Abstract
Chronic post-ischemic pain (CPIP) is an animal model of CRPS-I developed using a 3-hour ischemia-reperfusion injury of the rodent hind paw. The contribution of local endothelin to nociception has been evaluated in CPIP mice by measuring sustained nociceptive behaviours (SNBs) following intraplantar injection of endothelin-1 or -2 (ET-1, ET-2). The effects of local BQ-123 (ETA-R antagonist), BQ-788 (ETB-R antagonist), IRL-1620 (ETB-R agonist) and naloxone (opioid antagonist) were assessed on ET-induced SNBs and/or mechanical and cold allodynia in CPIP mice. ETA-R and ETB-R expression was assessed using immunohistochemistry and Western blot analysis. Compared to shams, CPIP mice exhibited hypersensitivity to local ET-1 and ET-2. BQ-123 reduced ET-1- and ET-2-induced SNBs in both sham and CPIP animals, but not mechanical or cold allodynia. BQ-788 enhanced ET-1- and ET-2-induced SNBs in both sham and CPIP mice, and cold allodynia in CPIP mice. IRL-1620 displayed a non-opioid antinociceptive effect on ET-1- and ET-2-induced SNBs and mechanical allodynia in CPIP mice. The distribution of ETA-R and ETB-R were similar in plantar skin of sham and CPIP mice, but both receptors were over-expressed in plantar muscles of CPIP mice. This study shows ETA-R and ETB-R have differing roles in nociception for sham and CPIP mice. CPIP mice exhibit more local endothelin-induced SNBs, develop a novel local ETB-R agonist-induced (non-opioid) analgesia, and exhibit over-expression of both receptors in plantar muscles, but not skin. The effectiveness of local ETB-R agonists as anti-allodynic treatments in CPIP mice holds promise for novel therapies in CRPS-I patients.
1. Introduction
Endothelins are a family of peptides with potent biologic effect in vascular and nonvascular cells. Two main peptides have been identified in the periphery, endothelin-1 (ET-1) and endothelin-2 (ET-2) [10], that play a role in nociception (see table 1). Another, peptide, endothelin-3 (ET-3), has been described, but its peripheral role in nociception is less clear. The effects of ET-1 and ET-2 are mediated by two receptors: ETA-R and ETB-R, which often produce opposite effects. At the vascular level, ETA-R activation induces vasoconstriction, while ETB-R activation induces vasodilatation. Endothelin binding sites are described at hair follicles, sebaceous and sweat glands, and arrector pili muscle [56], where they may have trophic effects. Finally, ETA-R and ETB-R are found at all levels of the nervous system: in the sciatic nerve and DRG [3, 43], as well as in the spinal cord and brain (for review, see [20]).
Table 1.
Review of the literature on the effect of local ETA-R or ETB-R ligands on nociception in rodent.
| Pain Model | agonist | antagonist | Reference | ||
|---|---|---|---|---|---|
| ETA-R | ETB-R | ETA -R | ETB- R | ||
| Formalin (paw) | 0 and ⇑ | 0 | ⇓ | [38] | |
| Carrageenan or LPS (knee) | 0 | ⇓ | [13] | ||
| Capsaicin (foot) | 0 | 0 | [39] | ||
| Serotonin (foot) | ⇓ | 0 | 0 | [39] | |
| Ovalbumin-induced nociception | ⇑ | ⇓ | ⇓ | [41] | |
| Carrageenan or CFA (foot) | ⇓ | 0 or ⇓ | [1] | ||
| Tumoral XC cells | ⇓ | 0 or ⇓ | [2] | ||
| Cancer pain | 0 | [55] | |||
| Carrageenan (knee) | ⇓ | 0 | ⇑ | [9] | |
| IL-12 induced hyperalgesia | 0 | ⇓ | [51] | ||
| IL-18 induced hyperalgesia | 0 | ⇓ | [50] | ||
| CCI | ⇓ | [26] | |||
| CION | ⇓ | ⇓ | [5] | ||
| Carrageenan (lips) | ⇓ | ⇓ | [6] | ||
| cancer pain | ⇓ | [47] | |||
| Post-incisional pain | ⇓ | [35] | |||
| Antigen-induced nociception | ⇓ | 0 | [52] | ||
| cancer pain | ⇓ | 0 | [16] | ||
| Ischemic pain | ⇓ | [48] | |||
| ET-1 in other pain model: | |||||
| ET-1 + carrageenan (knee) | ⇓ | ⇓ | [14] | ||
| ET-1 + Capsaicin | ⇓ | 0 | [39] | ||
| ET-1 + cancer pain | ⇓ | [55] | |||
| ET-1 + cancer pain | ⇓ | [16] | |||
| ET-1 + capsaicin | ⇓ | 0 or ⇓ | [33] | ||
| ET-1 + formalin | ⇓ | ⇓ | [34] | ||
| ET-induced nociception: | |||||
| ET-1 (s.c.) | ⇓ | 0 | [13] | ||
| ET-1 (foot) | ⇓ | ⇓ | 0 | [40] | |
| ET-1 (s.c.) | ⇓ | ⇑ | [23] | ||
| ET-1 (s.c.) | ⇓ | 0 | [30] | ||
| ET-1 (s.c.) | ⇓ | ⇓ | [2] | ||
| ET-1 (sciatic nerve) | ⇓ | [21] | |||
| ET-1, ET-2, ET-3 | 0 | ⇓ | [8] | ||
0 no effect on nociception; ⇓ decrease nociception; ⇑ increase nociception.
Locally injected, ET-1 induces spontaneous pain in human [19] and rodents [29]. These effects are independent of its vasoactive activity [11], but rather involve activation and sensitization of C-nociceptors [37], probably by increasing intracellular calcium [25]. In rodents, ET-1-induced nociception can be reduced by systemic or central morphine [11, 30], but is resistant to other pharmacological treatments (indomethacin, atenolol, dexamethasone, ibuprofen, acetaminophen; [8, 45]).
Complex regional pain syndrome (CRPS), with major nerve injury (type II) or without (type I), is characterized by spontaneous and stimulus-evoked pain, edema, vasomotor and sudomotor abnormalities, motor dysfunction, and trophic changes [49]. The symptoms typically occur in the distal part of the affected limb, sometimes following a relatively benign trauma. In patients with CRPS-I, the level of ET-1 has been shown to be increased locally in blister fluids [18]. However, neither plasma levels [15], nor cerebrospinal fluid levels [36] were increased. Increased local ET-1 levels in CRPS patients may have a key role in the development of the pathology. Thus, endothelins are a potential pharmacological target in CRPS-I.
We previously showed that a 3 h-ischemia/reperfusion (I/R) of the hind paw induced long-term mechanical allodynia or chronic post-ischemia pain (CPIP) [7]. The features occurring in CPIP rats (microvascular injury, chronic ischemia, chronic mechanical and cold allodynia, pharmacological profile, vasoconstrictor hypersensitivity and painful response to intradermal norepinephrine) are also similar to those described in patients with CRPS-I [27, 31, 53, 54] and suggest that mediators involved in microvascular injury may play a role in CRPS-I.
While the role of ETA-Rs in pain and analgesia are now well established [25], the contribution of ETB-Rs is still controversial and may be particularly relevant to the pathophysiology of CRPS. The present study was performed to establish whether peripheral endothelin receptors are involved in nociception in a rodent model of CRPS-I.
2. Materials and methods
2.1. Animals
Male Swiss CD1 mice (8 weeks old, Charles River, Quebec) arrived 7 days before experiments. All treatments and testing were performed blindly by a single experimenter using a randomized block design. These studies were approved by the Animal Care Committee at McGill University, and conformed to ethical guidelines of the Canadian Council on Animal Care.
