based on international normalized ratio (INR) and serum bilirubin on or after postoperative day 5.24
Patients were divided into four groups for the analysis of perioperative variables according to whether they had undergone a segmental resection, left hepatectomy, right hepatectomy or complex hepatectomy. Data for patients submitted to segmental resection, left lobectomy and resections of other segments in different combinations were combined for analysis within the ‘segmental resection’ group. Extended right hepatectomies and resections with concomitant biliary and/or vascular reconstruction were classified under ‘complex hepatectomies’.
The LiMAx algorithm for patient evaluation before hepatectomy has been described previously.23 Lack of data precluded the stratification of patients according to whether or not the LiMAx algorithm had been used. However, LiMAx was not used for clinical decision making in 2006 and 2007. The LiMAx algorithm was introduced to clinical practice in 2008 and 2009, and by 2010 LiMAx and the LiMAx algorithm had been fully integrated into the perioperative management of all patients undergoing elective hepatectomy at the study centre. Thus, to more clearly display any effects of the integration of the LiMAx algorithm on patient outcome and in order to minimize confounding factors, patients submitted to surgery in 2006 and 2007 were matched with patients submitted to surgery in 2010 and 2011 using propensity score matching. The propensity score for each patient was estimated by applying a logistic regression model based on eight variables: gender; age; ASA score; diagnosis; presence of cirrhosis; type of hepatic resection; performance of biliary or vascular resection, and duration of surgery. Patients operated in either period were matched with the counterpart from the opposite period with the closest estimated propensity score.
Statistical analysis
Categorical data are presented as frequencies and percentages, and numerical data are expressed as medians and interquartile ranges unless otherwise stated. To account for missing values (ASA score), multiple imputation for all participants to impute 10 values for each missing observation was performed and then combined with multivariable modelling estimates. Trends over time were analysed using the Cochran–Armitage test, which is based on a linear probability model. In the matched cohort, comparisons were performed using Wilcoxon signed rank tests and McNemar tests with respect to data distribution. A P-value of <0.05 was considered to indicate statistical significance. Statistical analysis was performed using IBM spss Statistics for Windows Version 21.0 (IBM Corp., Armonk, NY, USA) and R Version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics and extent of surgery
During the study period, 1302 adult patients underwent hepatectomy. Following the application of the exclusion criteria, 1170 consecutive patients submitted to elective hepatic resection of one or more segments were found to be eligible for analysis. Of the 68 patients who suffered in-hospital death, 48 (70.6%) met the criteria for PHLF and 20 (29.4%) did not. Of the 48 patients with PHLF, 25 patients died as a result of PHLF. One patient with PHLF underwent rescue liver transplantation but died subsequently. Patient demographics and data on the aetiologies of liver disease are shown in Table1. The proportion of patients undergoing laparoscopic resection during the study period was 0.9% (n = 11).
Table 1.
Patient demographics and diagnoses in 1170 patients submitted to elective hepatectomy of one or more segments between January 2006 and December 2011
| Variable | Value |
|---|---|
| Male, n (%) | 629 (53.8%) |
| Age, years, median (range) | 63 (52–70) |
| ASA class, n (%) | |
| I | 39 (3.3%) |
| II | 524 (44.8%) |
| III | 515 (44.0%) |
| IV | 92 (7.9%) |
| Cirrhosis, n (%) | 127 (10.9%) |
| Diagnosis, n (%) | |
| Malignant | 999 (85.4%) |
| Colorectal liver metastases | 343 (34.3%) |
| Hepatocellular carcinoma | 185 (18.5%) |
| Hilar cholangiocarcinoma | 173 (17.3%) |
| Others | 298 (29.8%) |
| Benign | 152 (13.0%) |
| Others | 19 (1.6%) |
ASA, American Society of Anesthesiologists.
Perioperative variables for individual years are summarized in Table2. The number of procedures (P < 0.001) and the proportion of complex hepatectomies (P = 0.034) increased significantly. The use of preoperative procedures such as portal vein embolization to enhance future remnant liver volume and liver function increased (P < 0.001). In addition, the proportion of patients with liver cirrhosis undergoing surgery increased (P = 0.039), whereas disease severity in those patients as indicated by Model for End-stage Liver Disease (MELD) scores remained stable (P = 0.747).
