Abstract
Understanding the underpinnings of social responsiveness and theory of mind (ToM) will enhance our knowledge of autism spectrum disorder (ASD). We hypothesize that higher-order relational reasoning (higher-order RR: reasoning necessitating integration of relationships among multiple variables) is necessary but not sufficient for ToM, and that social responsiveness varies independently of higher-order RR. A pilot experiment tested these hypotheses in n = 17 children, 3–14, with and without ASD. No child failing 2nd-order RR passed a false belief ToM test. Contrary to prediction, Social Responsiveness Scale scores did correlate with 2nd-order RR performance, likely due to sample characteristics. It is feasible to translate this comparative cognition-inspired line of inquiry for full-scale studies of ToM, higher-order RR, and social responsiveness in ASD.
Keywords: Theory of mind, Relational reasoning, Analogical reasoning, Social responsiveness, Autism, Cognition
Introduction
This brief report about a small feasibility study demonstrates that we can translate a central idea in comparative social cognition for hypothesis-testing research in autism with high potential basic science and clinical impact. Social reciprocity—termed “social responsiveness” for the remainder of this paper—has been defined as “the extent to which a child engages in emotionally appropriate turn-taking social interaction with others” (Constantino and Todd 2000). Humans and chimpanzees are extremely social animals, and social responsiveness may be shared across these species (Marrus et al. 2011). Theory of mind (ToM) (Premack and Woodruff 1978) is a social cognitive ability that encompasses the attribution of psychological states to self and others. There is strong evidence that humans over the age of 4 can use ToM to make causal inferences about social behavior (Wellman et al. 2001).
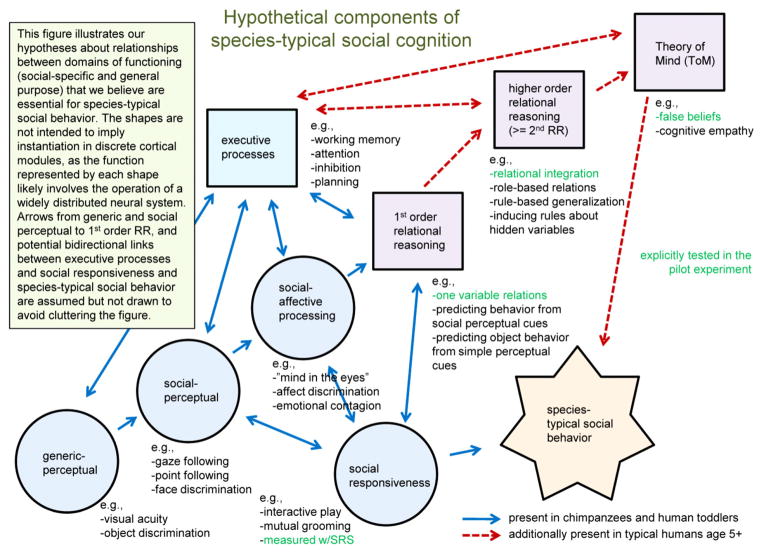
Understanding why humans alone (Penn and Povinelli 2007) possess these unique social cognitive abilities is one of the fundamental, unanswered questions of cognitive psychology and neuroscience. And, a comparative perspective allows us to ask whether characteristic social deficits in autism arise from atypical development of brain systems that we share with other primates, systems that are unique to humans, and/or the interfaces between such systems. Most believe that uniquely human social abilities1 are a result of language and/or highly specialized cognitive mechanisms (e.g., Premack 2004). Our hypothesis, on the contrary, is that human ToM is the result of a multi-layered interaction between a vast array of 1st-order social-cognitive mechanisms we share with other vertebrates, and higher-order relational reasoning abilities that are uniquely human (see Fig. 1).
Fig. 1.
