Abstract
We report the first study on pronoun use by an under-studied research population, children with autism spectrum disorder (ASD) exposed to American Sign Language (ASL) from birth by their deaf parents. Personal pronouns cause difficulties for hearing children with ASD, who sometimes reverse or avoid them. Unlike speech pronouns, sign pronouns are indexical points to self and other. Despite this transparency, we find evidence from an elicitation task and parental report that signing children with ASD avoid sign pronouns in favor of names. An analysis of spontaneous usage showed that all children demonstrated the ability to point, but only children with better-developed sign language produced pronouns. Differences in language abilities and self-representation may explain these phenomena in sign and speech.
Keywords: sign language, autism spectrum disorder, deafness, pronouns, language development
Personal pronouns, especially first- and second-person forms (e.g., I/me/my/mine andyou/you/your/yours in English) have long been known to cause difficulty for hearing children with autism spectrum disorder (ASD). Children with ASD sometimes reverse pronouns, referring to themselves as you or to others as me, especially at early ages (e.g., before age 3, Evans & Demuth, 2012; up to age 6, Kanner, 1943). Rarely, children with ASD persist in producing such reversals throughout childhood (Ritvo, Ritvo, Freeman, & Mason-Brothers, 1994). In one study, Tager-Flusberg (1994) found 220 reversal errors (13.15%) in a corpus of 1,673 spoken English pronouns produced by six children with ASD between the ages of 3;4 and 9;9. Typically-developing (TD) children also occasionally go through a phase of pronoun reversal between 19 and 28 months of age (e.g., Chiat, 1982; Oshima-Takane, 1992; Schiff-Myers, 1983).
The motivations for pronoun reversal have been debated at length in the literature, both for TD children and children with ASD. Kanner (1943) linked the phenomenon to echolalia, since children who parrot the utterances of others may reproduce pronouns exactly as they hear them without shifting reference. Others have argued that children who systematically refer to themselves as you may be treating the second-person pronoun as their name; this has been described for very young TD children (Charney, 1980; Clark, 1978; Evans & Demuth, 2012) and for children with ASD (Oshima-Takane & Benaroya, 1989). Reversal errors have often been interpreted as evidence of a pragmatic deficit in understanding how discourse roles, as encoded by personal pronouns, shift between speaker and listener in conversation (e.g., Charney, 1980; Chiat, 1982; Tager-Flusberg, 1994).
In addition to producing reversals, children with ASD sometimes use proper names in contexts where pronouns are normally expected. Jordan (1989) found that eight of 11 (72%) autistic children (ages 6;8-16;5) used their own name for self-reference instead of the pronoun me in a picture-identification task, while only four of 22 (18%) language-matched control children did so. Jordan speculated that the use of proper names could reflect the input from adults, who may intuit that spoken language pronouns are confusing to children. Using a similar task, Lee, Hobson, and Chiat (1994) reported that ten of 12 (83%) ASD participants (ages 8;4-19;6) referred to themselves by name, whereas just four of 12 (33%) non-ASD matched participants did so. Children in this older age range produced few reversal errors, suggesting that older children with ASD may have the ability, but not the propensity, to use pronouns. Lee, et al. speculated that pronoun avoidance could reflect psychological differences in how such children experience the self:
...autistic subjects’ use of names and not pronouns for photographs might have reflected a relatively detached, almost third-person attitude to these depictions of themselves and the experimenter. In contrast, nonautistic subjects seemed to identify with the photographs of themselves, and to see and care about the photographed person as me: The images were infused with the subjects’ and experimenter's sense of identity as well as formal identity. Autistic subjects seemed not to become engaged nor to confer “subjectivity” in this way. (p. 174).
If this account is correct, then the use of pronouns could reflect not just linguistic competence but also the psychological experience of selfhood. The formation of a self-representation typically emerges between 15 and 24 months (Lewis & Brooks-Gunn, 1979; Lewis & Ramsay, 2004) and is necessary for the development of social behaviors such as empathy (Bischof-Kohler, 1994), theory of mind (Lee, et al., 1994), and imitation (Asendorpf, 2002). There is evidence that self-representation ability is underdeveloped in some children with ASD (Carmody & Lewis, 2012), as indicated by mirror recognition and other-directed pretense.
In short, there are competing hypotheses about why children with ASD sometimes avoid pronouns, and why both TD and ASD children sometimes reverse pronouns. All studies to-date have focused on pronouns in spoken languages. In recent decades, a rapidly growing body of work has examined the signed languages of the deaf, which are full-fledged linguistic systems characterized by the hallmarks of human languages: for example, they are acquired naturally from birth by children exposed to them (Newport & Meier, 1985), they exhibit duality of patterning and syntactic recursion (Meier, 2002), and late-learners show critical period effects (Mayberry & Eichen, 1991; Mayberry, Lock, & Kazmi, 2002). A study of the use of sign language pronouns by TD and ASD deaf children could shed new light on the phenomena of pronoun reversal and avoidance. Moreover, there are interesting differences between signed and spoken pronouns which might impact their use in children with ASD.
Personal pronouns in ASL are indexical points to self or other (Klima & Bellugi, 1979); see Figure 1. Thus, they clearly pick out their intended referents,1 unlike spoken language pronouns whose phonological forms give no hint as to their referents. Despite this transparency, several reports indicate that some very young TD signing children appear to go through a phase of pronoun reversal. Petitto (1987) reported that two TD deaf children who were acquiring ASL from birth misused second-person pronouns to mean ‘me’ between the ages of 21 and 23 months. Jackson (1989) described a hearing child of Deaf parents who used the first-person possessive sign pronoun mine2 in lieu of the second-person possessive pronoun yours between 21 and 24 months. Finally, Pizzuto (1990) reported that a TD deaf child produced the first-person pronoun me to mean ‘you’ at 15 and 20 months. Not all deaf children go through such a phase, however; in a recent case study of the acquisition of pronouns in Greek Sign Language between 12 and 36 months of age, Hatzopoulou (2010) did not find any pronoun errors. Thus, some very young TD signing children sometimes produce reversed pronominal forms, despite their similarity to gestural points, which emerge by eight to ten months in TD children (Bates & Dick, 2002).
Figure 1.
The ASL signs i/me (left) and you (right). Photographs copyright Richard P. Meier.
