Abstract
A key feature of many eukaryotic cells, most prominently seen in developing neurons, is their ability to form and extend membrane protrusions. How protrusion formation is linked to exocytic membrane trafficking is largely unclear. In a recent paper published in Nature, Raiborg et al identify a crucial role in this process for dynamic membrane contact sites (MCSs) between the ER and endosomes. The MCSs are formed by endoplasmic reticulum (ER)-localized protein protrudin and the late endosomal kinesin adaptor FYCO1 and the small GTPase Rab7.
See also: C Raiborg et al (April 2015)
The ER forms a network of membrane tubules that extend through the entire cytoplasmic space. Whereas the rough ER defines sites of protein translation, the smooth ER mediates lipid biosynthesis and storage. ER tubules form contacts to a number of other organelles including mitochondria, the plasma membrane and endosomes. Such membrane contact sites (MCSs) are well known to regulate a diversity of cellular functions, including signalling and motor protein-dependent transport of organelles. In addition, MCSs serve as platforms for lipid exchange and possibly membrane growth. Yet, the precise architecture, function and regulation of individual MCSs remain poorly understood.
In a recent issue of Nature, Stenmark and colleagues (Raiborg et al, 2015) unravel the role of protrudin, a protein previously implicated in neurite outgrowth, in the establishment of contact sites between the ER and late endosomes (LEs). The study was prompted by the unusual domain organization of protrudin: the protein resides in the ER, but harbours a FYVE domain known to promote association with phosphatidylinositol 3-phosphate [PI(3)P], a lipid species usually found at the cytoplasmic surface of endosomes. The authors find that the FYVE domain of protrudin enables its association with endosomes, and the establishment of MCSs. Contact site formation also requires the binding of the low-complexity region of protrudin to active Rab7, a small GTPase recruited to LEs during endosomal maturation. Overexpression of both protrudin and a constitutively active Rab7 mutant triggered a massive reorganization of LEs that became extensively enwrapped by ER.
LAMP1- and Rab7-positive LEs normally accumulate in the perinuclear region. During their experiments, the authors noted that overexpression of protrudin caused the dispersion of LEs towards the cell periphery. This raised the question how protrudin achieves transport of LEs. A candidate factor to mediate protrudin-dependent transport of LEs is FYCO1, another Rab7 effector and PI(3)P-binding protein known to promote plus-end-directed vesicular transport along microtubules by recruiting the light chain of kinesin-1 (Pankiv et al, 2010). Interestingly, FYCO1 forms a complex with protrudin, and both proteins colocalize on ER-engulfed LEs. Further knockdown and rescue experiments established that FYCO1, protrudin and kinesin indeed are all required for LE translocation to the cell periphery (Fig1).
Figure 1. Model for the functions of protrudin and FYCO1 in LE translocation and neurite outgrowth.
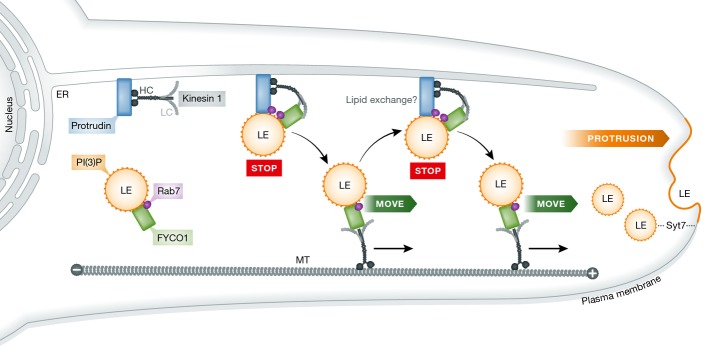
ER-localized protrudin forms contact sites with late endosomes (LEs) by coincident detection of Rab7 and PI(3)P (yellow) stopping LE movement (STOP). Kinesin-1 bound to protrudin is handed over to the LE protein FYCO1, which mediates plus-end (+)-directed LE movement (MOVE) along microtubules (MT) to the periphery. Synaptotagmin 7 (SYT7)-mediated LE fusion with the plasma membrane enables protrusion formation and neurite outgrowth.
To monitor the behaviour of protrudin-containing ER and individual FYCO1-positive LEs directly, Raiborg et al used live cell imaging and observed that the ER repeatedly associates with LEs. Furthermore, when protrudin meets endosomes decorated by FYCO1, these endosomes display slow random motility, but show enhanced plus-end-directed movement once FYCO1 is released from protrudin. Thus, MCSs formed between the ER and endosomes pause the net movement of LEs to the cell periphery. Protrudin has previously been shown to associate with kinesin-1 heavy chain (Matsuzaki et al, 2011). Could protrudin, thus, facilitate motor loading onto FYCO1-engaged LEs? Affinity purification experiments showed that overexpression of protrudin enhances the association of kinesin-1 with FYCO1, whereas depletion of protrudin prevents FYCO1–kinesin association. Hence, protrudin may promote the movement of endosomes by loading kinesin-1 onto FYCO1-positive LEs when ER–endosome contact sites are formed, and this allows for rapid movement of LEs towards the cell periphery as soon as the protrudin–FYCO1 complex is disassembled.
