Abstract
INTRODUCTION
The etiology of autism spectrum disorders (ASD) is believed to involve genetic and environmental components. The present study focused on the plasticizer, Bisphenol-A (BPA). The major pathway for BPA metabolism and excretion is via glucuronidation.
OBJECTIVES
To determine whether there was a relationship between BPA exposure and ASD.
METHODS
Urine specimens were collected from 46 children with ASD and 52 controls. Free and total BPA concentrations were determined by mass spectrometry. The fraction glucuronidated was calculated from the difference. A metabolomics study was done to investigate metabolite distribution in the urine.
RESULTS
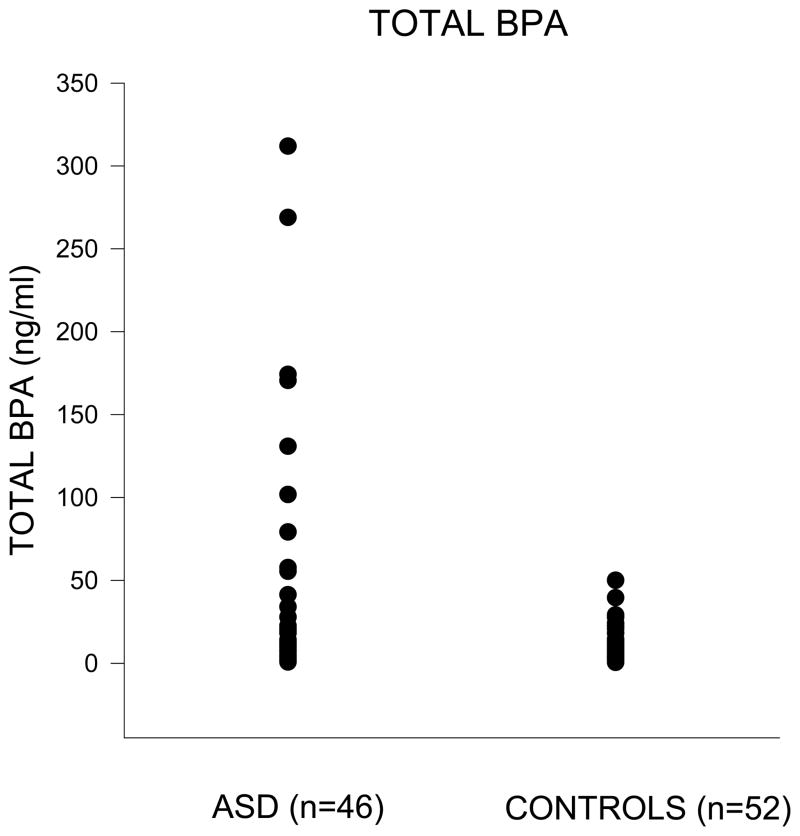
(i) Most of the BPA excreted in the urine was as the glucuronide. (ii) About 20% of the ASD children had BPA levels beyond the 90th percentile (> 50 ng/ml) of the frequency distribution for the total sample of 98 children. (iii) Mann-Whitney U tests and multiple regression analyses found significant differences (p <0 .05) between the groups in total and % bound BPA. (iv) The metabolomics analyses showed the number of absolute partial correlations ≥ |0.30| between metabolite concentrations and total BPA was ~3 times greater with the ASD group than the controls ( p <0 .001), and the number of absolute partial correlations ≥ |0.30| for % bound BPA was ~15 times higher with ASD ( p <0 .001).
CONCLUSION
The results suggest there is an association between BPA and ASD.
Keywords: Bisphenol A (BPA), Autism Spectrum Disorders, Glucuronidation, Phthalates, Plasticizers
INTRODUCTION
The etiology of autism spectrum disorders (ASD) is believed to be multifactorial. One popular hypothesis involves toxicant exposure acting upon genetically-sensitive individuals. ASD has strong associations with environmental factors and genetic components (Herbert, 2010; Institute of Medicine and Proceedings, 2008; Landrigan, 2010; Muhle et al., 2004). It is now generally accepted that chemicals introduced into the environment by human activity can have adverse effects on human health (Center et al., 2005; Herbert; NTP-CERHR, 2007; Pessah and Lein, 2008; Pessah et al., 2008). The present study focuses on one of these compounds, the common plasticizer Bisphenol-A (BPA). BPA is used in the manufacture of polycarbonate plastics, as an antioxidant in some plasticizers, in polyvinyl chloride (PVC) manufacture, and the Epoxy resins used to coat the inside of many food and beverage cans (FDA., 2010; Makris et al., 2013b; NIEHS, 2011; NTP-CERHR, 2007). Human exposure can come from direct contact with plastics, food wrapped in plastics, drinking water, flooring and the contamination of indoor air (Erickson, 2008; Kalkbrenner et al., 2010; Larsson et al., 2009; Makris et al., 2013a).
There is some recent indirect evidence linking exposure to plasticizers in general, including BPA to ASD. Epidemiological studies found that increased maternal prenatal exposure to BPA was linked to behavioral problems in the children some years later (Larsson et al., 2009; Miodovnik et al., 2011; Vom Saal et al., 2012). Autism per se was not documented, but the umbrella term ‘behavioral problems’ could include ASD (Miodovnik et al., 2011). A Swedish epidemiological study found that PVC flooring material in the home was associated with ASD (Larsson et al., 2009). Although not implicating plasticizers directly, Kalkbrenner et al. found perinatal exposure to air pollutants to be associated with ASD in 8 year-old children (Kalkbrenner et al., 2010). Indoor dust samples are often contaminated with BPA (Hwang et al., 2008).
The major pathway for BPA metabolism and excretion is via glucuronidation. The glucuronidation pathway makes a large variety of substances more water-soluble allowing for their subsequent elimination from the body upon urination. Most BPA excretion is as the glucuronide with a small amount as the sulfate (Liao and Kannan, 2012). Tracer studies have shown that more than 95% of a test dose of U-13C BPA is recovered in the urine (Volkel et al., 2002).
We recently published two studies of children with and without ASD comparing metabolites found in the urine specimens used for the present study (Ming et al., 2012; Stein et al., 2013). The first investigated exposure to another common plasticizer Diethylhexyl phthalate (DEHP) by measuring the excretion of its metabolites (Stein et al., 2013). We found glucuronidation to be decreased in the ASD group suggesting that the glucuronidation pathway was compromised in at least some children with ASD. The second was a metabolomics study of the urine specimens. Significant differences in the pattern of metabolite excretion in the urine between the control and ASD children were found; specifically there was evidence for abnormal amino acid metabolism, increased oxidative stress, and altered gut microbiomes in the ASD group (Ming et al., 2012).
The objectives of the present study were to determine whether: (i) there was a relationship between total BPA excretion and ASD, (ii) BPA detoxification via the glucuronidation pathway was compromised in children with ASD and (iii) integrate the three data sets, (BPA, DEHP metabolites and metabolomics) to examine the inter-relationships among them and with ASD.
