Abstract
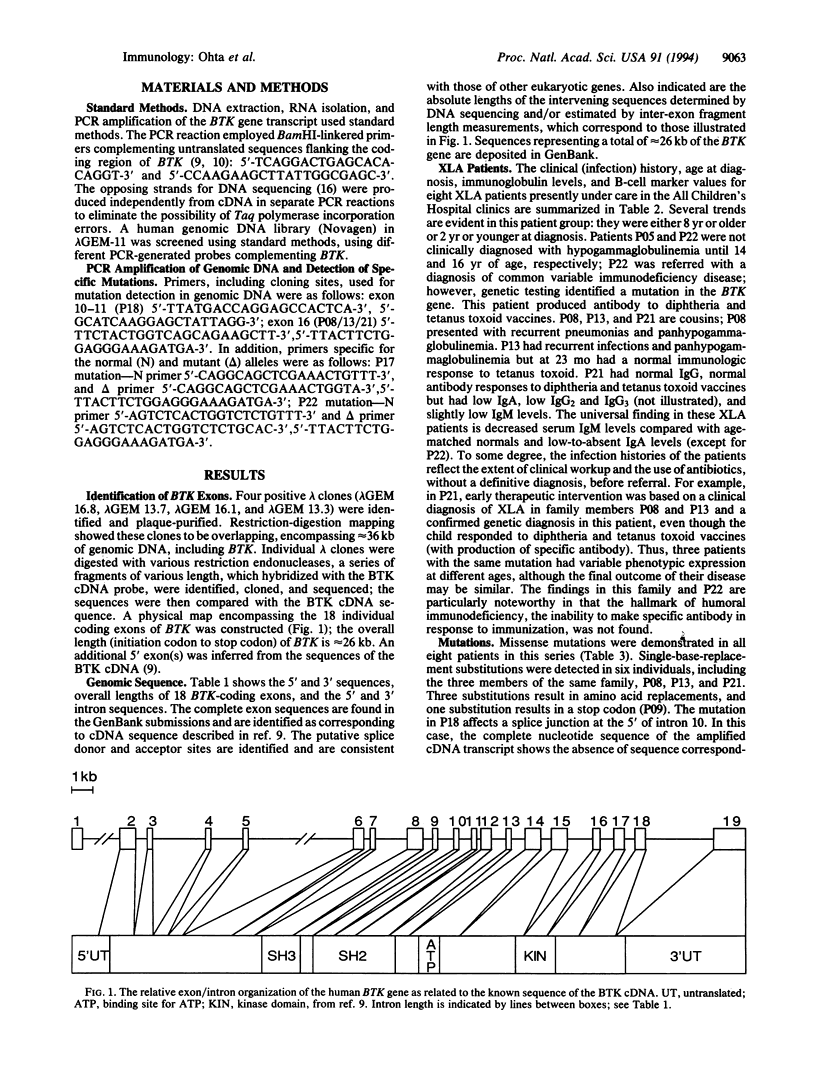
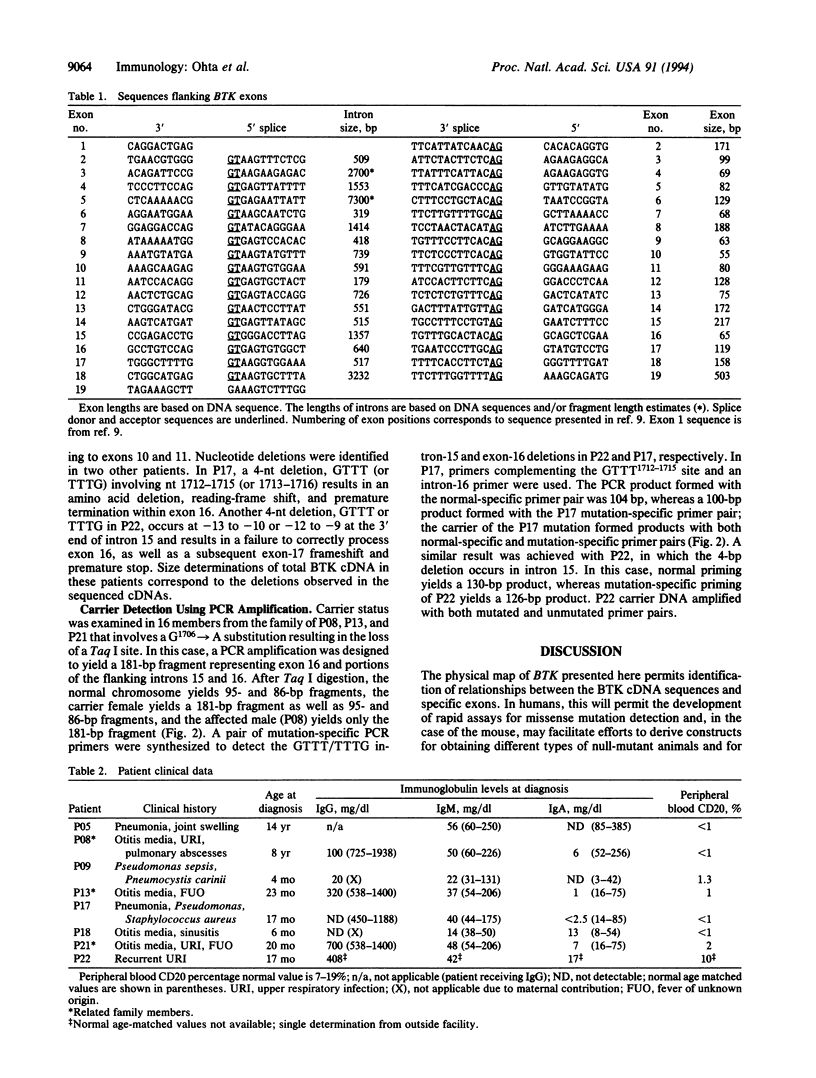
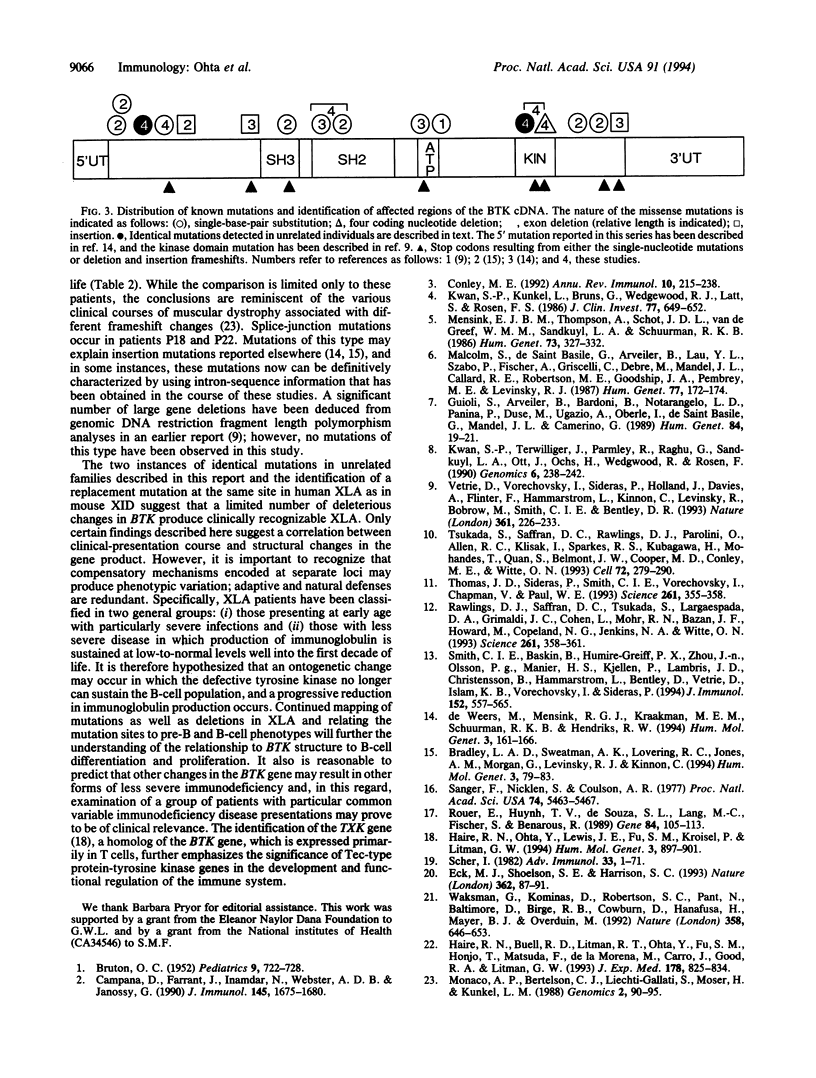
X chromosome-linked agammaglobulinemia is a life-threatening disease that involves a failure in normal development of B lymphocytes and is associated with missense mutations in BTK, a gene encoding a cytoplasmic tyrosine kinase (Bruton agammaglobulinemia tyrosine kinase, EC 2.7.1.112), a member of the Tec family of protein-tyrosine kinases. The genomic organization has been determined by using conventional restriction fragment mapping, extended DNA sequencing, and PCR fragment-sizing approaches. The DNA sequences of the 18 coding exons composing BTK and their flanking-region sequences are reported; an additional exon(s) encodes a 5' untranslated segment. Single-base-pair substitutions and 4-nt deletions resulted in amino acid replacement, premature termination, frameshift, and exon deletion in a group of X chromosome-linked agammaglobulinemia patients exhibiting different clinical presentations and courses. The nature of the mutations is interpreted in terms of the genomic organization of the BTK gene and the disease course in individual patients. Several examples are found in which the same mutation occurs in unrelated patients, and one of these mutations occurs at the same codon that is substituted in the murine form of BTK, resulting in X chromosome-linked immunodeficiency disease. Considerable variation in presentation and disease course in X chromosome-linked agammaglobulinemia appears associated with the nature and position of different missense mutations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUTON O. C. Agammaglobulinemia. Pediatrics. 1952 Jun;9(6):722–728. [PubMed] [Google Scholar]
- Bradley L. A., Sweatman A. K., Lovering R. C., Jones A. M., Morgan G., Levinsky R. J., Kinnon C. Mutation detection in the X-linked agammaglobulinemia gene, BTK, using single strand conformation polymorphism analysis. Hum Mol Genet. 1994 Jan;3(1):79–83. doi: 10.1093/hmg/3.1.79. [DOI] [PubMed] [Google Scholar]
