Abstract
We investigated roles for spinal neurons expressing the neurokinin-1 receptor (NK1R) and/or gastrin releasing peptide receptor (GRPR) in a mouse model of ovalbumin (OVA)-induced chronic atopic dermatitis (AD). Mice receiving repeated topical application of OVA exhibited atopic-like skin lesions and behavioral signs of chronic itch including spontaneous scratching, touch-evoked scratching (alloknesis), and enhancement of chloroquine-evoked scratching (hyperknesis). Substance P-saporin (SP-SAP) and bombesin-saporin (BB-SAP) were intrathecally injected into OVA-sensitized mice to neurotoxically ablate NK1R- or GRPR-expressing spinal neurons, respectively. SP-SAP diminished the expression of NK1R in the superficial spinal dorsal horn, and significantly attenuated all behavioral signs of chronic itch. BB-SAP reduced the spinal dorsal horn expression of GRPR and significantly attenuated hyperknesis, with no effect on spontaneous scratching or alloknesis. To investigate whether NK1R-expressing spinal neurons project in ascending somatosensory pathways, we performed a double-label study. The retrograde tracer, Fluorogold (FG), was injected into either the somatosensory thalamus or lateral parabrachial nucleus. In the upper cervical (C1-2) spinal cord, most neurons retrogradely labeled with FG were located in the dorsomedial aspect of the superficial dorsal horn. Of FG-labeled spinal neurons, 89-94% were double-labeled for NK1R. These results indicate that NK1R-expressing spinal neurons play a major role in the expression of symptoms of chronic itch, and give rise to ascending somatosensory projections. GRPR-expressing spinal neurons contribute to hyperknesis but not alloknesis or ongoing itch. NK1R-expressing spinal neurons represent a potential target to treat chronic itch.
Keywords: Atopic dermatitis, chronic itch, central sensitization, alloknesis, hyperknesis, substance P, neurokinin 1 receptor, gastrin releasing peptide receptor
Introduction
Chronic itch is thought to result from increased sensitivity of itch-signaling pathways, resulting in symptoms of spontaneously-occurring itch, itch in response to non-itchy light touch (“alloknesis”), and increased itch to a normally itchy stimulus such as an insect bite (“hyperknesis”). Under conditions of chronic itch such as atopic dermatitis (AD), it is hypothesized that peripheral and/or central itch-signaling neurons become sensitized to provide a stronger itch signal to the central nervous system. However, the cellular and molecular mechanisms that underlie this process are currently unknown.
Recent studies have revealed cellular and molecular mechanisms underlying acute itch [1; 12; 20]. A variety of chemicals can elicit itch via histamine-dependent and histamine-independent pathways [1]. Histaminergic and non-histaminergic itch require TRPV1 and TRPA1, respectively [18; 33]. In the spinal cord, glutamate as well as neuropeptides including substance P, gastrin releasing peptide (GRP), neuromedin B, and natriuretic polypeptide B (Nppb) are involved in the transmission of itch signals [6; 8; 21; 27; 36]. Neurons expressing the substance P neurokinin-1 receptor (NK1R) represent the majority of ascending somatosensory projection neurons from the spinal and medullary dorsal horn, and are implicated in acute itch [14; 29]. Gastrin releasing peptide receptor (GRPR)-expressing spinal neurons are required for both histaminergic and non-histaminergic itch [7; 28]. In the present study, we developed behavioral tools to assess itch sensitization in an animal model of AD. Using this model, we investigated roles for NK1R- and/or GRPR-expressing spinal neurons in chronic itch.