METHOD AND MATERIALS
SUBJECTS
Informed consent for these studies was obtained from the care-givers and was approved by the Institutional Review Board of the University of Medicine and Dentistry of New Jersey (now Rutgers University)-New Jersey Medical School. Subjects were recruited from the Pediatric Neurology and Pediatrics clinical practices at the University of Medicine and Dentistry of New Jersey (Rutgers), New Jersey Medical School. Spot urine specimens were collected from 46 children with ASD and 52 age-matched healthy controls between 10:00 a.m. and 4:00 p.m. (Ming et al., 2012). The samples were frozen and then stored at −70 °C within 2 hrs. of collection. All ASD subjects were under the care of the pediatric neurologist (X.M.) and the diagnoses were made by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV TR); almost 90% of the diagnoses were further confirmed by Autism Diagnostic Interview-Revise, and/or Autism Diagnostic Observation Scale-Generic criteria. Medical history and comorbidity data were collected for the ASD subjects. Because medical and psychiatric co-morbidities along with intellectual impairment are common among ASD children (Ming et al., 2008; Buck et al. 2014), we included ASD children with or without co-morbidity and all levels of intellectual performance. Children with ASD and a known genetic disorder (double syndrome such as Fragile X syndrome) were excluded. Control children were screened for medical and developmental disorders during their well-child visits in addition to chart review and only those free of any chronic or recurrent medical disorders were considered healthy and included in this study. All subjects were carefully screened for signs of infection or intercurrent illness on the day of specimen acquisition, and subjects with acute illness were excluded. The dietary intake history within 24 hours of sampling was recorded, including that of medication and vitamin intake. Daily plastics or BPA exposure in these subjects was not assessed, as accurate levels of exposure cannot be obtained from a diet history.
ANALYTICAL METHODOLOGY
The concentration of free and total BPA in the collected urine specimens was measured by isotope dilution-liquid chromatography mass spectrometry–mass spectrometry (ID-LC-MSMS) using a modification of the methodology described by Liao and Kannan (Liao and Kannan, 2012). The ID-LC-MSMS assay measures free BPA. In order to measure the total BPA present in the urine, the glucuronidated BPA had to be deconjugated. This was done by treating the urine with β-glucuronidase to remove the glucuronic acid residue from the glucuronidated BPA (Sigma-Aldrich, St. Louis MO).
1 ml urine and 100μl Internal Standard (ISTD – 13C BPA 100ng/ml, (Cambridge Isotope Labs, Andover, MA)) were combined in a test tube and vortexed. The contents were divided into two aliquots, 0.55 ml being withdrawn from the tube for Total BPA analysis and the remainder left behind for free BPA analysis.
FREE BPA
After adding 1.0 ml 1M Ammonium Acetate, the sample was extracted 3 times with 2 ml ethyl acetate. The combined extracts were washed with 4 ml Pico-Pure water, centrifuged for 2 minutes at 1500 rpm at 4° and the ethyl acetate layer transferred to a screw-cap tube. The samples were then gently dried under a stream of nitrogen (just to dryness) and reconstituted in 150 μl methanol. The residues were stored at 4° overnight prior to LC-MSMS analysis.
TOTAL BPA
A fresh solution containing ~250–300 units/ml of E. Coli β-glucuronidase in 1M Ammonium Acetate was prepared immediately before use. 1 ml of enzyme solution was added to each ‘Total BPA’ tube, capped and incubated at 37°overnight (~19 hours). The next day samples were extracted as per the Free BPA tubes, dried and reconstituted in 150μl methanol for injection. 10μl was injected into the LC-MSMS. A mixture of external standards consisting of 100 pg BPA and 100 pg 13C BPA was run after every 5 injections as was a water blank.
LC-MSMS CONDITIONS
Analyses were performed with an Agilent Technologies 1200 Series HPLC coupled with a 6410 Triple Quad MS. The LC column used was a Zorbax SB-C18 2.1 × 150mm (Agilent Technologies, Wilmington, DE) at 25°. The mobile phase was A: 10mM ammonium acetate, B: methanol. A gradient was used at 0.3 ml/min 0–2 min, 15% methanol, 2.5–5 min, 75% methanol, 10–18 min, 99% methanol. The MS/MS was operated in the negative ESI multiple reaction monitoring (MRM) mode with the following source parameters: Nitrogen gas temperature 340°, gas flow 10 L/min, nebulizer 40 psi, capillary 2500 volts. The MRM transitions monitored were: (227.2-->212.1 (unlabeled 12C BPA) and 239.2-->224.1 (U-13C BPA).
The resulting MRMs (actual MRM abundances for each time point) were then exported to an Excel spreadsheet and the 4 digitized scans averaged to better define the peaks. The averaged spectra were then exported to a chromatogram deconvolution program (Peakfit, SPSS, Chicago IL.) and the resolved areas under the MRM curves integrated offline. The reason for going this route is that some urine specimens (~50%) had a large contaminating peak partially adjacent to the small 12C BPA peak of interest, and the software provided by the LC-MSMS manufacturer was unable to satisfactorily resolve the two peaks. The limits of detection (LOD) were 0.07 ng/ml in water, and the limits of measurement (LOM) were 0.2 ng BPA/ml in urine. The LOD was the background water concentration.
The DEHP metabolite data, urine creatinine values and the metabolomics data were available from previously published studies (Ming et al., 2012; Stein et al., 2013). All three studies (BPA, DEHP metabolite and metabolomic) were done on the same urine specimen. There were four fewer subjects for the BPA analyses because of lack of sample (3 ASD, 1 control).
STATISTICAL ANALYSES
The skewness and kurtosis indices of the frequency distributions for the BPA (total, free and % bound) and metabolomic indices were calculated, and all were found to be positively skewed. The % bound BPA index was calculated by subtracting the % free BPA metabolite value from 100. Logarithmic (base 10) transformations were applied to all of these indices before using them in parametric analyses.
For the analyses using the metabolomic data, the raw areas counts for the metabolic indices were also log10 transformed to reduce positive skewness. Compounds were excluded if the number of detections for a particular metabolite (counts) was < 20 for both the ASD and control groups. No missing data were imputed. To test whether the mean number of absolute partial correlations (rps ≥ |0.30|) of the BPA indices for the ASD group with the metabolomic indices was higher than the mean number of absolute partial correlations (rps ≥ |0.30|) of the BPA indices in the control sample, sex, age, BMI, and creatinine were first controlled for by using absolute partial correlation analyses. The absolute rp of ≥ |0.30| was chosen as the lower limit for a medium effect size relationships between the BPA indices and the metabolomic data based on Cohen’s interpretative guidelines (Cohen, 1993). To minimize spurious relationships arising from calculating the 1,173 partial correlations between a possible 391 metabolomic and 3 BPA indices, the partial correlation analyses were restricted to metabolomics compounds in which there was data for at least 20 subjects for each group.