- Campana D., Farrant J., Inamdar N., Webster A. D., Janossy G. Phenotypic features and proliferative activity of B cell progenitors in X-linked agammaglobulinemia. J Immunol. 1990 Sep 15;145(6):1675–1680. [PubMed] [Google Scholar]
- Conley M. E. Molecular approaches to analysis of X-linked immunodeficiencies. Annu Rev Immunol. 1992;10:215–238. doi: 10.1146/annurev.iy.10.040192.001243. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Shoelson S. E., Harrison S. C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Guioli S., Arveiler B., Bardoni B., Notarangelo L. D., Panina P., Duse M., Ugazio A., Oberlé I., de Saint Basile G., Mandel J. L. Close linkage of probe p212 (DXS178) to X-linked agammaglobulinemia. Hum Genet. 1989 Dec;84(1):19–21. doi: 10.1007/BF00210664. [DOI] [PubMed] [Google Scholar]
- Haire R. N., Buell R. D., Litman R. T., Ohta Y., Fu S. M., Honjo T., Matsuda F., de la Morena M., Carro J., Good R. A. Diversification, not use, of the immunoglobulin VH gene repertoire is restricted in DiGeorge syndrome. J Exp Med. 1993 Sep 1;178(3):825–834. doi: 10.1084/jem.178.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haire R. N., Ohta Y., Lewis J. E., Fu S. M., Kroisel P., Litman G. W. TXK, a novel human tyrosine kinase expressed in T cells shares sequence identity with Tec family kinases and maps to 4p12. Hum Mol Genet. 1994 Jun;3(6):897–901. doi: 10.1093/hmg/3.6.897. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Kunkel L., Bruns G., Wedgwood R. J., Latt S., Rosen F. S. Mapping of the X-linked agammaglobulinemia locus by use of restriction fragment-length polymorphism. J Clin Invest. 1986 Feb;77(2):649–652. doi: 10.1172/JCI112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S. P., Terwilliger J., Parmley R., Raghu G., Sandkuyl L. A., Ott J., Ochs H., Wedgwood R., Rosen F. Identification of a closely linked DNA marker, DXS178, to further refine the X-linked agammaglobulinemia locus. Genomics. 1990 Feb;6(2):238–242. doi: 10.1016/0888-7543(90)90562-9. [DOI] [PubMed] [Google Scholar]
- Malcolm S., de Saint Basile G., Arveiler B., Lau Y. L., Szabo P., Fischer A., Griscelli C., Debre M., Mandel J. L., Callard R. E. Close linkage of random DNA fragments from Xq 21.3-22 to X-linked agammaglobulinaemia (XLA). Hum Genet. 1987 Oct;77(2):172–174. doi: 10.1007/BF00272387. [DOI] [PubMed] [Google Scholar]
