Abstract
Introduction
MRI of peripheral nerve and muscle in patients with ALS may be performed to investigate alternative diagnoses including multifocal motor neuropathy (MMN). MRI findings of peripheral nerve and muscle are not well described in these conditions, making interpretation of results difficult.
Methods
We examined systematically the peripheral nerve and muscle MRI findings in patients with ALS (n=60) and MMN (n=8).
Results
In patients with ALS and MMN, abnormal MRIs were common (85% and 75%, respectively) but did not correlate with disease severity. Peripheral nerve MRI abnormalities were similar in frequency (ALS: 58% vs. MMN: 63%) with most changes being of mild-to-moderate severity. Muscle MRI changes were more common in ALS (57% vs. 33%), and no muscle atrophy was seen in patients with MMN.
Discussion
MRI abnormalities of peripheral nerve and muscle in ALS and MMN are common and share some features.
Keywords: amyotrophic lateral sclerosis, multifocal motor neuropathy, MRI, MR neurography, ALS
Magnetic resonance imaging (MRI) of peripheral nerve and muscle in patients with amyotrophic lateral sclerosis (ALS) may be performed to investigate alternative diagnoses, especially in patients with atypical presentations. Multifocal motor neuropathy (MMN) is an important ALS mimic, since it is amenable to immunotherapy and has a much better prognosis. Little is known about known the typical peripheral nerve and muscle radiological findings in ALS and MMN, which makes interpretation of results difficult1-3. We present the experience of peripheral nerve and muscle MRI in a large cohort from a tertiary care center.
Methods
After obtaining approval from the Institutional Review Board, the Mayo Clinic electronic medical record (1/1/2002 – 12/31/2011) was queried for patients with either ALS or MMN diagnosed by a neuromuscular specialist who received either an MRI of brachial or lumbosacral plexus [MRI strength: 3 Tesla (64%), 1.5 Tesla (22%), unknown (14%)]. Patients with MMN had no sensory signs or symptoms, had clear response to intravenous immunoglobulin, and had variably elevated GM1 antibodies (5/8). Demographics and clinical characteristics were retrieved from the medical records in all patients. Manual muscle strength was abstracted from the medical record and was scored using a modified Neuropathy Impairment Score (mNIS), which is an additive score that grades muscles for weakness based a modified Mayo linear scale (0-normal strength, 1 – 25% weakness, 2 – 50% weakness, 3 – 75% weakness, 3.5 unable to overcome gravity, 3.75 – muscle flicker, 4 – plegia)4. In the upper limbs, shoulder abductors, elbow flexors, elbow extensors, finger extensors, finger flexors, finger abductors, and thenar muscles were scored (maximum score of 28 in a plegic limb). In the lower extremities, bilateral scoring was performed since MRI of lumbosacral plexus images bilateral plexi at our institution. Strength scoring was performed on bilateral hip flexors, knee extensors, knee flexors, ankle dorsiflexors, and ankle plantarflexors (maximum score of 40 in bilateral plegic limbs).
MRI of either brachial plexus or lumbosacral plexus was reviewed, and nerve and muscle abnormalities were graded by a radiologist (BMH) blinded to the neurological diagnosis. In order to quantify the severity of the MRI findings, an MRI composite score with a maximum score of 16 was calculated for each study. Nerve and muscle MRI abnormalities were graded separately based on T2 hyperintensity (0-normal, 1-mild, 2-moderate, 3-severe), nerve enlargement (muscle atrophy) (0-normal, 1-mild, 2-moderate, 3-severe), and pattern of involvement (focal-1, diffuse-2). Descriptive statistics are presented as median (range). Pearson 2-tailed correlation analyses and Student t-tests were performed using Prism 6.0 (GraphPad).
Results
Sixty patients with limb-onset ALS were identified who underwent MRI of either the brachial (n=36) or lumbosacral plexus (n=24). The median age at MRI was 58 years (range 23-77) and was performed at a median 12 months from onset of weakness (range 0-96 months). At the time of MRI, the median mNIS was 14.5 in upper limb patients (range 2-25), and 13.5 in the lower limb patients (range 0-35). Thirty-five patients had greater than 4 months clinical follow-up that documented disease progression (median follow-up: 16 months, range 4-74 months). Death was confirmed in 47 (78%) patients (median disease duration: 43 months, range 12-158 months). While it was often difficult to determine the exact reason for ordering MRI, 3 general reasons were most common: 1) it was early in the disease with mild weakness of undetermined etiology; 2) a strong alternative diagnosis was being considered (e.g. cancer, radiation plexopathy, CIDP/MMN); or 3) the study was part of an aggressive diagnostic work-up even when the diagnosis of ALS was suspected from the outset.