Could the transparency of reference exhibited by ASL pronouns aid learners in understanding their use? There is some evidence that linguistic symbols which have motivated, non-arbitrary (iconic or indexic) forms can be beneficial to both first-language (L1) and second-language (L2) learners. L1 learners tend to acquire signs with greater iconicity earlier in life (Vinson, Cormier, Denmark, Schembri, & Vigliocco, 2008) and are faster at matching signs and pictures when those pictures resemble iconic qualities of the sign (Thompson, Vinson, & Vigliocco, 2009). L2 learners remember iconic signs better than non-iconic signs (Beykirch, Holcomb, & Harrington, 1990). Similarly, hearing children with ASD seem better able to learn iconic signs than non-iconic signs (Konstantareas, Oxman, & Webster, 1978).
On the other hand, there is little evidence that children acquiring sign are aided by indexic forms, such as pronouns or the highly-transparent “agreement” verbs (Padden, 1983), which may move from a location associated with the subject and toward a location associated with the object. Thus, the signed equivalent to “I give you” looks very much like the act of me-giving-something-to-you. Since indexic forms are highly motivated and may be fundamentally gestural (Johnston, 2013), it might be expected that children would acquire such forms early and without error. However, the limited available data on the acquisition of personal pronouns in ASL suggests that errors sometimes do occur, as described above (Jackson, 1989; Petitto, 1987; Pizzuto, 1990). Data on the acquisition of other indexic forms such as agreement verbs has suggested that such forms are acquired relatively late (after age three) and that a characteristic error in the acquisition of both ASL and British Sign language is the omission of indexic elements (Casey, 2003; Meier, 1982, 1987, 2002; Morgan, Barriere, & Woll, 2006); a possible explanation is that omission of the indexic elements may yield forms that are morphologically simpler. Thus, TD children learning sign do not always use motivated forms to their advantage in the language acquisition process.
Sign language pronouns could also be difficult for ASD learners because they are identical or nearly identical to pointing gestures, which are often absent or delayed in children with ASD (Baron-Cohen, 1989; Camaioni, Perucchini, Muratori, & Milone, 1997; Camaioni, Perucchini, Muratori, Parrinini, & Cesari, 2003). In particular, “protodeclarative” pointing gestures, used to attract another's attention in order to share or comment (Baron-Cohen, 1989), may be absent or delayed. Therefore, it is possible to formulate conflicting predictions. On the one hand, signing children with ASD could be relatively advantaged in their use of pronouns compared to their non-signing peers who must contend with arbitrary linguistic forms. On the other hand, the social aspect of sign language pronouns could pose a significant challenge to children with ASD in the same way that protodeclarative points do.
Nearly all research to date on sign learning by ASD children has focused on minimally-verbal hearing children with severe forms of ASD. There have been very few studies of deaf children with ASD, particularly those exposed to ASL from birth by their deaf parents. Deaf children with ASD who are exposed natively to a signed language provide an important test case for understanding how language modality and first language acquisition interact in children with ASD. By studying how native-signing children with ASD acquire sign pronouns, we can gain greater insight into the nature and causes of pronoun use (or non-use) by hearing children with ASD.
We take a multifaceted approach to the investigation of how native-signing children with and without ASD use sign pronouns, employing (a) an experimental paradigm to elicit first- and second-person pronouns,3 (b) an analysis of spontaneous production of pronouns and non-pronominal points, and (c) a parental questionnaire about parent input and child pronoun use.
Method
Participants
Two groups of participants were tested: 1) signing children with ASD, and 2) typically-developing deaf children. All children were raised in households in which ASL was the primary language. Only children born to two signing parents were tested because we can be assured that such children were exposed to a rich linguistic environment beginning when they were neonates. All but one of the participants were born to deaf parents; the one child whose parents are hearing has four deaf grandparents (and thus his parents both had native exposure to ASL). All of the children were also deaf, except for one hearing child of two deaf parents. None of the children had received a cochlear implant or used amplification (hearing aids).
ASD group
Children were recruited via a video in ASL posted on social media (http://youtu.be/VeWmb6jLOgg), and research visits were conducted at the child's home or school. Fifteen children with ASD (11 male, four female) were included in the study and are reported here. Eleven additional children (eight male, three female) were recruited but were not included in the study. Six of these children did not have any expressive language and thus could not complete any of the tasks, three children completed all the tasks but neither the clinician's judgment nor the ADOS-2 supported an ASD diagnosis, and two children were not included because the parents of both children used Signed English4 and spoken English rather than ASL with their child, and both children responded to the tasks in English.
ASD diagnosis in deaf children is complicated by the fact that current gold-standard instruments were not designed for these children. In this study we utilized a combined approach of standardized instruments adapted into ASL and a clinical assessment conducted by a bilingual clinician. Children were included in the study if their ASD diagnosis was confirmed either clinically according to DSM-5 criteria (American Psychiatric Association, 2013) or by one or both of two standard diagnostic instruments: the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2012) and the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003). The ADOS-2 was given by two administrators who had attained research reliability on the instrument and were also proficient in ASL. Due to the unusual nature of the research population, several modifications in administration and scoring were made; these are described in the Appendix. Certain items were not scored (i.e., received an ‘8’) due to their inappropriateness for deaf children (e.g., Intonation of Vocalizations/Verbalizations). Thus, the scores of the children in our sample may underestimate autism severity, since the maximum possible score is lower than when the ADOS-2 is used in standard practice with hearing children. ADOS-2 scores were not used to disqualify subjects but rather as one piece of evidence for the presence or absence of an ASD.
Parents were asked to complete the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), a widely-used screening tool with high specificity and sensitivity in discriminating between children with and without ASD. Like the ADOS-2, the SCQ is not designed specifically for deaf, signing children. We eliminated two items that seemed inappropriate for such children: Item 23, which asks if children ever use gestures to communicate; and Item 38, which asks the rater to say whether or not the child usually looks up and pays attention when the rater enters a room and starts talking to the child. With regard to Item 23, it was unclear how deaf parents would interpret the term “gestures,” since both signs and gestures are produced with the hands. We did not think that the original intent of this question, which is clearly focused on non-linguistic communication, would be faithfully maintained when applied to deaf, signing children. Likewise, Item 38 did not seem like an effective probe of ASD in deaf children, since children who cannot hear are unlikely to perceive that someone is talking to them unless they are already looking at them or can see that person in their peripheral vision. Thus, deaf children's scores on the SCQ were again likely lower than those of their hearing peers. As with the ADOS-2, SCQ scores were not used to disqualify subjects but as additional data to help characterize the children. Of the 15 children eventually included in the study, six children scored above the standard cut-off criterion score of 15 and nine children scored below (one scored 12, two scored 10, three scored 9, one scored 8, and two scored 4). Taken alone, these scores would not support an ASD diagnosis in these nine children. However, seven of these nine children scored above threshold for ASD on the ADOS-2, the gold-standard behavioral observation. Because the SCQ is a parent-reported measure, and Deaf parents for whom written English is a second language may have had difficulty understanding some of the questions, the ADOS-2 scores were given priority and these children were included in the study.