Proposed relationships between basic and social perceptual-motor, executive processes, relational reasoning systems, ToM, and social behaviors
Relational reasoning (RR) is the ability to solve novel problems involving the relationship between variables. The most basic (1st-order) form of RR involves reasoning about a single, perceptual relationship: whether one object is larger than another or whether one conspecific is dominant to another. Raven’s Progressive Matrices (RPM) (Raven 1958), a quintessential test of fluid intelligence, measures a human subject’s ability to solve RR problems involving visual-spatial, logical, and geometric relationships (see Fig. 2); the order of the RR or RPM-like problem is defined by the number of variables that change. Research has shown that nearly every animal, from honeybees to apes, is capable of solving first-order RR problems involving simple perceptual relationships. 2nd-order, or two variable, RR problems involve the relationship between relationships: understanding that {‘dog’ is to ‘dog house’} is analogous to {‘bird’ is to ‘bird nest’} requires the ability to reason about the relationship between relationships. One must reason about relationships between relationships to solve a 2nd-order (two variable) RR problem because to do so necessitates recognizing the simultaneous ways that levels of the two variables are changing: e.g., object shape and color; type of animal and home. In a 3rd-order RR problem three variables change levels; and an example 0th-order RR problem would require selecting the texture or color that completes a uniform picture with a hole cut in it—zero (objects) variables change. The RPM-like problem in Fig. 2 (from Keith Holyoak) taxes a level of fluid intelligence (2nd-order RR) that we predict is necessary for ToM. To select the missing puzzle piece from the options below, one must integrate simultaneous changes in the relationships between levels of two variables (this is higher-order RR because changes occur in more than one variable): e.g., one must reason that both object shading (variable 1) and orientation (variable 2) change levels across and down the display. One classic ToM problem involves testing whether children can attribute false beliefs to others (Wimmer and Perner 1983). In the Wimmer and Perner false belief ToM task, the test is whether Child A thinks that Child B believes a cookie is still in a jar, when someone moved it to the cupboard while Child B was not looking. Prior to solution of the cookie transfer problem via attribution of hidden variables (mental states), Child A might represent it as a higher-order problem involving observable variables: predicted behavior of Child B (explore: jar/cupboard) is a function of Child B’s observed proximity to the cookie at time of switch (variable 1 = near/far) and the cookie’s location (variable 2 = same/moved). A non-ToM-based solution could be derived via computation of spatial contingencies of the two levels of the two variables: Child B would look in the jar if she was (variable 1=) not in the room when (variable 2=) switch occurred. Around age 4–5, Child A might then begin to accurately predict Child B’s behavior by inducing a rule involving the unobservable variable ‘belief’ after exposure to many different, specific instances of problems described by two observable variables (e.g., proximity to switch, location of cookie). By integrating the patterns of change across these two observable variables (higher-order RR) and arriving at a general solution, Child A may now represent the problem as: predicted behavior of Child B (look in wrong place) is a function of Child B’s false belief. The use of a hidden variable (B’s belief is not directly observable) reduces the dimensionality of the problem and is thus an extremely useful heuristic.
Fig. 2.
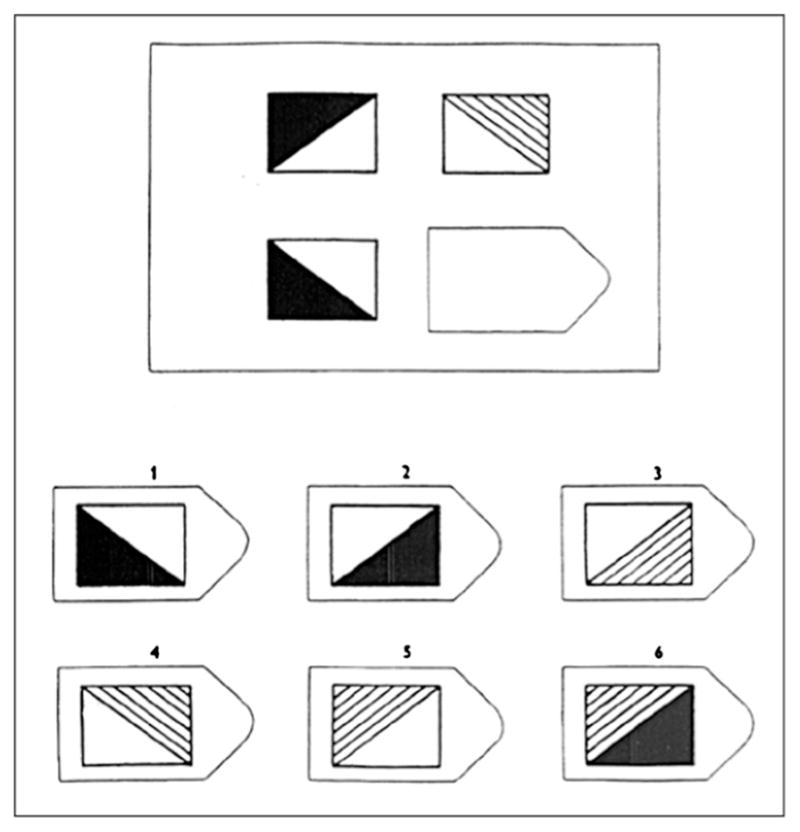
A higher-order RPM-like problem
To explain further, reasoning about another subject’s mental states often involves reasoning about the relationship between that subject’s internal beliefs and desires and events and objects in the external world. One cannot see these internal beliefs and desires nor can one see the relationship between these mental states and the events and objects in the world. Instead, these relationships must be inferred by observation of observable stimuli such as where the subject is looking, how the subject has behaved in the past, and the set of objects present in the world. Thus, in order to reason about another subject’s internal mental states one must be capable of reasoning about the relationship between perceptual relationships and mental relationships. Furthermore, having a “theory” about how minds work (which all typical humans do, at least in a “folk”, or commonsense fashion), requires the ability, in addition, to reason about the relationship between these unobservable mental relationships and hypothesized general principles of folk psychology. In other words, one must additionally posit that an induced unobservable mental state will cause another to behave in certain ways. In short, ToM arguably involves quite complex forms of higher-order RR. Yet, all typical members of our species are capable of this cognitive feat after the age of 4 (Wellman et al. 2001).