2.2. I/R injury
Chronic post-ischemia pain (CPIP) was generated following exposure to prolonged hind paw I/R injury. Mice were anesthetized over a 3 h period with an initial bolus (55 mg/kg, i.p.) and supplements (27.5 mg/kg, i.p) of sodium pentobarbital when required. After induction of anesthesia, a Nitrile 70 Durometer O-ring (O-rings West, Seattle, WA) with a 5/64 inch internal diameter was placed around the mouse’s left ankle joint for 3 h, as was initially described with larger O-rings in rats (see [7]). Sham mice were anesthetized, but no O-ring was placed on the ankle.
2.3. Behavioral Experiments
2.3.1. Behavioral Testing
Sustained Nociceptive Behaviors (SNBs) were assessed over 30 minutes after an intraplantar (i.pl.) injection of endothelin peptide or vehicle (10 μl). The total SNB score was calculated as: Time (paw elevation)/3 + Time (flinching)/2 + Time (biting, licking or scratching).
Mechanical allodynia
von Frey filaments were applied to the plantar surface of the hind paw in either ascending (after negative response) or descending (after positive response) force as necessary to determine the filament closest to the threshold of response. Each filament was applied for 4 seconds or until a flexion reflex occurred. The minimum stimulus intensity was 0.008 g and the maximum was 4 g. Based on the response pattern, and the force of the final filament (5th stimulus after first direction change), the 50% threshold to withdraw was calculated in grams (for more detail see [4]).
Cold allodynia was assessed by measuring the total time spent exhibiting sustained nociceptive behaviors (paw elevation + flinching + biting + licking + scratching) over 1 minute after a drop (25 μl) of acetone was gently applied on the plantar surface of the hind paw.
2.3.2. Experimental design
Experiment 1: Dose effect of ET-induced SNBs
Two days after I/R injury, sham and CPIP mice were habituated for 20 minutes to an observation chamber (12 cm × 12 cm). After the habituation period, the mice received a 10 μl i.pl. injection of vehicle or various doses of ET-1 (0.3, 1, 5, 10, 25, 50, 100, 200 and 400 pmol) or ET-2 (2, 10, 50, 100, 200, 400 and 600 pmol), and SNB score was determined over 30 min as described above (N=7–9/group). Doses higher than 200 pmol ET-1 or 400 pmol ET-2 cause undue stress and were therefore not included.
Experiment 2. Effect of ETB-R agonist or ETA/B-R antagonists on ET-induced SNBs
Two days after I/R injury, sham and CPIP mice first received a 10 μl i.pl. injection of vehicle, BQ-123 (5 or 10 nmol, ETA-R antagonist), BQ-788 (30 or 60 nmol, ETB-R antagonist) or IRL-1620 (50 or 200 pmol, ETB-R agonist) and then were habituated for 20 minutes. After habituation, they received a second 10 μl i.pl. injection of vehicle, ET-1 or ET-2. The doses selected for this experiment were based on the EC50 previously calculated for each condition (i.e. for ET-1: 70 pmol for sham and 7 pmol for CPIP, and for ET-2: 200 pmol for sham and 125 pmol for CPIP). Their 30 min SNB score was determined after this second i.pl. injection (N=7–8/group).
Experiment 3. Effect of ETB-R agonist or ETA/B-R antagonists on mechanical and cold allodynia
Two days after I/R injury, mechanical and cold sensitivities were assessed in both sham and CPIP mice: before, 30 and 60 min after mice received a 10 μl i.pl. injection of vehicle, BQ-123 (10 nmol), BQ-788 (60 nmol) or IRL-1620 (50 pmol) (N=7–8/group).
Experiment 4. Effect of naloxone on the anti-allodynic effect of IRL-1620 in CPIP mice
Two days after I/R injury, baseline mechanical sensitivity was assessed in four groups of CPIP mice. Mice then received a 5 μl i.pl. injection of naloxone (100 nmol) or vehicle, followed 5 min later by a second 5 μl i.pl. injection of IRL-1620 (50 pmol) or vehicle. Mechanical sensitivity was assessed again 30 min after the second treatment. The percentage of analgesia was calculated as the relative change between the two measures for each group ([(value post-treatment - value pre-treatment)/value pre-treatment) × 100], N=7–8 / group).
2.3.3. Drugs
Drugs used included sodium pentobarbital (Vetoquinol N.-A., Inc., Lavaltrie, QC), endothelin peptides 1 and 2, BQ-123, BQ-788, IRL-1620 (all obtained from Tocris, USA), and naloxone (Sigma-Aldrich, St. Louis, MO, USA). All drugs were dissolved in distilled water (vehicle).
2.4. Immunohistology
Distribution of ETA-R and ETB-R in the skin of sham and CPIP mice
Two days post procedure, 3 sham and 5 CPIP mice were perfused with intra-cardiac cold phosphate-buffered saline (PBS). The ipsilateral plantar skin was quickly removed and rapidly frozen in liquid nitrogen. Twelve-micron serial sections were cut with a micro-cryostat, mounted on gelatin-coated slides and kept frozen at −20 °C until further staining (slide 1, 4, 7, etc for ETA-R staining, slide 2, 5, 8, etc for H&E staining, slide 3, 6, 9, etc for ETB-R staining, see below).
For immunohistochemistry, all reagents, washes and incubation were done at 4°C. Sections were first rinsed 3 × 10 min in PBS, and then blocked for non-specific binding for 1 h with 10% normal donkey serum (Sigma-Aldrich, St. Louis, MO, USA), followed by an incubation overnight at 4°C with a monoclonal mouse-derived anti-NF200 (1:6000, Millipore, Billerica, MA, USA) and rabbit-derived anti-ETA-R (1:4000, Alomone, Jerusalem, Israel) or anti-ETB-R (1:2000, Alomone, Jerusalem, Israel) antibodies in 4% normal donkey serum. On the second day, after 3 × 10 min rinse in PBS, sections were incubated with a donkey anti-rabbit antibody conjugated to CY2 cytochrome (1:200) and a donkey anti-mouse antibody conjugated to CY3 cytochrome (1:200) for 90 min. Both secondary antibodies were from Jackson ImmunoResearch Laboratories (Mississauga, ON, Canada). Finally, slides were washed 3 × 10 minutes with PBS and cover-slipped with aqua-polymount. Controls were obtained by pre-incubating anti-ETA-R and anti-ETB-R antibodies with blocking peptides, or no incubation with the primary antibody (i.e. NF200 or ETA-R/ETB-R). All pictures were taken with 40 X or 60 X objectives on a BX-51 Olympus microscope equipped with a DP-71 camera.
Classical hematoxylin and eosin staining (H&E) was performed as follows: (1) 10 second wash in distilled water, (2) 5 minute immersion in hematoxylin reagent, (3) 5 minute wash in running tap water, (4) 30 second immersion in differentiation solution, (5) 1 minute wash in running tap water, (6) 30 second immersion in building solution, (7) 5 minute wash in running tap water, (8) 10 dips in 95% alcohol solution, (9) 2.5 minute immersion in eosin solution, (10) quick rinse in 70% alcohol solution. All reagents were provided as a staining kit by DAKO (Carpinteria, CA, USA). Finally, slides were cover-slipped with DPX (Sigma-Aldrich, St. Louis, MO, USA). All pictures were taken with a 40 X objective.
2.5. Western Blotting
Quantification of ETA-R and ETB-R in the skin and muscle of sham and CPIP mice
Two days post procedure, plantar skin and muscle from 6 sham and 8 CPIP mice were freshly removed and frozen on dry ice. Samples were then homogenized with 200 μl of RIPA buffer and protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA). After centrifugation (4000G, 20 min), the supernatant was collected for protein determination by the Lowry method. Protein samples (15 μg) were subjected to electrophoresis in 12% polyacrylamide SDS gels, and subsequently transferred onto PVDF membranes (GE Healthcare Canada, Baie d’Urfé, QC, Canada). Non-specific binding sites were blocked by incubation in 5% skim milk for 30 min. Membranes were incubated overnight at 4°C in rabbit-derived anti-ETA-R or ETB-R (1:200, Alomone, Jerusalem, Israel). Following washes in TBS-0.2% Tween 20, membranes were incubated for 1 h at room temperature in a HRP-conjugated goat polyclonal to rabbit IgG (1:10000, Abcam; Cambridge, MA, USA). Proteins were visualized using chemiluminescence substrate for peroxidase (ECL plus, GE Healthcare Canada, Baie d’Urfé, QC, Canada) and Kodak films (Biomax MS from Sigma-Aldrich, St. Louis, MO, USA). Membranes were then stripped (20 min at 50°C in a mix of 3.2 ml 1M Tris-HCl, 10 ml 10% SDS, 350 μl β-mercaptoethanol in distilled water). After block of nonspecific binding (skim milk 4%, 30 min), membranes were incubated overnight at 4°C in rabbit polyclonal to β-actin (1:6000, Abcam; Cambridge, MA, USA).