A chi-square test for independence with Yates’ correction for continuity was calculated to ascertain whether the percentages of boys and girls in the autistic and control samples were comparable. t tests for independence were performed to determine whether the mean ages, BMIs, creatinine levels and numbers of absolute partial correlations > |0.30| were comparable in both samples. The median concentrations of the untransformed total BPA, free BPA, and % bound BPA indices were first compared using Mann-Whitney U tests. Because the Ns for both samples were > 30, the resultant U values were converted into z statistics for interpretive purposes, and the effect sizes for the median differences were estimated with r, i.e., Z / square root of the sum of the Ns for both groups. Sex, age, BMI, and creatinine were also simultaneously entered as covariates in multiple regression analyses comparing the autistic and control samples’ adjusted means for the log10 transformed total BPA, free BPA, and % bound BPA indices.
No statistical correction, such as a Bonferroni adjustment, was made to alpha to control for the familywise error rate that was incurred in conducting the numerous statistical analyses that were made. Two-tailed tests of significance were employed, and Cohen’s d statistic was used to reflect the effect sizes of the adjusted mean differences (Cohen, 1993).
RESULTS
SUBJECTS
There were 46 (47%) children diagnosed with ASD and 52 (53%) control children for whom there were complete BPA assays. As Table 1 indicates, there was a lower percentage of girls than boys reflecting the known increased prevalence of ASD in males. However, the φ (phi) correlation in table 1 suggests that the effect size was small according to Cohen’s interpretative guidelines (Cohen, 1993).
Table 1.
Characteristics of ASD and Control subjects.
| Characteristic | ASD | Control | Statistic | P | Effect Size |
|---|---|---|---|---|---|
| N | 46 | 53 | |||
| Sex (% Boys) | 76 | 54 | x2Yates(1) = 4.34 | 0 .04* | φ =0.23 |
| (M ± SD) | (M ± SD) | ||||
| Age (years) | 10.09 ± 3.70 | 10.83 ± 4.01 | t(96) = 0.61 | 0.35 | d = 0.19 |
| BMI | 16.81 ± 2.38 | 16.51 ± 1.86 | t(101) = 0.65 | 0 .52 | d =0 .13 |
| Creatinine (ng ml−1) | 1.23 ± 0.10 | 1.13 ± 0.09 | t(101) = 0.67 | 0 .41 | d = 0.14 |
Values for total and free BPA were obtained for all of the 98 children. Most of the BPA excreted in the urine was as the glucuronide (% bound BPA M = 83.39, SD = 19.09). Although there were three values for free BPA at or close to the limits of measurement (0.26, 0.25, 0.15 ng/ml), the deconvoluted peaks for these three subjects were well defined. The medians along with the 25th and 75th percentiles for total BPA, free BPA and % bound BPA are presented in Table 2. The Mann-Whitney U tests found significant differences (ps <0 .05) between the medians of the groups for the total BPA and % bound BPA indices. The median total BPA was higher in the children with ASD than in the control children, and the median % bound BPA was lower in the children with ASD than the median in the control group. The groups were comparable with respect to their median levels of free BPA. The rs shown in table 2 indicate that the effects sizes for the total BPA and % bound BPA indices were small.
Table 2.
Comparisons of Bisphenol A Metabolites by Mann-Whitney U tests for Autistic (ASD) and Control Children.
| METABOLITE | ASD | CONTROL | U | z | p | r | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | MEDIAN | 25th % | 75th % | N | MEDIAN | 25th % | 75th % | |||||
| Total BPA (ng/ml) | 46 | 11.18 | 4.54 | 29.36 | 52 | 6.58 | 3.32 | 12.15 | 848 | −2.48 | 0.01 | −0.25 |
| Total BPA (ng/ml) | 37 | 9.66 | 4.33 | 13.61 | 51 | 6.41 | 3.28 | 11.50 | 811 | −1.12 | 0.26 | −0.12 |
| Free BPA(ng/ml) | 46 | 0.78 | 0.48 | 1.38 | 52 | 0.72 | 0.39 | 1.32 | 1061 | −0.96 | 0.34 | −0.10 |
| Free BPA(ng/ml) | 37 | 0.67 | 0.44 | 1.05 | 51 | 0.74 | 0.39 | 1.34 | 887 | −0.48 | 0.63 | −0.05 |
| Bound BPA (%) | 46 | 91.21 | 86.81 | 96.03 | 52 | 86.98 | 77.28 | 94.25 | 901 | −2.10 | 0.04 | −0.21 |
| Bound BPA (%) | 37 | 89.90 | 85.20 | 94.20 | 51 | 86.72 | 77.06 | 94.02 | 769 | −1.48 | 0.14 | −0.16 |
BPA = Bisphenol A, U = Mann-Whitney U Test statistic, r = effect size. Data in regular font are for the total data set (n=98), in italics are the data without the 10 subjects with very high total urinary BPA levels (n=88).
Similarly, table 3 shows the adjusted means, standard deviations, F statistics, and Cohen ds for the multiple regression analyses after controlling for gender, age, BMI, and creatinine levels, (Cohen, 1993). The overall pattern of results from the multiple regression analyses parallel those found with the nonparametric Mann-Whitney U tests for total BPA, Free BPA, and the % Bound BPA. The non-log10 transformed adjusted means and standards deviations are given in table 2 to facilitate clinical interpretation, but the F and d statistics are based on the log10 transformed indices.
Table 3.
Adjusted Means of the Bisphenol A Metabolites by type of sample after controlling for sex, age, BMI and Creatinine (n=98). The F and d statistics are based on the log10 transformed data, but the untransformed adjusted Ms and SDs are listed here for the metabolites to facilitate clinical interpretation. Data in regular font are for the total data set (n=98), in italics are the data without the 10 subjects with very high total urinary BPA levels (n=88).
| Metabolite | ASD | CONTROL | F (1,92) | p | d | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Madj | SD | N | Madj | SD | ||||
| Total BPA | 46 | 36.28 | 48.23 | 52 | 11.72 | 48.09 | 6.75 | 0.01 | 0.54 |
| Total BPA | 37 | 10.90 | 8.40 | 51 | 9.31 | 8.36 | 0.53 | 0.47 | 0.16 |
| Free BPA | 46 | 2.03 | 3.48 | 52 | 1.45 | 3.47 | 0.10 | 0.75 | 0.07 |
| Free BPA | 37 | 0.81 | 1.40 | 51 | 1.35 | 1.39 | 3.52 | 0.06 | 0.41 |
| % Bound BPA | 46 | 88.75 | 18.98 | 52 | 78.65 | 18.92 | 6.10 | 0.02 | 0.51 |
| % Bound BPA | 37 | 86.94 | 19.68 | 51 | 78.55 | 19.56 | 3.77 | 0.06 | 0.43 |
Although the log10 transformations were successful in reducing the positive skewness of the BPA indices, there were 10 (9 ASD and 1 control) children whose untransformed total BPA scores were beyond the 90th percentile (> 50 ng/ml) of the frequency distribution for the total sample of 98 children (figure 1). A total BPA score of 50 was also more than four standard errors of the mean (SEM = 4.96) above the mean total BPA score of 23.25 (SD = 49.11) for the 98 children. To determine whether these 10 children with high total BPA scores were responsible for any differences between the control and ASD children, the data in tables 2 to 5 are presented for all 98 children (regular font), and then without the 10 children (n=88, italics).