Materials and Methods
OVA sensitization and behavioral tests
Experiments were performed using adult male C57BL/6 mice (19-27 g) under a protocol approved by the UC Davis Animal Care and Use Committee. The procedure to sensitize mice with OVA was similar to that used previously with minor modification [26; 34]. Mice were given an intraperitoneal injection of ovalbumin (OVA; 100 μg; Sigma-Aldrich, St. Louis, MO), alum (1 mg; Sigma-Aldrich) and pertussis toxin (300 μg Life Technologies, Grand Island, NY) on the first day (Fig.1A). Five days later, they received a subcutaneous injection of 50 μg of ovalbumin or saline alone. Then, local sensitization was performed once a day from day 14 to day 39 after the first systemic sensitization. The local sensitization was conducted as follows. Fur on the rostral back was shaved with electric clippers. Then, gauze (1 × 1 cm) soaked with 0.1% OVA (100 μl) or saline (100 μl) was applied to the shaved skin area. The treated skin area was covered with a patch (Tegaderm, 3M Health Care, St. Paul, MN). The next day the patch was removed, and an identical piece of soaked gauze followed by Tegaderm patch was reapplied to the same skin area. This procedure was repeated daily up to day 39. Starting at day 14, mice were videotaped for one hour twice a week following the removal of the patch to count scratching behavior. The videotape was played back and the number of scratch bouts was counted by two independent observers blinded as to the treatment. After videotaping, alloknesis testing was conducted. Alloknesis was assessed as follows [3]: the mouse received five separate innocuous mechanical stimuli delivered using a von Frey filament (bending force: 0.7 mN) on the border of the gauze treatment area at five randomly selected sites. The presence or absence of a positive response, i.e., an immediate hindlimb scratch bout directed to the site of mechanical stimulation, was noted for each stimulus before the next one was given. The alloknesis score was the total number of positive responses elicited by the five stimuli, i.e., 0, 1, 2, 3, 4, or 5. The patch was left continuously on the rostral part of the back of the mouse, except during the measurement of scratching behavior. To assess hyperknesis, a 10 μl intradermal (id) injection of saline was injected within the treatment area one week after the ablation. One week later, mice received an id injection of either chloroquine (30 μg, Sigma-Aldrich) or histamine (35 μg, Sigma-Aldrich) within the treatment area (Fig. 1A).
Fig. 1.

A typical example of an OVA-treated mouse. A: timeline of treatments. B: photo taken on day 0 prior to the beginning of OVA treatment. C: OVA treatment day 25.
Saporin-injection
To neurotoxically ablate NK1R- or GRPR-expressing spinal neurons, OVA-treated mice received an intrathecal injection of either blank saporin (SAP, 400 ng/5 μl, Advanced Targeting Systems, San Diego, CA), substance P-SAP (SP-SAP, 100 ng/5 μl, Advanced Targeting Systems), or bombesin-SAP (BB-SAP, 400 ng/5 μl, Advanced Targeting Systems) under isoflurane anesthesia on treatment day 25. The same concentration of BB-SAP was used as in previous studies [17; 25; 28]. The SP-SAP or BB-SAP was administered intrathecally via lumbar puncture.
Immunohistochemistry
After completing behavioral testing, the animal was anesthetized with sodium pentobarbital and perfused transcardially with phosphate-buffered saline followed by 4% paraformaldehyde perfusion. The upper cervical spinal cord was harvested and post-fixed in 4% paraformaldehyde followed by 30% sucrose. Thirty-micrometer sections of the cervical spinal cord were immunostained with either anti-rabbit GRPR antibody (1:500; LS-A831, Medical & Biological Laboratories International, Woburn, MA) [16; 36] or anti-rabbit NK1R antibody (1:500; AB5060, Millpore, Billerica, MA) using Tyramide Signal Amplification kits (Life Technologies). Briefly, they were incubated with 0.2% Triton X-100 for 10 minutes at room temperature, followed by incubation with peroxidase quenching buffer (PBS + 3% H2O2) for either 30 minutes for GRPR antibody or 15 minutes for NK1R at room temperature. Then, they were incubated with 1% blocking reagent for 60 minutes at room temperature, followed by incubation with the primary antibody in 1% blocking reagent overnight at 4°C. The next day, sections were incubated with the horseradish peroxidase (HRP) conjugate secondary antibody solution for 45 minutes at room temperature, followed by incubation with the tyramide working solution for 7 minutes at room temperature. Coverslips were mounted with ProLong® Gold Antifade Mountant (Life Technologies). Images were captured using a fluorescence microscope (Nikon Eclipse Ti; Technical Instruments, San Francisco CA).
