Abstract
Background
Existing therapies for recurrent or refractory histiocytoses, including Langerhans cell histiocytosis (LCH), juvenile xanthogranuloma (JXG), and Rosai-Dorfman disease (RDD), have limited effectiveness. We report our experience with using clofarabine as therapy in children with recurrent or refractory histiocytic disorders, including LCH (11 patients), systemic JXG (4 patients), and RDD (3 patients).
Methods
Patients treated with clofarabine for LCH, JXG, or RDD by Texas Children’s Hospital physicians or collaborators between May 2011 and January 2013 were reviewed for response and toxicity.
Results
Patients were treated with a median of 3 chemotherapeutic regimens prior to clofarabine. Clofarabine was typically administered at 25 mg/m2/day for five days. Cycles were administered every 28 days for a median of six cycles (range: 2–8 cycles). Seventeen of eighteen patients are alive. All surviving patients showed demonstrable improvement after 2–4 cycles of therapy, with eleven (61%) complete responses, four (22%) partial responses, and two patients still receiving therapy. Five patients experienced disease recurrence, but three of these subsequently achieved complete remission. All patients with JXG and RDD had complete or partial response at conclusion of therapy. Side effects included neutropenia in all patients. Recurring but sporadic toxicities included prolonged neutropenia, severe vomiting, and bacterial infections.
Conclusion
Clofarabine has activity against LCH, JXG, and RDD in heavily pretreated patients, but prospective multi-center trials are warranted to determine long-term efficacy, optimal dosing, and late toxicity of clofarabine in this population.
Keywords: histiocytosis, Langerhans cell histiocytosis, juvenile xanthogranuloma, clofarabine, Rosai-Dorfman disease
Introduction
The histiocytic disorders are a heterogeneous group of proliferative neoplasms originating from monocyte and macrophage lineages. The most common of these, Langerhans cell histiocytosis (LCH), is characterized by heterogeneous lesions containing pathologic dendritic cells that express CD207 (Langerin), along with mixed inflammatory infiltrate [1]. LCH lesions primarily involve bone, skin, lymph nodes, lungs, liver, thymus, spleen, bone marrow, and/or the central nervous system (CNS) [2,3].
Refractory and recurrent multi-system LCH poses a significant therapeutic challenge. Patients with risk-organ (liver, spleen, or bone marrow) disease have estimated five year overall survival of approximately 85 percent but often require intensive therapy, including bone marrow and/or solid organ transplantation [4–6]. Responses to regimens including cladribine (2-chlorodeoxyadenosine, or 2-CdA), cytarabine, or combinations have been reported, though durable responses are not consistent [7–11]. More intensive therapeutic regimens such as combined cladribine/cytarabine are also associated with substantial acute toxicity and delayed immune reconstitution [9,12].
Additionally, no standard treatment exists for patients with high-risk manifestations of other more rare histiocytic disorders, including juvenile xanthogranuloma (JXG) and Rosai-Dorfman disease (RDD; also known as sinus histiocytosis with massive lymphadenopathy). Patients with systemic and/or central nervous system JXG have been successfully treated with vinca alkaloid- and steroid-containing regimens. However, significant morbidity and death have been reported in patients with disseminated systemic or central nervous system disease despite therapeutic intervention [13]. RDD is generally self-limited but may require therapy when significant morbidity or life-threatening complications, such as airway obstruction or organ compromise, occur. No clinical trials have been conducted for therapy in RDD, but patients have been treated with methotrexate and 6-mercaptopurine, or with cladribine [14–16].
Clofarabine has been suggested as a potential agent for treating refractory or recurrent LCH [17]. Clofarabine is a second-generation purine analog, similar in structure to cladribine and fludarabine, except that its biochemical modifications enhance its resistance to deamination and degradation. It is converted in vivo to its active form, clofarabine 5’-triphosphate, which subsequently inhibits DNA polymerase and ribonucleotide reductase [18]. Though only a small percentage of the drug penetrates the blood-brain barrier, cytotoxic concentrations of the drug are achieved in cerebrospinal fluid [19]. It is approved for use in the United States for treatment of relapsed acute lymphoblastic leukemia in children, and it is being tested in multiple clinical trials of hematologic malignancies [20,21].
Clofarabine has been reported to have some effectiveness in treating recurrent risk-organ LCH [17,22]. Here, we conduct a retrospective review of the use of clofarabine in recurrent or refractory risk-organ LCH, as well as non-risk-organ LCH, JXG and RDD.
Methods
Patients
Patient charts were reviewed in accordance with IRB-approved protocols at Baylor College of Medicine. All patients treated at Texas Children’s Hospital from May 2011 to January 2013 with this regimen were reviewed. Additionally, all patients known to the Texas Children’s Hospital group from collaborating institutions who were treated with clofarabine were offered study enrollment. Patients treated at other institutions were consented by Baylor College of Medicine researchers for chart review and for release of information.
Statistics
Disease response was assessed and reported using the categories established in the Histiocyte Society Evaluation and Treatment Guidelines [23]: Better/complete resolution, non-active disease (NAD); Better/regression, active disease (AD) better; Intermediate/mixed, new lesions in one site, regression in another site; Intermediate/stable, unchanged disease; or worse, progressive disease. Progression free survival was calculated using the log-rank (Mantel-Cox) test; data were censored at time of last follow-up. Progression was defined as disease classifiable as intermediate or worse response after administration of clofarabine therapy. Data analyses and Kaplan-Meier curves were conducted using Prism version 5.0d (GraphPad, La Jolla, California).