A clinical psychologist (a native signer of ASL expert in diagnosing ASD) reviewed all of the data available for the children whose ADOS-2 scores were under threshold for ASD. She considered each child's SCQ, videotaped ADOS-2, experimental data collection session, and educational or medical records, if available. Two children, subjects ASD-M2 and ASD-M5, were included in the study despite scoring under the cut-off on the ADOS-2 because the psychologist judged these children to meet DSM-5 criteria for ASD after conducting this review.
A summary of characteristics of all of the children recruited for the ASD group of the study can be seen in Table 1, along with the reason for their eventual inclusion or exclusion.
Table 1.
Participants Recruited with a Suspected ASD and Rationale for Inclusion/Exclusion
| Reason for inclusion/ exclusion |
Subject ID | Age | Sex | Hearing status |
Parental hearing status |
ADOS-2 module |
ADOS-2 score |
SCQ score |
TONI-4 standard score |
ASL RST standard score |
ASL RST raw score |
ASL RST language equivalent |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Included; met ADOS-2 criteria for ASD (i.e., scored ≥ 8 for Modules 1 and 2 or ≥ 7 for Module 3) | ASD-M1 | 5;1 | M | Deaf | Deaf | 2 | 11 | 15* | 88 | 92 | 9 | 4;0 |
| ASD-F1 | 7;1 | F | Deaf | Deaf | 1 | 18 | 8 | 98 | 70 | 1 | <3;0 | |
| ASD-M3 | 8;5 | M | Deaf | Hearing (CODA**) | 2 | 14 | 19 | 100 | 95 | 22 | 7;0 | |
| ASD-M4 | 9;5 | M | Deaf | Deaf | 1 | 18 | 31 | 80 | 70 | 2 | <3;0 | |
| ASD-M6 | 9;6 | M | Deaf | Deaf | 3 | 11 | 4 | 86 | 84 | 16 | 5;0 | |
| ASD-M7 | 9;8 | M | Deaf | Deaf | 2 | 16 | 15 | 117 | 79 | 12 | 4;6 | |
| ASD-M8 | 10;2 | M | Hearing (CODA) | Deaf | 1 | 15 | 14 | 69 | 70 | 3 | <3;0 | |
| ASD-M9 | 10;10 | M | Deaf | Deaf | 3 | 8 | 9 | 102 | 90 | 22 | 7;0 | |
| ASD-M10 | 11;0 | M | Deaf | Deaf | 3 | 8 | 9 | 100 | 99 | 31 | 11;0 | |
| ASD-F2 | 11;1 | F | Deaf | Deaf | 3 | 14 | 18 | 104 | 98 | 30 | 10;0 | |
| ASD-F3 | 11;8 | F | Deaf | Deaf | 1 | 15 | 12 | 87 | 78 | 15 | 5;0 | |
| ASD-M11 | 12;7 | M | Deaf | Deaf | 1 | 13 | 4 | 96 | 81 | 19 | 6;0 | |
| ASD-F4 | 14;4 | F | Deaf | Deaf | 3 | 16 | 10 | 100 | 96 | 31 | 11;0 | |
| Included; met DSM-5 clinical criteria for ASD | ASD-M2 | 5;3 | M | Deaf | Deaf | 1 | 1 | 10 | 100 | 84 | 3 | <3;0 |
| ASD-M5 | 9;0 | M | Deaf | Deaf | 3 | 4 | 9 | 92 | 104 | 31 | 11;0 | |
| Excluded; did not meet clinical or ADOS-2 criteria for ASD | ASD-F5 | 13;3 | F | Deaf | Deaf | 3 | 5 | 18 | 90 | 93 | 29 | 10;0 |
| ASD-F6 | 9;6 | F | Deaf | Deaf | - | - | 23 | 98 | 102 | 29 | 10;0 | |
| ASD-M12 | 11;3 | M | Deaf | Deaf | 3 | 5 | 12 | 116 | 103 | 34 | 13;0 | |
| Excluded; could not complete tasks | ASD-M13 | 6;9 | M | Deaf | Deaf | - | - | 19 | - | - | - | - |
| ASD-F7 | 10;5 | F | Deaf | Deaf | - | - | 17 | - | - | - | - | |
| ASD-M14 | 5;3 | M | Hearing (CODA) | Deaf | 1 | 19 | 15 | - | - | - | - | |
| ASD-M15 | 5;1 | M | Deaf | Deaf | 1 | 18 | 26 | - | - | - | - | |
| ASD-M16 | 5;5 | M | Deaf | Deaf | - | - | 23 | - | - | - | - | |
| ASD-M17 | 4;5 | M | Deaf | Deaf | 1 | 21 | 27 | - | - | - | - | |
| Excluded; used spoken and/or signed English instead of ASL | ASD-M18 | 6;0 | M | Hard-of-hearing | Hard-of-hearing | 3 | 7 | 21 | 100 | 78 | 3 | <3;0 |
| ASD-M19 | 12;6 | M | Hearing (CODA) | Hard-of-hearing | 3 | 20 | 19 | 101 | 79 | 17 | 5;6 | |
Cut-off for hearing children on the SCQ > 15.
CODA (Child Of Deaf Adults) is an acronym referring to a hearing person who has deaf parents.
TD group
Eighteen typically-developing deaf children (8 male, 10 female) participated. TD children were recruited through schools for the deaf, and the study was conducted in those schools. All children had at least one deaf parent and had been exposed to ASL from birth. The children were screened using the SCQ. All scored well below the clinical cut-off; the group mean was 2.39 (SD = 2.35; range = 0-7), which was significantly lower than that of the ASD group (M = 12.5; SD = 6.8; range 4-31), Mann-Whitney U = 9.0,5 p < .001; Cohen's d = 1.99.6 A summary of characteristics of the TD deaf children included in the study can be seen in Table 2.
Table 2.