Fluorogold injection
To retrogradely label spinoparabrachial and spinothalamic projection neurons, Fluorogold (Fluorochrome, LLC, Denver, CO) was injected into the lateral parabrachial nucleus or thalamus, respectively. Mice were anesthetized with sodium pentobarbital (65 mg/kg ip). The head was fixed in a stereotaxic frame, the calvarium was exposed by midline scalp incision, and a craniotomy made for introduction of a microinjection cannula into the thalamus (AP:1.8, ML:1.0, DV:-3.6 and AP:1.8, ML:1.6, DV:-3.6) or parabrachial nucleus (AP:5.0, ML:1.27, DV:-3.75 and AP:5.2, ML:1.27, DV:-3.75). The craniotomy consisted of a small burr hole (<2 mm diameter) made through the skull using an electric drill over the intended injection site as determined using stereotaxic coordinates. Eighty nL of Fluorogold was injected at each site. After injection of tracer, the cannula was removed, and the incision was closed with Vetbond (tissue adhesive, 3M). Mice were given an analgesic (Buprenorphrine, 0.05 mg/kg s.c.; Cardinal Health, Dublin OH) at the conclusion of surgery, and again twice daily, for 1 day post- surgery. Animals receiving the tracer injection were then used 1 week later for immunohistochemistry.
Data analysis
Between-group comparisons were made by one-way or two-way ANOVA followed by the Bonferroni post-test. In all cases p<0.05 was considered to be significant.
Results
OVA treatment induced erythema as well as excoriation. Fig. 1 shows macroscopic views of the skin before and 25 days after OVA treatment. Naive mice exhibited little spontaneous scratching (Fig. 2A). By day 21, counts of spontaneous scratch bouts had increased significantly to a plateau in OVA-treated mice (Fig. 2A). The low level of spontaneous scratching in saline-treated control mice increased only slightly over the same period (Fig. 2A). Naive mice exhibited an alloknesis score of 0 (Fig. 2B). The alloknesis score started to increase by day 18, and was significantly higher by day 25 in OVA-sensitized mice. There was no significant change in the mean alloknesis score over time in saline-treated mice (Fig. 2B). The number of scratch bouts evoked by acute id injection of chloroquine was significantly greater in OVA-sensitized vs. control mice (Fig. 3). In contrast, there was no significant difference in the number of histamine-evoked scratch bouts between OVA-sensitized and control mice. Selective sensitization of the histamine-independent pathway has been observed in other studies [2; 24]. OVA-sensitized mice appear to be a useful model to investigate the neural mechanisms of itch and itch sensitization in AD.
Fig. 2.

Time-dependent changes in scratch bouts and alloknesis score in OVA-sensitized mice. A) Spontaneous scratching was measured on pretreatment day 0, and OVA treatment days 14, 18, 21, 25, 28, 32, 35, and 39. Black dots (●) and white squares (□) show, respectively, OVA (OVA-treated) and Control (saline-treated) groups. Error bars are S.E.M. *p< 0.05, significantly different from day 0 (two-way ANOVA followed by Bonferroni test, F (9,100)=3.584). B) As in A for alloknesis score. Error bars are S.E.M. *p< 0.05, significantly different from day 0 (two-way ANOVA followed by Bonferroni test, F (9,220)=6.417).
Fig. 3.

Stimulus-selective hyperknesis in OVA-sensitized mice. Either histamine or chloroquine was intradermally injected into the nape of the neck. Following the injection scratch bouts were counted over a 30 min period. White, gray, and black columns show, respectively, naive, saline-treated, and OVA-treated groups. Error bars are S.E.M. *p< 0.05, significant difference from naïve (one-way ANOVA followed by Bonferroni test, F=0.882 for the histamine-treated group and F=4.337 for the chloroquine-treated group, n=6)).