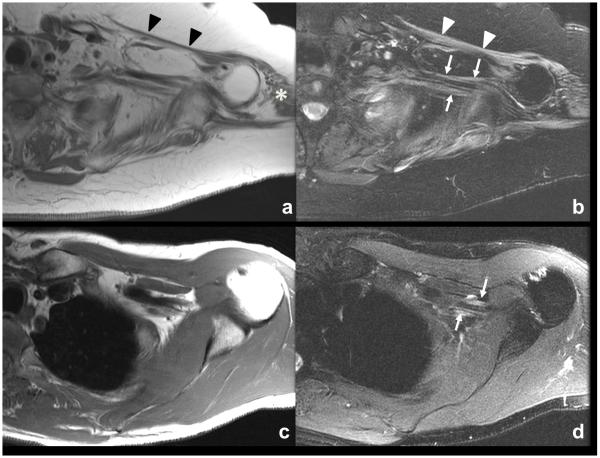
In patients with ALS, an abnormal MRI of either peripheral nerve or muscle was seen in 51/60 (85%). Abnormalities were restricted to peripheral nerve in 17 patients, to muscle in 16 patients, and combined nerve and muscle abnormalities in 18 patients. Peripheral nerve MRI abnormalities (Figure A, B) were characterized by T2 hyperintensity in 36 of 60 (mild=22, moderate=9, severe=5) and enlargement in 18 of 60 (mild=17, moderate=1, severe=0) that was observed in either a focal (n=3) or diffuse (n=33) distribution within the plexus. Gadolinium contrast was administered in 45 of 60 patients, and contrast enhancement of nerve was seen in only one patient. In this case, there was focal peripheral gadolinium enhancement of the medial cord of the brachial plexus in a patient later found to have an SOD1 AV4 mutation. Muscle MRI abnormalities were characterized as T2 hyperintensity in 30 of 60 (mild=17, moderate=10, severe=3) with atrophy in 21/60 (mild=12, moderate=8, severe=1) observed in either a focal (n=17) or diffuse (n=17) distribution.
Figure.
MRI findings in ALS (A, B) and MMN (C, D). Axial T1-weighted (A) and T2-weighted fat saturated (B) MR images of the left brachial plexus in a patient with ALS. Diffuse atrophy of the left shoulder girdle musculature with intramuscular edema consistent with chronic atrophy and ongoing denervation change, including the pectoralis (A and B, arrowheads) and deltoid (A, asterisk) The brachial plexus demonstrates diffuse mild thickening and increased T2-weighted signal of the nerves as seen in B (arrows). Axial T1-weighted (C) and T2-weighted fat saturated (D) MR images of the left brachial plexus in a patient with MMN. No abnormal muscle findings are identified about the left shoulder. The left brachial plexus demonstrates mild thickening and increased T2-weighted signal (D, arrows).
An MRI composite score was derived from the attributes described above in order to investigate correlations with clinical features. The median MRI composite score in the brachial plexus was 5 (range 0-12), and in the lumbosacral plexus it was 3 (range 0-10). While the MRI composite score correlated positively with the time from disease onset to MRI (r2=0.11, P=0.009), it did not correlate with age at MRI, overall length of disease (time from disease onset until death or last clinical follow-up), or severity of weakness as measured by the mNIS at the time of MRI.
For comparison, MRIs were reviewed in 8 patients with MMN (7 brachial plexus and 1 lumbosacral plexus) (Figure C, D). An abnormal MRI of either peripheral nerve or muscle was seen in 6 (75%), which was restricted to peripheral nerve in 4 patients, to muscle in 1 patient, and a combination of nerve and muscle in 1 patient. Peripheral nerve abnormalities were characterized as T2 hyperintensity in 5 of 8 (mild=2, moderate=2, severe=1) with enlargement in 2 of 6 (mild=2, moderate=0, severe=0) that was observed in a diffuse fashion (5 of 8). Muscle MRI findings included T2 hyperintensity in 2 of 6 (mild=1, moderate=1, severe=0), both of which were focal. No muscle atrophy was seen in any of the MRIs from patients with MMN. The median MRI composite score in MMN patients was 3.5 (range 0-6), which was not significantly different than in ALS (P=0.10).