Figure 1.
Table 5.
Mean Total Number of Absolute Partial Correlations (r ≥ |.30|) of BPA metabolites with 340 metabolomic counts after controlling for sex, age, BMI, and Creatinine by type of sample. Total number of matched metabolomic compounds for metabolite =340, d = effect size The partial correlations were calculated for the log10 transformations of the three BPA with 340 compounds detected in the metabolomic analyses that were represented by at least 20 data points in both the autistic and control samples. Data in regular font are for the total data set (n=98), in italics are the data without the 10 subjects with very high total urinary BPA levels (n=88).
| METABOLITE | GROUP | N | M | SD | Welch’s t’ | (df) | d |
|---|---|---|---|---|---|---|---|
| Total BPA (ng/ml) | ASD | 46 | 39 | 49 | 8.44** | 588 | 0.70 |
| CONTROL | 52 | 12 | 32 | ||||
| Free BPA (ng/ml) | ASD | 46 | 8 | 27 | 3.46** | 617 | 0.28 |
| CONTROL | 52 | 16 | 37 | ||||
| % Bound BPA | ASD | 46 | 59 | 49 | 19.10** | 447 | 1.81 |
| CONTROL | 52 | 4 | 20 | ||||
| Total BPA (ng/ml) | ASD | 37 | 46 | 50 | 8.35** | 637 | 0.66 |
| CONTROL | 51 | 18 | 38 | ||||
| Free BPA (ng/ml) | ASD | 37 | 9 | 28 | 2.70* | 641 | 0.21 |
| CONTROL | 51 | 16 | 36 | ||||
|
% Bound BPA Control |
ASD | 37 | 62 | 49 | 20.02** | 456 | 1.88 |
| CONTROL | 51 | 4 | 21 |
P < .01,
p < 0.001
As Tables 2 and 3 indicate, the median of the BPA total values for the ASD group was approximately twice the median for the control group (table 2) when the 10 outliers were included and the adjusted mean BPA total value for the ASD group was approximately three times the adjusted mean for the control group (table 3). Although the larger difference in the adjusted BPA total means might be assumed to be attributable to the controlling for sex, age, BMI, and creatinine, the difference was mostly produced by the use of the log 10 transformation which corrected for the extreme BPA scores and made the frequency distributions of the adjusted BPA total values approach those for normally distributed data. The adjustments afforded by sex, age, BMI, and creatinine were trivial. For example, the correlations of sex (0 = Male, 1 = Female) and age (yrs.) with the log 10 transformed BPA total scores were, respectively, 0.01 and −0.14 for the total sample of 98 and 0.14, and −0.03 for the sample of 88 after the 10 outliers were excluded. None of these correlations were significant enough to afford much adjustment.
The median and adjusted mean differences between the groups that had been found for total BPA and % bound BPA, with the Mann-Whitney U analyses and the multiple regression analyses disappeared when the 10 subjects with the very high BPA levels were excluded. For comparable total BPA concentrations, there was no difference in the fraction glucuronidated between the ASD and control groups.
BPA AND DEHP METABOLITES
Our previous study on DEHP metabolites found four of them to be associated with ASD, Free mono Methylethyl phthalate (MEHP), % glucuronidated MEHP, free 5-oxo-mono Methylethyl phthalate (5-oxo-MEHP) and % glucuronidated 5-carboxy MEPP (5-cx MEPP, (Stein et al., 2013). Although both BPA and DEHP metabolites showed associations with ASD, only weak, but statistically significant correlations between total BPA and MEHP, and 5-oxo MEHP were found in the present study. This was probably because different plastics have different mixes of plasticizers. Table 4 shows that the free BPA and % bound BPA were positively (ps <0 .05) related, respectively, to free MEHP and % bound MEHP for the 41 ASD children from whom both BPA and phthalate data were available, total BPA was only positively associated with % bound MEHP in the 47 control children for whom both BPA and phthalate data were available. The magnitudes of the rps for these significant relationships represent only medium effect sizes.
Table 4.
Partial correlations among BPA and Phthalate metabolites after controlling for sex, age, BMI and Creatinine. Data in regular font are for the total data set (n=98), in italics are the data without the 10 subjects with very high total urinary BPA levels (n=88).
| GROUP | PHTHALATE | BPA Total (ng/ml) | Free BPA (ng/ml) | % Bound BPA | |||
|---|---|---|---|---|---|---|---|
| rp | rp | rp | rp | rp | rp | ||
| ASD | N | 41 | 32 | 41 | 32 | 41 | 32 |
| Free MEHP | −0.02 | −0.18 | .35* | .36* | −0.20 | −0.25 | |
| Free 5-OXO | 0.22 | 0.22 | −0.26 | −0.28 | 0.27 | 0.25 | |
| % Bound MEHP | 0.06 | 0.20 | −0.22 | −0.29 | .32* | .38* | |
| % Bound 5-OXO | 0.22 | 0.16 | −0.26 | −0.20 | 0.14 | 0.08 | |
| CONTROL | N | 47 | 46 | 47 | 46 | 47 | 46 |
| Free MEHP | 0.11 | 0.11 | −0.27 | −0.27 | 0.10 | 0.10 | |
| Free 5-OXO | 0.12 | 0.16 | −0.10 | −0.18 | −0.21 | 0.23 | |
| % Bound MEHP | 0.34* | 0.46** | −0.22 | −0.36* | 0.16 | 0.20 | |
| % Bound 5-OXO | −0.07 | −0.02 | −0.05 | −0.12 | 0.18 | 0.20 | |
p < 0.05,
p<0.01.
METABOLOMICS
Only compounds that were detected in 20 or more children per group were used in the analyses of the metabolomics data set. There were 340 (87%) metabolites that met the criterion of being detected in more than 20 children in each group out of the initial set of 391 compounds that were detected. The number of partial correlations ≥ |0.30| for the three BPA indices by type of sample are displayed in table 5 for the total sample. The number of absolute partial correlations ≥ |0.30| for total BPA was approximately 3 times higher than that for the control group ( p <0 .001), and the number of absolute partial correlations ≥ |0.30| for % bound BPA was approximately 15 times higher than that for the control group (p<0.001). In contrast, for free BPA the total number of correlations was smaller, but the number of free partial absolute correlations for the control group was approximately twice as high as for ASD group (p<0.001).
