Abstract
Gray matter loss in cortical regions is a normal ageing process for the healthy brain. There have been few studies on the process of ageing of the brain in chronic neurological disorders. In this study, we evaluated changes in the cortical thickness by age in 92 female subjects (46 migraine patients, and 46 healthy controls) using high field MRI. The results indicate that in contrast to healthy subjects migraineurs show lack of thinning in the insula by age. The functional significance of the lack of thinning is unknown, but may contribute to the overall cortical hyperexcitability of the migraine brain since the region is tightly involved in a number of majo brain networks involved in interoception, salience, nociception, and autonomic function, including the default mode network.
Keywords: Migraine, Headache, Women, Ageing, Aging, MRI, Imaging, Insula
Introduction
Cortex is the substrate for many of the abnormalities observed in the migraine even during the migraine-free periods. As such it represents a potential target for alterations in processes that may affect gray matter thickness. A number of studies have already provided evidence for structural changes or abnormalities in the cortex [1, 2] in migraine. Such evidence include cortical abnormalities in the frontal [3], and parietal lobes [3, 4], anterior cingulate cortex [5–7], hippocampus [8], primary somatosensory cortex [9], and insula [5, 6, 10, 11].
While multiple cortical and subcortical brain regions may be involved in migraine pathophysiology (e.g., thalamus), the insula is in a prominent position (involved in interoception [14], emotion [15], autonomic function [16, 17], salience [18], and multiple sensory modalities [19, 20]) to be part of the cortical expression and integration of many of the symptoms that occur during migraine. These include headache pain and autonomic processes [23–25]. In humans, insular involvement in migraine has been reported in a number of functional imaging studies [26–30]. We recently reported that insula was thicker in migraineurs vs. healthy controls and this effect was greater in female migraineurs [11]. Sex-related changes in women may result from an innate genetic or epigenetic process in female migraineurs. Alternatively it may relate to sex related hormonal changes that may result in the observed prevalence [31].
One way to evaluate the changes in the brain is to determine if the morphology as measured by cortical thickness changes with age since aging is a process that involves several changes in the brain including cortical thinning. Therefore, we hypothesized that we would observe differences in the brain of female migraineurs over time compared with healthy controls. Such changes may correlate with putative regions of the structure based on afferent drive (sensory), autonomic, or interoceptive function (see [22]). While a number of brain areas show volumetric changes in migraineurs [3, 4, 11, 32], reports of sex-related measures in female migraineurs are novel and evaluation of age-related changes may confer a basis for new understanding of the neurobiology of the brain morphometric changes associated with migraine. The underlying rationale is that the expected changes in cortical regions with age normally correlates with a reduction of the volume or thinning in the brain cortical regions; a normal process that occurs non-uniformly across the cortex with aging [33]. Here we evaluated cortical thickness in female migraineurs compared with healthy controls. Our findings suggest that in migraineurs the insula maintains its cortical thickness with age, whereas in healthy controls it becomes progressively thinner with time [34]. This is particularly of interest since among all cortical areas, the insula does have an accelerated relative gray matter loss that is approximately double the global cortex (i.e., whole cortical) in healthy subjects [34].
Methods
In this study, we measured cortical thickness in a cohort of female migraine patients and a cohort of female age-matched healthy control subjects in an age range of 20 to 65 years using high-resolution structural imaging of the brain in a 3.0 Tesla Magnetic Resonance Imaging (MRI) scanner. The institutional review board at McLean Hospital approved the study and it met the criteria for experimentation of pain in human subjects (http://history.nih.gov/research/downloads/helsinki.pdf). Informed consent was obtained from all subjects prior to participation in the study.
Subjects
Ninety-two age-matched female subjects (N=46 migraine patients, and N=46 healthy control) underwent an imaging session at McLean Hospital. Twenty-two subjects (N=11 migraine patients, and N=11 healthy control) had participated in our previous study [11]. Patients were selected according to the International Classification for Headache (ICHD-II) [35] definition for episodic migraine and the diagnosis of the migraine was confirmed by a neurologist (DB or SS) prior to the scanning. In order to be included in the study, they had to have suffered from migraines for more than three years. All of the participants were screened for depression and all had Beck Depression Inventory II (BDI-II) scores < 25. All of the participants were screened by a urine test for opioids or barbiturates before the MRI scan. Subjects were not scanned if they showed positive results on any of the tests. The healthy participants had no significant history of pain and had no history of any chronic pain, psychiatric or neurological disorder or any other major disease. All of the participating patients in the study were migraine free at least 48 hours before the MRI scan and had refrained from taking any medication before the scan. A detailed migraine history was gathered from the patients that included information on the average frequency and laterality of the migraine attacks over the year preceding the scan. For the latter the patients were asked to indicate the percentage of the time that they felt the pain on either side (left or right) of their head.