Table 2.
Patient demographics and types of hepatic resection by year in 1170 patients submitted to elective hepatectomy of one or more segments
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | P-value | |
|---|---|---|---|---|---|---|---|
| Patients, n | 175 | 194 | 198 | 204 | 187 | 212 | <0.001 |
| Age, years, median (IQR) | 62 (53–68) | 64 (53–70) | 63 (53–70) | 65 (54–71) | 63 (52–69) | 60 (50–71) | 0.527 |
| ASA class, n (%) | |||||||
| I | 4 (2.3%) | 3 (1.5%) | 7 (3.5%) | 11 (5.4%) | 8 (4.3%) | 6 (2.8%) | 0.280 |
| II | 79 (45.1%) | 82 (42.3%) | 83 (41.9%) | 96 (47.1%) | 89 (47.6%) | 95 (44.8%) | |
| III | 79 (45.1%) | 89 (45.9%) | 94 (47.5%) | 83 (40.7%) | 81 (43.3%) | 89 (42.0%) | |
| IV | 13 (7.4%) | 20 (10.3%) | 14 (7.1%) | 14 (6.9%) | 9 (4.8%) | 22 (10.4%) | |
| Cirrhosis, n (%) | 12 (6.9%) | 16 (8.2%) | 23 (11.6%) | 26 (12.7%) | 26 (13.9%) | 24 (11.3%) | 0.039 |
| MELD score, median (IQR) | 8 (7–11) | 7 (6–8) | 7 (7–9) | 7 (7–8) | 7 (6–7) | 7 (6–8) | 0.747 |
| Portal vein embolization, n (%) | 6 (3.4%) | 16 (8.2%) | 23 (11.6%) | 17 (8.3%) | 34 (18.2%) | 24 (11.3%) | <0.001 |
| Preoperative drainage, n (%) | 8 (4.6%) | 18 (9.3%) | 13 (6.6%) | 13 (6.4%) | 19 (10.2%) | 17 (8.0%) | 0.063 |
| Type of resection, n (%) | |||||||
| Segmental resection | 39 (22.3%) | 29 (14.9%) | 42 (21.2%) | 65 (31.9%) | 39 (20.9%) | 51 (24.1%) | 0.125 |
| Left hepatectomy | 31 (17.7%) | 34 (17.5%) | 31 (15.7%) | 33 (16.2%) | 19 (10.2%) | 37 (17.5%) | 0.347 |
| Right hepatectomy | 54 (30.9%) | 61 (31.4%) | 58 (29.3%) | 39 (19.1%) | 48 (25.7%) | 44 (20.8%) | 0.003 |
| Complex hepatectomy | 51 (29.1%) | 70 (36.1%) | 67 (33.8%) | 67 (32.8%) | 81 (43.3%) | 80 (37.7%) | 0.034 |
| PHLF, n (%) | 43 (24.6%) | 45 (23.2%) | 44 (22.2%) | 42 (20.6%) | 28 (15.0%) | 19 (9.0%) | <0.001 |
| APACHE II score, median (IQR) | 13 (10–17) | 13 (10–16) | 13 (10–17) | 12 (9–15) | 12 (8–15) | 12 (9–17) | 0.128 |
| ICU stay, days, median (IQR) | 2 (1–4) | 1 (1–2) | 1 (1–4) | 1 (1–2) | 1 (1–3) | 1 (1–2) | 0.138 |
| Postoperative LoS, days, median (IQR) | 14 (10–23) | 14 (10–26) | 14 (10–25) | 13 (8–22) | 15 (9–28) | 12 (8–19) | 0.083 |
| Hospital death, n (%) | 12 (6.9%) | 11 (5.7%) | 13 (6.6%) | 11 (5.4%) | 11 (5.9%) | 10 (4.7%) | 0.387 |
| Liver failure-related death, n (%) | 7 (4.0%) | 6 (3.1%) | 6 (3.0%) | 4 (2.0%) | 2 (1.1%) | 2 (0.9%) | 0.014 |
Trends over time were tested for significance using the Cochran-Armitage test.