Deficits in either ToM or RR [an essential component of fluid intelligence: see (Blair 2006)] may lead to abnormal social behavior. For example, children with autism may have normal or even superior RR (Dawson et al. 2007; Hayashi et al. 2008), but these same children may have severely impaired social responsiveness and, as a group, may perform poorly on experimental tests of ToM (e.g., Baron-Cohen et al. 1985; Colle et al. 2007; and for review see: Boucher 2012). This dissociation shows that ToM in humans is not solely dependent on higher-order RR. On the other hand, children with Down syndrome may exhibit quasi-normal social behavior but fail to pass tests of ToM because of their intellectual disability (Zelazo et al. 1996). This pattern suggests that higher-order RR may be a necessary, but not sufficient, condition for normal ToM in humans. Penn, Holyoak, and Povinelli recently presented this theoretical position in detail in (Penn et al. 2008).
Typical humans are capable of solving 2nd-order RR problems by approximately age 5, which is also (perhaps not coincidentally) the approximate age of false belief ToM consolidation in typical children. The correlation between age of consolidation for false belief ToM and 2nd-order RR has never been directly tested. We propose that the relationship between 2nd-order RR and ToM is not just a correlation, but that 2nd-order RR is a necessary, but not sufficient, cognitive pre-requisite for ToM, and independent of social responsiveness. We are encouraged by recent work in autism that suggests a potential dependency of ToM on other domain-general cognitive abilities (Pellicano 2007, 2010; Zelazo et al. 2002) and work in subjects with Down syndrome showing degrees to which these functions may be separable from social adaptive functioning (Molloy et al. 2009). However, we are not aware that anyone has explicitly tested the necessity of higher-order RR for ToM and the independence of social responsiveness from RR and ToM. If higher-order RR proves necessary for ToM, then future research can address a potentially central question in ASD, namely why higher-order RR is not sufficient for ToM in ASD.
Methods
Subjects
This feasibility test involved study of a heterogeneous group (n = 17 total) of children with (n = 6; 5 male; mean ± S.D. age = 12.1 ± 1.6 years) and without (n = 11; 2 male; age = 9.0 ± 3.2) autism spectrum disorder (ASD) to test a range of RR and ToM abilities and SRS scores. Three more children with ASD were tested. We excluded one of these three subjects (a boy) from the analyses because he reported looking at the RR problem answer key. Two more (one boy and one girl) were excluded for reasons described below. Recruitment and the experiment were performed according to an IRB approved protocol. Participants were recruited from existing studies and the local community. Pre-screening involved a brief medical/medication history, pedigree and demographics.
The ratio of males to females will be balanced in the future, full-scale study, where effects of sex may also be explored in a larger sample.
Assessments
Non-ASD participants had no clinical diagnosis of ASD; each participant with ASD had a community MD or PhD DSM-IV-TR clinical diagnosis of an ASD and tested positive on the Autism Diagnostic Observation Schedule (ADOS: Lord et al. 2000) and/or Autism Diagnostic Interview Revised (ADI-R: Lord et al. 1994). Subjects were assessed with the age-appropriate version of the Social Responsiveness Scale [SRS: (Constantino and Gruber 2005)].
General Procedures
We constructed a ToM and RR battery that would engage typical children as young as 3.5 years old, while challenging bright, older children (total time ≤ 20 min). “Passing” a false belief (or ToM control problem) meant getting 2/2 such problems correct. The child viewed each RR problem, selected his/her answer (laminated answer cut-outs were affixed with VELCRO® in pseudorandom positioning on a separate sheet), and stuck it with VEL-CRO® in the empty box. After the experiment, we debriefed the children about the strategies they used for the false belief ToM problem and one pre-specified 2nd-order RR problem.