2.6. Statistics
All data are expressed as mean +/− S.E.M. In experiment 1, EC50s were calculated with a non-linear regression profile for curve fitting (Prism 4.0), and data are expressed as EC50 in pmol (+/− 95% confidence intervals). In experiment 2, effects of ETB-R agonist or ETA/B-R antagonist on ET-1/2-induced SNBs were analyzed with ANOVA followed by a Dunnett’s test for multiple comparisons (comparison to vehicle pretreated group). In experiments 3 and 4, the time course of cold and mechanical sensitivities were analyzed with repeated measures 2 way-ANOVA. When a significant time or treatment effect was detected, but the interaction between the two factors was not significant, values before and after treatments were compared for each treatment group by a 1 way-ANOVA followed by a Dunnett’s test for multiple repeated comparisons. In experiment 4, the percentage of analgesia was analyzed with 2-way ANOVA followed by a Bonferroni’s post hoc test. In Western blot experiments, the comparison between sham and CPIP levels of ETA-R and ETB-R was performed using an unpaired t-test.
3. Results
3.1. Behavior
Experiment 1: Dose effect curves of ET-induced SNBs
Both ET-1 (Fig. 1A) and ET-2 (Fig. 1B) injected into the hind paw induced SNBs in sham and CPIP mice. The calculated EC50 was significantly lower in CPIP than in sham mice for both peptides (ET-1-induced SNB, EC50= 61.5 (47.5 to 79.6) pmol for sham and 6.2 (4.6 to 8.3) pmol for CPIP, Fig. 1A; ET-2-induced SNB, EC50= 182.3 (139.4 to 238.4) pmol for sham and 125.6 (95.1 to165.9) pmol for CPIP, Fig. 1B). Above the dose of 100 pmol for ET-1 and 200 pmol for ET-2, CPIP mice exhibited stress-related behaviours (freezing). For this reason, these doses are not shown and were not included in the EC50 calculation.
Fig. 1. Dose response curve of ET-induced SNB.
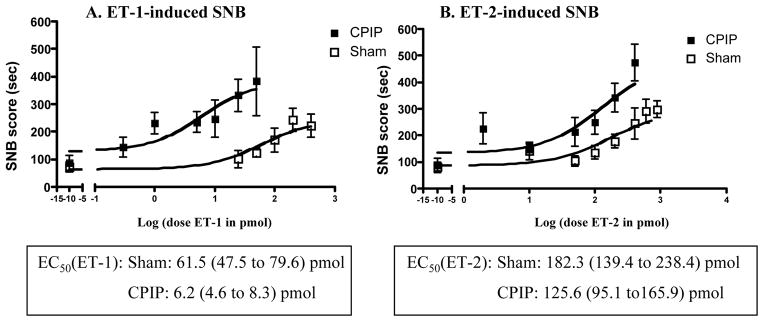
Two days after I/R injury, sham and CPIP mice received a 10 μl i.pl. injection of (A) endothelin-1 (ET-1, from 0.3 pmol to 400 pmol or vehicle), (B) endothelin-2 (ET-2, from 2 pmol to 600 pmol) or (C) vehicle. The x-axis is expressed as the log dose in pmol. The total sustained nociceptive behavior score (tot SNB score) was determined over a 30 minute time period. The EC50 values were calculated using a non-linear regression profile for the curve fit (Prim 4.0), and data are expressed EC50 in pmol (+/− 95% Confidence Intervals). N=7–9/group. Dose response curves of the SNBs to both ET-1 and ET-2 were shifted to the left in CPIP, as compared to sham, mice.
These data show that CPIP mice are more sensitive to ET-1- and ET-2-induced SNBs than sham control mice. They exhibited a significant leftward shift of the dose-effect curve for both peptides. They were 10 times more sensitive to local ET-1, and almost 2 times more sensitive to ET-2 than sham mice.
Experiment 2. Effect of an ETB-R agonist or ETA/B-R antagonists on ET-1/2-induced SNBs
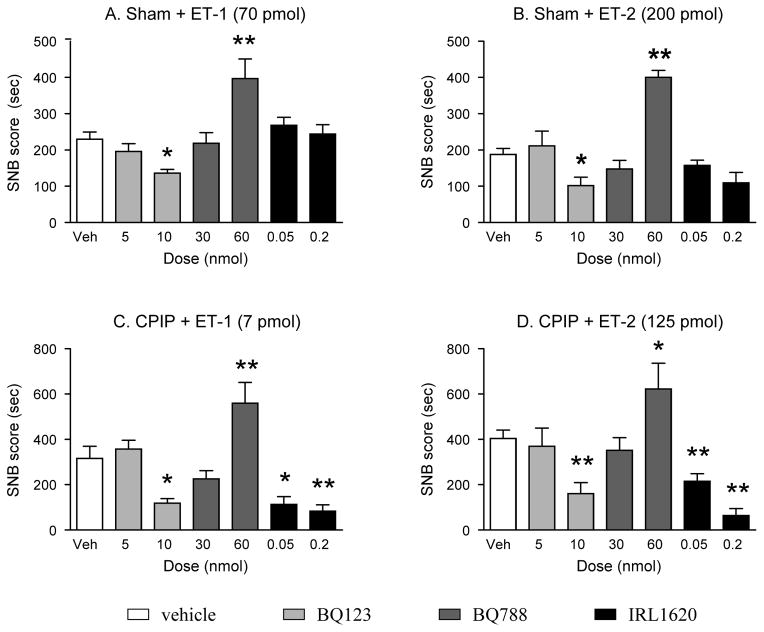
ET doses were selected that induced SNBs in both sham (ET-1: 70 pmol and ET-2: 200 pmol) and CPIP (ET-1: 7 pmol and ET-2: 135 pmol) mice (Fig. 2A–D). Mice pretreated with 10 nmol of the ETA-R antagonist BQ-123, but not 5 nmol, had significantly lower SNB scores than their respective controls, in all conditions (i.e., CPIP or sham injected with ET-1 or ET-2). On the other hand, pretreatment with a medium dose of ETB-R antagonist BQ-788 (30 nmol) did not affect the total SNB scores in any condition, while a larger dose (60 nmol) significantly enhanced the ET-induced SNB in all conditions. Finally, sham mice pretreated with the ETB-R agonist IRL-1620, exhibited the same ET-1 or ET-2-induced SNB scores as their respective controls, whereas CPIP mice pretreated with IRL-1620 (50 or 200 pmol) exhibited significantly reduced SNB scores compared to their respective controls (Fig. 2A–D). (F(6, 41060)=7.077, p<0.0001 for Fig. 2A, F(6, 65160)=10.58,, p<0.0001 for Fig. 2B, F(6, 217900)= 11.15, p<0.0001 for Fig. 2C, and F(6, 242900)= 9.091, p<0.0001 for Fig. 2D). Since the lower dose was as effective as the higher dose, for further experiments, only 50 pmol of IRL-1620 was used.
Fig. 2. Effect of ETB-R agonist or ETA/B-R antagonists on ET-induced SNB.