P-values in bold are significant at P < 0.05.
APACHE II, Acute Physiology and Chronic Health Evaluation II; ASA, American Society of Anesthesiologists; ICU, intensive care unit; IQR, interquartile range; LoS, length of stay; MELD, Model for End-stage Liver Disease; PHLF, post-hepatectomy liver failure.
Further changes were analysed by stratifying patients into four groups based on the extent of resection (Table3). Over the study period there was a progressive trend towards reduced rates of PHLF in all groups. In particular, the proportion of complex hepatectomies increased (P = 0.034), and rates of PHLF and postoperative liver failure-related mortality declined (P = 0.001 and P = 0.023, respectively). No significant trends could be detected with respect to APACHE II score at postoperative ICU admission, length of ICU stay or median postoperative hospital LoS in any of the groups.
Table 3.
Surgery-related characteristics of patients grouped by type of resection for the study period
| Type of partial resection | Variable | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | P-value |
|---|---|---|---|---|---|---|---|---|
| Segmental resection | Patients, n | 39 | 29 | 42 | 65 | 39 | 51 | 0.125 |
| Cirrhosis, n (%) | 5 (12.8%) | 3 (10.3%) | 7 (16.7%) | 13 (20.0%) | 11 (28.2%) | 14 (27.5%) | 0.015 | |
| Operating time, min, median (IQR) | 209 (158–235) | 198 (163–249) | 181 (152–218) | 179 (141–227) | 212 (166–260) | 171 (135–215) | 0.342 | |
| PHLF, n (%) | 7 (17.9%) | 1 (3.4%) | 2 (4.8%) | 4 (6.2%) | 1 (2.6%) | 1 (2.0%) | 0.007 | |
| APACHE II score, median (IQR) | 14 (9–15) | 10 (7–13) | 12 (9–15) | 13 (10–15) | 11 (8–15) | 11 (8–17) | 0.699 | |
| ICU stay, days, median (IQR) | 1 (1–4) | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.926 | |
| Postoperative LoS, days, median (IQR) | 11 (8–15) | 10 (8–19) | 10 (8–13) | 8 (7–13) | 10 (7–15) | 10 (7–15) | 0.803 | |
| Hospital deaths, n (%) | 4 (10.3%) | 0 | 1 (2.4%) | 2 (3.1%) | 1 (2.6%) | 1 (2.0%) | 0.130 | |
| Liver failure-related death, n (%) | 2 (5.1%) | 0 | 0 | 0 | 0 | 1 (2.0%) | 0.272 | |
| Left hepatectomy | Patients, n | 31 | 34 | 31 | 33 | 19 | 37 | 0.347 |
| Cirrhosis, n (%) | 4 (12.9%) | 5 (14.7%) | 5 (16.1%) | 4 (12.1%) | 4 (21.1%) | 3 (8.1%) | 0.655 | |
| Operating time, min, median (IQR) | 235 (203–318) | 232 (175–294) | 249 (169–294) | 137 (170–270) | 235 (190–260) | 191 (154–237) | 0.007 | |
| PHLF, n (%) | 3 (9.7%) | 5 (14.7%) | 3 (9.7%) | 4 (12.1%) | 0 | 1 (2.7%) | 0.086 | |
| APACHE II score, median (IQR) | 12 (10–15) | 13 (11–16) | 13 (9–17) | 10 (8–15) | 11 (9–14) | 11 (8–18) | 0.312 | |
| ICU stay, days, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–1) | 0.073 | |
| Postoperative LoS, days, median (IQR) | 12 (8–17) | 12 (10–19) | 11 (9–18) | 11 (10–20) | 11 (7–14) | 10 (7–13) | 0.028 | |