Stimuli
The ToM problems employed a five location (opaque containers with lids) variant of the classic false belief task with six conditions (designed by Derek Penn): (1) Static Control (child must select the container where s/he saw the experimenter place the object, which was a small toy star—a very basic control condition); (2) informed static position control (child must select the container where the doll saw the experimenter place the object—additionally tests if the child can extrapolate to the doll); (3) informed position change control (object is placed in view of the doll; the doll’s vision is not blocked, and the object is switched to another container; child must indicate where the doll will now look—importantly, we hypothesize that this condition does not require higher-order RR because it does not test ToM); (4) false belief (object is placed in view of doll; doll’s vision is blocked, and the object is switched to another container; child must indicate where the doll will now look—tests ToM and requires higher-order RR); (5) completely uninformed control (doll never sees the object being put in container—another basic control); and (6) Removed (same as condition 4, except the object is removed from the experimental field of view instead of being switched to another container). Two instances of each problem were presented in pseudo-random order (one block of the 6 conditions using a Buzz Lightyear toy as the doll, the other using Mr. Potato Head). A 12 problem RR set was constructed that contained problems varying in order [as defined in the Introduction and in (Crone et al. 2009; Kroger et al. 2002)] from 0 to 4, number of elements (4 or 9), and the number of rules and tokens of each rule, as described in the Carpenter et al. taxonomy of Raven’s Advanced Progressive Matrices (Carpenter et al. 1990). Per this taxonomy, we define each RR problem to contain a number of items, or elements, relating to one another through some operation or rule (e.g., additive progression, stretching), where each rule can occur one or more times, each instance identified as a token. Figure 3 shows a 3rd-order, 2 rule, 2 token, 4 element problem with the indicated answer. These 12 RR problems were presented to each child in pseudorandom sequence in blocks of 6 problems. The children alternated ToM and RR problem blocks, with the sequence, RR or ToM first, counter-balanced across subjects.
Fig. 3.
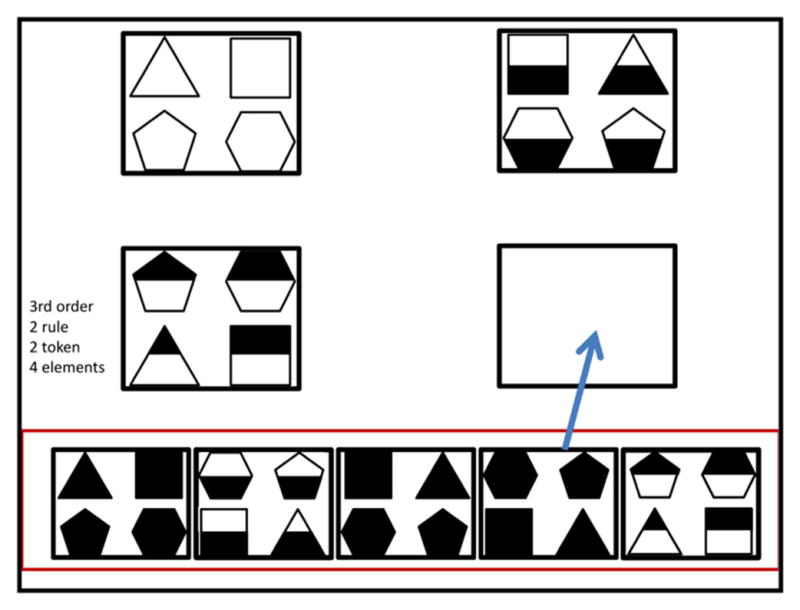
One of the more challenging RR problems in the set
Analyses
The children’s performances (passing versus failing, defined as above) for the ToM problems were compared against the number of each order of RR problem solved. We also explored ToM and RR performance in relationship to age, diagnosis, and SRS. Non-parametric statistical tests were performed using IBM SPSS Statistics Version 22 (International Business Machines Corp.). We predict the following: (1) children will pass 1st-order relational reasoning at a younger age than 2nd-order relational reasoning, (2) more children with ASD will fail False Belief, (3) higher-order relational reasoning will not prove necessary for passing Informed Position Change, and crucially, (4) there will be a strong relation between passing 2nd-order RR and passing False Belief.2