To investigate the role of NK1R- and GRPR-expressing spinal neurons in itch and its sensitization, mice were treated with either SP-SAP or BB-SAP on day 25 of OVA treatment, at a time when the number of spontaneous scratch bouts and the alloknesis score had both reached a plateau. Fig. 4 shows an image of the dorsal horn of the upper cervical spinal cord. BB-SAP or SP-SAP treatment resulted in significant reductions in expression of NK1R- or GRPR-immunoreactive spinal neurons, respectively (Fig. 4). SP-SAP treatment resulted in a significant reduction in ongoing (spontaneous) scratching (Fig. 5). In contrast, BB-SAP treatment did not significantly affect the number of spontaneous scratch bouts. SP-SAP treatment resulted in a significant reduction in the alloknesis score, while BB-SAP did not affect the alloknesis score (Fig. 6). Both BB-SAP and SP-SAP treatments significantly reduced chloroquine-evoked scratching behavior, compared to OVA-sensitized mice receiving blank-SAP (Fig. 7). Blank-SAP did not result in any changes in spontaneous, or, touch- or chloroquine-evoked scratching compared to OVA-sensitized mice not receiving SAP.
Fig. 4.

A typical example of gastrin-releasing peptide receptor (GRPR) and neurokinin 1 receptor (NK1R) expression in the upper cervical spinal cord of saporin-treated mice. A) GRPR expression in the spinal cord of mouse treated with blank saporin. B) Reduced GRPR expression in the spinal cord of mouse treated with bombesin saporin. C) NK1R expression in the spinal cord of mouse treated with blank saporin. D) Reduced NK1R expression in the spinal cord of mouse treated with substance P saporin.
Fig. 5.

Substance P saporin reduced ongoing (spontaneous) scratching in ovalbumin (OVA)-sensitized mice. Saporin was intrathecally injected on the day 25 in OVA-sensitized mice. White, black, and gray columns show, respectively, SAP (blank saporin-treated), BB-SAP (bombesin saporin-treated), and SP-SAP (substance P saporin-treated) groups. Error bars are S.E.M. *p< 0.05, significant difference from SAP (one-way ANOVA followed by Bonferroni test, F=3.438, n=6).
Fig. 6.

Substance P saporin reduced alloknesis score in ovalbumin (OVA)-sensitized mice. Saporin was intrathecally injected on day 25 in OVA-sensitized mice. White, black, and gray columns show, respectively, SAP (blank saporin-treated), BB-SAP (bombesin saporin-treated), and SP-SAP (substance P saporin-treated) groups. Error bars are S.E.M. *p< 0.05, significantly difference from SAP. Two-way ANOVA followed by Bonferroni test, F(2,30)=8.010, n=6.
Fig. 7.

Bombesin saporin and substance P saporin reduced chloroquine-evoked scratching in ovalbumin (OVA)-sensitized mice. Saporin was intrathecally injected on day 25 in OVA-sensitized mice. Chloroquine was intradermally injected in OVA-treated skin. Striped, white, black, and gray columns show, respectively, no treatment, SAP (blank saporin-treated), BB-SAP (bombesin saporin-treated), and SP-SAP (substance P saporin-treated) groups. Bar graph plots mean number of scratch bouts recorded over a 30-min period following id injection of chloroquine with vehicle-associated scratching subtracted. Error bars are S.E.M. *p< 0.05, significantly difference from no treatment group. One-way ANOVA followed by Bonferroni test (F=9.848, n=6).