| Hospital deaths, n (%) | 1 (3.2%) | 2 (5.9%) | 0 | 2 (6.1%) | 0 | 0 | 0.260 | |
| Liver failure-related death, n (%) | 0 | 1 (2.9%) | 0 | 0 | 0 | 0 | 0.399 | |
| Right hepatectomy | Patients, n | 54 | 61 | 58 | 39 | 48 | 44 | 0.003 |
| Cirrhosis, n (%) | 2 (3.7%) | 7 (11.5%) | 7 (12.1%) | 4 (10.3%) | 5 (10.4%) | 2 (4.5%) | 0.975 | |
| Operating time, min, median (IQR) | 230 (199–261) | 222 (189–259) | 221 (175–287) | 244 (193–283) | 244 (100–293) | 219 (171–269) | 0.295 | |
| PHLF, n (%) | 11 (20.4%) | 14 (23.0%) | 12 (20.7%) | 8 (20.5%) | 6 (12.5%) | 4 (9.1%) | 0.056 | |
| APACHE II score, median (IQR) | 15 (9–19) | 14 (11–17) | 13 (11–17) | 12 (10–16) | 12 (8–16) | 14 (9–19) | 0.338 | |
| ICU stay, days, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–3) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 0.556 | |
| Postoperative LoS, days, median (IQR) | 14 (10–20) | 11 (9–19) | 13 (9–22) | 10 (8–15) | 12 (9–20) | 10 (8–14) | 0.253 | |
| Hospital death, n (%) | 2 (3.7%) | 4 (6.6%) | 3 (5.2%) | 0 | 1 (2.1%) | 3 (6.8%) | 0.841 | |
| Liver failure-related death, n (%) | 1 (1.9%) | 1 (1.6%) | 2 (3.4%) | 0 | 1 (2.1%) | 0 | 0.487 | |
| Complex hepatectomy | Patients, n | 51 | 70 | 67 | 67 | 81 | 80 | 0.034 |
| Cirrhosis, n (%) | 1 (2.0%) | 1 (1.4%) | 4 (6.0%) | 5 (7.5%) | 6 (7.4%) | 5 (6.3%) | 0.177 | |
| Operating time, min, median (IQR) | 346 (255–407) | 340 (283–381) | 317 (266–390) | 330 (272–366) | 348 (282–450) | 313 (228–385) | 0.498 | |
| PHLF, n (%) | 22 (43.1%) | 25 (35.7%) | 27 (40.3%) | 26 (38.8%) | 21 (25.9%) | 13 (16.3%) | <0.001 | |
| APACHE II score, median (IQR) | 13 (11–17) | 13 (9–16) | 15 (10–19) | 13 (9–16) | 12 (9–16) | 12 (10–16) | 0.359 | |
| ICU stay, days, median (IQR) | 4 (2–8) | 1 (1–3) | 3 (1–6) | 1 (1–6) | 1 (1–2) | 1 (1–3) | 0.170 | |
| Postoperative LoS, days, median (IQR) | 20 (14–32) | 24 (15–41) | 21 (14–35) | 21 (14–50) | 23 (16–41) | 17 (11–27) | 0.131 | |
| Hospital death, n (%) a | 5 (9.8%) | 5 (7.1%) | 9 (13.6%)a | 7 (10.4%) | 9 (11.1%) | 6 (7.5%) | 0.896 | |
| Liver failure-related death, n (%) | 4 (7.8%) | 4 (5.7%) | 4 (6.0%)a | 4 (6.0%) | 1 (1.2%) | 1 (1.3%) | 0.023 |
One patient, who received a liver transplant, was excluded.
Trends over time were tested for significance using the Cochran–Armitage test.
P-values in bold are significant at P < 0.05.
APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; IQR, interquartile range; LoS, length of stay; PHLF, post-hepatectomy liver failure.
Effects in the matched patient cohort
Of the 369 patients who underwent hepatic resection in 2006 and 2007 and the 399 patients who underwent hepatic resection during 2010 and 2011, 313 pairs were matched. After propensity score matching, analysis yielded results similar to those observed in all patients, with reduced rates of PHLF and reduced postoperative mortality caused by liver failure (Table4).
Table 4.