Results
Patients
Patients were diagnosed with LCH (n=11), JXG (n=4), or RDD (n=3) (Table I). Patients had a median age of 22 months at diagnosis and were treated with a median of 3 separate treatment regimens prior to initiation of therapy with clofarabine. Clofarabine was started in patients with persistent disease or new lesions. Common therapies given prior to clofarabine included vinblastine/prednisone, cladribine, and cytarabine (Table I). One patient received pretreatment with combined high-dose cladribine and cytarabine [9]. One infant patient with congenital central nervous system JXG started clofarabine as initial therapy.
TABLE I.
Patient Characteristics
| Number of patients 18 | Disease location | |||
| Male | 8 | LCH (N = 11) | ||
| Female | 10 | Multiple site | 11 | |
| Single organ system | 4 | |||
| Diagnosis | Multiple organ system | 7 | ||
| LCH | 11 | CNS or CNS risk | 5 | |
| JXG | 4 | Risk organ involvement | 3 | |
| RDD | 3 | Liver | 1 | |
| Spleen | 2 | |||
| Age at diagnosis | Bone marrow | 2 | ||
| Median | 22 months | Lung without risk organ | 2 | |
| Range | 2 months–11 years | JXG (N = 4) | ||
| Multiple site | 4 | |||
| Age at start of clofarabine | Single organ system | 1 | ||
| Median | 3 years 2 months | Multiple organ system | 3 | |
| Range | 2 months-18 years | CNS disease | 3 | |
| RDD (n = 3) | ||||
| Time from diagnosis to clofarabine start | Multiple organ system | 2 | ||
| Median | 14 months | Orbital | 2 | |
| Range | 0 months–10 years | |||
| Pretreatment regimen | ||||
| Number of treatment regimens prior to clofarabine | Vinblastine +/− prednisone | 14 | ||
| Cladribine | 13 | |||
| Median | 3 | Cytarabine +/− vincristine | 11 | |
| Range | 0–8 | Cladribine + Cytarabine | 1 | |
| Time to last follow up | ||||
| Median | 10.5 months | |||
| Range | 4–24 months | |||
Clofarabine was started in fifteen of eighteen (83%) patients at a dose of 25 mg/m2/day for 5 days as published previously [17]. Two patients started at a dose of 50 mg/m2/day, given severe burden of disease and poor response to previous chemotherapy. One patient, a two-month-old infant, was administered weight-based dosing of clofarabine (0.8 mg/kg/day). Cycles of therapy were administered every 28 days, unless delayed by toxicity, and all patients received at least two cycles. Patients had repeat imaging of their lesions after two cycles and (in most cases) every two cycles thereafter. Intended duration of therapy was six cycles for most patients, except for those patients who did not experience a complete response at this time point. All patients had multifocal disease (Table I).
With median follow up of 10.5 months after initiation of clofarabine, 15/18 patients demonstrated improved disease after two cycles of therapy, with one patient having a complete response at this time point (Table II). All patients with JXG and RDD demonstrated improvement at this time point. Three patients with LCH demonstrated mixed response (some lesions better, some lesions worse). One such patient had response of existing lesions but developed new marrow disease, and alternate therapy was pursued. Clofarabine was continued, and subsequent disease improvement seen, in the remaining seventeen patients. Eleven patients (61%) experienced complete response with a total of 2–8 cycles (median 6 cycles). Four patients (22%) achieved better/regression response but did not achieve NAD. In two of these four patients, subsequent disease progression occurred after clofarabine was discontinued, while two patients have maintained good disease control with continued low-dose therapy (25 mg/m2/day × 2 days/month). Two additional patients, with demonstrated regression, are still receiving primary therapy.
TABLE II.