Brain Imaging
The subjects underwent a MRI scan session of structural imaging under a 3.0T Siemens MRI scanner. 3D MPRAGE pulse sequence (TR/TE/TI=2100/2.74/1100ms, FA=12, 128 sagittal slices, res = 1.33 × 1.0 × 1.0mm3) was used for collecting the data.
Data Analysis
Images were processed offline using automatic parcellation tools of the Freesurfer image analysis software (http://surfer.nmr.mgh.harvard.edu/). These tools enable detection of sub-millimeter changes in cortical thickness. First, an atlas-based Bayesian segmentation procedure [36] was used to label subcortical and cortical tissue classes followed by motion corrected and the non-brain tissue was removed [37]. Following to the subcortical segmentation [36, 38], the gray matter/white matter boundary was tessellated. Then an automated topology correction was performed [39, 40] followed by surface deformation [41] to reconstruct the cortex that allowed vertex-wise measurements on the cortical thickness. The data for each subject was resampled to an average brain and smoothed (10mm full-width half maximum Gaussian Kernel) for group comparison. Lower smoothing kernels (ex. <5mm) were avoided to reduce the potential of preserving smaller clusters with low significance.
This method of reconstruction of the cortical thickness surfaces uses both intensity and continuity information from the entire 3D image volume in segmentation as well as deformation procedures to reconstruct cortical thickness. The maps are created using spatial intensity gradients across tissue classes and therefore are not simply reliant on absolute signal intensity. Therefore while the image voxel resolution of the data used in this study was 1×1×1.3mm, the produced cortical thickness maps are not restricted to the voxel resolution of the data [41] and thus are capable of detecting sub-millimeter differences between groups [41]. This capability has been validated by acquiring images of autopsy of the brains and comparing the measures obtained from imaging to the measures obtained using traditional neuropathology methods. The differences between the two methods were reported to be statistically indistinguishable [42].
Statistical Analysis
Group level statistical analysis was performed using the Freesurfer QDEC analysis tool such that a general linear model was fitted at each surface vertex with diagnosis (healthy or migraine) as a grouping factor, age as covariate and total intracranial volume as nuisance covariate to account for differences in the cranium size. The z-statistic surface maps were then cluster-wise corrected for multiple comparisons [43] by method of Monte-Carlo simulation [44] (10,000 iterations) with cluster-forming threshold set to p < 0.05. The significance of the clusters from the analysis were then tested against an empirical null distribution of maximum cluster size created by synthesized z-distributed data across 10,000 permutations, producing clusters wise p-values corrected for multiple comparisons. In addition, a region of interest (ROI) was created from these clusters in each hemisphere that was resampled back to each subject’s native anatomical space to measure the average cortical thickness in the corresponding ROIs for each subject. In the patient cohort the correlation of these measures with the disease duration was assessed.
Overlap between Structural Differences and Insular Functional Organization
Once the statistical contrast maps of the cortical changes with age were generated, a functional map of the insula [22, 45] was overlaid on the results in order to define overlaps between the structural differences and to delineate the potential correlation of the changes with putative functions of the insula. While this is an approximation, the overlap segregates the major functional components of the insula as is currently understood in the literature [21, 22]. The functional map is based on resting state functional connectivity where insular voxels were segregated in to clusters that were associated with brain regions associated with these functions [45]. Three major functional areas are used here.
Results
Subjects
Forty-six migraine patients both with and without aura were included in this study. Per the inclusion criteria of the study, these patients were free from other chronic/functional pain disorders and migraine was the only chronic pain disorder that they were suffering from at the time of the study. They also did not have any major medical illness history (such as seizure disorder, diabetes, alcoholism, cardiac disease, psychiatric problems; drug or alcohol addiction, respiratory problems, liver disease, etc.). All of the patients were migraine-free at least 48 hours before their imaging visits and the visits were rescheduled if they had a migraine during that time frame. The demographics of the subjects participating in the study are presented in Table 1. Frequency of migraine attacks in this cohort of patients ranged from low (1–2/attacks per month) to intermediate (2 < and < 6) to high (>8 attacks per month) with a comparable number of patients across all frequency categories (see Table 2). The patients ranged in age from 20 to 61 year. The breakdown of the subjects per age group is presented in Table 3. Consistent with the pattern of the migraine disease prevalence in the population, in our patient cohort the maximum number belonged to the cohort of patients <29 yrs. of age. The migraineurs had suffered from migraine for an average duration of 15.6±9.5 (mean ± SD) years. There was a significant correlation (R2=0.804, p-value=2.4e-10) between the age and the disease duration. The majority of the patients were taking abortive medication such as Advil, Excedrin, and Triptans such as Imitrex, Maxalt and Zomig for their migraines. Some of the patients were on preventive medications such as Topomax. All of the patients skipped taking any medication for 24 hours prior their scan to avoid any potential acute drug effects on the imaging data.
Table 1.