Previous studies suggest that GRPR-expressing spinal neurons are interneurons [32; 35]. We hypothesized that GRPR-expressing spinal neurons may be upstream of itch signaling mediated through NK1R-expressing spinal neurons. To investigate the extent to which NK1R-expressing spinal neurons project in ascending sensory pathways, or do not project and are presumptive interneurons, we injected the retrograde tracer Fluorogold into the thalamus or lateral parabrachial nucleus. Spinothalamic projection neurons were largely located contralateral to the thalamic injection, while spinoparabrachial projection neurons were distributed bilaterally and predominantly in the superficial dorsal horn of the upper cervical (C1-C2) segments examined. Examples of retrogradely-labeled neurons are shown in Fig. 8. The average numbers of spinoparabrachial tract neurons or spinothalamic tract neurons per section were 5.1±0.4 (SEM, n=5) or 3.1±0.3 (SEM, n=5), respectively. Importantly, 89% (87/98) of spinoparabrachial tract neurons retrogradely labeled by Fluorogold were double-labeled for NK1R. Similarly, 94% (58/62) of spinothalamic tract neurons retrogradely labeled by Fluorogold were double-labeled for NK1R.
Fig. 8.

Double-labeling of spinoparabrachial projection neurons with NK1R. A) Fluorescence microscope image of superficial dorsal horn of C2 spinal cord section, showing neurons retrogradely-labeled with Fluorogold (blue). Inset shows injection site of Fluorogold. Abbreviations: LC, locus coeruleus; LPB, lat. parabrachial n., MPB, med. parabrachial n.; SCP, superior cerebellar peduncle; Vc, trigeminal subnucleus caudalis. B) Fluorescence microscope image showing NK1R-immunopositive neurons (green) in superficial dorsal horn (same section as in A). C) Merged image of A and B. Inset shows high magnification of neuron to which the yellow arrow points. White arrows in A-C point to neurons exhibiting double-labeling (teal in C).
Discussion
OVA-sensitized mice appear to represent a useful model to examine the neuronal mechanisms underlying itch and its sensitization in AD for the following reasons. 1) OVA-sensitized mice exhibited an increase in ongoing (spontaneous) scratching over an 18 day period, suggesting that chronic itch occurs in this model. 2) OVA-sensitized mice exhibited alloknesis and hyperknesis, which are signs of itch sensitization and are observed in human patients with AD. Additionally, alloknesis developed at a delay after the increase in spontaneous scratching. This supports the idea that alloknesis occurs as a result of itch sensitization. 3) Skin symptoms observed macroscopically, while not severe, were characterized by erythema and excoriation.
Here we show that chloroquine-evoked scratching was significantly enhanced in OVA-sensitized mice, while histamine-evoked scratching was not significantly affected. There was a strong trend toward enhancement of histamine-evoked scratching, similar to that observed previously in mice treated to produce dry skin pruritus [2]. Although we cannot completely exclude the possibility of sensitization of the histamine-dependent pathway based on the current data, selective sensitization of a histamine-independent pathway has been reported in previous studies [2, 13]. In humans, nerve growth factor induced sensitization of cowhage- but not histamine-evoked itch [24]. This sensitization correlated with mechanical but not heat hyperalgesia. In a mouse model of chronic dry skin itch, we previously found that scratching evoked by either serotonin or the PAR2/MrgprC11 (protease-activated receptor 2/Mas-related G-protein coupled receptor C11) agonist SLIGRL was enhanced, while histamine-evoked scratching was not enhanced [2]. Responses of cultured DRG neurons to either serotonin or the PAR2/MrgprC11 agonist were enhanced [2], while their responses to histamine were not significantly affected, suggesting that peripheral sensitization of the histamine-independent pathways plays a major role in hyperknesis and enhanced itch signals that are presumably conveyed to GRPR-expressing spinal neurons.
Using OVA-sensitized mice, we investigated whether spinal neurons that express molecular receptors of the neuropeptides gastrin releasing peptide and substance P are involved in itch sensitization in this mouse model of AD. Here we show that SP-SAP ablated NK1R-expressing neurons in the spinal dorsal horn and significantly attenuated behavioral signs of ongoing itch, alloknesis, and hyperknesis, suggesting that NK1R-expressing spinal neurons have a major role in itch sensitization in AD. We previously found that NK1R-expressing spinal neurons are involved in conveying acute itch signaling as well [14]. Intracisternal injection of SP-SAP ablated NK1R-expressing spinal neurons in the superficial medullary dorsal horn and significantly reduced scratching evoked by intradermal injection of serotonin in rats [14]. NK1R-expressing spinal neurons in the superficial dorsal horn of the rat cervical spinal cord are known to give rise to ascending projections [29]. The present study confirms that a large percentage of ascending projection neurons express NK1R in the mouse cervical spinal cord. NK1R-expressing spinal neurons plausibly relay itch information to the brain under conditions of both acute and chronic itch.