Characteristics of Typically-Developing Deaf Participants
| Subject ID | Age | Sex | Hearing status | Parental hearing status | SCQ score | TONI-4 standard score | ASL RST standard score | ASL RST raw score | ASL RST language equivalent |
|---|---|---|---|---|---|---|---|---|---|
| TD-F1 | 6;7 | F | Deaf | Deaf | 0 | 98 | 109 | 26 | 8;0 |
| TD-F2 | 7;7 | F | Deaf | Deaf | 1 | 96 | 116 | 35 | 13;0 |
| TD-F3 | 7;7 | F | Deaf | Deaf | 1 | 94 | 106 | 27 | 9;0 |
| TD-F4 | 7;7 | F | Deaf | Deaf | 0 | 106 | 116 | 35 | 13;0 |
| TD-F5 | 7;7 | F | Deaf | Deaf | 3 | 86 | 106 | 27 | 9;0 |
| TD-M1 | 7;9 | M | Deaf | Deaf | 0 | 106 | 116 | 35 | 13;0 |
| TD-F6 | 8;7 | F | Deaf | Deaf | 1 | 98 | 105 | 29 | 10;0 |
| TD-M2 | 8;7 | M | Deaf | Deaf | 6 | 90 | 110 | 33 | 12;0 |
| TD-M3 | 8;10 | M | Deaf | Deaf | 1 | 127 | 111 | 34 | 13;0 |
| TD-F7 | 9;7 | F | Deaf | Deaf | 0 | 95 | 111 | 36 | >13;0 |
| TD-M4 | 9;7 | M | Deaf | Deaf | 2 | 99 | 111 | 36 | >13;0 |
| TD-M5 | 9;11 | M | Deaf | Deaf | 3 | 107 | 116 | 40 | >13;0 |
| TD-M6 | 9;11 | M | Deaf | Deaf | 6 | 121 | 111 | 36 | >13;0 |
| TD-F8 | 10;3 | F | Deaf | Deaf | 7 | 112 | 110 | 37 | >13;0 |
| TD-F9 | 11;2 | F | Deaf | Deaf | 4 | 98 | 107 | 37 | >13;0 |
| TD-F10 | 11;6 | F | Deaf | Deaf | 0 | 101 | 105 | 35 | 13;0 |
| TD-M7 | 12;2 | M | Deaf | Deaf | 5 | 95 | 100 | 33 | 12;0 |
| TD-M8 | 12;9 | M | Deaf | Deaf | 3 | 99 | 91 | 26 | 8;0 |
Matching
The two groups were matched for chronological age and non-verbal intelligence. The TD group was four months younger on average (Mage = 9;4, SD = 1;9, range 6;7-12;9) than the ASD group (Mage = 9;8, SD = 2;6, range 5;1-14;4); however, this difference was not significant (Mann-Whitney U = 119.0, p = .56, ns).
The Test of Nonverbal Intelligence, Fourth Edition (TONI-4; Brown, Sherbenou, & Johnsen, 2010) was used to estimate general intellectual ability. This test has been used in research with deaf children (e.g., Schick, de Villiers, de Villiers, & Hoffmeister, 2007), has been validated for use with children with ASD, and requires little or no verbal instruction. The TD group scored slightly higher on the TONI-4 (M = 101.6; SD = 10.3, range = 86-127) than the ASD group (M = 95.1, SD = 11.5, range = 69-117); however, this difference was not significant (Mann-Whitney U = 110.5, p =.37, ns).
Children were also tested for sign language comprehension level using the ASL Receptive Skills Test (ASL RST; Enns, Zimmer, Boudreault, Rabu, & Broszeit, 2013), which measures children's understanding of ASL grammar and is appropriate for use with children ages 3 to 13. The ASL RST consists of two parts, a 20-item vocabulary check and a 42-item multiple-choice video-presented receptive skills test. The test was presented on a MacBook Pro laptop computer placed on the table in front of the seated child. For each item, the Deaf female sign model produces a sentence in ASL on the screen. Four pictures depicting possible meanings of the signed sentence then appear on the screen. Children are asked to point at the picture matching the meaning of the signed sentence. The sentences increase in difficulty as the test goes on. Testing is discontinued after five consecutive incorrect responses. The Receptive Skills Test assesses the following grammatical structures: (1) number/distribution, (2) negation, (3) noun/verb distinction, (4) spatial verbs, (5) size and shape specifiers, (6) handling classifiers, (7) role shift, and (8) conditional sentences. The TD children's mean standard language score was significantly higher (M = 108.7, SD = 6.3; range 91-116) than that of the ASD children (M = 86, SD = 11.3; range 70-104), Mann-Whitney U = 7.0, p < .001; Cohen's d = 2.5. This is unsurprising since by definition ASD entails deficits in language and communication. Thus, the two groups were not matched for language.
Procedures
Pronoun elicitation
At least two prior studies (Lee, et al., 1994; Jordan, 1989) used picture identification tasks to elicit first- and second-person pronouns in children with ASD. We sought to replicate portions of these studies with deaf children using sign. The procedures were adapted from the picture identification task described in Lee, et al. (1994), but were modified to be conducted in ASL.
To elicit the first-person pronoun, the experimenter sat across from the child, and took a picture of the child using an iPad. The experimenter then showed the picture to the child and asked in ASL “Who is this?” This question consisted of two signs, the sign who and an indexical point at the picture. Thus, the question itself contained a sign that resembles the sign pronouns me and you, but that was directed at the iPad rather than at any person. This procedure differed from the past studies in that it used an iPad rather than photographs printed on paper.
The experimenter then elicited the second-person pronoun by showing the child a picture of the experimenter on the iPad (which had been taken earlier) and asking again in ASL “Who is this?”
Spontaneous production of points and pronouns
We used the ADOS-2 evaluation as a naturalistic language sample in order to gain a broader understanding of how the children with ASD spontaneously used sign pronouns outside of the elicitation task. We documented all pointing behaviors, whether or not the points functioned as linguistic pronouns, since children with ASD tend to point less than TD children in some contexts (e.g., Baron-Cohen, 1989).
Data were coded independently by two separate raters (a deaf native signer and a hearing signer), and were then compared and compiled. All points (defined as instances of an extended index finger directed at a person, object, or other location in space), pronouns, and name signs7 produced by the children were transcribed. Each token was classified as a personal pronoun, a non-pronominal point, or a name sign. Personal pronouns were defined as points to a person, either self or other, or to a location in space meant to index a person. We also transcribed two other types of pronouns: possessive and reflexive. These signs point to the location associated with a person, but do so with a flat handshape (in the case of possessives) or a fisted handshape (in the case of reflexives). Non-pronominal points were defined as points to present objects; the index finger could optionally touch the object. Name signs, which varied with each child, were signs that were confirmed as referring to the child, the researcher, or another person known to the child and that were otherwise not conventional ASL signs. Disagreements were resolved through consensus.