Demographic Data Of the Study Subjects
| Cohort | Subjects | Gender | Age Range | Age | Duration |
|---|---|---|---|---|---|
| Migraine | 46 | Female | 20 to 61 | 34.7 (10.4) | 15.6 (9.5) |
| Control | 46 | Female | 20 to 65 | 34.1 (10.6) | - |
Key: Age and age range values are reported as mean (standard deviation).
Table 2.
Frequency and Dominant Side of Migraine Attacks in Patients
| Frequency (per month) | Subjects | Right | Left | Both |
|---|---|---|---|---|
| Low (< 2) | 13 (27.5%) | 6 | 2 | 5 |
| Intermediate (2 < and < 6) | 17 (37.5%) | 7 | 3 | 7 |
| High (> 8) | 16 (35%) | 3 | 5 | 8 |
|
| ||||
| Total | 46 (100%) | 16 (35%) | 10 (22.5%) | 20 (42.5%) |
Table 3.
Breakdown of Subjects per Age Bracket
| Age | Migraine | Healthy |
|---|---|---|
| 20 – 29 | 18 | 20 |
| 30 – 39 | 11 | 11 |
| 40 – 49 | 11 | 11 |
| 50+ | 6 | 4 |
|
| ||
| Total | 46 | 46 |
Cortical Thickness
The results of the comparison are shown in Figure 1 and Table 4. The data indicate a significant difference in the effect of ageing on the insula in migraine patients as compared to healthy subjects (left (p < 0.046) and more predominantly in the right side (p < 0.002). The overlap between these clusters and the resting-state based functional connectivity map of the insula is presented in Figure 2. There was also higher cortical thickness in migraineurs in other areas including the superior frontal gyrus (p < 0.026), paracentral gyrus (p < 0.011), temporal pole (p < 0.044), and precuneus (p < 0.046). These results are presented in Figure 3, Table 5. There were no differences between the groups in the whole brain gray matter (GM) volume (p=0.151). There was significant correlation of GM with the main effect of age independent of group (main effect of age: p<0.016) but there was no age-by-group interaction on whole brain GM volume (p=0.358). In the patient group, there was no correlation between the mean insular thickness and disease duration in ROIs defined based on the regions showing contrast in correlation with age in migraine vs. healthy control cohort (LH: P=0.187, RH: P=0.291). In addition, there was no correlation between GM volume and the migraine duration (p=0.776) accounting for differences in age.
Figure 1. Cortical Thickness Maps.
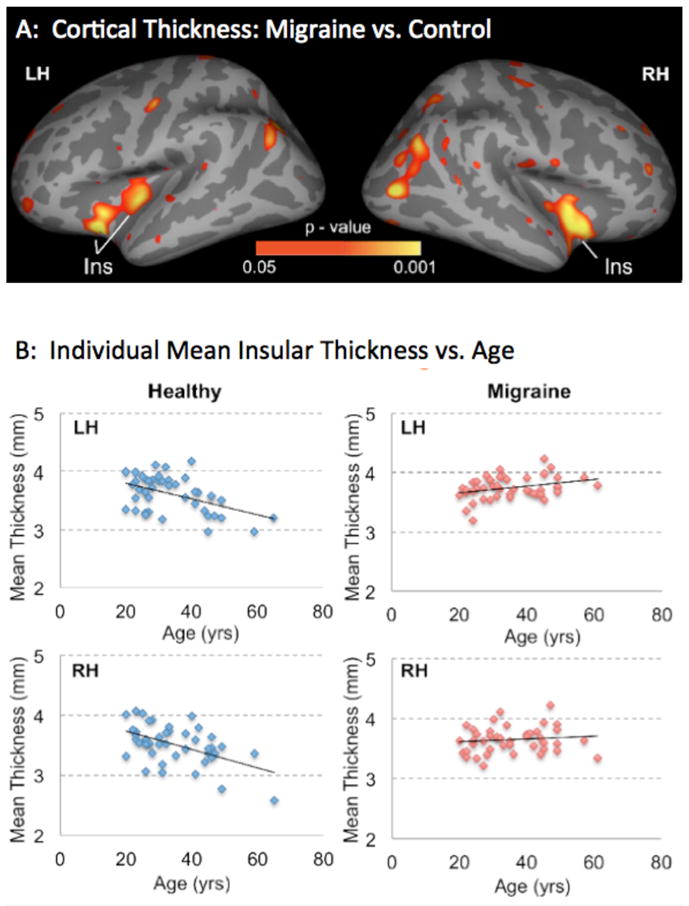
A: Maps showing changes in cortical thickness in migraineurs vs. controls. Significant clusters from vertex-wise cortical thickness comparisons conducted on female migraineurs (N=46) vs. age- and gender- matched healthy control subjects (N=46) are shown. Clusters represent areas showing a significant difference in interaction between age and cortical thickness correlation between the two cohorts, with red-yellow clusters in insula showing regions that showed reduction (negative correlation) with age in healthy control subjects and no reduction in migraineurs. The results are Monte Carlo corrected for multiple comparisons. LH: Left Hemisphere; RH: Right Hemisphere; Ins: Insula.