Two days after I/R injury, sham (A and B) and CPIP (C and D) mice received a 10 μl i.pl. injection of vehicle (white histogram), BQ-123 (light grey histogram, 5 or 10 nmol, ETA-R antagonist), BQ-788 (dark grey histogram, 30 or 60 nmol, ETB-R antagonist) or IRL-1620 (black histogram, 50 or 200 pmol, ETB-R agonist). Twenty minutes later, they received a 10 μl i.pl. injection of endothelin-1 (ET-1, A: 70 pmol for sham and C: 7 pmol for CPIP) or endothelin-2 (ET-2, B: 200 pmol for sham and D: 125 pmol for CPIP). The total sustained nociceptive behavior score (SNB score) was determined over a period of 30 minutes. N=7–8/group (* p<0.05, ** p<0.01, one-way ANOVA followed by a Dunnett’s t-test). BQ-123 and IRL-1620 reduced, and BQ-788 enhanced, ET-1/2-induced SNBs in CPIP mice, while BQ-123 also reduced ET1/2-induced SNBs in shams.
Thus, ET-1- and ET-2-induced SNBs were reduced by local pretreatment with an ETA-R antagonist and increased by local pretreatment with an ETB-R antagonist in both sham and CPIP mice. Conversely, local pretreatment with an ETB-R agonist significantly reduced ET-1- and ET-2-induced SNBs in CPIP mice only.
Experiment 3. Effect of an ETB-R agonist or ETA/B-R antagonists on mechanical and cold allodynia
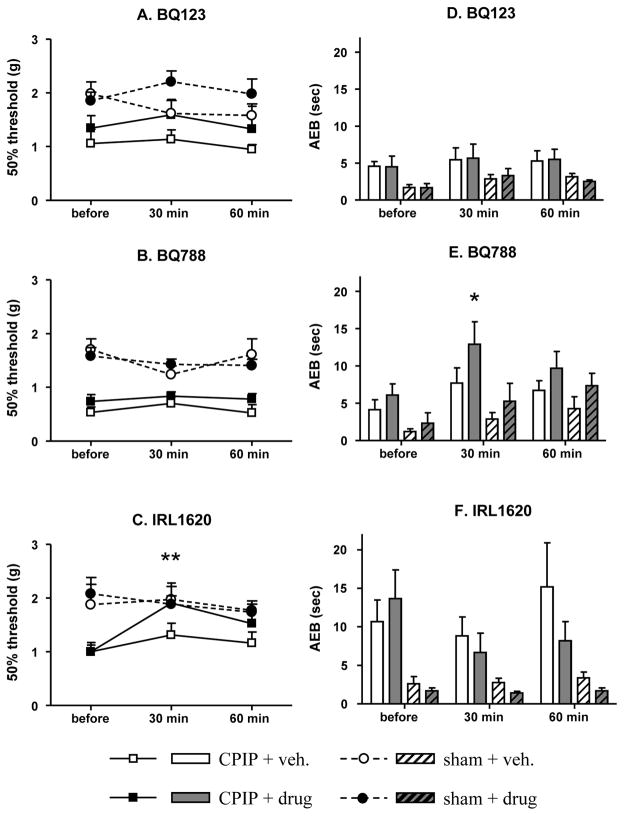
CPIP mice exhibited significantly lower 50% withdrawal thresholds to mechanical stimuli than sham mice (Fig. 3A–C) (F(3, 0.5147)=19.23, p=0.0018 for Fig. 3A, F(3, 0.6774)=27.36, p=0.0007 for Fig. 3B, and F(3, 0.3751)= 6.093, p=0.0298 for Fig. 3C). Whereas local treatment with BQ-123 or BQ-788 did not affect mechanical thresholds in sham or CPIP mice, IRL-1620 reduced mechanical allodynia in CPIP mice (F(2, 1.448)=7.624, p=0.0073) (Fig. 3A–C) without affecting mechanical thresholds in sham mice.
Fig. 3. Effect of ETB-R agonist or ETA/B-R antagonists on mechanical and cold allodynia.
Two days after I/R injury, mechanical (A, B and C) and cold (D, E, F) sensitivities were assessed in both sham and CPIP mice: before, 30 and 60 min after mice received a local 10 μl i.pl. treatment with vehicle, BQ-123 (10 nmol), BQ-788 (60 nmol) or IRL-1620 (50 pmol). N=7–8 / group. Only IRL-1620 significantly elevated the 50% threshold in CPIP mice (* p<0.05, ** p<0.001, One-way ANOVA followed by a Dunnett for multiple comparison test).
All groups of CPIP mice exhibited more nociceptive behaviors than sham mice during the acetone test (Fig. 3D–F) (F(3, 6.891)=97.44, p<0.0001 for Fig. 3D, F(3, 24.08)=9.073, p=0.0057 for Fig. 3E, and F(3, 71.18)= 11.97, p=0.0061 for Fig. 3F). Local treatment with BQ-123 or IRL-1620 did not affect cold allodynia. However, local treatment with BQ-788 (60 nmol) significantly enhanced cold allodynia in CPIP (F(2, 81.43)=4.870, p=0.0283), but did not affect cold responses in sham (F(2, 44.35)=1.552, n.s.), mice.
Neither the ETA-R nor the ETB-R antagonist reduced mechanical allodynia in CPIP mice when locally injected. Consistent with the result on ET-1/2-induced SNBs, local treatment with the ETB-R agonist IRL-1620 reduced mechanical allodynia in CPIP mice, but had no effect on cold allodynia.
Experiment 4. Effect of naloxone on the anti-allodynic effect of IRL-1620 in CPIP mice
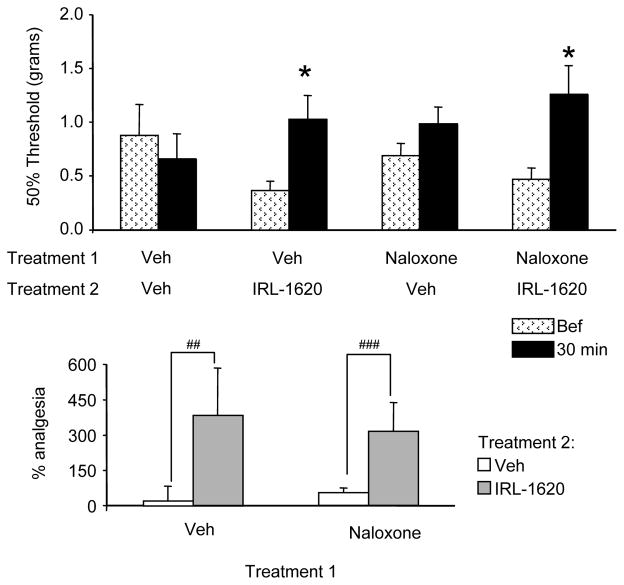
I.pl. naloxone on its own did not affect 50% withdrawal thresholds in CPIP mice (Fig 4A–B). IRL-1620 produced an anti-allodynic effect in mice, regardless of the pretreatment received (i.e. vehicle or naloxone, Fig. 4A). While there was a significant effect of treatment group (Fig. 4B), this effect did not depend on the first treatment (i.e. vehicle or naloxone; 2 way ANOVA, F(1, 3042)=0.02236, n.s.), but rather depended on the second treatment (i.e. vehicle or IRL-1620; 2 way ANOVA, F(1, 816700)=6.002, p=0.0203). Furthermore, there was no interaction between the two treatments. Thus, naloxone had no effect on IRL-1620-induced analgesia in CPIP mice.
Fig. 4. Effect of naloxone on the anti-allodynic effect of IRL-1620 in CPIP mice.
Top graph: two days after I/R injury, baseline mechanical sensitivity was assessed in four groups of CPIP mice (patterned histogram). Then, mice received a 5 μl i.pl. injection of naloxone (100 nmol) or vehicle, followed 5 min later by a 5 μl i.pl. injection of IRL-1620 (50 pmol) or vehicle. Mechanical sensitivity was assessed 30 min after the second treatment (black histogram). Bottom graph: the percentage of analgesia was calculated as the relative change between the two measures for each group. The significant elevation of the 50% threshold (A), or % analgesia (B), by IRL-1620 in CPIP mice was unaffected by naloxone treatment. N=7–8 / group (* p<0.05, paired t-test (compared to the value before treatment; ## p<0.05, ### p<0.01, 2-way ANOVA followed by a Bonferroni’s multiple comparison test).