Patient Descriptions
| Patient # | Diagnosis | Age at diagnosis (years, months) |
Age at clofarabine initiation (years, months) |
Gender | Sites of disease | Previous therapies | Clofarabine dosec |
Total number of cycles |
Response after 2 cycles |
Response after completion of therapy |
Progression or disease recurrence? (time from start of therapy to relapse) |
Months from start of therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LCH | 0 y 5 m | 1 y 1 m | M | Skin Bone marrow Skull Spleen |
VBL/pred; 2-CdA |
25 × 2 cycles; 20 × 2 cyclesd |
4 | Intermed./ mixed |
Better/ regression (ongoing) |
No | 5 |
| 2 | LCH | 0 y 6 m | 1 y 7 m | M | Skull (multifocal) C1 Otic |
VBL/pred; VCR/AraC/pred; Etoposide |
25 | 6 | Better/ regression |
NAD | No | 23 |
| 3 | LCH | 0 y 7 m | 2 y 7 m | M | Pelvic bone (multifocal) Skin |
VBL/pred +/− PO MTX; VBL/IV MTX; VCR/AraC; 2-CdA |
25 | 3 | Better/ regression |
NAD | No | 14 |
| 4 | LCH | 0 y 8 m | 2 y 1 m | F | Mastoid, sphenoid, temporal bones |
VBL/pred; 2-CdA; VCR/AraC |
25 | 6 | Better/ regression |
NAD | No | 24 |
| 5 | LCH | 1 y 2 m | 2 y 1 m | F | Liver/spleen Lymph node Skin Bone (multifocal) Lung |
VBL/pred; VCR/AraC |
25 × 3 cycles; 50 × 3 cycles |
6 | Intermed./ Mixed |
Better/ regression |
Yes (11 months) |
15 |
| 6 | LCH | 2 y 0 m | 2 y 10 m | F | Skull (multifocal) Lungs Thymus |
VBL/pred; MTX/6MP/pred; 2-CdA; VCR/AraC |
25 | 8 | Better/ regression |
NAD | No | 17 |
| 7 | LCH | 2 y 4 m | 3 y 6 m | F | Skull: frontal, parietal |
VBL/pred; AraC |
25 × 1 cycle; 19 × 5 cycles |
6 | Better/ regression |
NAD | No | 10 |
| 8 | LCH | 3 y 5 m | 6 y 4 m | M | Bone (multifocal; pelvic) |
Surgical curettage; VBL/pred; 2-CdA; VCR/AraC |
25 | 8 | Better/ regression |
NAD | No | 20 |
| 9 | LCH | 6 y 3 m | 7 y 1 m | F | Multifocal bone | VBL/pred; 2-CdA |
25e | 4 | NAD | NAD | No | 6 |
| 10 | LCH | 16 y 5 m | 17 y 9 m | M | Lung Pituitary |
Ara-C | 25 | 2 | Better/ regression |
Better/ regression (ongoing) |
No | 4 |
| 11 | LCH/JXGa | 0 y 8 m | 3 y 7 m | F | Bone (multifocal) Bone marrow Otic Skull Skin Perineum |
Oral MTX; VBL/pred/MTX; VCR/AraC; Thalidomide; 2-CdA/AraC |
25 | 2 | Intermed./ Mixed |
Intermed./ Mixed |
Yes† (2 months) |
2 (died) |
| 12 | JXG | 0 y 2 m | 0 y 2 m | M | Brain (CNS tumor with obstructive hydrocephalus) |
None | 0.8 mg/kg/day × 5 days |
6 | Better/ regression |
Better/ regressionb |
No | 11 |
| 13 | JXG | 1 y 3 m | 1 y 10 m | M | Brain, Lungs Kidneys Bone (multifocal) Skin |
VBL/pred; 2-CdA |
25 | 8 | Better/ regression |
Better/ regressionb |
No | 15 |
| 14 | JXG | 1 y 7 m | 1 y 9 m | F | Liver Spleen Kidneys Bone marrow Skin |
2-CdA | 50 × 1 cycle; 25 × 5 cycles |
6 | Better/ regression |
NAD | Yes (7 months) |
15 |
| 15 | JXG | 3 y 6 m | 5 y 10 m | F | CNS (pituitary, pons) Skin |
Ara-C; VBL/Ara-C/MTX; 2-CdA |
50 × 3 cycles | 3 | Better/ regression |
NAD | No | 8 |
| 16 | RDD | 11 y 2 m | 18 y 6 m | F | Orbital (with propotosis) Bone marrow Pterygopalatine fossa |
Pred/MTX/6MP; 2-CdA; AraC |
25 | 6 | Better/ regression |
NAD | No | 17 |
| 17 | RDD | 11 y 6 m | 14 y | M | Bone (multifocal) Otic Lymph nodes |
VBL/MTX/ 6MP/pred; Curettage/XRT; imatinib, sorafenib; 2-CdA |
25 (5 days/ cycle in cycles 1–4, 4 days/cycle in cycles 5– 6) |
6 | Better/ regression |
NAD | Yes (11 months) |
15 |
| 18 | RDD | 8 y 0 m | 18 y 0 m | F | Orbital (with proptosis) |
Prednisone; VBL/MTX; CSA/pred; VBL/celecoxib; XRT; MMF/rituximab; 2-CdA; Dasatinib |
25 | 4 | Better/ regression |
Better/ regression |
Yes | 7 |
VBL, vinblastine; pred, prednisone; VCR, vincristine; AraC, cytarabine; 2-CdA, cladribine; MTX, methotrexate; IV, intravenous; 6MP, 6-mercaptopurine; CSA, cyclosporine; XRT: radiation therapy; MMF: mycophenolate mofetil; Intermed: Intermediate.
Patient was initially diagnosed with LCH but had change in histology to comorbid LCH/JXG after clofarabine therapy.
Patient receiving ongoing subsequent lower-dose clofarabine maintenance therapy for improved but residual disease.
mg/m2/day × 5 days/cycle, unless specified.
Patient had intermediate/mixed response after 2 cycles, then received cytarabine 75 mg/m2/day × 5 days in addition to clofarabine with subsequent improvement.
Patient received 5 days/cycle of clofarabine therapy in cycles 1–2, 2 days/cycle in cycles 3–4 due to prolonged neutropenia.