GRPR-expressing spinal neurons play a critical role in the transmission of itch signals [28]. BB-SAP treated mice did not show an increase in spontaneous scratching following repeated treatment with diphenylcyclopropenone, while blank-saporin treated mice exhibited an increase in spontaneous scratching [28], suggesting that GRPR-expressing spinal neurons participate in the development of itch sensitization. In contrast, in the present study BB-SAP treatment in OVA-sensitized mice resulted in a reduction in chloroquine-evoked hyperknesis, but not in spontaneous scratching or alloknesis. This suggests that GRPR-expressing spinal neurons are not essential for the maintenance of itch sensitization, and that different neuronal pathways underlie ongoing itch, alloknesis and hyperknesis. The latter is supported by our recent study showing that the κ- opioid receptor agonist, nalfurafine, inhibited spontaneous scratching, but not alloknesis, in chronic dry skin-associated itch in mice [4].
Both SP-SAP and BB-SAP treatment resulted in a significant reduction in hyperknesis. Hyperknesis observed in the OVA-treated animals might be explained by peripheral sensitization of primary afferent pruriceptors. We previously reported that sensory DRG neurons innervating a region of chronic dry skin treatment exhibited enhanced responses to serotonin and SLIGRL but not histamine [2], consistent with peripheral sensitization of pruriceptors signaling non-histaminergic itch. In OVA-treated mice, activation of sensitized pruriceptors by chloroquine would elicit enhanced responses in both NK1R- and GRPR-expressing spinal neurons, either in parallel or serially, to result in hyperknesis.
Here we show that animals treated with SP-SAP, but not BB-SAP, exhibited significant reductions in alloknesis and ongoing scratching behavior. A speculative explanation for this is a selective central sensitization of spinal neurons expressing NK1R, but not neurons expressing GRPR (Fig. 9). NK1R-expressing spinal neurons receive polysynaptic input from mechanoreceptor afferents (Fig. 9A)[31]. Central sensitization would result in enhanced responsiveness of the NK1R-expressing neurons to low-threshold mechanical stimulation, similar to the enhanced mechanical sensitivity observed in lumbar spinal neurons following their activation by intradermal histamine [5]. The heightened mechanically-evoked response of these neurons, most of which project in spinothalamic or spinoparabrachial pathways, presumably signals alloknesis. Alternatively, disinhibition of polysynaptic mechanical inputs can contribute to alloknesis. Polysynaptic mechanical inputs are constitutively inhibited by GABAergic and glycinergic inhibitory interneurons (Fig. 9B)[11; 31]. Central sensitization of NK1R-expressing spinal neurons could also account for ongoing (spontaneous) scratching behavior, which was significantly reduced following SP-SAP treatment. Here we show that neurotoxic ablation of GRPR-expressing neurons did not result in reduced alloknesis or ongoing scratching behavior. This implies that GRPR-expressing neurons are presynaptic to (i.e., upstream of) NK1R-expressing neurons in the spinal itch-signaling pathway. It also implies that GRPR-expressing spinal neurons did not become sensitized, even though they were required for the expression of hyperknesis which we speculate to be mediated by peripheral sensitization of pruriceptors (see the preceding paragraph). Input from sensitized pruriceptors is a logical trigger for the development of central itch sensitization, characterized by ongoing (spontaneous) scratching as well as alloknesis. Thus, it is unclear why NK1R- but not GRPR-expressing spinal neurons were required for the full expression of itch sensitization (i.e., spontaneous scratching and alloknesis). Speculatively, NK1R-expressing neurons developed a greater degree of central sensitization than GRPR-expressing neurons, achieving a sufficient level of activity to generate ongoing (spontaneous) scratching behavior. Ablation of GRPR-expressing neurons might disrupt inhibitory (e.g., Bhlhb5 interneurons [23]) and/or excitatory (e.g., testicular orphan nuclear receptor 4 (TR4) [32]) interneuronal networks, resulting in facilitation and/or disinhibition that would contribute to the sensitization of postsynaptic NK1R-expressing neurons (Fig. 9B). Reestablishment of GABAergic interneurons by transplantation of precursors of cortical inhibitory interneurons rescued itch-related phenotypes of Bhlhb5 knockout mice [13], presumably due to restoration of spinal inhibition.