3.2. Distribution of ETA-R and ETB-R in the skin of sham and CPIP mice
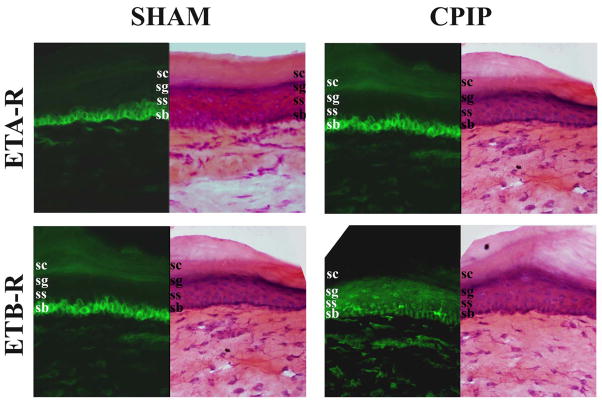
In mouse skin processed for immunostaining with antibodies against ETA-R and ETB-R, a strong signal was detected. However, no obvious difference could be observed between sham and CPIP skin (Figs 5,6).
Fig. 5. Distribution of ETA-R and ETB-R in the skin of sham and CPIP mice.
Two days post I/R injury, the skin of sham (left panels) and CPIP (right panels) mice was incubated with anti-ETA-R (1:4000, top panels, green) or anti-ETB-R (1:2000, bottom panels, green) antibodies, or stained with hemathoxiline and eosin (H&E). All pictures were taken with a 40 X objective. ETA-R staining was predominantly found in the deeper stratum basalis (s.b.) layer and ETB-R in the medium strati granulosum (s.g.) and spinosum (s.s.) layers. No or poor staining was observed in the external stratum corneum (s.c.) There are no obvious changes in ETA-R or ETB-R staining between sham and CPIP mice.
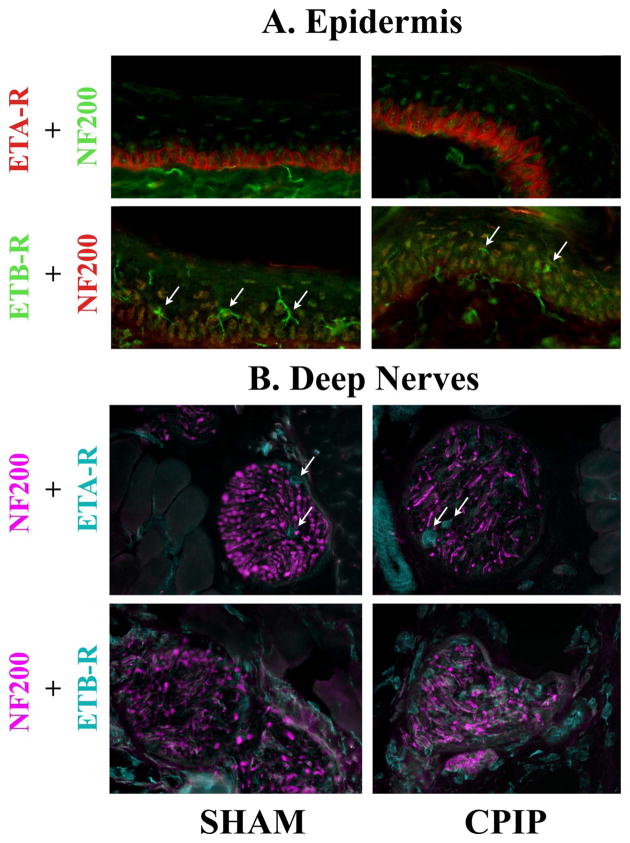
Fig. 6. Fine cellular distribution of ETA-R and ETB-R in the epidermis and peripheral nerves of sham and CPIP mice.
Two days post I/R injury, the epidermis (A) and deep nerves (B) of the skin of sham (left column) and CPIP (right column) mice was incubated with anti-NF200 antibody (1:6000) and anti-ETA-R (1:4000) or anti-ETB-R (1:2000) antibodies. All pictures were taken with a 60 X objective. ETA-R staining was observed at the surface membrane of the keratinocytes from the s.b. layer. ETB-R staining appears within s.g. and s.s. layers, with more intensively stained star-shaped cells found mainly in the s.s. layer, which are most likely Langerhans cells (arrow in A). ETA-R and ETB-R staining was also observed in filamentous structures co-migrating within the NF200-IR fiber bundle. Finally, ETA-R staining was observed on blood vessels (arrows) within the bundle. In the epidermis and in deeper peripheral nerves, ETA-R and ETB-R immunoreactivity shows a similar pattern of distribution in CPIP and sham mice.
Epidermal cells
In the epidermis, both ETA-R and ETB-R antibodies poorly stained the external stratum corneum (s.c.) layer. ETA-R staining was predominantly found in the deeper stratum basalis (s.b.) layer (Fig. 5), mostly on keratinocytes (Fig. 6A). ETB-R staining showed a denser labeling in the medium strati granulosum (s.g.) and spinosum (s.s.) layers than in the stratum basalis (s.b.) layer (Fig. 5). At higher magnification, ETB-R staining appeared as a uniform background within s.g. and s.s. layers, with more intensively stained star-shaped cells found mainly in the s.s. layer, which are most likely Langerhans cells (Fig. 6A, arrow).
Peripheral nervous system
Deep nerve bundles traveling between muscle fibers and innervating both skin and muscles were carefully examined in both CPIP and sham skin. No obvious differences were detected between the two conditions. However, we noticed that in CPIP skin preparations ETB-R staining was slightly more intense within the nerve bundle than in sham skin preparation. In deep nerve bundles, ETA-R staining was observed as filamentous background, and as more intensively stained independent tubular shaped structures coursing between NF200 positive fibers (Fig. 6B, arrow). One or two of these tubular structures was observed within each large deep nerve bundle and were relatively large diameter. We interpret these tubular structures as stained blood vessels supplying larger nerves (arteries or capillaries). ETB-R staining was observed on fibers or bundles co-labeled with NF200, and we interpret this as staining structures within nerve fibers. (Fig. 6)
In the epidermis and in deeper peripheral nerves, ETA-R and ETB-R immunoreactivity showed a similar pattern of distribution in CPIP and sham mice. Epidermal free nerve endings could not be observed with ETA-R or ETB-R antibodies.
3.3. Western Blotting
Quantification of ETA-R and ETB-R in skin and muscle of sham and CPIP mice
To quantify ETA-R and ETB-R in skin and muscle, sham and CPIP hind paw tissues were analyzed by Western blotting. ETA-R and ETB-R displayed relative molecular masses of 61 kDa and 59 kDa, respectively, under our experimental conditions (Fig. 7A). The skin of CPIP mice did not exhibit significant changes in the expression of either ETA-R or ETB-R, as compared to skin in sham mice (Fig. 7B). However, in the plantar muscles of CPIP mice, the density of both the ETA-R and ETB-R bands were significantly greater than in sham tissues (Fig. 7C).
Fig. 7. Quantification of ETA-R and ETB-R in the skin and muscle of sham and CPIP mice.