All of these mean differences in the partial absolute correlation counts remained after the 10 children with very high total BPA concentrations were excluded (table 5). The elimination of the 10 children reduced the median and adjusted mean differences in total BPA excretion and the % bound between the ASD and control groups to non-significance, but increased the magnitudes of the partial correlations by yielding less skewed frequency distribution.
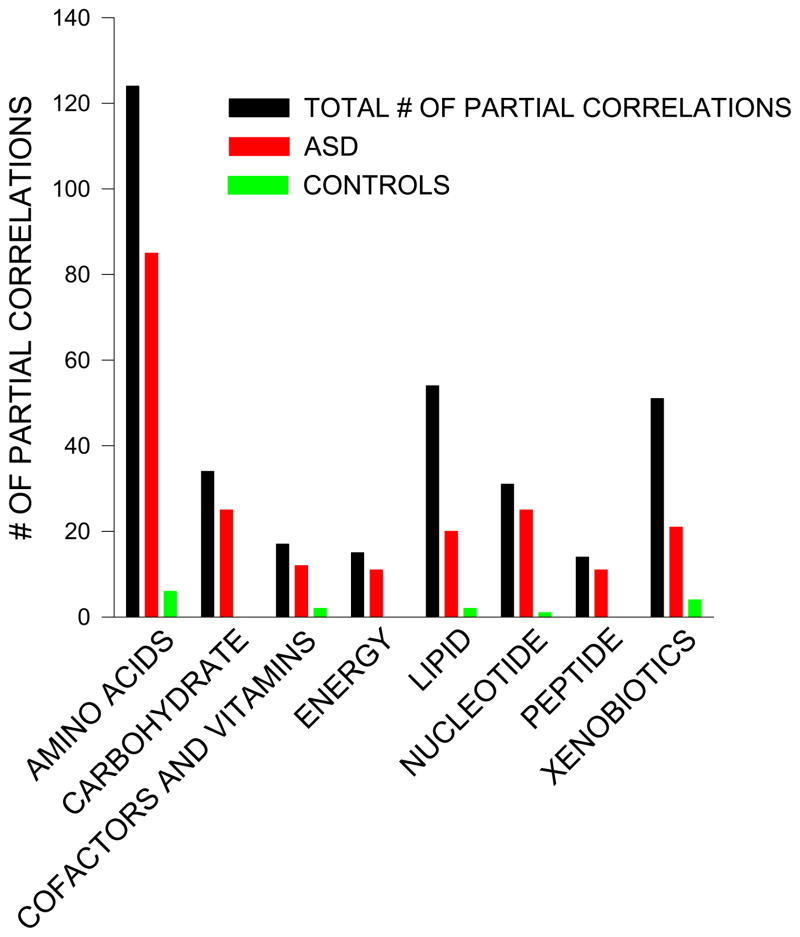
Table 6 (Total BPA) and table 7 (% bound BPA) tabulate the 340 metabolites according to their frequency distributions in the ‘Super-Pathways’ for each group along with the number of absolute partial correlations ≥ |0.30| that were found for the ASD and control groups. The tables show that both the number and the percentages of partial absolute correlations found for the ASD group were higher for the seven ‘super-pathways’ than the number and percentages of the partial absolute correlations found for the control children. The percentage is defined as the number of absolute partial correlations ≥ |.30| divided by the total number of compounds in that particular ‘Super Pathway’ where there were at least 20 data points in both the autistic and control samples. For clarity, some of the data from table 7 (% bound BPA and without the 10 outliers) are shown as a figure (figure 2).
Table 6.
Number of Absolute Partial Correlations ≥ |.30| for Total BPA with 340 metabolomic metabolites by type of Super Metabolic Pathway for ASD and Control Samples. Total number of matched metabolomic compounds for each sample = 340. Column 1 gives the name of the ‘Super-Pathway’ and in parentheses the number of compounds detected within that group. Columns 2 and 7 (‘Correlations’) gives the sum of the number of correlations with at least 20 data points for the ASD plus control samples. % is the number of Absolute Partial Correlations ≥ |.30| divided by the number of correlations (column 2 or 7). Data in regular font are for the total data set (n=98), in italics are the data without the 10 subjects with very high total urinary BPA levels (n=88). Total rps is the total number absolute partial correlations detected.
| SUPER PATHWAY | Total rps | ASD (n=46) | CONTROLS (n=52) | Total rps | ASD (n=37) | CONTROLS (n=51) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | N | % | N | % | |||
| AMINO ACID (124) | 49 | 37 | 76 | 12 | 24 | 85 | 63 | 74 | 22 | 26 |
| CARBOHYDRATE (34) | 25 | 19 | 76 | 6 | 24 | 26 | 19 | 73 | 7 | 27 |
| COFACTORS & VITAMINS (17) | 12 | 10 | 83 | 2 | 17 | 15 | 12 | 80 | 3 | 20 |
| ENERGY (15) | 6 | 6 | 100 | 0 | 0 | 8 | 6 | 75 | 2 | 25 |
| LIPID (54) | 26 | 20 | 77 | 6 | 23 | 30 | 20 | 67 | 10 | 33 |
| NUCLEOTIDE (31) | 22 | 21 | 95 | 1 | 5 | 26 | 21 | 95 | 1 | 5 |
| PEPTIDE (14) | 9 | 6 | 67 | 3 | 33 | 10 | 7 | 70 | 3 | 30 |
| XENOBIOTICS (51) | 22 | 12 | 55 | 10 | 45 | 23 | 10 | 43 | 13 | 57 |
| TOTAL 340) | 171 | 131 | 77 | 40 | 23 | 231 | 158 | 72 | 61 | 28 |
Table 7.
Number of Absolute Partial Correlations ≥ |.30| for % bound BPA with 340 metabolomic metabolites by type of Super Metabolic Pathway for ASD and Control Samples. Total number of matched metabolomic compounds for each sample = 340. Column 1 gives the name of the ‘Super-Pathway’ and in parentheses the number of compounds detected within that group. Columns 2 and 7 (‘Correlations’) gives the sum of the number of correlations with at least 20 data points for the Autistic plus control samples. % is the number of Absolute Partial Correlations ≥ |.30| divided by the number of correlations (column 2 or 7). Data in regular font are for the total data set (n=98), in italics are the data without the 10 subjects with very high total urinary BPA levels (n=88). Total rps is the total number absolute partial correlations detected.