Parental questionnaire
Parents of the ASD participants were asked to complete a written questionnaire via email subsequent to the testing session. The questionnaire was designed to determine two things: (a) whether elicited behavior was consistent with the child's spontaneous behavior, and (b) whether sign names has been modeled to children with ASD by parents or teachers, since parents sometimes use names instead of pronouns in their utterances to young children (Smiley, Chang, & Allhoff, 2011). It is worth noting that unlike in spoken language, sign names are not typically used in direct address; the ASL equivalent of a command such as “Sally, do your homework” would almost certainly omit the name Sally, instead substituting a pronoun or an attention-getter such as the sign hey (Hoza, 2011). It is therefore likely that deaf children have fewer opportunities to see their sign name than hearing children have to hear their spoken name.
The questionnaire included the following questions:
-
1)
Does your child ever refer to him/herself with his/her sign name? For example, instead of signing i want eat he/she might sign joey want eat.
-
2)
Do you or your spouse ever use your child's sign name when signing to him/her? For example, instead of signing you want cookie, you or your spouse might sign joey want cookie.
-
3)
Does your child ever refer to you or your spouse with the sign mom/mommy (or dad/daddy) when signing to you or your spouse? For example, instead of signing you give toy, he/she might sign mommy give toy.
-
4)
Do you or your spouse ever refer to yourself with the sign mom/mommy (or dad/daddy) when signing to your child? For example, instead of signing i give you toy, you or your spouse might sign mommy give you toy.
Results
Pronoun elicitation
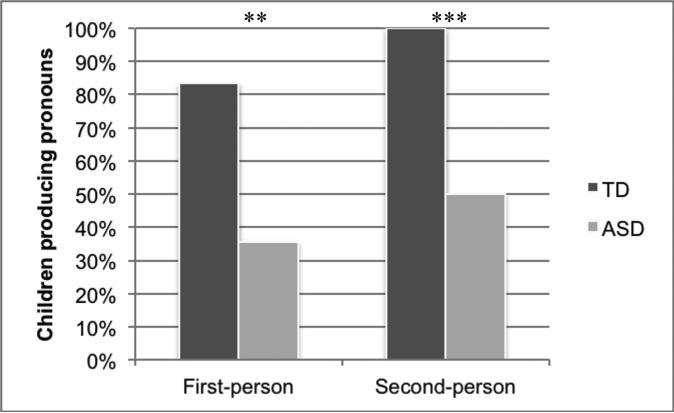
Each child's responses to the two pronoun elicitation tasks are shown in Table 3; the results are summarized in Figure 2. One child with ASD (ASD-F1) did not respond to either task; thus, this child was excluded for the purposes of analysis. On the first-person task, 15 of 18 TD children and 5 of 14 ASD children produced the ASL pronoun me (i.e., they pointed to themselves); this difference between the TD and ASD groups was significant (Fisher's Exact Test, p < .01, one-tailed, odds ratio = 9.0). None of the children produced a reversed pronoun (i.e., you in lieu of me). The three TD children and nine ASD children who did not produce the pronoun me each produced their name sign or fingerspelled their English name. Two children with ASD and one TD child produced their name twice, once as a lexical name sign and once as a fingerspelled name. The one hearing child with ASD produced his name sign while simultaneously uttering his English name. Three TD children and one child with ASD produced both the first-person pronoun and their name sign.
Table 3.
Participants' Responses in the Pronoun Elicitation Tasks
| ASD subjects | Age | First-person response | Second-person response | TD subjects | Age | First-person response | Second-person response |
|---|---|---|---|---|---|---|---|
| ASD-M1 | 5;1 | name sign | name sign & pronoun | TD-F1 | 6;7 | name sign & pronoun | pronoun |
| ASD-M2 | 5;3 | name sign | MAN | TD-F2 | 7;7 | pronoun | pronoun |
| ASD-F1 | 7;1 | No response | No response | TD-F3 | 7;7 | name sign | pronoun |
| ASD-M3 | 8;5 | pronoun | pronoun | TD-F4 | 7;7 | pronoun | pronoun |
| ASD-M4 | 9;0 | pronoun | pronoun | TD-F5 | 7;7 | name sign & fingerspelling | pronoun |
| ASD-M5 | 9;5 | fingerspelling | MAN | TD-M1 | 7;9 | pronoun | pronoun |
| ASD-M6 | 9;6 | pronoun | pronoun | TD-F6 | 8;7 | pronoun | pronoun |
| ASD-M7 | 9;8 | fingerspelling | MAN | TD-M2 | 8;7 | pronoun | pronoun |
| ASD-M8 | 10;2 | name sign | name sign | TD-M3 | 8;10 | pronoun | pronoun |
| ASD-M9 | 10;10 | pronoun & name sign | pronoun & fingerspelling | TD-F7 | 9;7 | name sign | pronoun & fingerspelling |
| ASD-M10 | 11;0 | fingerspelling | fingerspelling | TD-M4 | 9;7 | pronoun | pronoun |
| ASD-F2 | 11;1 | pronoun | pronoun | TD-M5 | 9;11 | pronoun & fingerspelling | pronoun |
| ASD-F3 | 11;8 | name sign | DOCTOR | TD-M6 | 9;11 | pronoun | pronoun |
| ASD-M11 | 12;7 | name sign & fingerspelling | pronoun | TD-F8 | 10;3 | name sign & pronoun | pronoun |
| ASD-F4 | 14;4 | name sign & fingerspelling | fingerspelling | TD-F9 | 11;2 | pronoun | pronoun |
| TD-F10 | 11;6 | pronoun | pronoun | ||||
| TD-M7 | 12;2 | pronoun | pronoun | ||||
| TD-M8 | 12;9 | pronoun | pronoun |
Figure 2.