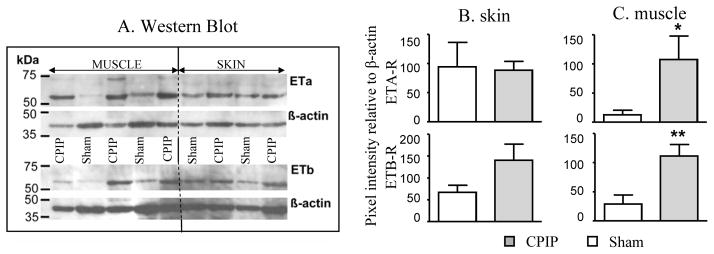
Western blot analysis of plantar skin and muscles extracts from sham and CPIP mice. The top membrane was incubated with an anti-ETA-R antibody (1:200, top picture; β-actin, bottom picture), and the bottom membrane was incubated with an anti-ETB-R antibody (1:200, top picture; β-actin, bottom picture). The five first lanes received supernatant from homogenized muscles; the four last lanes received supernatant from homogenized skin. A) The same lane reflects extracts from the same animal. B and C show the quantification by densitometry of the pixel intensity relative to β-actin for ETA-R and ETB-R for both plantar skin (B) and muscle (C). There was a significant upregulation of both ETA-R and ETB-R in CPIP muscle (A & C), but not skin (A & B) (* p < 0.05, ** P < 0.01 unpaired t-test).
Therefore, consistent with the immunohistochemistry results, the skin of CPIP mice did not exhibit significant changes in the level of ETA-R or ETB-R in comparison to sham mice. However, an increase of both ETA-R and ETB-R was detected in the plantar muscles of CPIP mice.
4. Discussion
Consistent with previous studies in both rodents and humans, we found that both ET-1 and ET-2 induce SNBs when locally injected in the mouse hind paw [8, 20, 29, 44]. Two days post-I/R injury, CPIP mice exhibited significantly more ET-1- and ET-2-induced SNBs than shams, with a significant leftward shift of the dose response curve for each. In various pain models (cancer, neuropathy and inflammation), local ET-1 induces more SNBs than in sham animals, and increases mechanical hypersensitivity (see Table 1). But to our knowledge, the present study is the first to demonstrate a leftward shift of the dose effect curve in a pathological condition. We suspect CPIP mice are particularly sensitive to ET-induced SNBs, greater so than other pain models. Our previous work shows that the hypersensitivity of CPIP mice to local ET-2-induced SNB is unique to this pain model. Unlike CPIP mice, mice with neuropathic (chronic constriction injury and spared nerve injury) or inflammatory (complete Freund’s adjuvant model) pain display the same level of local ET-2-induced SNBs as uninjured control mice ([32], unpublished data).
In both sham and CPIP mice, ET-1- and ET-2-induced SNBs seem to be mediated by local ETA-Rs, and were reduced by local pretreatment with BQ-123, an ETA-R antagonist. This observation is in line with current literature (see Table 1). However, BQ-123 was unable to alleviate mechanical or cold allodynia in CPIP mice. Others have tried to use peripheral ETA-Rs as targets for analgesics, but review of the literature does not allow convincing conclusions (see Table 1).
In the current study, local pretreatment with BQ-788 (60 nmol), an ETB-R antagonist, enhanced ET-1- and ET-2-induced SNBs in both sham and CPIP mice. The effects of local ETB-R antagonists on ET-induced SNBs seem controversial (see Table 1); however, these studies used a wide range of doses for BQ-788. Indeed, three studies demonstrate decreased ET-1-induced SNBs with local ETB-R antagonists (doses from 0.03 to 30 pmol), three studies show no effect (10 nmol for all of them), and only one study shows an increase of ET-1-induced SNBs (60 nmol). Our results mirror the literature since only the 60 nmol dose of BQ-788 enhanced ET-induced SNBs in CPIP mice. Moreover, local BQ-788 (60 nmol) enhanced cold allodynia in CPIP mice, but did not affect mechanical sensitivity.
In experiments here, local pretreatment with IRL-1620, an ETB-R agonist, reduced both ET-1- and ET-2-induced SNBs and mechanical allodynia in CPIP mice. Sham mice were not affected by this treatment. Again, few studies have shown analgesic effects of local ETB-R agonists on ET-1-induced nociception or tactile hypersensitivity in various pain models (Table 1). Taken together with earlier studies, the current results suggest that activating peripheral ETB-Rs produces analgesia in pathological pain conditions in rodents. Further support for an anti-nociceptive effect of ETB-R activation comes from studies showing that ETB-R-deficient mice are hypersensitive to mechanical stimuli [3].
It has been demonstrated that ET-1 is equipotent on both subtypes of endothelin receptors, while ET-2 has three times higher affinity for ETB-R than ETA-R [22]. This difference of affinity between ETA-R and ETB-R for the two peptides might explain why ET-1 is more potent at inducing SNBs than ET-2. The present study shows that in sham mice the ET-1 EC50 is 3 times lower than the ET-2 EC50, and this ratio is increased to 20 times in CPIP mice. The leftward shift of the dose response curves in CPIP mice for both ET-1- and ET-2-induced SNBs, as well as the analgesic effects of IRL-1620 observed only in CPIP mice, suggest there may be a selective increase of local ET receptors in CPIP mice. To test this hypothesis, we assessed the distribution (using immunohistochemistry) and quantity (using Western blotting) of ETA-R and ETB-R in the skin of CPIP compared to sham mice. However, our results demonstrate that the level of expression of endothelin receptors and their distribution in skin is unaffected by I/R injury. A change in affinity of endothelin for its receptors is also unlikely since in patients with critical limb ischemia the levels of ET-1 are increased, but not the number or affinity of ETA/ETB-Rs [10].
Khodorova et al. [24] argued that ETB-R agonist-mediated analgesia was mediated by a local opioidergic pathway, since it was reversed by local naloxone treatment. They suggested that ETB-R activity stimulated the release of β-endorphin from keratinocytes. Confirming Khodorova et al. [24] findings, we find ETB-R-immunoreactivity (IR) predominantly in the strati granulosum and spinosum layers of the mouse epidermis, in both sham and CPIP mice. These layers have been demonstrated to be mostly composed of keratinocytes that co-express ETB-R and β-endorphin [24]. Our results show that the deepest of these layers also express ETA-R, and CPIP and sham mice exhibit the same distribution of ETA-R/ETB-Rs. However, at the dose we used (50 pmol), IRL-1620-induced analgesia in CPIP is unlikely to be mediated by β-endorphin released from keratinocytes, since it was unaffected by local naloxone. In this regard, the present study does not confirm Khodorova findings, but the ETB-R agonist doses we used in CPIP mice were smaller than those used by Khodorova et al. [23]. Thus, we do not expect the release of β-endorphin by keratinocytes to be the primary mechanism of IRL-1620-induced analgesia.
In the deep nerve bundles projecting into the epidermis, we found a slight but consistent staining with ETA-R and ETB-R antibodies, but the epidermal free nerve endings derived from these ETA-R-ir or ETB-R-ir deep nerve bundles were never stained for ETA-R or ETB-R. The skin of CPIP and sham mice exhibited a similar distribution of ETA-R and ETB-R within the dermis, and deep nerve bundles. Pomonis et al. [43] showed that ETA-R–ir was present on rat dorsal root ganglion neurons and their axons, while ETB-R–ir was localized on satellite and Schwann cells in dorsal root ganglia. However, Berti-Mattera et al. [3] found both receptors were expressed in sciatic nerves. Previous studies demonstrated the presence of endothelin receptors within peripheral nerve, but whether or not they are localized directly on nociceptor endings is unknown. Gokin et al. [17] showed that subcutaneous ET-1 injection selectively excited C- and Aδ-, but not Aβ-, fibers in the rat sciatic nerve. The ET-1-induced activation of these fibers was abolished by local BQ-123 treatment. This experiment clearly demonstrated the direct pro-nociceptive effects of ETA-R activation. Unfortunately, these authors did not test the ability of ETB-R ligands to reverse or augment ET-1-induced nociceptor activation. Although Plant et al. [42] showed co-localization of ETA-R and TRPV1 in dorsal root ganglia neurons, further studies are needed to characterize the exact distribution of ETA-R and ETB-R on nociceptor endings, and their role in nociception.