| SUPER PATHWAY | Total rps | ASD (n=46) | CONTROLS (n=52) | Total rps | ASD (n=37) | CONTROLS (n=51) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | N | % | N | % | |||
| AMINO ACID (124) | 84 | 79 | 94 | 5 | 6 | 91 | 85 | 93 | 6 | 7 |
| CARBOHYDRATE (34) | 26 | 26 | 100 | 0 | 0 | 25 | 25 | 100 | 0 | 0 |
| COFACTORS & VITAMINS (17) | 15 | 13 | 87 | 2 | 13 | 14 | 12 | 86 | 2 | 14 |
| ENERGY (15) | 11 | 11 | 100 | 0 | 0 | 11 | 11 | 100 | 0 | 0 |
| LIPID (54) | 22 | 20 | 91 | 2 | 9 | 22 | 20 | 91 | 2 | 9 |
| NUCLEOTIDE (31) | 27 | 26 | 96 | 1 | 4 | 26 | 25 | 96 | 1 | 4 |
| PEPTIDE (14) | 9 | 9 | 100 | 0 | 0 | 11 | 11 | 100 | 0 | 0 |
| XENOBIOTICS (51) | 21 | 17 | 81 | 4 | 19 | 25 | 21 | 84 | 4 | 16 |
| TOTAL (340) | 215 | 201 | 93 | 14 | 7 | 225 | 210 | 93 | 15 | 7 |
Figure 2.
The largest number of absolute partial correlations was found in the amino acids super pathway. Table 8 shows the distribution of partial correlations by amino acid. Most of the partial correlations were found with the essential amino acids with the majority being in the ASD group.
Table 8.
Comparison of distributions of partial correlations for amino acid metabolites with BPA between ASD and control children.
| AMINO ACID SUB-PATHWAYS | TOTAL # OF CORRELATIONS | # OF PARTIAL CORRELATIONS |<| 0.3 ASD GROUP | # OF PARTIAL CORRELATIONS |<| 0.3 CONTROL GROUP | % OF PARTIAL CORRELATIONS |<| 0.3 ASD GROUP | % OF PARTIAL CORRELATIONS |<| 0.3 CONTROL GROUP |
|---|---|---|---|---|---|
| ESSENTIAL AAs | |||||
| Cysteine, methionine, SAM, Taurine metabolism | 3 | 3 | 0 | 100 | 0 |
| Threonine metabolism | 1 | 1 | 0 | 100 | 0 |
| Histidine metabolism | 8 | 4 | 0 | 50 | 0 |
| Lysine metabolism | 9 | 4 | 1 | 44 | 11 |
| Phenylalanine & tyrosine metabolism | 23 | 9 | 1 | 39 | 4 |
| Tryptophan metabolism | 15 | 9 | 0 | 60 | 0 |
| Valine, Leucine and Isoleucine metabolism | 25 | 9 | 0 | 36 | 0 |
| TOTALS | 84 | 39 | 2 | 46 | 2 |
| NON-ESSENTIAL AAs | |||||
| Alanine and aspartate metabolism | 8 | 1 | 0 | 13 | 0 |
| Glutamate metabolism | 9 | 1 | 1 | 11 | 11 |
| Glycine, Serine and Threonine metabolism | 4 | 1 | 0 | 25 | 0 |
| Urea cycle; Arginine and, Proline metabolism | 10 | 3 | 1 | 30 | 10 |
| TOTALS | 31 | 6 | 2 | 19* | 6 |
p<0.01 vs control for the % of partial correlations (columns 5 and 6).
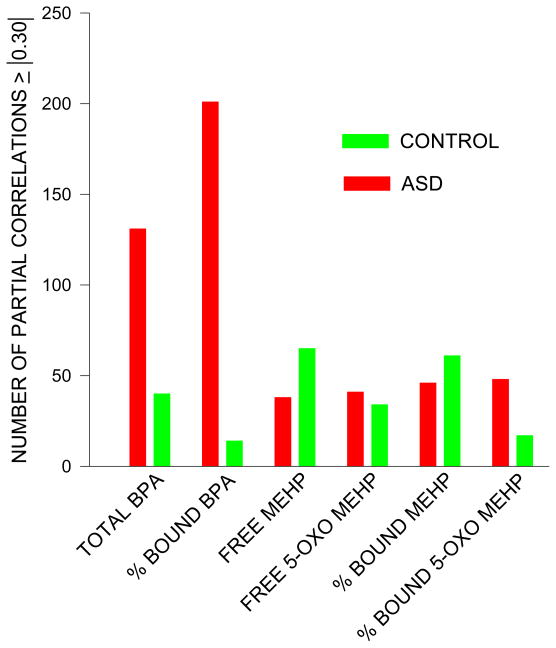
In contrast to BPA, the total numbers of partial absolute correlations for the four phthalate metabolites shown in figures 2 and 3 were much lower. There were no manifest striking visual differences in the frequencies of these counts across the seven super pathways between the ASD and control children for the phthalate metabolites.
Figure 3.
DISCUSSION
Previous studies found epidemiological evidence suggesting a relationship between maternal BPA exposure and ASD (Kalkbrenner et al., 2010; Larsson et al., 2009; Miodovnik et al., 2011; Vom Saal et al., 2012). This is the first study to demonstrate an ongoing association between children with ASD and BPA exposure. Review of the total data set (n=98) showed increases in both total BPA and the % glucuronidated. In our study, there was a subset of children who had very high urinary BPA concentrations (ASD group, 9/46, control group 1/52). When the 10 children with very high urinary total BPA levels were excluded from the statistical analyses, there were no longer any statistically significant differences in either total BPA or the fraction of BPA glucuronidated. With the DEHP metabolites, there were no outliers; all of the values were within 2 SD of the mean. Using the same criteria as for the DEHP metabolite analyses, it is reasonable to conclude that there were no ASD related differences in BPA conjugation. The results from the DEHP metabolite analyses were different. There was decreased glucuronidation with ASD suggesting a compromised glucuronidation pathway in (some) children with ASD. If both DEHP metabolites and BPA are associated with ASD, they appear to do so by different pathways.
According to the metabolomic data, the percentage of absolute partial correlations ≥ |0.30| was much higher in the ASD group for total BPA than in the control group (table 5). A similar result was found with the % bound with or without including the 10 subjects with the very high total BPA levels. The differences in the percentages of partial correlations shown in tables 6 and 7 were broadly distributed being mostly concentrated in the area of intermediary metabolism. Proportionately, there were fewer differences in the percentages of partial correlations > |0.30| in the domain of xenobiotics. These results are found even when there are no differences in total BPA exposure or the fraction glucuronidated (tables 6 and 7).
Table 8 explored one of the super-pathways in more detail. Both essential and non-essential amino acids are needed for protein synthesis. They also have other roles in metabolism. Specifically, non-essential amino acids have major roles in intermediary metabolism either directly (e.g., Glutamine and Alanine for N transport) or as large scale precursors of widely used metabolites (e.g., Glutamate and Aspartate for TCA cycle intermediates, Glycine/serine, a source of 1C units, Arginine as part of the urea cycle). Because of their critical importance in intermediary metabolism, there are multiple redundancies in their synthesis and degradation pathways. In contrast, the non-protein synthesis roles of the essential amino acids are limited to being precursors of quantitatively small amounts of hormones (e.g., Histidine for Histamine, Tryptophan for Serotonin, Methionine for Polyamines etc.) and have complex metabolic pathways with little redundancy. The present data set suggests that BPA predominantly affects essential amino metabolism where there is less opportunity for compensation via collateral mechanisms for a metabolic step compromised by BPA.