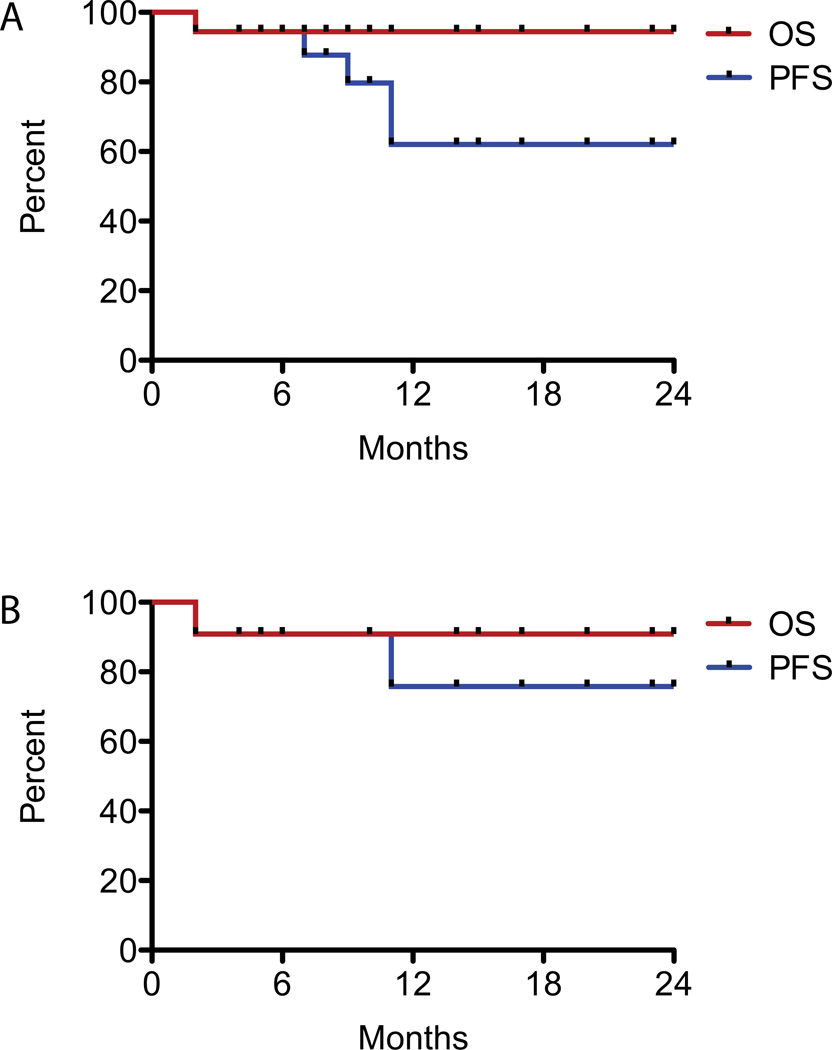
To date, five of eighteen patients (2 LCH, 1 JXG, 2 RDD) have experienced disease progression or recurrence after clofarabine therapy. One patient progressed on therapy; the other four recurred after therapy cessation. Estimated one year progression free survival is 62% and overall survival is 94% (Figure 1A). Three patients achieved subsequent complete disease response after recurring post clofarabine: two patients (#5, #14) experienced complete remission upon reintroduction of clofarabine (in one case in combination with cytarabine), and a patient with Rosai-Dorfman disease (#17) remains disease free after curettage of new, isolated bone lesions.
Figure 1.
Kaplan-Meier curves of estimated progression-free survival (PFS, in blue) and overall survival (OS, in red) of patients treated with clofarabine, from time of clofarabine initiation, in A) all histiocytic disorders and B) LCH. Patients were censored at progression/death or time of last follow up.
LCH
Eleven LCH patients were treated and three had risk-organ involvement. Of the eight patients without risk-organ disease, two had lung plus other sites, four patients had CNS or CNS-risk lesions, and two patients had multifocal disease limited to bone. Overall, eight patients with LCH (73%) experienced disease improvement after two cycles of therapy; the other three patients had mixed response but two of these had improvement of all lesions with continued therapy. Seven patients have maintained a complete response after completion of therapy. Two patients experienced progressive disease, and two patients are still receiving therapy. Estimated one-year PFS for the LCH cohort is 76% and OS is 91% (Figure 1B).
One patient with risk-organ disease (#1) demonstrated regression of all lesions on therapy after four cycles of clofarabine therapy; two patients with risk-organ disease recurred. Patient #11 presented with rash at 4 months of life, and was diagnosed 4 months later with LCH. Over the next three years, she developed severely exudative, denuded scalp and profound urethritis requiring bladder catheterization. At age 3 years she developed rapidly progressive disease with new orbital and skull lesions, cervical lymphadenopathy, and bone marrow involvement of LCH. She had intermediate/stable disease after six months of cladribine and cytarabine and was switched to clofarabine. She received two cycles of therapy (Table II) complicated by chemotherapy-associated fever, pancytopenia requiring transfusion support, Bacillus bacteremia, and Micrococcus bacteremia. She had improvement of all lesions but developed new leg pain and lesions in her bilateral iliac crests, as well as hypermetabolic state with rapid weight loss. Bone marrow biopsy after two cycles of clofarabine showed pathology consistent with concomitant LCH (CD1a+, CD207+ histiocytes) and JXG (population of CD68+, CD1a−, S100− cells). She developed hemophagocytic syndrome and started etoposide and dexamethasone therapy. Over the next two months she had continued disease progression and died.