Consistent with our immunohistochemistry results, the Western blot studies showed that the skin levels of ETA-R and ETB-R were not different between sham and CPIP mice. Alternatively, we found a strong difference of expression of both receptors in the plantar muscle between sham and CPIP mice, with an upregulation of both ETA-R and ETB-R in CPIP mice. Other studies also demonstrated clear changes in expression of ETA-R and/or ETB-R in neuropathic pain conditions in rodents. After chronic constriction injury of the sciatic nerve in rodents, Klass et al. [26] have shown increased ETA-R and decreased ETB-R levels at the injury site. Moreover, Berti-Mattera et al. [3] found a decrease of ETB-R in sciatic nerve, but not in DRG of diabetic rats, while ETA-R levels were unchanged. More recently, Rey et al. [46] showed that both ETA-R and ETB-R are found on the sympathetic innervation of blood vessels, and that ETB-R but not ETA-R, was increased after repeated short-term I/R injury in cat. Further investigation of sensory innervation of muscle after I/R injury are needed.
CPIP rats have persistent capillary no-reflow in their hind paw muscles and exhibit abnormal collapsed capillaries [27]. They also exhibit enhanced vasoconstrictive and painful responses to intrarterial and intracutaneous norepinephrine, respectively [54], and a reduction in allodynia after sympatholytic treatments [53], suggesting they have sympathetically-maintained pain (SMP). The presence of ETA-Rs and ETB-Rs on sympathetic innervation of blood vessels, and the reported over-expression of ETA-R and ETB-R in muscle after I/R injury [28, 46] suggest there may be a contribution of endothelin receptors to SMP in CRPS-I.
CONCLUSIONS
This study clearly shows that the involvement of both ETA-R and ETB-R in nociception differs between sham and CPIP mice. CPIP mice are more sensitive to local endothelin-induced SNBs, develop mechanisms of local ETB-R agonist-induced analgesia, and exhibit over-expression of both receptors in plantar muscles, but not skin. The effectiveness of local ETB-R agonists as anti-allodynic treatments in CPIP mice holds promise for novel therapies in CRPS-I patients. However, further investigations are needed to characterize the mode of action of IRL-1620-induced analgesia. It is possible that IRL-1620 produces analgesia due to a vasodilator action that might aid hind paw reperfusion in CPIP mice, although additional studies are needed to test this hypothesis. The levels of ET-1 and ET-2, as well as the specific localization of ETA-R and ETB-R within the muscle of CPIP mice also need to be further examined.
Summary.
Mice with chronic post-ischemia pain exhibit more local endothelin-induced nociceptive behaviors, a novel local ETB receptor agonist-induced analgesia, and over-expression of both ETA and ETB receptors in plantar muscles, but not skin.
Acknowledgments
The authors wish to thank Dr. Alfredo Ribeiro-da-Silva and Manon Saint-Louis, for help with immunohistochemistry, Dr. Louis-Etiennes Lorenzo and Anna Taylor for their expertise in microscopy. They also wish to thank Jennifer Peleshok for assistance with protein measurement Dr Julie Desbarats for loan of equipment, and Dr Theodore Price and Dr Gary Bennett for their fruitful discussions. This work was supported by grants from CIHR and the Louise and Alan Edwards Foundation to TJC. MM was supported by an AstraZeneca/AECRP postdoctoral fellowship.
Footnotes
5. Conflict of Interest Statement: The authors declare there are no conflicts of interest.
References
- 1.Baamonde A, Lastra A, Fresno MF, Llames S, Meana A, Hidalgo A, Menéndez L. Implantation of tumoral XC cells induces chronic, endothelin-dependent, thermal hyperalgesia in mice. Cell Mol Neurobiol. 2004;24:269–81. doi: 10.1023/B:CEMN.0000018621.58328.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baamonde A, Lastra A, Villazón M, Bordallo J, Hidalgo A, Menéndez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–51. doi: 10.1007/s00210-003-0841-1. [DOI] [PubMed] [Google Scholar]
- 3.Berti-Mattera LN, Gariepy CE, Burke RM, Hall AK. Reduced expression of endothelin B receptors and mechanical hyperalgesia in experimental chronic diabetes. Exp Neurol. 2006;201:399–406. doi: 10.1016/j.expneurol.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 5.Chichorro JG, Zampronio AR, Rae GA. Endothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp Biol Med (Maywood) 2006;231:1136–40. [PubMed] [Google Scholar]
- 6.Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123:64–74. doi: 10.1016/j.pain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hind paw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.da Cunha JM, Rae GA, Ferreira SH, de Cunha FQ. Endothelins induce ETB receptor-mediated mechanical hypernociception in rat hind paw: roles of cAMP and protein kinase C. Eur J Pharmacol. 2004;501:87–94. doi: 10.1016/j.ejphar.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Daher JB, Souza GE, D’Orléans-Juste P, Rae GA. Endothelin ETB receptors inhibit articular nociception and priming induced by carrageenan in the rat knee-joint. Eur J Pharmacol. 2004;496:77–85. doi: 10.1016/j.ejphar.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Dashwood MR, Tsui JC. The effect of acute ischemia on ET-1 and its receptors in patients with underlying chronic ischemia of the lower limb. Exp Biol Med (Maywood) 2006;231:802–5. [PubMed] [Google Scholar]
- 11.Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9:2279–83. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- 12.Davenport AP, Maguire JJ. Endothelin. Handb Exp Pharmacol. 2006;176:295–329. doi: 10.1007/3-540-32967-6_9. [DOI] [PubMed] [Google Scholar]
- 13.De-Melo JD, Tonussi CR, D’Orléans-Juste P, Rae GA. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998;77:261–9. doi: 10.1016/S0304-3959(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 14.De-Melo JD, Tonussi CR, D’Orléans-Juste P, Rae GA. Effects of endothelin-1 on inflammatory incapacitation of the rat knee joint. J Cardiovasc Pharmacol. 1998;31:S518–20. doi: 10.1097/00005344-199800001-00149. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg E, Erlich T, Zinder O, Lichinsky S, Diamond E, Pud D, Davar G. Plasma endothelin-1 levels in patients with complex regional pain syndrome. Eur J Pain. 2004;8:533–8. doi: 10.1016/j.ejpain.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Fujita M, Andoh T, Saiki I, Kuraishi Y. nvolvement of Endothelin and ET(A) Endothelin Receptor in Mechanical Allodynia in Mice Given Orthotopic Melanoma Inoculation. J Pharmacol Sci. 2008;106:257–63. doi: 10.1254/jphs.fp0072051. [DOI] [PubMed] [Google Scholar]
- 17.Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21:5358–66. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet Disord. 2006;7:91. doi: 10.1186/1471-2474-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett. 2007;418:117–21. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Hans G, Deseure K, Adriaensen H. Endothelin-1-induced pain and hyperalgesia: A review of pathophysiology, clinical manifestations and future therapeutic options. Neuropeptides. 2008;42:119–32. doi: 10.1016/j.npep.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Houck CS, Khodorova A, Reale AM, Strichartz GR, Davar G. Sensory fibers resistant to the actions of tetrodotoxin mediate nocifensive responses to local administration of endothelin-1 in rats. Pain. 2004;110:719–26. doi: 10.1016/j.pain.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal J, Sanghia R, Das SK. ndothelin receptor antagonists: an overview of their synthesis and structure-activity relationship. Mini Rev Med Chem. 2005;5:381–408. doi: 10.2174/1389557053544010. [DOI] [PubMed] [Google Scholar]