What is the physiological function of the protrudin–FYCO1 complex? Protrudin, as the name suggests, has previously been shown to promote the formation of cellular protrusions (Shirane & Nakayama, 2006). Accordingly, the authors find protrudin to induce epithelial cell protrusions and to facilitate extension of neurites containing FYCO1-positive LEs at their tips in neuroendocrine PC12 cells. Similar to protrudin-mediated peripheral dispersion of LEs, neurite outgrowth depends on complex formation between protrudin and FYCO1 as well as their ability to associate with kinesin (Fig1). It also requires the calcium sensor synaptotagmin 7 (SYT7), which mediates fusion of LEs with the plasma membrane in agreement with previous data (Arantes & Andrews, 2006).
Taken together, the study by Raiborg et al reveals an unanticipated role of ER–LE contacts in the formation of membrane protrusions and in neurite outgrowth, a process of pivotal importance not only for development but also for neuronal regeneration after axonal injury (Bradke et al, 2012). The study also raises a number of interesting questions. For instance, what exactly drives handover of kinesin from protrudin to FYCO1 at ER–LE contacts? Moreover, MCSs are known to serve as platforms for lipid exchange (Rowland & Voeltz, 2012), and such exchange is of importance for membrane expansion, that is during neurite outgrowth as recently shown for ER–plasma membrane contacts in primary neurons (Petkovic et al, 2014). Stenmark and colleagues observed that the outward movement of LEs is stopped as soon as protrudin and FYCO1 meet. It is thus conceivable that—besides their role in recruiting kinesin-1 motors—this halt may allow for the recruitment of lipid transfer proteins, for example cholesterol-binding proteins. Such a mechanism could aid cholesterol efflux from LEs/lysosomes, which occurs during LE maturation. The fundamental importance of this process becomes apparent in lysosomal storage diseases, which develop when cholesterol transfer is impaired (van der Kant & Neefjes, 2014). In line with this possibility, low cholesterol content of LEs/lysosomes facilitates the association of plus-end-directed motor proteins (Rocha et al, 2009), suggesting that positioning of LEs/lysosomes is correlated with their maturation stage. Interestingly, lysosomal positioning has also been shown to control mTOR activity and autophagy (Korolchuk et al, 2011). Whether the mechanism elucidated by Raiborg et al is relevant for this process remains open.
The fusion of lysosomes with the plasma membrane has not only been implicated in neurite outgrowth, but also underlies plasma membrane repair, and the release of lytic granule contents by cytotoxic T lymphocytes (Luzio et al, 2007). The Ca2+-sensor SYT7 has been implicated in all of these fusion events. Therefore, it will be of interest to investigate whether protrudin- and FYCO1-dependent MCSs regulate these processes as well.
Lastly, as the ER makes extensive MCSs with a variety of organelles, that is mitochondria (Rowland & Voeltz, 2012) or other types of endosomes (Rowland et al, 2014), it will be interesting to see how precisely the ER regulates the function of these organelles.
References
- Arantes RM, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26:4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- van der Kant R, Neefjes J. Small regulators, major consequences - Ca(2)(+) and cholesterol at the endosome-ER interface. J Cell Sci. 2014;127:929–938. doi: 10.1242/jcs.137539. [DOI] [PubMed] [Google Scholar]
- Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, O'Kane CJ, Deretic V, Rubinsztein DC. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Matsuzaki F, Shirane M, Matsumoto M, Nakayama KI. Protrudin serves as an adaptor molecule that connects KIF5 and its cargoes in vesicular transport during process formation. Mol Biol Cell. 2011;22:4602–4620. doi: 10.1091/mbc.E11-01-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjorkoy G, Johansen T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic M, Jemaiel A, Daste F, Specht CG, Izeddin I, Vorkel D, Verbavatz JM, Darzacq X, Triller A, Pfenninger KH, Tareste D, Jackson CL, Galli T. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat Cell Biol. 2014;16:434–444. doi: 10.1038/ncb2937. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, VBucci C, Brech A, Johansen T, Stenmark H. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. [DOI] [PubMed] [Google Scholar]
- Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. ER contact sites define the position and timing of endosome fission. Cell. 2014;159:1027–1041. doi: 10.1016/j.cell.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane M, Nakayama KI. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314:818–821. doi: 10.1126/science.1134027. [DOI] [PubMed] [Google Scholar]