Fig. 9.
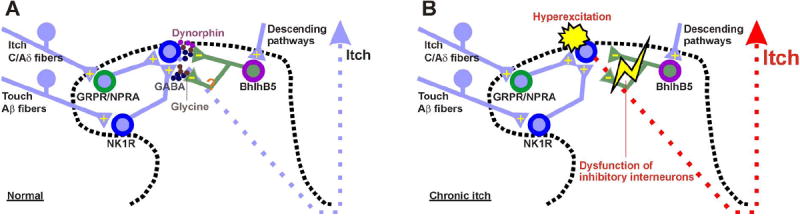
Schematic diagram. A: baseline conditions. B: OVA-sensitized condition. See text for further explanation.
We observed retrogradely labeled neurons to be located predominantly in the superficial dorsal horn at upper cervical (C1-2) and caudal medullary levels following Fluorogold injections in the lateral parabrachial nucleus or ventral posteromedial thalamus, confirming recent studies in mice [15], and rats [9; 10]. Using a double-label strategy, we observed that 89% of spinoparabrachial and 94% of spinothalamic projections neurons co-expressed NK1R. The high incidence of NK1R-expression in spinoparabrachial and spinothalamic tract projection neurons is consistent with previous studies using rats [30]. Using antidromic stimulation to functionally characterize ascending projection neurons in rats, 27% of trigeminothalamic [22] and 62% of trigeminoparabrachial [19] neurons responded to intradermal injection of the itch mediator, serotonin, as well as additional itch (chloroquine, histamine) and pain mediators (capsaicin, mustard oil). We conclude that a substantial fraction of ascending spinothalamic and spinoparabrachial projection neurons that express the NK1R function to convey itch information to higher centers in the brain.
Few GRPR-expressing spinal neurons coexpress NK1R in the dorsal horn [35], implying that GRPR-expressing spinal neurons are interneurons. This is consistent with a previous study using TR4 knockout mice [32]. In these mice, a dramatic loss of GRPR-expressing spinal neurons was observed, while projection neurons were preserved. Presumably, NK1R-expressing spinal neurons are downstream of GRPR-expressing spinal neurons.
The present study reveals a major role for NK1R-expressing spinal neurons in chronic itch. GRPR-expressing spinal neurons are important for the development, but not the maintenance, of itch sensitization. Desensitization of NK1R-experssing spinal neurons might be a useful approach to treat chronic itch, as an alternative to blocking peripheral molecular targets.
Summary.
Mice sensitized with ovalbumin developed dermatitis and behavioral signs of chronic itch that are mediated by ascending spinal projection neurons that express the neurokinin-1 receptor.
Acknowledgments
The work was supported by grants from the National Institutes of Health DE013685, AR057194 and AR063228 and National Eczema Association grant.
Footnotes
None of the authors declares any conflict of interest.