B: Insula Thickness vs. Age in Migraineurs and Healthy Controls. Scatterplots of thinning in the insula regions identified by comparing migraineurs and healthy control subjects regressed by age. For these scatterplots, the z-statistic maps were thresholded at p < 0.01 and regions of interest were created in the insula of both hemispheres. The plots show the average cortical thickness values corresponding to each subject of each cohort that are plotted against the age.
Table 4.
Significant Clusters from Comparisons for Correlation with Age (age * group)
| Region | Side | Max | VtxMax | Size (mm2) | TalX | TalY | TalZ | CWP | CI | NVtxs |
|---|---|---|---|---|---|---|---|---|---|---|
| Insula | L | 4.553 | 39768 | 250.81 | −29.2 | 13.7 | −11.5 | 0.04640 | [0.04370 0.04910] | 647 |
| Insula | L | 3.030 | 43392 | 225.49 | −35.5 | −17.5 | −6.0 | 0.04640 | [0.04370 0.04910] | 491 |
| Insula | R | 3.409 | 107356 | 494.32 | 37.0 | −5.7 | −9.8 | 0.00280 | [0.00210 0.00350] | 1156 |
Key: Max = maximum −log10(p-value) in the cluster; VtxMax = vertex number at the maximum; Size = Surface area of cluster; Tal(XYZ) = Talairach (MNI305) coordinate of the maximum; CWP = cluster-wise p-value; CI = 90% confidence interval for CWP. NVtxs = Number of vertices.
Figure 2. Overlap of the Insular Functional Organization Map and the Ageing Cortical Thickness Correlation Contrast Map.
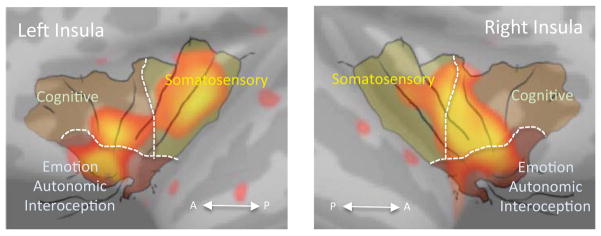
The functional overlay map (with black lines and segments delineated by dotted lines), adapted from [22] is overlaid over the insular cortex. While an approximation, it helps segregate the observed changes in cortical thickness with three main functional regions in the structure: the somatosensory region, cognitive region and a third region for emotion, autonomic function and interoception. A: Anterior; P: Posterior.
Figure 3. Maps showing Differences in Cortical Thickness.
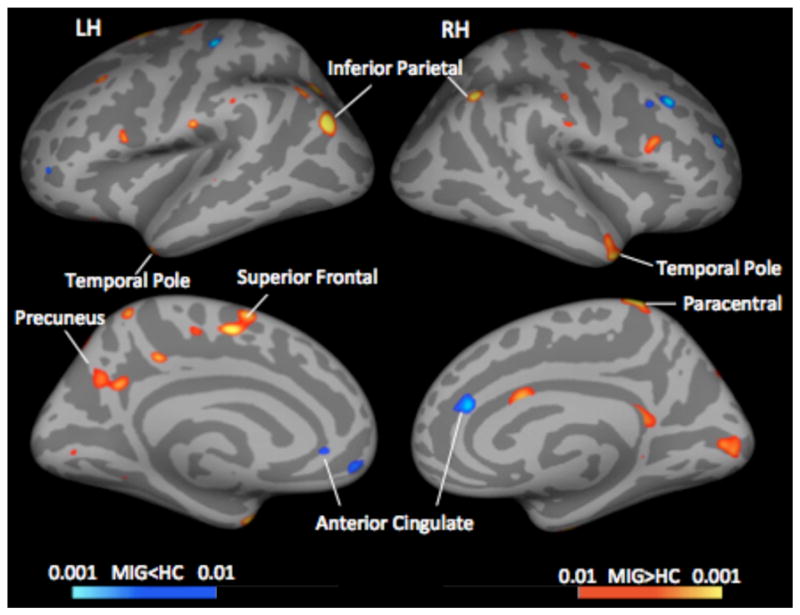
Significant clusters from vertex-wise cortical thickness comparisons conducted on female migraineurs vs. age- and gender- matched healthy subjects. Red-yellow colors represent areas with thicker cortex in migraineurs, blue-light blue areas with thinner cortex. LH: Left Hemisphere; RH: Right Hemisphere; MIG: Migraine; HC: Healthy Control.
Table 5.