Results
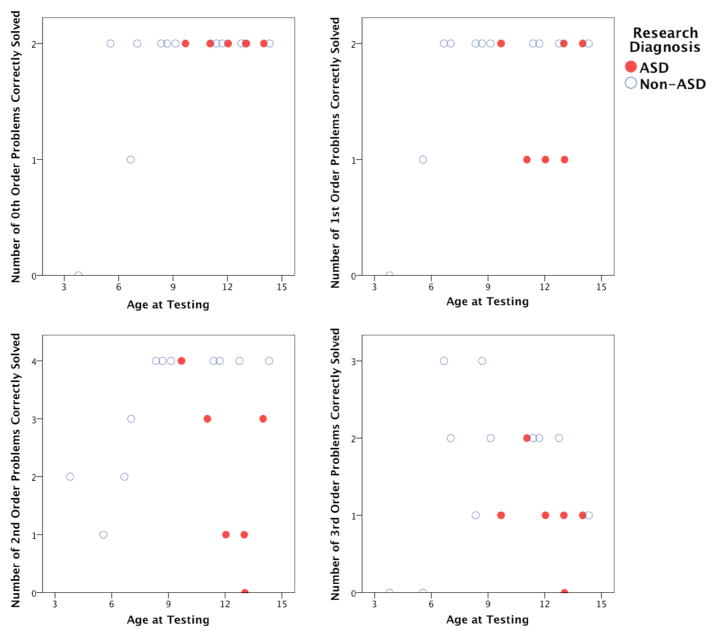
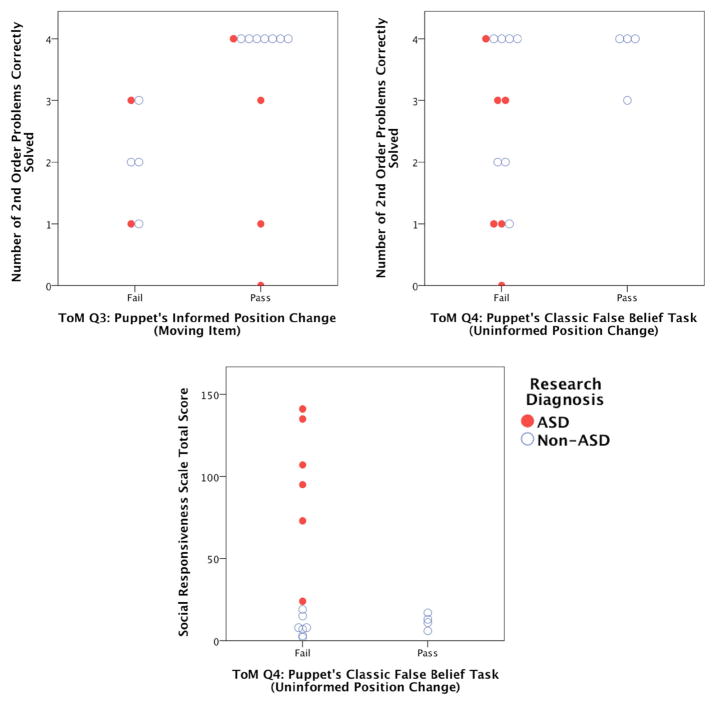
No child under 7 passed False Belief (doll’s vision is blocked; does require 2nd-order RR), and all children with ASD failed. Figure 4, top, left and right panels show the relationships between age and the number of 0th- and 1st-order RR problems solved, respectively (red dots for children with ASD, blue circles for non-ASD children). The bottom two panels in this figure show the relationships between age and the number of 2nd- and 3rd-order RR problems solved, respectively. The jump in 2nd-order RR success rate between ages 5 and 8 for the non-ASD children is grossly consistent with reported findings (Crone et al. 2009; Richland et al. 2006). Figure 4’s depiction of age-related change in relational reasoning ability was confirmed with a Friedman test across the children’s percent correct for each order (0th, 1st, 2nd, and 3rd) of RR problem attempted [χ2(3) = 22.623, p = 0.000]. A Wilcoxon signed-rank test showed that the difference between 2nd-and 1st-order RR showed a trend towards significance with this small pilot sample (Z = −1.513, p = 0.13). Figure 5, top, left panel illustrates that it was possible for some children who got most of the 2nd-order problems wrong to pass Informed Position Change, supporting the notion that higher-order RR is not necessary for reasoning about the puppet’s behavior with respect to changes in object location when the correct answer requires no mentalistic (false belief) attribution. Importantly, Fig. 5, top, right panel illustrates that regardless of age and diagnosis, only children who correctly solved a majority of 2nd-order RR problems passed False Belief. Some did well on 2nd-order RR but failed False Belief, but no child who systematically failed 2nd-order RR passed False Belief. These relationships were confirmed with binomial tests of the probability of obtaining the observed (or fewer) number of children passing Informed Position Change or False Belief. Of the 6 children who failed the majority of 2nd-order RR problems (<3 problems correct), 2 passed Informed Change [exact binomial p = 0.344], supporting our hypothesis that 2nd-order RR is not necessary for Informed Position Change success. Of the 6 children who failed the majority of 2nd-order RR problems, 0 passed False Belief [exact binomial p = 0.016], supporting our hypothesis that 2nd-order RR is necessary for False Belief success.
Fig. 4.
Panels depict subject age versus the number of each order of RR problem correctly solved. Please see text for details
Fig. 5.