- 23.Khodorova A, Fareed MU, Gokin A, Strichartz GR, Davar G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci. 2002;22:7788–96. doi: 10.1523/JNEUROSCI.22-17-07788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–61. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 25.Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klass M, Hord A, Wilcox M, Denson D, Csete M. role for endothelin in neuropathic pain after chronic constriction injury of the sciatic nerve. Anesth Analg. 2005;101:1757–62. doi: 10.1213/01.ANE.0000180766.74782.7E. [DOI] [PubMed] [Google Scholar]
- 27.Laferrière A, Millecamps M, Xanthos DN, Xiao WH, Siau C, de Mos M, Sachot C, Ragavendran JV, Huygen FJ, Bennett GJ, Coderre TJ. Cutaneous tactile allodynia associated with microvascular dysfunction in muscle. Mol Pain. 2008;4:49. doi: 10.1186/1744-8069-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maddahi A, Edvinsson L. Enhanced expressions of microvascular smooth muscle receptors after focal cerebral ischemia occur via the MAPK MEK/ERK pathway. BMC Neurosci. 2008;9:85. doi: 10.1186/1471-2202-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKelvy AD, Mark TR, Sweitzer SM. Age- and sex-specific nociceptive response to endothelin-1. J Pain. 2007;8:657–66. doi: 10.1016/j.jpain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Menéndez L, Lastra A, Hidalgo A, Baamonde A. Nociceptive reaction and thermal hyperalgesia induced by local ET-1 in mice: a behavioral and Fos study. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:28–34. doi: 10.1007/s00210-002-0655-6. [DOI] [PubMed] [Google Scholar]
- 31.Millecamps M, Coderre TJ. Rats with chronic post-ischemia pain exhibit an analgesic sensitivity profile similar to human patients with complex regional pain syndrome - type I. Eur J Pharmacol. 2008;583:97–102. doi: 10.1016/j.ejphar.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millecamps M, Coderre TJ. Role of peripheral endothelin receptors in an animal model of CRPS-type I. Society for Neuroscience, Annual meeting; San Diego. 2007 Nov. 4; p. Abstract# 181.4. [Google Scholar]
- 33.Motta EM, Chichorro JG, Rae GA. Role of ET(A) and ET(B) endothelin receptors on endothelin-1-induced potentiation of nociceptive and thermal hyperalgesic responses evoked by capsaicin in rats. Neurosci Lett. 2009a;457:146–150. doi: 10.1016/j.neulet.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 34.Motta EM, Chichorro JG, D’Orléans-Juste P, Rae GA. Roles of endothelin ETA and ETB receptors in nociception and chemical, thermal and mechanical hyperalgesia induced by endothelin-1 in the rat hindpaw. Peptides. 2009b;30:918–925. doi: 10.1016/j.peptides.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Mujenda FH, Duarte AM, Reilly EK, Strichartz GR. Cutaneous endothelin-A receptors elevate post-incisional pain. Pain. 2007;133:161–73. doi: 10.1016/j.pain.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munts AG, Zijlstra FJ, Nibbering PH, Daha MR, Marinus J, Dahan A, van Hilten JJ. Analysis of cerebrospinal fluid inflammatory mediators in chronic complex regional pain syndrome related dystonia. Clin J Pain. 2008;24:30–4. doi: 10.1097/AJP.0b013e318156d961. [DOI] [PubMed] [Google Scholar]
- 37.Namer B, Hilliges M, Orstavik K, Schmidt R, Weidner C, Torebjörk E, Handwerker H, Schmelz M. Endothelin 1 activates and sensitizes human C-nociceptors. Pain. 2008;137:41–9. doi: 10.1016/j.pain.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Piovezan AP, D’Orléans-Juste P, Tonussi CR, Rae GA. Endothelins potentiate formalin-induced nociception and paw edema in mice. Can J Physiol Pharmacol. 1997;75:596–600. [PubMed] [Google Scholar]
- 39.Piovezan AP, D’Orléans-Juste P, Tonussi CR, Rae GA. Effects of endothelin-1 on capsaicin-induced nociception in mice. Eur J Pharmacol. 1998;351:15–22. doi: 10.1016/s0014-2999(98)00281-7. [DOI] [PubMed] [Google Scholar]
- 40.Piovezan AP, D’Orléans-Juste P, Souza GE, Rae GA. Endothelin-1-induced ET(A) receptor-mediated nociception, hyperalgesia and oedema in the mouse hind-paw: modulation by simultaneous ET(B) receptor activation. Br J Pharmacol. 2000;129:961–8. doi: 10.1038/sj.bjp.0703154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piovezan AP, D’Orléans-Juste P, Frighetto M, Souza GE, Henriques MG, Rae GA. Endothelins contribute towards nociception induced by antigen in ovalbumin-sensitised mice. Br J Pharmacol. 2004;141:755–63. doi: 10.1038/sj.bjp.0705663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plant TD, Zöllner C, Mousa SA, Oksche A. Endothelin-1 potentiates capsaicin-induced TRPV1 currents via the endothelin A receptor. Exp Biol Med (Maywood) 2006;231:1161–4. [PubMed] [Google Scholar]
- 43.Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;21:999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raffa RB, Jacoby HI. Endothelin-1, -2 and -3 directly and big-endothelin-1 indirectly elicit an abdominal constriction response in mice. Life Sci. 1991;48:PL85–90. doi: 10.1016/0024-3205(91)90130-4. [DOI] [PubMed] [Google Scholar]
- 45.Raffa RB, Schupsky JJ, Lee DK, Jacoby HI. Characterization of endothelin-induced nociception in mice: evidence for a mechanistically distinct analgesic model. J Pharmacol Exp Ther. 1996;278:1–7. [PubMed] [Google Scholar]
- 46.Rey S, Corthorn J, Chacón C, Iturriaga R. Expression and immunolocalization of endothelin peptides and its receptors, ETA and ETB, in the carotid body exposed to chronic intermittent hypoxia. J Histochem Cytochem. 2007;55:167–74. doi: 10.1369/jhc.6A7079.2006. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, Jordan RC. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. 2007;11:406–14. doi: 10.1016/j.ejpain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Seo HS, Kim HW, Roh DH, Yoon SY, Kwon YB, Han HJ, Chung JM, Beitz AJ, Lee JH. A new rat model for thrombus-induced ischemic pain (TIIP); development of bilateral mechanical allodynia. Pain. 2008;139:520–532. doi: 10.1016/j.pain.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 49.Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–33. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 50.Verri WA, Jr, Schivo IR, Cunha TM, Liew FY, Ferreira SH, Cunha FQ. Interleukin-18 induces mechanical hypernociception in rats via endothelin acting on ETB receptors in a morphine-sensitive manner. J Pharmacol Exp Ther. 2004;310:710–7. doi: 10.1124/jpet.103.063990. [DOI] [PubMed] [Google Scholar]
- 51.Verri WA, Jr, Molina RO, Schivo IR, Cunha TM, Parada CA, Poole S, Ferreira SH, Cunha FQ. Nociceptive effect of subcutaneously injected interleukin-12 is mediated by endothelin (ET) acting on ETB receptors in rats. J Pharmacol Exp Ther. 2005;315:609–615. doi: 10.1124/jpet.105.089409. [DOI] [PubMed] [Google Scholar]
- 52.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Liew FY, Ferreira SH, Cunha FQ. Antigen-induced inflammatory mechanical hypernociception in mice is mediated by IL-18. Brain Behav Immun. 2007;21:535–43. doi: 10.1016/j.bbi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Xanthos DN, Coderre TJ. Sympathetic vasoconstrictor antagonism and vasodilatation relieve mechanical allodynia in rats with chronic postischemia pain. J Pain. 2008;9:423–433. doi: 10.1016/j.jpain.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xanthos DN, Bennett GJ, Coderre TJ. Norepinephrine-induced nociception and vasoconstrictor hypersensitivity in rats with chronic post-ischemia pain. Pain. 2008;137:640–651. doi: 10.1016/j.pain.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuyama H, Koakutsu A, Fujiyasu N, Tanahashi M, Fujimori A, Sato S, Shibasaki K, Tanaka S, Sudoh K, Sasamata M, Miyata K. Effects of selective endothelin ET(A) receptor antagonists on endothelin-1-induced potentiation of cancer pain. Eur J Pharmacol. 2004;492:177–82. doi: 10.1016/j.ejphar.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 56.Zhao YD, Springall DR, Wharton J, Polak JM. Autoradiographic localization of endothelin-1 binding sites in porcine skin. J Invest Dermatol. 1991;96:152–4. doi: 10.1111/1523-1747.ep12515951. [DOI] [PubMed] [Google Scholar]