Significant Clusters from Cortical Thickness Difference Comparisons (main effect: group)
| Region | Side | Max | VtxMax | Size (mm2) | TalX | TalY | TalZ | CWP | CI | NVtxs |
|---|---|---|---|---|---|---|---|---|---|---|
| Superiorfrontal | L | 3.261 | 12423 | 1233.57 | −5.6 | −5.3 | 48.3 | 0.02630 | [0.02430 0.02840] | 2504 |
| Precuneus | L | 2.588 | 112936 | 1112.79 | −9.4 | −54.9 | 27.3 | 0.04640 | [0.04370 0.04910] | 2404 |
| Paracentral | R | 4.035 | 32954 | 280.44 | 12.1 | −36.4 | 71.0 | 0.01130 | [0.0100 0.01270] | 682 |
| Temporal Pole | R | 2.921 | 130556 | 497.38 | 45.7 | 7.6 | −30.5 | 0.04440 | [0.04180 0.04700] | 749 |
Key: Max = maximum −log10(p-value) in the cluster; VtxMax = vertex number at the maximum; Size = Surface area of cluster; Tal(XYZ) = Talairach (MNI305) coordinate of the maximum; CWP = cluster-wise p-value; CI = 90% confidence interval for CWP. NVtxs = Number of vertices.
Lateralization
In Table 2, the breakdown of the dominant sides per each frequency category and also for the whole cohort is presented. The dominant side was defined as the side that most of the attacks started from. For instance if a patient had 60% of the attacks starting on the right and 40% on the left, the dominant side for that patient was labeled as the right side. We found that the laterality of the headache symptoms was unilateral to the predominant cortical changes (right side) in the low frequency (<2 attacks/month, Right: 45.5% vs. Left: 18.2%) and medium frequency (between 2 to 6 attacks/month, Right: 40% vs. Left: 20%) migraineurs that disappeared in the high frequency migraineurs (>8 attacks/month, R: 21.4% vs. L: 28.6%).
Functional Overlap
The insular regions that showed contrast in correlation with age between migraineurs and healthy subjects spread along the anterior-inferior to superior-posterior direction of the insula and overlapped with all three functional regions defined according to the functional connectivity of the insula [45] i.e. somatosensory region, cognitive region and the emotion/autonomic/interoception region. However the relative spatial distribution was more prominent in the region for emotion/autonomic/interoception compared to the other two regions bilaterally.
Discussion
In this study, we examined the effect of ageing on the migraine brain in women ranging between the ages of 20 to 65 yrs. The findings of this study suggest that there is a lack of cortical thinning normally observed with ageing in female migraineurs compared with healthy controls. This is specially of interest since studies in healthy subjects show that the relative gray matter loss rate of the insular cortex is approximately double of that seen in other cortical areas during ageing [34]. This, in addition to our previous findings [11], further emphasizes a role for insula in migraine pathophysiology.
Cortical Changes associated with Normal Aging
Several MRI cross-sectional studies in healthy subjects have reported a consistent pattern of cortical reduction in the insula with age [34, 46–50]. The mechanisms responsible for cortical changes with ageing are not completely understood. Post-mortem examination of the cortex using serologic methods [49] have shown that thinning of the frontal and temporal regions are not associated with neuronal loss (the total neuronal number remained relatively constant between 56 to 103 years of age), but instead with loss of neuronal and dendritic architecture.
Cortical Abnormalities and Migraine
Cortical changes have been reported in migraine patients during their ictal [54–56] and interictal [10, 57] periods including the insula [5, 6, 10, 11]. Functional imaging studies have shown consistent and bilateral increases in insula activation as well as greater intrinsic connectivity between the insula and the default mode network in migraine vs. healthy controls [26, 29, 58, 59]. However, a minority of studies have also shown opposite changes to those noted above. Specifically, interictal insula [27], a bilateral decrease in activity [28] and in grey matter volume [6] in migraineurs. The relationship between activity and gray matter volume in the insula varies on the location and state of disease (see [52]).
Abnormality in the Insular Thickness Changes by Age in Migraine
The findings suggest that there is a lack of cortical thinning normally observed with ageing in female migraineurs compared with healthy controls. Furthermore, the regions observed in our study (see Figure 2) implicate that the multiple afferent inputs and outputs of the structure involve all three of the examples shown in the figure i.e., somatosensory, cognitive and emotional/autonomic/interoceptive Normal thinning mechanisms of the insula may also be the result of lack of dendritic pruning. Alternatively,, the large and elongated nerve cells that are found only in the insula and anterior cingulate cortex (ACC) known as von Economo neurons (VEN) may have different ageing-related characteristics in the insula compared to the frontal and temporal regions of the brain. It should be noted however similar age-related differences were not observed in the ACC [18, 58].
Disease vs. Aging Effects
Most of the migraine patients suffer form disease for most of their lives. The same applied to our patient cohort and there was a significantly strong correlation between age and duration of disease. Similar pattern of lack of change in the insular thickness with the disease duration was observed in the areas with abnormal aging pattern in migraine patients. From studying the cortical changes with age in migraine patients in comparisons to healthy subjects in this study it can not be determined whether the changes observed with migraine are a result of afferent drive or alterations in the interictal state (e.g., pain and increased somatosensory cortical thickness [9]) and as such affected by disease duration, or an underlying phenotypic characteristic of migraine in a genetically/epigenetically predisposed brain. Moreover cellular level mechanisms responsible for changes in the brain due to migraine may be different from those responsible for aging. Further studies are needed to identify the mechanisms.