Top panels show the number of 2nd-order RR problems correctly solved for children who pass/fail Informed Position Change (left) and False Belief (right). The bottom panel shows SRS scores for children who pass/fail False Belief. Please see text for details
All of the reported 17 subjects appeared to readily understand the ToM and RR problem instructions and executed the mechanics of the tasks from the first trial forward. We excluded 2 children for protocol-related reasons. Their data is available upon request. The non-ASD participant mean ± S.E.M. SRS score was 10 ± 2; ASD mean was 96 ± 18; these means were significantly different [t(5.0913) = 4.837; p = 0.005]. Social Responsiveness Scale (SRS) scores did correlate with 2nd-order RR performance (r = −0.505, p = 0.039). The bottom, right panel, however, shows the relationship between False Belief problem success and SRS; the mean SRS score for children passing False Belief was 12 ± 2; that for children failing False Belief was 49 ± 15. These means were different [t(12.553) = 2.492; p = 0.028]. Restricting to the non-ASD children, only, the mean SRS score for children passing False Belief was 12 ± 2; that for children failing false belief was 9 ± 2. These means were not different [t(9) = −0.814; p = 0.437]. Potential patterns with respect to the number of matrix elements, rules, or tokens of each rule and False Belief success will be explored in the planned large-scale follow-up study.
Discussion
Our results from this study are encouraging, despite the small sample size and obvious limitations. Importantly, this pilot experiment demonstrates feasibility for future full-scale studies of the relationships between high-order RR, ToM, and social responsiveness, including additional tests of interdependency with joint attention-related behaviors and aspects of language, in children with and without ASD. We have hypothesized, in a diagnosis-, age-, and species-independent manner, that higher-order RR is necessary but not sufficient for ToM, and that social responsiveness will be largely independent of ToM and higher-order RR. The results of this pilot experiment are consistent with our central hypothesis: higher-order RR may be necessary for ToM. Though contrary to our other hypothesis, the observed relationship between SRS and 2nd-order RR should be interpreted in consideration of the observed pattern of false belief and RR performance across diagnostic groups at this small sample size. We did not match groups on IQ for this pilot study. The full-scale study would employ different forms of IQ matching (crystallized, fluid), better decoupling diagnosis (subjects with ASD having high SRS scores) from RR performance to allow a more balanced assessment of potential relationships between SRS and RR ability. Adding subjects with sub-clinical ASD symptoms would provide a more continuous range of SRS scores.
Theoretical Context
This experiment tests hypotheses put forth in a recent theoretical paper (Penn et al. 2008); and see (Povinelli 2012) for experiments in chimpanzees and humans involving related tests of causal reasoning about object weight. Studying the necessity of higher-order RR for ToM is by no means a simple attempt to refine existing executive functioning accounts of ToM (Andrews et al. 2003; Bloom and German 2000; Grant et al. 2004; Perner and Leekam 2008; Scott et al. 1999; Zelazo et al. 1996). We agree that normal executive functioning is necessary for ToM, but we also specifically seek to understand whether higher-order RR is necessary (but not sufficient), in addition, for ToM. An individual with normal executive functioning (e.g., working memory and inhibitory control) might fail ToM tasks if some other system necessary for higher-order relational representations and/or first-order social-perceptual representations (e.g., related to gaze following) is dysfunctional.
Future Directions and Implications for Basic Science
A full-scale study might similarly employ a modified version of a classic false belief ToM paradigm and pictorial relational reasoning problems and also include measures of social behavior, joint attention (JA; which we additionally hypothesize may explain why higher-order RR is not sufficient for ToM in autism), general intelligence, receptive and expressive language, and basic executive functioning (e.g., controlled measures of memory, attention, planning, and inhibition) to relate to patterns of ToM and RR performance in a sample of children that is heterogeneous with respect to ToM and relational reasoning abilities and general social competency. Including subjects with intellectual disability would dissociate chronological age from RR and ToM, important, as our hypothesis is age-independent. By including subjects with intellectual disability, a future experiment could show whether some un-measured maturational variable accounts for the presumed RR-ToM relationship. E.g., a 10-year-old with Down syndrome (and excellent social responsiveness/no ASD) might fail ToM problem solution because of delayed acquisition of 2nd-order RR. Yet, a 10-year-old with ASD and superior fluid intelligence might fail ToM for other (yet-to-be-determined) reasons. If our hypotheses hold in larger studies, subsequent experiments could include more encompassing tests. Analyses might involve path models including combinations of the above variables. Our hypothesis is age-independent and applies to any ToM task that unequivocally requires the attribution of unobservable causal variables. Future research might demonstrate sensitivity to higher-order relational structure in toddlers. For example, one might test infants using artificial grammar-like paradigms (to assess infant sensitively to relational structures varying in order) and purported mental state attribution tasks. Future developmental and cross-species functional neuroimaging studies (e.g., see Vincent et al. 2007) could probe for human-unique (supporting higher-order RR and ToM) and evolutionarily conserved (supporting social responsiveness) brain systems that account for similarities and differences in social cognitive abilities across species and across human development.