- Mensink E. J., Thompson A., Schot J. D., van de Greef W. M., Sandkuyl L. A., Schuurman R. K. Mapping of a gene for X-linked agammaglobulinemia and evidence for genetic heterogeneity. Hum Genet. 1986 Aug;73(4):327–332. doi: 10.1007/BF00279095. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993 Jul 16;261(5119):358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Rouer E., Van Huynh T., Lavareda de Souza S., Lang M. C., Fischer S., Benarous R. Structure of the human lck gene: differences in genomic organisation within src-related genes affect only N-terminal exons. Gene. 1989 Dec 7;84(1):105–113. doi: 10.1016/0378-1119(89)90144-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Smith C. I., Baskin B., Humire-Greiff P., Zhou J. N., Olsson P. G., Maniar H. S., Kjellén P., Lambris J. D., Christensson B., Hammarström L. Expression of Bruton's agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol. 1994 Jan 15;152(2):557–565. [PubMed] [Google Scholar]
- Thomas J. D., Sideras P., Smith C. I., Vorechovský I., Chapman V., Paul W. E. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993 Jul 16;261(5119):355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Vorechovský I., Sideras P., Holland J., Davies A., Flinter F., Hammarström L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993 Jan 21;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Waksman G., Kominos D., Robertson S. C., Pant N., Baltimore D., Birge R. B., Cowburn D., Hanafusa H., Mayer B. J., Overduin M. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature. 1992 Aug 20;358(6388):646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]
- de Weers M., Mensink R. G., Kraakman M. E., Schuurman R. K., Hendriks R. W. Mutation analysis of the Bruton's tyrosine kinase gene in X-linked agammaglobulinemia: identification of a mutation which affects the same codon as is altered in immunodeficient xid mice. Hum Mol Genet. 1994 Jan;3(1):161–166. doi: 10.1093/hmg/3.1.161. [DOI] [PubMed] [Google Scholar]