Functional Overlap in Regions of Insular Morphometric Changes
Functional neuroanatomy of the insula has been reviewed elsewhere recently [22, 61–64]. Based on The insula can been divided into functional sub-regions ranging from at least two or three to nine in number [45, 61, 63]. These sub-regions may not be completely distinct and there may also be functional overlaps among them, however the converging knowledge from all of these studies suggests that there is an anterior to posterior functional gradient within the insula. We found an overlap in all three functional subregions, bilaterally, most prominently centered on the transition zone from the anterior region to the exterior region. We argue that the abnormal plasticity observed in the insula may provide an enhanced convergence zone [66] for integration of salience [62], interoception [14], pain and autonomic changes associated with migraine attacks [23–25].
Non-Age Related Differences in Other Cortical Regions
We also observed bilateral higher cortical thickness in temporal pole, precuneus, superior frontal and paracentral gyrus of the migraineurs compared to healthy subjects that were independent of age.
The temporal pole is thought to play a variety of roles in social and emotional processing [67, 68]. Studying this part of the cortex, we have identified functional and structural alteration in the temporal lobe of migraine patients vs. healthy controls, both at the ictal and interictal stage [55]. We also found that these alterations are associated with increased migraine frequency (i.e., between the high vs. low frequency migraine groups, [10]. We also found that these alterations are associated with increased migraine frequency (i.e., between the high vs. low frequency migraine groups, [10]. We have previously reported alterations in cortical thickening of precuneus in female migraineurs relative to male migraineurs and healthy control subject [11]. While the precuneus is involved in complex functions (see [67]), its exact role in migraine is unclear. The superior frontal gyrus is involved in cognitive functions [68] and observed changes may correlate with alterations in cognition associated with pain in migraine attacks. The rationale for this is accumulated data on altered cognition in chronic pain [69] or altered attention to pain [70]. The role of paracentral gyrus in migraine remains unclear.
Lateralization
The literature supports the notion of a predominant right-sided changes in the insula: (1) the right hemisphere is the specialized side in responding to novel versus routine events [75]; (2) the right hemisphere is selectively involved in processing negative emotions [76], and (3) migraine is associated with robust autonomic changes [77] and the right anterior insula is involved in cortical regulation of autonomic function [78]. Our results would suggest that sensitivity to the migraine attacks may similarly be related to right sided processing in interoception [79], allostasis [10] and salience [29] that may occur in migraineurs.
Cortical Changes and other Pain Disorders
Most pronounced pain processing regions in the brain with age-related decreases in volume include the prefrontal cortex and hippocampus [78]. Brain regional gray matter decreases have been reported in a number of chronic pain conditions: dorsolateral prefrontal cortex and thalamic gray matter decreases in chronic back pain [79], Insula and cingulate cortex gray matter decreases in irritable bowel syndrome [80] as well as decrease gray matter density in fibromyalgia [81]. Abnormal gray matter ageing in chronic pain patients suffering from temporomandibular disorder has also been reported [82]. Interestingly, this study also reports age related gray matter reductions in two regions in the brain (dorsal striatum and the premotor cortex) in healthy control subjects that are not seen in the patients [82].
Caveats
Cohort Size
The numbers of subjects in this study compared to the numbers in the majority of literature on ageing is relatively small. However, finding the comparable pattern and rate of cortical reduction in healthy subjects validates the findings and the comparability of the data to bigger size studies.
Age-related Changes in Male Migraineurs
This study was limited to studying age-related changes in female migraineurs by design. Similar trends may also be observed in male migraineurs and that and any potential sex-related differences in brain aging pattern remains to be assessed.
Genetic Determination of Insula Cortex in Migraine
Genetic predisposition cannot be ruled out as a basis for the observed changes. Although an ‘episodic migraine phenotype’ was defined in our inclusion criteria, a genetic background may be a contributory factor (see [85, 86]).
Hormonal Differences
The effects of the hormones on the observed changes in our study cannot be assessed or completely ruled out. However given earlier studies on the effect of menstrual cycle phase [86] or usage of oral contraceptives/hormone therapy [87] on brain plasticity, insular regions reported in this study seem to be not affected by such plasticity modulatory effects.
Medication Effects
Patients were predominantly on non-steroidals and triptans. It is not clear whether these drugs may have direct or indirect neurotrophic effects. While serotonin has a profound effect on cortical neurons in animal models [90], the CNS penetration of triptans is limited.
Age distribution
Although we evaluated 46 subjects the distribution was not equal amongst different ages with relatively few in the older age group (i.e., 50+).
Conclusion
Based on our finding of abnormal pattern of changes in the insula by age and based on the other structural and functional abnormalities in the insula in migraine [5, 6, 10, 11], its anatomical location and connectivity, we propose the insula, as a major hub for cortical integration, plays an important role in migraine pathophysiology. This pattern of changes may be observed in other forms of chronic illnesses including chronic pain and future studies in other patient groups could provide more insight on this matter.