Percentage of TD and ASD children who produced pronouns in the pronoun elicitation task
On the second-person task, all 18 TD children and 7 of 14 ASD children produced the ASL pronoun you (i.e., they pointed to the experimenter). There was a significant difference between the TD and ASD groups (Fisher's Exact Test, p = .001, one-tailed, odds ratio = ∞). None of the children produced a reversed pronoun (i.e., me). One TD child and two ASD children produced the experimenter's name sign along with the pronoun. Three children with ASD produced the experimenter's name sign or fingerspelled name only. Three ASD children produced the ASL sign man (or perhaps the formationally-similar sign father) and one ASD child produced the ASL sign doctor.8
We analyzed the relationship between performance on this task and overall receptive language level, mental age, and chronological age across both groups. Children were assigned a composite score in which one point was given for production of the first-person pronoun and one point was given for production of the second-person pronoun. A Pearson Product-Moment correlation found that pronoun production was strongly positively correlated with ASL comprehension; r(30) = .67, p < .001. Pronoun production was also moderately correlated with non-verbal intelligence; r(30) = .35, p < .05, but not with chronological age; r(30) = −0.1, ns.
Children (both TD and ASD) who produced the first-person pronoun at least once (N = 20) scored higher as a group on the ASL Receptive Skills Test (M = 105.3, SD = 9.5) than the 12 (ASD and TD) children who only produced names and did not produce the first-person pronoun (M = 89.3, SD = 14.3). A one-way ANOVA using production of the first-person pronoun as a group factor and ASL-RST standard score as the dependent measure found that this difference was significant, F(1, 30) = 14.53, p <.001.
Although the TD and ASD groups could not be matched for overall language level, we were able to match a subset of each group on language. The six highest-scoring children with ASD were matched to the six lowest-scoring TD children (see Table 4). The mean raw score of the six highest-scoring ASD children was 27.8, while the mean raw score of the six lowest-scoring TD children was 28.0; an unpaired t-test found no difference between the groups, p = .94, ns.
Table 4.
Performance of Language-Matched Subsample on the Pronoun Elicitation Tasks
| ASD subjects | ASL RST raw score | First-person response | Second-person response | TD subjects | ASL RST raw score | First-person response | Second-person response |
|---|---|---|---|---|---|---|---|
| ASD-M3 | 22 | pronoun | pronoun | TD-F1 | 26 | pronoun & name sign | pronoun |
| ASD-M5 | 31 | pronoun | pronoun | TD-F3 | 27 | name sign | pronoun |
| ASD-M9 | 22 | pronoun & name sign | pronoun & fingerspelling | TD-F5 | 27 | name sign & fingerspelling | pronoun |
| ASD-M10 | 31 | fingerspelling | fingerspelling | TD-F6 | 29 | pronoun | pronoun |
| ASD-F2 | 30 | pronoun | pronoun | TD-M2 | 33 | pronoun | pronoun |
| ASD-F4 | 31 | name sign & fingerspelling | fingerspelling | TD-M8 | 26 | pronoun | pronoun |
| Mean score | 27.8 | 28.0 | |||||
| SD | 4.5 | 2.7 | |||||
When we compared the performance of these 12 children on the pronoun elicitation task, we found that the matched groups performed similarly on the first-person task. Three children with ASD produced the first-person pronoun only, one produced the first-person pronoun plus his name sign, and two produced their name sign only. Likewise, three TD children produced the first-person pronoun only, one produced the first-person pronoun plus her name sign, and two produced their name sign only. There was a difference in the results on the second-person task: all six TD children produced the second-person pronoun only, while only three children with ASD produced the second-person pronoun only. One child with ASD produced the second-person pronoun and fingerspelled the investigator's name, and two children with ASD fingerspelled the investigator's name only. Thus, children with ASD produced fewer second-person pronouns than TD children (but not first-person pronouns), even when matched for receptive language level. However, due to the small sample size, this difference approached, but did not reach, the level of significance (Fisher's Exact Test, p = .09, one-tailed).
Spontaneous production of pronouns and points
All of the children with ASD were included except for one child (subject ASD-M9) whose video recording was damaged and could not be analyzed. Since only children in the ASD group received an ADOS-2 evaluation, the TD children could not be included in this analysis. Length of the ADOS evaluation varied by subject; the shortest lasted 25 minutes, while the longest lasted 90 minutes. The average duration of the ADOS evaluation was about 46 minutes.
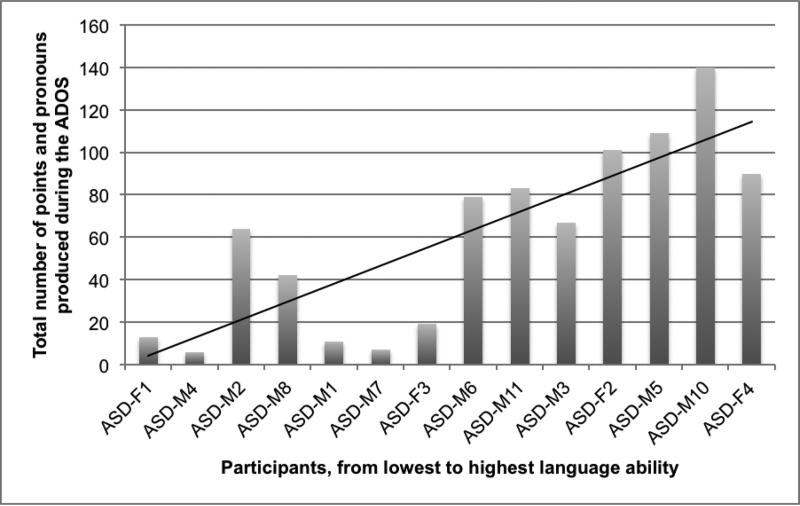
Every child produced pointing signs, with even the children with the lowest language scores pointing at least five tokens/instances. Children were ranked in order from 1 to 14 based on their performance on the ASL-RST. Results of the analysis of spontaneous production of pronouns and points on the ADOS-2 are shown in Figure 3. A Spearman Rank Correlation Coefficient found that overall production of pointing signs (personal pronouns as well as points to objects) was strongly correlated with ASL comprehension, r(12) = .81, p < .001.
Figure 3.
Overall production of pointing signs (pronouns and referential points) on the ADOS-2 was strongly correlated with level of ASL comprehension, r(12) = .81, p < .001
However, the distribution of these pointing signs differed dramatically across subjects. The participants were divided into two groups based on overall language ability as reflected by performance on the ASL-RST and the clinician's module selection on the ADOS-2: a low-language group (from ASD-F1 to ASD-M11 on Figure 3) and a high-language group (from ASD-M3 to ASD-F4). The mean raw score of the low-language group on the ASL-RST was 8.78 correct items out of 42 (SD = 7.03; range 0-19) as compared to 29.0 (SD = 3.94; range 22-31) for the high-language group; this difference was significant (Mann-Whitney U = 0.0, p < .01, Cohen's d = 3.6). The nine children with lower language scores produced 324 total pointing signs (M = 36.1, SD = 31.7), whereas the five children with higher language scores produced 507 pointing signs (M = 101.4, SD = 26.7).