The results with BPA are different from the findings with DEHP metabolites in this population and using the same urine samples. Like BPA, DEHP metabolites are excreted as a mixture of the free and glucuronidated forms. For BPA, there were ASD associated related differences in the total amount excreted, but not in the fraction glucuronidated. With DEHP metabolites, there were no differences in the total amount of metabolites excreted, the difference was in the fraction glucuronidated.
There were only weak correlations between free BPA and free MEHP, and with % bound MEHP and % bound BPA in the ASD group (table 4). Some correlation is to be expected because both BPA and DEHP excretion reflect exposure to plastics in general. In our earlier study on DEHP metabolites, it was not possible to discern whether one or more of the DEHP metabolites was directly associated with ASD or the decreased efficiency of the glucuronidation pathway for DEHP metabolites was a marker for compromised metabolism of some other compound metabolized by this pathway (Stein et al., 2013). Only BPA showed large differences in the number of absolute partial correlations between the ASD and control groups. The differences between BPA and phthalates imply that while both are associated with ASD, they do so by different mechanisms.
There is a very extensive literature on the multiple effects of BPA in animal models (IOM., 2008; NIEHS, 2011; NTP-CERHR, 2007, Hengstler et al., 2011). In animals, BPA functions as an endocrine disruptor of steroid mediated processes. Endocrine disruptors can interfere with multiple ongoing metabolic processes within the body as well as causing permanent changes in gene expression during development (Dolonoy et al., 2007; Richter et al., 2007; Rubin, 2011; Thayer et al., 2012; Vom Saal et al., 2012; Wolstenholme et al., 2012).
Unlike the urinary excretion of a plasticizer which will reflect the immediate antecedent diet, metabolic patterns are likely to persist. Metabolic homeostasis is maintained over extended time periods. Therefore it is plausible that the observed metabolic differences started during development and persist into childhood. There is widespread redundancy within metabolic pathways. So minor perturbations can be accommodated and are not likely to affect overall health, but subtle effects such as behavioral changes which are not metabolic health threatening could exist within an otherwise healthy individual. Animal studies have shown that fetal metabolic abnormalities associated with BPA persist into adulthood (NTP-CERHR, 2007; Richter et al., 2007; Rubin, 2011; Cabaton et al., 2013). The animal effects have been ascribed to BPA acting as an endocrine disruptor.
A recent study by Cabaton examined the effect of exposure to BPA in the perinatal period on the metabolome of the pups (Cabaton et al., 2013). They concluded that ‘Low doses of BPA disrupt global metabolism, including energy metabolism and brain function, in perinatally exposed CD-1 mouse pups. Metabolomics can be used to highlight the effects of low doses of endocrine disruptors by linking perinatal exposure to changes in global metabolism’ (Cabaton et al., 2013). Our results are similar, except that we found them in humans, and an association with ASD. An implication from the present study is that there might be benefit to pregnant women and to children with ASD by reducing their exposure to BPA (Rudel et al., 2012).
STUDY LIMITATIONS
There are a number of limitations to this study.
The relatively small number of subjects precludes examining for heterogeneities in the data set. Yet ASD is known to be a multi-factorial disease. A larger study might have revealed heterogeneities.
The study assumes that home and socio-economic environments were comparable across the two groups. Although the subjects were drawn from the same catchment area, no socioeconomic data were collected. Points (i) and (ii) suggest caution before extrapolating the total BPA exposure data to other localities.
A minor pathway for BPA metabolism (<10%) is sulfation (Liao and Kannan, 2012). The BPA total measurements do not account for any sulfated BPA.
Although we did screen during the selection process for drug use, actual measurement would have been preferable. Drug use could interfere with the glucuronidation pathway. Some data are available from the metabolomic profiles. Traces of Acetaminophen were detected in 2 control and 4 ASD subjects, Topirmate (3 ASD), Citrolopram (1 ASD), Fluoxetime (1 ASD), Ranitidine (1 ASD) and Ibuprofen (1 control).
-
Reliance on single spot urine specimen. The literature data on the reproducibility of BPA excretion in single urine specimens is conflicting. A recent study reported poor reproducibility between BPA exposure measured one to three years apart (Townsend et al., 2013). Shorter duration studies either found poor or moderate correlations (Braun et al., 2011a; Mahalingaiah et al., 2008; Makris et al., 2013a; Nepomnaschy et al., 2009; Ye et al., 2011). For studies for other purposes, we had collected some pairs of urine specimens from ASD children 4 to 6 weeks apart. When analyzed for total BPA, the correlation between samples pairs approached significance (n=35, r=0.29, p=0.093, two tailed). Collectively, the observations show that over short periods of time total BPA levels correlate, with the correlation progressively weakening as the time interval between collection points increases.
Time of collection could be of concern for interpretations based on total BPA, but not for the % bound BPA parameter or the metabolomics data. For % bound BPA, it is immaterial where the (free) BPA came from. The requirement is that the BPA enters the body, is processed and leaves the body. Being products of metabolism, the % bound parameter and the metabolomic data are more robust measurements than the total BPA excreted measurement. The metabolomics data reflect intermediary metabolism. Unlike exposure to an environmental toxinogen where there might be temporal variation, intermediary metabolism does not fluctuate grossly on a day to day basis. The persistence of metabolic changes are likely due to the genetic background of the individual and the response to external environmental exposures.
Conceivably, the present results could be distorted by the antecedent diet and time of day the urine specimens were collected. Urine specimens were collected randomly between 10 am and 4 pm while the child was in clinic. At the time of the visit a dietary intake history for the previous 24 hours was obtained, including medication and vitamin intake. Children with unusual diets or on medication were excluded from the study. To further assess whether urine sampling and dietary intakes were comparable between the two groups, we examined the mass spectrometer determinations of the abundances of the 20 amino acids detected. Amino acid excretion in urine varies with nutritional state. There were no differences between the groups (p=0.60, n=20, ns).
CONCLUSIONS
The results suggest there is an association between BPA and ASD.