The other recurrence occurred in a patient (#5) with risk-organ involvement and substantial lymphadenopathy. After failing vinblastine/prednisone and cytarabine/vincristine therapy, she received clofarabine at standard dose for three cycles with mixed response. She achieved disease regression with three monthly cycles of clofarabine at 50 mg/m2/day for five days and continued on twice-monthly clofarabine. She developed skin, lymph node, hepatosplenic, and otic recurrence eleven months after starting treatment, but subsequently achieved complete remission with two cycles of clofarabine 25 mg/m2/day plus cytarabine 500 mg/m2/day for 5 days/cycle.
JXG
Four patients (#12–15 on this study) were diagnosed with JXG. Three patients presented with central nervous system disease, and one presented with high-risk systemic disease. All patients experienced significant clinical improvement following treatment with clofarabine.
Patient #12 presented at birth with congenital hydrocephalus without mass lesion detected on MRI at that time. He had a seizure at two months of life, and MRI showed 2.3 × 2.1 × 2.3 cm enhancing mass (Figure 2A) in the right dorsolateral midbrain. Brain biopsy was consistent with JXG. Several other additional CNS nodules were detected radiographically. The patient was started on clofarabine as front-line therapy, dose modified for weight-based dosing (0.8 mg/kg/day×5 days) due to the child being an infant. After two cycles of therapy his tumors regressed (Figure 2B). While neutropenic, he experienced a pseudomeningocele infection with methicillin-sensitive Staphylococcus aureus, which resolved with intravenous antibiotic therapy. After six cycles of therapy, his cerebellar lesions continued to regress, though he had persistent lesions in the brainstem. He received clofarabine at lower cumulative dose (25 mg/m2/day × 2 days/month) for another 6 months, with continued regression of the brainstem lesions.
Figure 2.
Brain MRI axial T1 post-contrast images of patients with JXG. A,B: 2-month-old patient (#12) with central nervous system JXG. A) prior to clofarabine. B) post two cycles of clofarabine. Minimal residual tumor was present. C–E: 1-year-old patient (#13) treated with clofarabine for multisystem JXG, including CNS involvement. C) pretreatment image. D) persistent disease after therapy with vinblastine/prednisone and cladribine. E) near resolution of disease after 6 cycles of clofarabine.
Patient #13 presented at 15 months of age with episodes of perioral cyanosis and near-syncope, as well as numerous annular, erythematous, hyperpigmented skin lesions 2 cm in diameter. Skin biopsy confirmed the diagnosis of JXG. MRI brain demonstrated numerous enhancing parenchymal masses with low T2 signal and significant diffusion restriction (Figure 2C), consistent with CNS involvement by JXG. Twelve-week courses of vinblastine/prednisone (according to LCH-III) and later cladribine demonstrated minimal response (Figure 2D), so the patient was switched to clofarabine 25 mg/m2/day for 5 days/cycle. After eight cycles of therapy he showed resolution of most CNS lesions (Figure 2E), and better/regression response of skin and lung disease. He received lower-dose therapy (clofarabine 25 mg/m2/day × 2 days/cycle) for 2 cycles with continued regression of lung lesions, then with no progression off-therapy for 5 months.
Patient 14 presented with a two-month history of rash followed by fever, night sweats, weight loss, progressive lethargy, lymphadenopathy, organomegaly, anemia, and thrombocytopenia. Bone marrow biopsy showed infiltration by fascin+, Factor XIIIa+ histiocytes consistent with JXG [24]. Computerized tomography (CT) imaging studies demonstrated infiltrative disease in the liver, spleen, and kidneys. She received two cycles of cladribine (5 mg/m2 daily for 5 days). While receiving cladribine, she developed progressive, massive cervical lymphadenopathy, confirmed to be JXG on lymph node biopsy, with persistent bone marrow and worsening liver disease. Given the rapidly progressive nature of her disease, she was started on clofarabine 50 mg/m2/day, in combination with cytarabine 1 g/m2/day, for 5 days/cycle [25]. Over the next several weeks her cervical lymphadenopathy and organomegaly resolved. She experienced prolonged neutropenia after this cycle, later developing Serratia marcescens bacteremia and a perirectal phlegmon with myositis and rectal fistula. After marrow recovery and resolution of infection, she received clofarabine at 25 mg/m2/day × 5 days/cycle for five additional cycles with complete disease resolution and no further serious toxicity. She had isolated cutaneous recurrence 7 months off therapy, which resolved with one course of clofarabine at lower dose (10 mg/m2/day × 2 days), and is now disease free.
Patient #15 presented with skin rash and ocular mass. She received two courses of cytarabine with no response, followed by vinblastine, cytarabine, and methotrexate for 3 months with enlargement of pituitary mass. She experienced better/regression response with cladribine therapy for 9 months, with reduction in volume of ocular mass by 70%. While on therapy, however, she developed a new pontine lesion. She was started on clofarabine at 25 mg/m2/day × 5 days/cycle with resolution of pontine lesions after 3 cycles of therapy. The patient had no active disease eight months post initiation of clofarabine.