The two groups of children produced different kinds of pointing signs (Table 5). We differentiated between pointing at, or touch-pointing, an object or an image in a book (referential points) and pointing at self, other, or non-present referent (pronouns). Since the time of each ADOS assessment varied across subjects, we calculated the mean percentage of pronouns versus points to objects produced by each subject. On average, 87% (SD = 17%) of the pointing signs produced by children in the lower-language group were referential points to objects, while 13% (SD = 17%) were pronouns. By contrast, only 54% (SD = 20%) of the pointing signs produced by children in the higher-language group were referential points to objects, while 46% (SD = 20%) were pronouns. An unpaired t-test revealed that this difference was significant; p < .01.
Table 5.
Number of Pronouns and Points (Mean Proportion in Parentheses) Produced by Children in the Low- and High-Language Groups
| Subject | 1st-person | 2nd-person | 3rd-person | Points to objects | Total | |
|---|---|---|---|---|---|---|
| Low-language group | ASD-F1 | 0 (.00) | 0 (.00) | 0 (.00) | 13 (1.00) | 13 (1.00) |
| ASD-M4 | 1 (.17) | 0 (.00) | 0 (.00) | 5 (.83) | 6 (1.00) | |
| ASD-M2 | 0 (.00) | 0 (.00) | 0 (.00) | 64 (1.00) | 64 (1.00) | |
| ASD-M8 | 5 (.12) | 2 (.05) | 0 (.00) | 35 (.83) | 42 (1.00) | |
| ASD-M1 | 6 (.55) | 0 (.00) | 0 (.00) | 5 (.45) | 11 (1.00) | |
| ASD-M7 | 1 (.14) | 0 (.00) | 0 (.00) | 6 (.86) | 7 (1.00) | |
| ASD-F3 | 1 (.05) | 0 (.00) | 0 (.00) | 18 (.95) | 19 (1.00) | |
| ASD-M6 | 3 (.04) | 0 (.00) | 2 (.03) | 74 (.94) | 79 (1.00) | |
| ASD-M11 | 1 (01) | 1 (01) | 0 (.00) | 81 (.98) | 83 (1.00) | |
| Total (mean) | 18 (.12) | 3 (.01) | 2 (.00) | 301 (.87) | 324 (1.00) | |
| Subject | 1st-person | 2nd-person | 3rd-person | Points to objects | Total | |
|---|---|---|---|---|---|---|
| High-language group | ASD-M3 | 34 (.51) | 2 (.03) | 9 (.13) | 22 (.33) | 67 (1.00) |
| ASD-F2 | 14 (.14) | 3 (.03) | 1 (.01) | 83 (.82) | 101 (1.00) | |
| ASD-M5 | 38 (.35) | 2 (.02) | 3 (.03) | 66 (.61) | 109 (1.00) | |
| ASD-M10 | 52 (.37) | 5 (.04) | 3 (.02) | 80 (.57) | 140 (1.00) | |
| ASD-F4 | 57 (.63) | 0 (.00) | 0 (.00) | 33 (.37) | 90 (1.00) | |
| Total (mean) | 195 (.40) | 12 (.02) | 16 (.04) | 284 (.54) | 507 (1.00) | |
Of the personal pronouns produced by the higher-language group, first-person pronouns accounted for the majority (87.4% of pronouns; 195 tokens, M = 39.0 per subject, SD = 16.9); second-person pronouns accounted for 5.4% (12 tokens, M = 2.4 per subject, SD = 1.8) and third-person pronouns accounted for 7.2% (16 tokens, M = 3.2 per subject, SD = 3.5). Of the pronouns produced by the lower-language group, first-person pronouns also dominated, with 78% of all occurrences (18 tokens; M = 2.0 per subject, SD = 2.2); second-person pronouns accounted for 13% (3 tokens; M = 0.3 per subject, SD = 0.7); and third-person pronouns for 9% (2 tokens; M = 0.2 per subject, SD = 0.7). Two children in the lower-language group produced no personal pronouns at all, while three children in this group produced a single pronoun (the first-person pronoun me in all cases).
Children also differed in the type of pronouns produced. In the lower-language group, only one child produced a possessive form; Child ASD-M1 produced one token of my. However, children in the higher-language group produced 50 tokens of the possessive pronoun my, in addition to 145 tokens of me.
Finally, we analyzed whether any of the first- or second-person pronoun tokens could be considered reversals by considering each token in its discourse context. Two independent raters examined each token and judged whether or not the pronoun was reversed. Only two possible instances of pronoun reversal were found, both in echoed utterances produced by Child ASD-M5 (who had the second-lowest language score of all children). The two reversals occurred in the following contexts:
-
(1)Examiner: you tell
- Child: you tell
-
(2)Examiner: you like play with me?
- Child: me
This child tended to echo most utterances, so it does not appear that he intended to refer to himself or to the ADOS administrator. Thus, these examples are qualitatively unlike reported pronoun reversals by speaking children with ASD in which those children seemingly intended to refer to themselves using the pronoun you (Evans & Demuth, 2012; Tager-Flusberg, 1994).
Parental questionnaire
Fourteen parents of the 15 children with ASD completed the questionnaire. In response to Question 1 (“Does your child ever refer to him/herself with his/her sign name?”), eight parents (57%) reported that their children sometimes referred to themselves with their sign name, either currently or in the past. Four of these eight parents (50%) reported that their children had previously referred to themselves with sign names rather than pronouns, but had since learned how to use pronouns. The mother of an 11-year-old boy specified that this had occurred until age six, but then stopped. The mother of a 12-year-old boy reported that her son had stopped referring to himself with his sign name at approximately age nine. Similarly, the mother of an 11-year-old girl reported that her child's use of her own sign name had faded away since about the age of nine.
With regard to Question 2 (“Do you or your spouse ever use your child's sign name when signing to him/her?”), five parents (36%) reported using their children's name sign when addressing them. All five of these parents also responded affirmatively to Question 1. Three parents who responded affirmatively to Question 1 did not report using their children's sign name in direct address. In other words, five of eight (62.5%) parents whose children referred to themselves with name signs rather than pronouns also reported using their children's name signs in direct address.