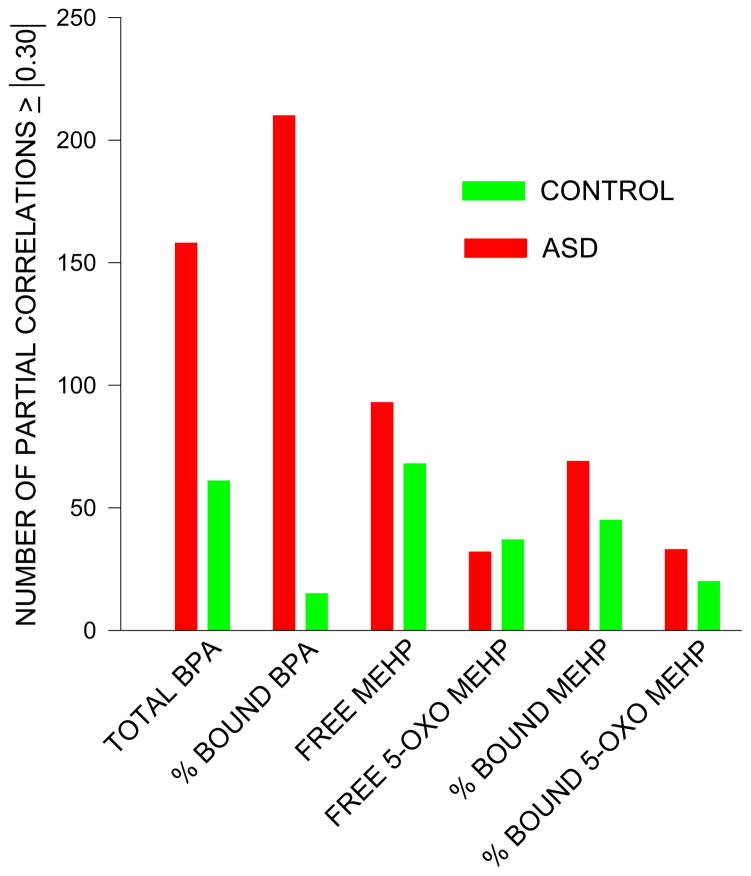
Figure 4.
Acknowledgments
Supported by grants CAUT13APL016 from the NJ Governor’s Council for Medical Research and Treatment of Autism, NIH grant #RES015316A and Department of Defense contract # W81XWH-08-1-0729
Footnotes
CONFLICT OF INTEREST: None of the authors have any conflicts of interest.
References
- FDA, (US Food and Drug Administration. Bisphenol A (BPA) 2010 Available from http://www.fda.gov/NewsEvents/Public/Health/Focus/uscm064437.htm.
- Braun JM, Hauser R. Bisphenol A and Children’s Health. Curr Opin Pediatr. 2011;23:233–239. doi: 10.1097/MOP.0b013e3283445675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011a;119 :131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011b;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck TR, Viskochil J, Farley M, Coon H, McMahon WM, Morgan J, Bilder DA. Psychiatric Comorbidity and Medication Use in Adults with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2014 Jun 24; doi: 10.1007/s10803-014-2170-2. 2014, epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton NJ, Canlet C, Wadia PR, Tremblay-Franco M, Gautier R, Molina J, Sonnenschein C, Cravedi JP, Rubin BS, Soto AM, Zalko D. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ Health Perspect. 2013;121:586–593. doi: 10.1289/ehp.1205588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control. Third National Report on Human Exposure to Environmental Chemicals. CDC, DHSS; Washington, DC: 2005. [Google Scholar]
- Cohen J. A Power Primer. Psychological Bulletin. 1993;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Dolonoy D, Huang D, Jirtle RL. Maternal nutrient supplementation counter-acts bisphenolA induiced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:130556–113061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120:978–983. doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson BE. Bisphenol A under scrutiny. Chemical and Engineering News. 2008;86:36–39. [Google Scholar]
- Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Volkel W, Wollin KM, Gundert-Remy U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Critical reviews in toxicology. 2011;41:263–291. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Current Opinion in Neurology. 2010;23:103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- Hwang HM, Park EK, Young TM, Hammock BD. Occurrence of endocrine-disrupting chemicals in indoor dust. Science of the Total Environment. 2008;404:26–35. doi: 10.1016/j.scitotenv.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, Proceedings, Washington DC. Autism and the Environment. National Academy Press; Washington, D.C: 2008. [Google Scholar]
- Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21:631–641. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ. What causes autism? Exploring the environmental contribution. Current Opinion in Pediatrics. 2010;22:219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Kannan K. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environ Sci Technol. 2012;46:5003–5009. doi: 10.1021/es300115a. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116:173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris KC, Andra SS, Herrick L, Christophi CA, Snyder SA, Hauser R. Association of drinking-water source and use characteristics with urinary antimony concentrations. J Expo Sci Environ Epidemiol. 2013a;23:120–127. doi: 10.1038/jes.2012.104. [DOI] [PubMed] [Google Scholar]
- Makris KC, Andra SS, Jia A, Herrick L, Christophi CA, Snyder SA, Hauser R. Association between water consumption from polycarbonate containers and bisphenol A intake during harsh environmental conditions in summer. Environ Sci Technol. 2013b;47:3333–3343. doi: 10.1021/es304038k. [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Malek J, Jani N, Wagner GC. Autism Spectrum Disorders and Identified toxic Land Fills: Co-Occurence Across States. Environmental Health Insights. 2008;2:55–59. doi: 10.4137/EHI.S830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Stein TP, Barnes V, Rhodes N, Guo L. Metabolic perturbance in autism spectrum disorders: a metabolomics study. J Proteome Res. 2012;11:5856–5862. doi: 10.1021/pr300910n. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS. Enodcrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Baird DD, Weinberg CR, Hoppin JA, Longnecker MP, Wilcox AJ. Within-person variability in urinary bisphenol A concentrations: measurements from specimens after long-term frozen storage. Environ Res. 2009;109:734–737. doi: 10.1016/j.envres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEHS. Report on Carcinogens. 12. U.S. Department of Health and Human Services Public Health Service National Toxicology Program, Research; Triangle Park, North Carolina: Washington, DC: 2011. pp. 156–158. [Google Scholar]
- NTP-CERHR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of BPA. CDC, DHSS; Washington, DC: 2007. http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Lein PJ. Evidence for environmental susceptbility in autism: what we need to know about gene x environment interations. In: Zimmerman A, editor. Autism: current theories and evidence. Humana Press; New York: 2008. [Google Scholar]
- Pessah IN, Seegal RF, Lein PJ, LaSalle J, Yee BK, Van De Water J, Berman RF. Immunologic and neurodevelopmental susceptibilities of autism. Neurotoxicology. 2008;29:532–545. doi: 10.1016/j.neuro.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. Journal of Steroid Biochemistry & Molecular Biology. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environmental Health Perspectives. 119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TP, Schluter MD, Steer RA, Ming XU. Autism and Phthalate Metabolite Glucuronidation. J Autism and Dev Disorders. 2013;43:2677–2685. doi: 10.1007/s10803-013-1822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health. 2013;12:80. doi: 10.1186/1476-069X-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Molecular & Cellular Endocrinology. 2012;354:74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119:983–988. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]