RDD
Three patients with Rosai Dorfman disease were treated with clofarabine. The first patient (#16) presented at 11 years of age with a three-month history of proptosis; subsequent imaging demonstrated an infiltrative neoplasm of the bilateral orbits with adjacent decreased T1-weighted bone marrow signal (Figure 3A). Orbital biopsy confirmed the diagnosis of Rosai-Dorfman disease. Numerous chemotherapy treatments given over a seven-year span (Table II) led to minimal regression. After receiving no therapy for 18 months, the patient developed worsening headaches, and progressive disease was demonstrated by MRI. She was started on clofarabine 25 mg/m2/day × 5 days/cycle. After six cycles of therapy, she had complete resolution of her disease (Figure 3B), with preservation of normal muscular architecture. She remained disease and symptom free seventeen months from start of clofarabine.
Figure 3.
MRI T2 orbital images of 18-year-old patient (#16) with orbital Rosai-Dorfman disease. A) orbital disease with proptosis evident despite multiple treatment courses. Normal orbital musculature not visualized. B) Complete resolution of orbital disease and proptosis, with restoration of normal orbital muscular architecture.
A second patient (#17) presented at age 11 with a nine-month history of left foot pain and a lesion in his left talus, which was confirmed as RDD on biopsy. He had stable disease with vinblastine-based therapy and radiation. He developed a new nodule of the external ear canal eleven months after diagnosis, at which time cladribine was started. He had no active disease after six months of cladribine therapy. Approximately one year after completing cladribine, he developed increasing foot pain and left navicular erosion, becoming nonambulatory. He also developed new femur lesions, otitis externa, and new lymphadenopathy. He started clofarabine therapy with complete disease response after six cycles and resumed normal ambulation. Routine post-therapy MRI screening demonstrated new navicular lesions, which were biopsy-confirmed recurrence of Rosai-Dorfman disease. He underwent subsequent curettage and bone grafting with resolution of symptoms.
A third patient (#18) presented at age 8 with bilateral proptosis and orbital masses. Initial biopsies were misdiagnosed as idiopathic orbital inflammation. The patient had no response to multiple chemotherapeutic agents or radiotherapy (Table II). After three years of observation, she had radiographic and clinical progression with decreased vision in the left eye. She was treated with mycophenolate before undergoing bilateral orbitotomy and debulking, wherein the diagnosis of Rosai Dorfman disease was made. After this diagnosis, she subsequently had no response to rituximab, cladribine, methotrexate, or dasatinib. Clofarabine was started ten years after symptom presentation, and for the first time she had demonstrable shrinkage of the tumors and improvement of proptosis. She completed four cycles of therapy, but four months after clofarabine was discontinued, her masses progressed.
Toxicity
Toxicity data were available for 17 patients, with median follow up 10.5 months. The most common toxicities were hematologic. All patients developed grade 4 neutropenia (absolute neutrophil count less than 500/µl). Four patients developed an occurrence of prolonged neutropenia lasting greater than one week (range 22–75 days). Prolonged neutropenia occurred twice in the setting of clofarabine 25 mg/m2/day dosing, but otherwise occurred with higher dose therapy (50 mg/m2) or when clofarabine and cytarabine were given in combination. Seven of seventeen (41%) patients required platelet transfusions, and 11/17 (64%) required red blood cell transfusions. Five patients (29%) developed grade 3 or higher emesis; one patient required dose reduction of clofarabine due to the severity of symptoms. Five patients developed grade 3 bacterial infections requiring IV antibiotics. One patient had therapy-associated fever. One patient had evidence of capillary leak syndrome, developing peripheral edema and hypoalbuminemia associated with two cycles of therapy. No patients have developed myelodysplastic syndrome or second malignancies.
Dose adjustments
Two dose adjustments were made for toxicity. Patient #14 required dose decrease from 50 mg/m2/day to 25 mg/m2/day due to prolonged neutropenia after the first cycle, with continued good clinical response at the lower dose. Patient #7 required 20% dose reduction after the first cycle due to severe emesis, with subsequent complete response.
Two dose adjustments were made for initial intermediate/mixed response. Patient #5 underwent dose increase from 25 mg/m2/day to 50 mg/m2/day due to intermediate/mixed response, with subsequent regression in all lesions. Patient #1 had addition of cytarabine with dose reduction of clofarabine to 20 mg/m2/day due to intermediate/mixed response, and subsequently experienced regression of all lesions but profoundly prolonged neutropenia (75 days).
Discussion
In this retrospective review of clofarabine therapy in patients with refractory or relapsed histiocytic disorders, we identify disease response classifiable as better/complete resolution or better/regression in seventeen of eighteen patients. Though estimated 1-year PFS was 62%, these results were achieved in a heavily pretreated population, with a median of three prior therapy failures. Moreover, even when patients had disease progression or recurrence, retreatment with additional clofarabine (or in one case, surgery) resulted in subsequent complete response in three of five patients.
Impressive responses were seen in patients diagnosed with JXG and RDD, including those with central nervous system involvement and those with heavily pretreated disease. Of note, the patients on this study with central nervous system JXG had life-threatening morbidity including seizures, hydrocephalus, or brainstem involvement. Two of the patients received maintenance clofarabine therapy (25 mg/m2/day × 2 days/month) because of residual lesions and are now off treatment. Larger numbers of patients will be needed to assess durability and extent of response in CNS-specific lesions. Two of the three patients with Rosai-Dorfman disease recurred off therapy after significant responses, raising the question of whether these patients need longer-term or maintenance therapy to prevent recurrence.