Clinical Relevance: Intervention
There is evidence that broad training in executive functioning in autism can improve ToM performance (Fisher and Happe 2005). Holyoak et al. have described how specific supports to RR can benefit achievement in math (Richland et al. 2007), and others have demonstrated that RR ability, itself, is sensitive to culturally-based differences in teaching (Richland et al. 2010) and improves with practice (Mackey et al. 2011). Deficits in joint attention are characteristic in autism [for review, see: (Bruinsma et al. 2004)], and several recent studies have demonstrated the benefit of JA-based interventions for children with autism (e.g., Kasari et al. 2010, 2012). If our ideas are correct, future interventions targeting both RR (to provide the cognitive substrate for ToM) and JA might enhance outcomes in autism beyond those seen with existing interventions.
Clinical Relevance: Early Risk Assessment
Enhancing our understanding of the cognitive architecture of general and social intelligence and social responsiveness will better enable future imaging studies to assess risk of subsequent autistic social deficits in young children with global cognitive delays. These envisioned advances could be profoundly important for infants diagnosed with Down syndrome, Fragile X, and other intellectual disability disorders that have significant rates of co-morbid autism.
Conclusion
Our objective is to understand more about the cognitive architecture that supports social functioning and its neural instantiation. We believe that this endeavor is of fundamental importance to our understanding of human developmental psychology and the relationship between human and nonhuman cognition, and it may provide novel insights into autism, which could lead to new assessments and treatments.
Acknowledgments
We would like to thank Derek C. Penn, Keith Holyoak, John N. Constantino, Dorothy K. Grange, Lawrence McEvoy, and Victoria Sorrentino for their significant contributions to this work. Britt Gott assisted with manuscript preparation. Funding included K12 EY016336 (Pruett), JSMF Centennial Fellowship (Povinelli), and The Drs. John R. (Sr.) and Patricia O. Pruett Fund (for research in Theory of Mind and for undergraduate training).
Footnotes
We do not in any way imply that there is anything less human about individuals with autism, young children, or those with intellectual disability because of limitations in certain forms of RR. Indeed, part of the supporting evidence for our work includes noting that some individuals with autism demonstrate above-average fluid reasoning. RR is only a small part of what makes all humans human.
Our prediction that no child failing higher-order relational reasoning could pass False Belief was tested by assessing the exact binomial probability of n children failing higher-order relational reasoning but passing False Belief.
Adjustment for unequal variances.
Contributor Information
John R. Pruett, Jr., Email: pruettj@psychiatry.wustl.edu, Department of Psychiatry, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8134, St. Louis, MO 63110, USA
Sridhar Kandala, Department of Psychiatry, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8134, St. Louis, MO 63110, USA.
Steven E. Petersen, Department of Neurology, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8111, St. Louis, MO 63110, USA. Department of Psychology, Washington University, One Brookings Drive, St. Louis, MO 63130, USA
Daniel J. Povinelli, Department of Biology, University of Louisiana, P.O. Box 42451, Lafayette, LA 70504, USA
References
- Andrews G, Halford GS, Bunch KM, Bowden D, Jones T. Theory of mind and relational complexity. Child Development. 2003;74(5):1476–1499. doi: 10.1111/1467-8624.00618. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behavioral Brain Sciences. 2006;29(2):109–125. doi: 10.1017/S0140525X06009034. discussion 125–160. [DOI] [PubMed] [Google Scholar]
- Bloom P, German TP. Two reasons to abandon the false belief task as a test of theory of mind. Cognition. 2000;77(1):B25–B31. doi: 10.1016/s0010-0277(00)00096-2. [DOI] [PubMed] [Google Scholar]
- Boucher J. Putting theory of mind in its place: Psychological explanations of the socio-emotional-communicative impairments in autistic spectrum disorder. Autism. 2012;16(3):226–246. doi: 10.1177/1362361311430403. [DOI] [PubMed] [Google Scholar]
- Bruinsma Y, Koegel RL, Koegel LK. Joint attention and children with autism: A review of the literature. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(3):169–175. doi: 10.1002/mrdd.20036. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Shell P. What one intelligence test measures: A theoretical account of the processing in the Raven Progressive Matrices Test. Psychological Review. 1990;97(3):404–431. [PubMed] [Google Scholar]
- Colle L, Baron-Cohen S, Hill J. Do children with autism have a theory of mind? A non-verbal test of autism vs. specific language impairment. Journal of Autism and Developmental Disorders. 2007;37(4):716–723. doi: 10.1007/s10803-006-0198-7. [DOI] [PubMed] [Google Scholar]
- Constantino J, Gruber C. Social responsiveness scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. American Journal of Psychiatry. 2000;157(12):2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Developmental Science. 2009;12(1):55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M, Soulieres I, Gernsbacher MA, Mottron L. The level and nature of autistic intelligence. Psychological Science. 2007;18(8):657–662. doi: 10.1111/j.1467-9280.2007.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher N, Happe F. A training study of theory of mind and executive function in children with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2005;35(6):757–771. doi: 10.1007/s10803-005-0022-9. [DOI] [PubMed] [Google Scholar]