Acknowledgments
The work was funded by grants from NINDS (R01-NS073997 and K24-NS064050) to DB. Salary support in part to NM by Boston Children’s Hospital Office of Faculty Development Fellowship and the Department of Anesthesiology, Perioperative and Pain Medicine, Boston Children’s Hospital, Harvard Medical School.
Footnotes
There are no conflicts of interest in respect to this work.
References
- 1.Schwedt TJ, Dodick DW. Advanced neuroimaging of migraine. Lancet neurology. 2009;8(6):560–8. doi: 10.1016/S1474-4422(09)70107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May A. New insights into headache: an update on functional and structural imaging findings. Nature reviews. Neurology. 2009;5(4):199–209. doi: 10.1038/nrneurol.2009.28. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett. 2008;440(2):92–6. doi: 10.1016/j.neulet.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz N, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48(7):1044–55. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28(6):598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 6.Valfre W, et al. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–17. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Wilcke T, et al. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28(1):1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 8.Maleki N, et al. Common hippocampal structural and functional changes in migraine. Brain structure & function. 2012 doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DaSilva AF, et al. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69(21):1990–5. doi: 10.1212/01.wnl.0000291618.32247.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maleki N, et al. Concurrent functional and structural cortical alterations in migraine. Cephalalgia: an international journal of headache. 2012;32(8):607–20. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maleki N, et al. Her versus his migraine: multiple sex differences in brain function and structure. Brain: a journal of neurology. 2012;135(Pt 8):2546–59. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. The Journal of comparative neurology. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 13.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. The Journal of comparative neurology. 1982;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 14.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 15.Phan KL, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 16.Beissner F, et al. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Critchley HD, et al. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton Neurosci. 2011;161(1–2):34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Legrain V, et al. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93(1):111–24. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007;128(1–2) doi: 10.1016/j.pain.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. 2012;195:123–63. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 21.Borsook D, et al. Migraine Mistakes: Error Awareness. Neuroscientist. 2013 doi: 10.1177/1073858413503711. [DOI] [PubMed] [Google Scholar]
- 22.Klein TA, Ullsperger M, Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Frontiers in human neuroscience. 2013;7:14. doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari MD. Headache: the changing migraine brain. Lancet Neurol. 2013;12(1):6–8. doi: 10.1016/S1474-4422(12)70290-9. [DOI] [PubMed] [Google Scholar]
- 24.Goadsby PJ, et al. Neurobiology of migraine. Neuroscience. 2009;161(2):327–41. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. 2013;154(Suppl 1) doi: 10.1016/j.pain.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahra A, et al. Brainstem activation specific to migraine headache. Lancet. 2001;357(9261):1016–7. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, et al. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia. 2010;30(1):53–61. doi: 10.1111/j.1468-2982.2009.01890.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Wilcke T, et al. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia: an international journal of headache. 2008;28(1):1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 29.Xue T, et al. Intrinsic Brain Network Abnormalities in Migraines without Aura Revealed in Resting-State fMRI. PLoS One. 2012;7(12):e52927. doi: 10.1371/journal.pone.0052927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, et al. A PET-CT study on the specificity of acupoints through acupuncture treatment in migraine patients. BMC Complement Altern Med. 2012;12:123. doi: 10.1186/1472-6882-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borsook D, et al. Sex and the Migraine Brain. Neurobiol Disease. 2014 doi: 10.1016/j.nbd.2014.03.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprenger T, Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr Opin Neurol. 2012;25(3):252–62. doi: 10.1097/WCO.0b013e3283532ca3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terribilli D, et al. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiology of aging. 2011;32(2):354–68. doi: 10.1016/j.neurobiolaging.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieve SM, et al. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ICHD-II . The International Classification of Headache Disorders: 2nd edition. Cephalalgia: an international journal of headache. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 36.Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 37.Segonne F, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 40.Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 41.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosas HD, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 43.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 44.Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33(4):1093–103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral cortex. 2011;21(7):1498–506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowell ER, et al. Mapping cortical change across the human life span. Nature neuroscience. 2003;6(3):309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 47.Good CD, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 48.Jernigan TL, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of aging. 2001;22(4):581–94. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 49.Abe O, et al. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiology of aging. 2008;29(1):102–16. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Kalpouzos G, et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiology of aging. 2009;30(1):112–24. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 51.Freeman SH, et al. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. Journal of neuropathology and experimental neurology. 2008;67(12):1205–12. doi: 10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borsook D, Erpelding N, Becerra L. Losses and gains: chronic pain and altered brain morphology. Expert Rev Neurother. 2013;13(11):1221–34. doi: 10.1586/14737175.2013.846218. [DOI] [PubMed] [Google Scholar]