Our experience suggests that eradication of disease prior to cessation of therapy is necessary for progression-free survival, although this study is not powered to determine this definitively. Of the five patients with disease progression, one LCH patient required alternate therapy after 2 cycles, two patients (1 LCH, 1 RDD) progressed after having residual disease at the end of clofarabine therapy, and two patients (1 JXG, 1 RDD) recurred after complete remission (CR). One patient (JXG) had skin-only recurrence that resolved with one course of additional low-dose clofarabine. Additionally, two patients with improved but residual disease in this cohort are receiving clofarabine maintenance therapy at lower cumulative dose after 6–8 cycles of full dose therapy. Lower dose therapy was administered in some patients due to concerns regarding risk of prolonged drug exposure resulting in long-term secondary complications, such as myelodysplastic syndrome or leukemia. It remains to be seen whether a maintenance approach will effectively eradicate residual disease, prevent recurrence, and/or mitigate risk of second malignancy.
While clofarabine has promise as a novel agent in risk-organ and multisystem LCH, it was not universally curative either in our cohort or in previously published studies [22]. The two LCH patients with eventual disease progression in our series had multiple risk-organ involvement and a heavy disease burden, highlighting the continued need for novel treatment strategies in children with risk-organ LCH.
The acute toxicity profiles in this study are consistent with other therapeutic approaches for refractory histiocytoses [7,22]. Neutropenia and transfusion dependence occurred as expected. While high-grade bacterial infections occurred in five patients, no patients experienced septic shock or need for ICU admission once clofarabine was started. Due to risk of prolonged neutropenia, we recommend against use of clofarabine doses higher than 25 mg/m2/day or clofarabine combination therapy except in patients with severe disease burden or poor response to lower doses. Pulmonary edema and systemic inflammatory response syndrome have been reported with clofarabine, but occurred in only one of our patients, possibly due to the fact that this patient population has not been heavily pretreated with total body irradiation or busulfan-containing regimens that may predispose patients to these side effects.
This study’s limitations include small numbers of patients, retrospective analysis, and lack of long-term follow-up for outcomes and toxicity, including potential for secondary malignancy. The optimal dosing, schedule, and duration of clofarabine therapy in these patients also remain to be defined. While the manufacturer recommended dose for leukemia therapy is 50 mg/m2/dose × 5 days per cycle, this study suggests lower doses may be adequate to treat refractory LCH, JXG, and RDD. The minimum effective dose remains to be tested. Furthermore, clinicians must weigh the risks of persistent active disease with the risks of therapy for an individual patient. For example, while non-risk organ LCH is rarely fatal, persistent active disease can cause significant morbidity including diabetes insipidus and other endocrinopathies, impaired growth, hearing loss, chronic pain, or neurodegeneration [26]. Additionally, persistent LCH and non-Langerhans cell histiocytoses with risk-organ involvement is generally fatal. This retrospective study suggests that clofarabine may have activity against refractory LCH, JXG and RDD. Prospective multi-center trials are warranted to determine efficacy, optimal dosing, and long-term toxicity of clofarabine for these patients.
Acknowledgements
This work was supported by grants from the National Institutes of Health, U.S.A. (K12 CA090433 to S.J.S.; R01 CA154489 to C.E.A., K.L.M.; P50 CA126752 to C.E.A.), the Histio CURE Foundation (TXCH Histiocytosis Program grant to K.L.M. and C.E.A.), Baylor College of Medicine (Junior Faculty Seed Grant to C.E.A.), Texas Children’s Hospital (Pediatric Pilot Award to C.E.A.), and the American Society of Hematology (Scholar Award to C.E.A.)
Footnotes
Conflict of interest statement: the authors have no conflicts of interest.