References
- 1.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151(2):378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012;132(7):1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens MI, Piecha D, Steppan S, Carstens E. Nalfurafine Suppresses Pruritogen- and Touch-evoked Scratching Behavior in Models of Acute and Chronic Itch in Mice. Acta Derm Venereol. 2015;95(2):147–150. doi: 10.2340/00015555-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama T, Nagamine M, Carstens MI, Carstens E. Behavioral model of itch, alloknesis, pain and allodynia in the lower hindlimb and correlative responses of lumbar dorsal horn neurons in the mouse. Neuroscience. 2014;266:38–46. doi: 10.1016/j.neuroscience.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J Neurophysiol. 2013;109(3):742–748. doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Role of spinal bombesin-responsive neurons in nonhistaminergic itch. J Neurophysiol. 2014;112(9):2283–2289. doi: 10.1152/jn.00409.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain. 2014;155(1):80–92. doi: 10.1016/j.pain.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Khater KM, Kerr R, Todd AJ. A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol. 2008;511(1):1–18. doi: 10.1002/cne.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515(6):629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardoni R, Takazawa T, Tong CK, Choudhury P, Scherrer G, Macdermott AB. Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn. Ann N Y Acad Sci. 2013;1279:90–96. doi: 10.1111/nyas.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. 2014;17(2):175–182. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braz JM, Juarez-Salinas D, Ross SE, Basbaum AI. Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J Clin Invest. 2014;124(8):3612–3616. doi: 10.1172/JCI75214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21(4):303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson S, Truong H, Giesler GJ., Jr Quantitative analysis of spinothalamic tract neurons in adult and developing mouse. J Comp Neurol. 2010;518(16):3193–3204. doi: 10.1002/cne.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16(2):174–82. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han N, Zu JY, Chai J. Spinal bombesin-recognized neurones mediate more nonhistaminergic than histaminergic sensation of itch in mice. Clin Exp Dermatol. 2012;37(3):290–295. doi: 10.1111/j.1365-2230.2011.04314.x. [DOI] [PubMed] [Google Scholar]
- 18.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen NA, Giesler GJ., Jr Response characteristics of pruriceptive and nociceptive trigeminoparabrachial tract neurons in the rat. J Neurophysiol. 2015;113(1):58–70. doi: 10.1152/jn.00596.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15(1):19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340(6135):968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser HR, Giesler GJ., Jr Characterization of pruriceptive trigeminothalamic tract neurons in rats. J Neurophysiol. 2014;111(8):1574–1589. doi: 10.1152/jn.00668.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65(6):886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rukwied RR, Main M, Weinkauf B, Schmelz M. NGF sensitizes nociceptors for cowhage- but not histamine-induced itch in human skin. J Invest Dermatol. 2013;133(1):268–270. doi: 10.1038/jid.2012.242. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki A, Adhikari S, Andoh T, Kuraishi Y. BB2 bombesin receptor-expressing spinal neurons transmit herpes-associated itch by BB2 receptor-independent signaling. Neuroreport. 2013;24(12):652–656. doi: 10.1097/WNR.0b013e32836352d8. [DOI] [PubMed] [Google Scholar]
- 26.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101(8):1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448(7154):700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 28.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325(5947):1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. The European journal of neuroscience. 2000;12(2):689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- 31.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26(6):1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, Yamanaka H, Rice D, Basbaum AI. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78(2):312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14(5):595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yatsuzuka R, Inoue T, Jiang S, Nakano Y, Kamei C. Development of new atopic dermatitis models characterized by not only itching but also inflammatory skin in mice. Eur J Pharmacol. 2007;565(1-3):225–231. doi: 10.1016/j.ejphar.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 35.Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, Liu XY, Barry DM, Wan L, Liu ZC, Li H, Turkoz A, Ma K, Cornelius LA, Kopan R, Battey JF, Jr, Zhong J, Chen ZF. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest. 2013;123(11):4769–4780. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao ZQ, Wan L, Liu XY, Huo FQ, Li H, Barry DM, Krieger S, Kim S, Liu ZC, Xu J, Rogers BE, Li YQ, Chen ZF. Cross-Inhibition of NMBR and GRPR Signaling Maintains Normal Histaminergic Itch Transmission. J Neurosci. 2014;34(37):12402–12414. doi: 10.1523/JNEUROSCI.1709-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