Distribution of parameters within the cohorts of patients submitted to elective hepatectomy of one or more segments matched using propensity score matching
| 2006–2007 | 2010–2011 | P-value | |
|---|---|---|---|
| Patients, n | 313 | 313 | |
| Age, years, mean ± SEM | 60 ± 0.72 | 60 ± 0.73 | 0.691 |
| ASA class, n (%) | |||
| I | 7 (2.2%) | 8 (2.6%) | 0.538 |
| II | 140 (44.7%) | 139 (44.4%) | |
| III | 137 (43.8%) | 146 (46.6%) | |
| IV | 29 (9.3%) | 20 (6.4%) | |
| Type of resection, n (%) | |||
| Segmental resection | 53 (16.9%) | 68 (21.7%) | 0.790 |
| Left hepatectomy | 52 (16.6%) | 46 (14.7%) | |
| Right hepatectomy | 97 (31.0%) | 73 (23.3%) | |
| Complex hepatectomy | 111 (35.5% | 126 (40.3% | |
| PHLF, n (%) | 77 (24.6%) | 35 (11.2%) | <0.001 |
| ICU stay, days, mean ± SEM | 5 ± 0.73 | 3 ± 0.56 | 0.001 |
| Postoperative LoS, days, mean ± SEM | 22 ± 1.27 | 18 ± 0.95 | 0.004 |
| Hospital deaths, n (%) | 19 (6.1%) | 16 (5.1%) | 0.735 |
| Liver failure-related death, n (%) | 12 (3.8%) | 3 (1.0%) | 0.035 |
P-values in bold are significant at P < 0.05.
ASA, American Society of Anesthesiologists; ICU, intensive care unit; LoS, length of stay; PHLF, post-hepatectomy liver failure; SEM, standard error of the mean.
Discussion
Over the 6-year study period, despite an increase in the frequency at which complex hepatectomies were performed, reductions in the rates of PHLF and postoperative liver failure-related mortality were observed. The analysis of data for the propensity score-matched cohort suggests the integration of the LiMAx algorithm may have been a major factor contributing to the improved outcomes.
The preoperative identification of candidates in whom liver surgery will be safe remains difficult, particularly in patients with pre-existing hepatic dysfunction.25 As the present authors have previously suggested, the accurate preoperative planning of the intervention using LiMAx, a 13C-based test for the determination of maximal liver function capacity, along with preoperative volume/function analysis, enables surgeons to calculate future remnant liver function. The clinical decision tree presented here might allow surgeons to offer individual and safer treatment strategies.23
The current study shows that complex hepatectomies involving biliary or vascular reconstruction were performed increasingly over the study period. Despite more complex procedures, the decline in rates of PHLF and, in particular, the reduction in the number of postoperative liver failure-related deaths in the overall cohort demonstrate that the proposed system provides a valid estimation of individual operative risk. Similarly, the increase in rates of portal vein embolization, the rise in the proportion of patients with cirrhosis eligible for surgery and the concomitant reduction in rates of PHLF further support improved patient management and optimized preoperative assessment.
In order to correct for the changes in surgical practice over the years, a propensity score-matched analysis was performed to more adequately estimate effects related to the full implementation of the LiMAx algorithm in clinical preoperative work-up by the year 2010. This demonstrated a reduction in postoperative liver failure-related death and PHLF, which suggests that the LiMAx algorithm is of benefit to patients considered for surgery.
A major strength of this study is that the present analysis is based on all consecutive and unselected patients submitted to partial hepatic resection of one or more segments in an attempt to overcome a potential selection bias. Charité Universitätsmedizin Berlin represents one of 437 reference hospitals that continuously report a distinct set of data (e.g. diagnoses, procedures, case-related costs) to the German Institute for Remuneration in Hospitals [Institut für das Entgeltsystem im Krankenhaus (InEK)] in order to facilitate a yearly calculation of revenues of the German hospital system. Thus, the accuracy and validity of the underlying medical controlling data are assured. A downside of the use of such data is that only a distinct set of parameters routinely recorded by the controlling and strategy office in the perioperative work-up were suitable for analysis and distinct clinical parameters (e.g. operative blood loss, time of pedicle clamping) could not be determined in this study.