- Grant CM, Riggs KJ, Boucher J. Counterfactual and mental state reasoning in children with autism. Journal of Autism and Developmental Disorders. 2004;34(2):177–188. doi: 10.1023/b:jadd.0000022608.57470.29. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Kato M, Igarashi K, Kashima H. Superior fluid intelligence in children with Asperger’s disorder. Brain and Cognition. 2008;66(3):306–310. doi: 10.1016/j.bandc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Kasari C, Gulsrud A, Freeman S, Paparella T, Hellemann G. Longitudinal follow-up of children with autism receiving targeted interventions on joint attention and play. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(5):487–495. doi: 10.1016/j.jaac.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Gulsrud AC, Wong C, Kwon S, Locke J. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. Journal of Autism and Developmental Disorders. 2010;40(9):1045–1056. doi: 10.1007/s10803-010-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cerebral Cortex. 2002;12(5):477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Hill SS, Stone SI, Bunge SA. Differential effects of reasoning and speed training in children. Developmental Science. 2011;14(3):582–590. doi: 10.1111/j.1467-7687.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- Marrus N, Faughn C, Shuman J, Petersen SE, Constantino JN, Povinelli DJ, Pruett JR., Jr Initial description of a quantitative, cross-species (chimpanzee-human) social responsiveness measure. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(5):508–518. doi: 10.1016/j.jaac.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy CA, Murray DS, Kinsman A, Castillo H, Mitchell T, Hickey FJ, Patterson B. Differences in the clinical presentation of Trisomy 21 with and without autism. Journal of Intellectual Disability Research. 2009;53(2):143–151. doi: 10.1111/j.1365-2788.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- Pellicano E. Links between theory of mind and executive function in young children with autism: Clues to developmental primacy. Developmental Psychology. 2007;43(4):974–990. doi: 10.1037/0012-1649.43.4.974. [DOI] [PubMed] [Google Scholar]
- Pellicano E. Individual differences in executive function and central coherence predict developmental changes in theory of mind in autism. Developmental Psychology. 2010;46(2):530–544. doi: 10.1037/a0018287. [DOI] [PubMed] [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: Explaining the discontinuity between human and nonhuman minds. Behavioral and Brain Sciences. 2008;31(2):109–130. doi: 10.1017/S0140525X08003543. discussion 130–178. [DOI] [PubMed] [Google Scholar]
- Penn DC, Povinelli DJ. On the lack of evidence that non-human animals possess anything remotely resembling a ‘theory of mind’. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2007;362(1480):731–744. doi: 10.1098/rstb.2006.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner J, Leekam S. The curious incident of the photo that was accused of being false: Issues of domain specificity in development, autism, and brain imaging. The Quarterly Journal of Experimental Psychology (Colchester) 2008;61(1):76–89. doi: 10.1080/17470210701508756. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ. World without weight: Perspectives on an alien mind. New York: Oxford University Press; 2012. [Google Scholar]
- Premack D. Psychology. Is language the key to human intelligence? [Comment] Science. 2004;303(5656):318–320. doi: 10.1126/science.1093993. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? The Behavioral and Brain Sciences. 1978;4:515–526. [Google Scholar]
- Raven JC. Standard progressive matrices. Manual. New York: Psychological Corporation; 1958. [Google Scholar]
- Richland LE, Chan TK, Morrison RG, Au TK. Young children’s analogical reasoning across cultures: Similarities and differences. Journal of Experimental Child Psychology. 2010;105(1–2):146–153. doi: 10.1016/j.jecp.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Richland LE, Morrison RG, Holyoak KJ. Children’s development of analogical reasoning: Insights from scene analogy problems. Journal of Experimental Child Psychology. 2006;94(3):249–273. doi: 10.1016/j.jecp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Richland LE, Zur O, Holyoak KJ. Mathematics. Cognitive supports for analogies in the mathematics classroom. Science. 2007;316(5828):1128–1129. doi: 10.1126/science.1142103. [DOI] [PubMed] [Google Scholar]
- Scott FJ, Baron-Cohen S, Leslie A. ‘If pigs could fly’: A test of counterfactual reasoning and pretence in children with autism. British Journal of Developmental Psychology. 1999;17:349–362. [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: The truth about false belief. Child Development. 2001;72(3):655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13(1):103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Burack JA, Benedetto E, Frye D. Theory of mind and rule use in individuals with Down’s syndrome: A test of the uniqueness and specificity claims. Journal of Child Psychology and Psychiatry. 1996;37(4):479–484. doi: 10.1111/j.1469-7610.1996.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Jacques S, Burack JA, Frye D. The relation between theory of mind and rule use: Evidence from Persons with autism-spectrum disorders. Infant and Child Development. 2002;11:171–195. [Google Scholar]