Discussion
We report that in patients with ALS, MRI abnormalities are found frequently in peripheral nerve and muscle and do not correlate with disease severity. When compared with MMN, the MRI nerve abnormalities in ALS patients were similar in frequency (60% vs. 63%), with most changes being mild-to-moderate severity in both. Changes in muscle on MRI were more common in ALS (57% vs. 33%), and no muscle atrophy was seen in the patients with MMN, which may be a useful clue in patients with longstanding disease.
A question raised by these data is what causes the nerve and muscle MRI signal changes in ALS patients? For the muscle changes, it is likely that denervation atrophy is the primary process, which is well known to cause MRI changes5. Supporting this, atrophy was only seen in patients with ALS, not in MMN where muscle atrophy is often less evident. We propose that the nerve MRI signal changes reflects ongoing axonal degeneration with macrophage infiltration into nerve and mild nerve edema, leading to T2 hyperintensity and mild enlargement6. This finding has been reported in the cauda equina in patients with fulminant ALS7.
This study increases substantially the scant literature on peripheral nerve and muscle MRI findings in ALS and MMN1-3, and these results should help the practicing neurologist interpret MRI reports in these patients. Based on our findings; however, we do not feel that routine peripheral nerve and muscle MRI is helpful in distinguishing between ALS and MMN, and furthermore is not a robust biomarker of ALS disease severity.
Acknowledgements
The authors wish to acknowledge the contributions of Zheyan Chen for technical assistance during early versions of the project.
Dr. Staff is funded by NIH grant (K08 - CA 169443).
Abbreviations
- MRI
magnetic resonance imaging
- ALS
amyotrophic lateral sclerosis
- MMN
multifocal motor neuropathy
- mNIS
modified Neuropathy Impairment Score
Footnotes
Dr. Amrami reports no disclosures.
Dr. Howe reports no disclosures.
References
- 1.Briani C, Cacciavillani M, Lucchetta M, Cecchin D, Gasparotti R. MR neurography findings in axonal multifocal motor neuropathy. J Neurol. 2013;260(9):2420–2422. doi: 10.1007/s00415-013-7052-6. [DOI] [PubMed] [Google Scholar]
- 2.Du R, Auguste KI, Chin CT, Engstrom JW, Weinstein PR. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112(2):362–371. doi: 10.3171/2009.7.JNS09414. [DOI] [PubMed] [Google Scholar]
- 3.Van Es HW, Van den Berg LH, Franssen H, Witkamp TD, Ramos LM, Notermans NC, Feldberg MA, Wokke JH. Magnetic resonance imaging of the brachial plexus in patients with multifocal motor neuropathy. Neurology. 1997;48(5):1218–1224. doi: 10.1212/wnl.48.5.1218. [DOI] [PubMed] [Google Scholar]
- 4.Dyck PJ, Sherman WR, Hallcher LM, Service FJ, O’Brien PC, Grina LA, Palumbo PJ, Swanson CJ. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Annals of neurology. 1980;8(6):590–596. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- 5.Kamath S, Venkatanarasimha N, Walsh MA, Hughes PM. MRI appearance of muscle denervation. Skeletal Radiol. 2008;37(5):397–404. doi: 10.1007/s00256-007-0409-0. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen C, Haughton VM, Ho KC, An HS, Myklebust JB, Hasegawa T, Xu R, Harb JM. Contrast enhancement in spinal nerve roots: an experimental study. AJNR Am J Neuroradiol. 1995;16(2):265–268. [PMC free article] [PubMed] [Google Scholar]
- 7.Young NP, Laughlin RS, Sorenson EJ. Gadolinium enhancement of the lumbar roots in a case of ALS. Amyotroph Lateral Scler. 2010;11(1-2):207–209. doi: 10.3109/17482960802642161. [DOI] [PubMed] [Google Scholar]