Several limitations of this analysis should be mentioned. Although the most significant improvements in operative technique were reported around the turn of the millennium, the potential impacts of any effects based on general improvements in surgical technique, anaesthetic care or intensive care nursing cannot be excluded. However, hepatectomy was performed following a common surgical approach (Appendix S1, online) and the vast majority of surgical procedures (70.8%) were performed by three experienced liver surgeons. Thus it would seem that any bias arising from the use of different surgical techniques is unlikely. It is the authors’ opinion that the improved outcomes are likely to be associated with the integration of the LiMAx algorithm in routine work-up. Unfortunately, data on the number of patients to whom surgery was denied based on actual LiMAx data were not available. Hence, the current study provides only a low level of evidence for the diagnostic accuracy of LiMAx as a screening tool. Randomized controlled trials would be beneficial to more clearly study the implications for patient management of using a preoperative clinical decision tree, such as that proposed, but these are difficult to perform.
In conclusion, the integration of the LiMAx algorithm seems to have played an important role in optimizing risk assessment prior to hepatic surgery.
Acknowledgments
The authors gratefully acknowledge the support of the staff of the Charité Medical Controlling and Strategy Office in the provision of data. The authors would also like to thank the staff of the Institute for Biostatistics and Clinical Epidemiology, Charité Universitätsmedizin Berlin for assistance with statistical analyses.
Conflicts of interest
MS is the inventor of the LiMAx test and has a capital interest in Humedics GmbH, Berlin, Germany, the company marketing the LiMAx test. MJ received a research grant in the context of the d-LIVER European Commission Framework Programme.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Standard operative and perioperative procedures.
References
- Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. doi: 10.1097/00000658-199610000-00009. discussion 520–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- Baer HU, Stain SC, Dennison AR, Eggers B, Blumgart LH. Improvements in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg. 1993;217:20–27. doi: 10.1097/00000658-199301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–4299. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PD. Clinical applications of 13CO2 measurements. Fed Proc. 1982;41:2698–2701. [PubMed] [Google Scholar]
- Armuzzi A, Candelli M, Zocco MA, Andreoli A, De Lorenzo A, Nista EC, et al. Review article: breath testing for human liver function assessment. Aliment Pharmacol Ther. 2002;16:1977–1996. doi: 10.1046/j.1365-2036.2002.01374.x. [DOI] [PubMed] [Google Scholar]
- Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- Stremmel W, Wojdat R, Groteguth R, Zoedler M, Ebener T, Niederau C, et al. Liver function tests in a clinical comparison. Z Gastroenterol. 1992;30:784–790. [PubMed] [Google Scholar]
- Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119–125. doi: 10.1097/SLA.0b013e3181ad85b5. [DOI] [PubMed] [Google Scholar]
- Lock JF, Malinowski M, Seehofer D, Hoppe S, Rohl RI, Niehues SM, et al. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg. 2012;397:1297–1304. doi: 10.1007/s00423-012-0972-2. [DOI] [PubMed] [Google Scholar]
- Jara M, Bednarsch J, Valle E, Lock JF, Malinowski M, Schulz A, et al. Reliable assessment of liver function using LiMAx. J Surg Res. 2015;193:184–189. doi: 10.1016/j.jss.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Jara M, Luttgert K, Orr J, Lock JF, Schott E, et al. Enzymatic liver function capacity correlates with disease severity of patients with liver cirrhosis: a study with the LiMAx test. Dig Dis Sci. 2014;59:2983–2991. doi: 10.1007/s10620-014-3250-z. [DOI] [PubMed] [Google Scholar]
- Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB. 2010;12:139–146. doi: 10.1111/j.1477-2574.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Wanless IR, Sweeney G, Dhillon AP, Guido M, Piga A, Galanello R, et al. Lack of progressive hepatic fibrosis during long-term therapy with deferiprone in subjects with transfusion-dependent beta-thalassemia. Blood. 2002;100:1566–1569. doi: 10.1182/blood-2002-01-0306. [DOI] [PubMed] [Google Scholar]
- Kumar M, Sakhuja P, Kumar A, Manglik N, Choudhury A, Hissar S, et al. Histological subclassification of cirrhosis based on histological–haemodynamic correlation. Aliment Pharmacol Ther. 2008;27:771–779. doi: 10.1111/j.1365-2036.2008.03653.x. [DOI] [PubMed] [Google Scholar]
- Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological–hemodynamic correlation in cirrhosis – a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111–117. doi: 10.1016/j.jhep.2005.07.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Standard operative and perioperative procedures.