- 53.Metz AE, et al. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106(7):2423–8. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bramanti P, et al. Ictal and interictal hypoactivation of the occipital cortex in migraine with aura. A neuroimaging and electrophysiological study. Funct Neurol. 2005;20(4):169–71. [PubMed] [Google Scholar]
- 55.Moulton EA, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex. 2011;21(2):435–48. doi: 10.1093/cercor/bhq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang J, et al. Altered cortical activation in adolescents with acute migraine: a magnetoencephalography study. J Pain. 2013;14(12):1553–63. doi: 10.1016/j.jpain.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierelli F, et al. Abnormal sensorimotor plasticity in migraine without aura patients. Pain. 2013;154(9):1738–42. doi: 10.1016/j.pain.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 58.Afridi SK, et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain: a journal of neurology. 2005;128(Pt 4):932–9. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- 59.Eck J, et al. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152(5):1104–13. doi: 10.1016/j.pain.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 60.Allman JM, et al. The von Economo neurons in apes and humans. American journal of human biology: the official journal of the Human Biology Council. 2011;23(1):5–21. doi: 10.1002/ajhb.21136. [DOI] [PubMed] [Google Scholar]
- 61.Kurth F, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain structure & function. 2010;214(5–6):519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cauda F, et al. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55(1):8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 64.Kelly C, et al. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 2012;61(4):1129–42. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peltz E, et al. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage. 2011;54(2):1324–35. doi: 10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Ibanez A, Gleichgerrcht E, Manes F. Clinical effects of insular damage in humans. Brain Struct Funct. 2010;214(5–6):397–410. doi: 10.1007/s00429-010-0256-y. [DOI] [PubMed] [Google Scholar]
- 67.Dupont S. Investigating temporal pole function by functional imaging. Epileptic Disord. 2002;4(Suppl 1):S17–22. [PubMed] [Google Scholar]
- 68.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(Pt 7):1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 69.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 70.du Boisgueheneuc F, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129(Pt 12):3315–28. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 71.Pincus T, Morley S. Cognitive-processing bias in chronic pain: a review and integration. Psychol Bull. 2001;127(5):599–617. doi: 10.1037/0033-2909.127.5.599. [DOI] [PubMed] [Google Scholar]
- 72.Van Damme S, et al. Keeping pain in mind: a motivational account of attention to pain. Neurosci Biobehav Rev. 2010;34(2):204–13. doi: 10.1016/j.neubiorev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Spasojevic G, et al. Morphology and digitally aided morphometry of the human paracentral lobule. Folia Morphol (Warsz) 2013;72(1):10–6. doi: 10.5603/fm.2013.0002. [DOI] [PubMed] [Google Scholar]
- 74.Cavanna AE. The precuneus and consciousness. CNS spectrums. 2007;12(7):545–52. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- 75.Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. The Behavioral and brain sciences. 2005;28(4):575–89. doi: 10.1017/S0140525X05000105. discussion 589–633. [DOI] [PubMed] [Google Scholar]
- 76.Hecht D. Depression and the hyperactive right-hemisphere. Neuroscience research. 2010;68(2):77–87. doi: 10.1016/j.neures.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Havanka-Kanniainen H, Tolonen U, Myllyla VV. Autonomic dysfunction in migraine: a survey of 188 patients. Headache. 1988;28(7):465–70. doi: 10.1111/j.1526-4610.1988.hed2807465.x. [DOI] [PubMed] [Google Scholar]
- 78.Meyer S, et al. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004;15(2):357–61. doi: 10.1097/00001756-200402090-00029. [DOI] [PubMed] [Google Scholar]
- 79.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 80.Farrell MJ. Age-related changes in the structure and function of brain regions involved in pain processing. Pain medicine. 2012;13(Suppl 2):S37–43. doi: 10.1111/j.1526-4637.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- 81.Apkarian AV, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(46):10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis KD, et al. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70(2):153–4. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- 83.Kuchinad A, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(15):4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moayedi M, et al. Abnormal gray matter aging in chronic pain patients. Brain research. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 85.Panizzon MS, et al. Genetic and environmental influences of white and gray matter signal contrast: a new phenotype for imaging genetics? Neuroimage. 2012;60(3):1686–95. doi: 10.1016/j.neuroimage.2012.01.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winkler AM, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3):1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dolman AJ, et al. Phenotype Matters: The Absence of a Positive Association between Cortical Thinning and Chronic Low Back Pain when Controlling for Salient Clinical Variables. Clin J Pain. 2013 doi: 10.1097/AJP.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pletzer B, et al. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 89.Boccardi M, et al. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a Voxel-based morphometry study. Menopause. 2006;13(4):584–91. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- 90.Altamura C, et al. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb Cortex. 2007;17(6):1394–401. doi: 10.1093/cercor/bhl051. [DOI] [PubMed] [Google Scholar]
- 91.Tfelt-Hansen PC. Does sumatriptan cross the blood-brain barrier in animals and man? J Headache Pain. 2010;11(1):5–12. doi: 10.1007/s10194-009-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