References
- 1.Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184(8):4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grois N, Fahrner B, Arceci RJ, et al. Central nervous system disease in Langerhans cell histiocytosis. J Pediatr. 2010;156(6):873–881. doi: 10.1016/j.jpeds.2010.03.001. 881 e871. [DOI] [PubMed] [Google Scholar]
- 3.Allen CE, McClain KL. Langerhans cell histiocytosis: a review of past, current and future therapies. Drugs Today (Barc) 2007;43(9):627–643. doi: 10.1358/dot.2007.43.9.1088823. [DOI] [PubMed] [Google Scholar]
- 4.Kudo K, Ohga S, Morimoto A, et al. Improved outcome of refractory Langerhans cell histiocytosis in children with hematopoietic stem cell transplantation in Japan. Bone Marrow Transplant. 2010;45(5):901–906. doi: 10.1038/bmt.2009.245. [DOI] [PubMed] [Google Scholar]
- 5.Gadner H, Minkov M, Grois N, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121(25):5006–5014. doi: 10.1182/blood-2012-09-455774. [DOI] [PubMed] [Google Scholar]
- 6.Ronceray L, Potschger U, Janka G, et al. Pulmonary involvement in pediatric-onset multisystem Langerhans cell histiocytosis: effect on course and outcome. J Pediatr. 2012;161(1):129–133. doi: 10.1016/j.jpeds.2011.12.035. e121-123. [DOI] [PubMed] [Google Scholar]
- 7.Weitzman S, Braier J, Donadieu J, et al. 2'-Chlorodeoxyadenosine (2-CdA) as salvage therapy for Langerhans cell histiocytosis (LCH). results of the LCH-S-98 protocol of the Histiocyte Society. Pediatr Blood Cancer. 2009;53(7):1271–1276. doi: 10.1002/pbc.22229. [DOI] [PubMed] [Google Scholar]
- 8.Imamura T, Sato T, Shiota Y, et al. Outcome of pediatric patients with Langerhans cell histiocytosis treated with 2 chlorodeoxyadenosine: a nationwide survey in Japan. Int J Hematol. 2010;91(4):646–651. doi: 10.1007/s12185-010-0558-0. [DOI] [PubMed] [Google Scholar]
- 9.Bernard F, Thomas C, Bertrand Y, et al. Multi-centre pilot study of 2-chlorodeoxyadenosine and cytosine arabinoside combined chemotherapy in refractory Langerhans cell histiocytosis with haematological dysfunction. Eur J Cancer. 2005;41(17):2682–2689. doi: 10.1016/j.ejca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Egeler RM, de Kraker J, Voute PA. Cytosine-arabinoside, vincristine, and prednisolone in the treatment of children with disseminated Langerhans cell histiocytosis with organ dysfunction: experience at a single institution. Med Pediatr Oncol. 1993;21(4):265–270. doi: 10.1002/mpo.2950210406. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto A, Ikushima S, Kinugawa N, et al. Improved outcome in the treatment of pediatric multifocal Langerhans cell histiocytosis: Results from the Japan Langerhans Cell Histiocytosis Study Group-96 protocol study. Cancer. 2006;107(3):613–619. doi: 10.1002/cncr.21985. [DOI] [PubMed] [Google Scholar]
- 12.Apollonsky N, Lipton JM. Treatment of refractory Langerhans cell histiocytosis (LCH) with a combination of 2-chlorodeoxyadenosine and cytosine arabinoside. J Pediatr Hematol Oncol. 2009;31(1):53–56. doi: 10.1097/MPH.0b013e31817e4a32. [DOI] [PubMed] [Google Scholar]
- 13.Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma. Pediatr Blood Cancer. 2008;51(1):130–133. doi: 10.1002/pbc.21523. [DOI] [PubMed] [Google Scholar]
- 14.Horneff G, Jurgens H, Hort W, et al. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): response to methotrexate and mercaptopurine. Med Pediatr Oncol. 1996;27(3):187–192. doi: 10.1002/(SICI)1096-911X(199609)27:3<187::AID-MPO10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Tasso M, Esquembre C, Blanco E, et al. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612–615. doi: 10.1002/pbc.20668. [DOI] [PubMed] [Google Scholar]
- 16.Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52. [PubMed] [Google Scholar]
- 17.Rodriguez-Galindo C, Jeng M, Khuu P, et al. Clofarabine in refractory Langerhans cell histiocytosis. Pediatr Blood Cancer. 2008;51(5):703–706. doi: 10.1002/pbc.21668. [DOI] [PubMed] [Google Scholar]
- 18.Parker WB, Shaddix SC, Chang CH, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5'-triphosphate. Cancer Res. 1991;51(9):2386–2394. [PubMed] [Google Scholar]
- 19.Berg SL, Bonate PL, Nuchtern JG, et al. Plasma and cerebrospinal fluid pharmacokinetics of clofarabine in nonhuman primates. Clin Cancer Res. 2005;11(16):5981–5983. doi: 10.1158/1078-0432.CCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 20.Jeha S, Kantarjian H. Clofarabine for the treatment of acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2007;7(2):113–118. doi: 10.1586/14737140.7.2.113. [DOI] [PubMed] [Google Scholar]
- 21.Hijiya N, Barry E, Arceci RJ. Clofarabine in pediatric acute leukemia: current findings and issues. Pediatr Blood Cancer. 2012;59(3):417–422. doi: 10.1002/pbc.24112. [DOI] [PubMed] [Google Scholar]
- 22.Abraham A, Alsultan A, Jeng M, et al. Clofarabine salvage therapy for refractory high-risk langerhans cell histiocytosis. Pediatr Blood Cancer. 2013;60(6):E19–E22. doi: 10.1002/pbc.24436. [DOI] [PubMed] [Google Scholar]
- 23.Minkov M, Grois N, McClain K, et al. Histiocyte Society Evaluation and Treatment Guidelines. [Accessed 1 June 2013];2009 Apr; www.histiocytesociety.org/document.doc?id=290. [Google Scholar]
- 24.Blouin P, Yvert M, Arbion F, et al. Juvenile xanthogranuloma with hematological dysfunction treated with 2CDA-AraC. Pediatr Blood Cancer. 2010;55(4):757–760. doi: 10.1002/pbc.22629. [DOI] [PubMed] [Google Scholar]
- 25.Faderl S, Gandhi V, O'Brien S, et al. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105(3):940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 26.Haupt R, Nanduri V, Calevo MG, et al. Permanent consequences in Langerhans cell histiocytosis patients: a pilot study from the Histiocyte Society-Late Effects Study Group. Pediatr Blood Cancer. 2004;42(5):438–444. doi: 10.1002/pbc.20021. [DOI] [PubMed] [Google Scholar]