With regard to Question 3 (“Does your child ever refer to you or your spouse with the sign mom/mommy (or dad/daddy) when signing to you or your spouse?”), seven parents (50%) reported that their child used the signs mom/dad, even in direct discourse. Three of these parents specified that such use was inconsistent or only in certain situations, while one parent indicated that this had occurred until age 6, but had since stopped. Five of these seven parents (71%) also responded affirmatively to Question 1, indicating that children who used name signs to refer to others in direct discourse also used names to refer to themselves. However, two parents (28.5%) reported that their children used the signs mom/dad but did not refer to themselves with their sign name.
With regard to Question 4 (“Do you or your spouse ever refer to yourself with the sign mom/mommy (or dad/daddy) when signing to your child?”), eight parents (57%) reported referring to themselves with the sign mom/dad. One parent wrote, “we would try to say ‘I, me, your, my & you’ at first.... If [our son] did not seem to catch on, we would repeat using [his name sign]. After he caught on, I would repeat ‘Mommy, me, I are the same. You, your means [son's name].’” Another parent remarked, “Yes we do refer to ourselves as ‘mommy’ or ‘daddy’ because that is how he understands what we are talking about.”
Discussion
Before discussing our results, we note that six children were not included in the study because they had such limited expressive sign language that they could not complete any of the tasks. These children, who are listed in Table 1 (ASD-M13, ASD-M14, ASD-M15, ASD-M16, ASD-M17, and ASD-F7), all had SCQ scores well above the threshold level for autism risk (M = 21.2, SD = 4.9; range 15-27), and ranged in age from 4;5 to 10;5 (M = 6;3, SD = 2;2). Three of the children received the ADOS-2, and all scored well above threshold for autism classification; the other three children did not receive the ADOS-2 because the data collection session had been scheduled first and their non-performance of the tasks excluded them from the study. Although we do not otherwise report results from these children, we note the lack of expressive sign language in some deaf children with ASD, a population that has received scant attention in the literature (for prior reports of such children with hearing parents, see Jure, Rapin, & Tuchman, 1991; Meinzen-Derr et al., 2014; Roper, Arnold, & Monteiro, 2003). It is estimated that up to 30% of hearing children with ASD show minimal expressive language (Tager-Flusberg & Kasari, 2013). In our sample, six of 23 (26%) children with suspected or confirmed ASD demonstrated minimal expressive sign language. Since all children had Deaf, signing parents, this failure to acquire expressive language cannot be due to a lack of exposure to sign. Although our sample is small, our results would indicate that a similar proportion of deaf children and hearing children with ASD are minimally verbal.
Pronoun elicitation
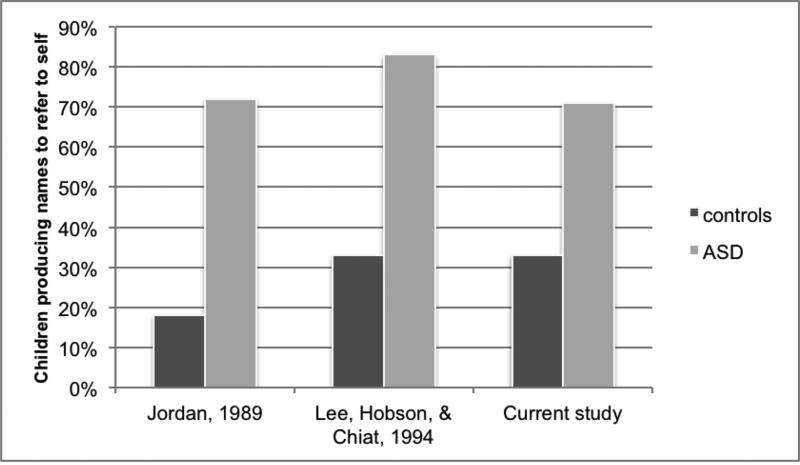
Deaf children with ASD differed significantly from TD deaf children in their performance on the first- and second-person pronoun elicitation task (though the matched subsamples performed similarly on the first, but not the second-person task). Children with ASD were less likely than TD children to produce a sign pronoun, and instead tended to refer to themselves and the experimenter by sign name. These results are nearly identical to those of two prior studies with hearing ASD children on similar tasks (Figure 4). This is surprising in light of the fact that sign language pronouns are transparent indexical points to self and to other. It is even more striking when one considers that the experimenter's question (“Who is this?”) contains an indexical point to the picture, thus modeling the very form that the answer should contain. Moreover, unlike prior studies, children witnessed the act of taking the photograph, which could have aided children in performing the first-person task.
Figure 4.
Percentage of ASD participants and controls in three different studies who labeled a picture of themselves with their own name, with or without a pronoun
The language-matched subsample performed more similarly than the groups overall. Therefore, it appears likely that language level, independent of ASD, plays some role in the use of a name rather than a pronoun.
Both groups produced more pronouns on the second-person task than on the first-person task; however, this difference was not significant (Fisher's Exact Test, p = .55, ns). A plausible explanation is that children were less likely to know or remember the experimenter's name, and thus using the pronoun may have been the most available strategy for answering this question. It is also worth noting that on this task, four children with ASD responded with nouns (three with the sign man and one with the sign doctor). It is unclear from these responses if the children recognized that the person in the picture was indeed the experimenter seated in front of them. By contrast, it is clear that all children understood the first-person question and recognized themselves in the picture, responding with a correct answer, whether a pronoun or a sign name.
None of the children produced a reversed pronoun on either the first- or second-person task. This could be due in part to the small number of opportunities each child had to produce a pronoun in this task. Alternatively, it could be that the children in our sample were past the age in which pronoun reversals are typically observed. However, pronoun reversals may be less related to chronological age than to the child's stage of language development: Tager-Flusberg (1994) found that some hearing children with ASD and low language (MLU < 4) reversed pronouns up to about age nine. Although MLU was not calculated for the children in our sample, the ASL-RST gives normed age equivalents based on a standardization sample of 203 deaf children in the United States and Canada. Eleven of the children with ASD had language age equivalents under age nine, and four of these children had language age equivalents of three or under. Therefore, we believe that many of the children in our sample could be comparable to hearing children with ASD who show pronoun reversals.
Spontaneous production of pronouns and points
All children produced points on the ADOS-2, including children with very low language scores. Thus, the lack of pointing at self or other in the pronoun elicitation tasks by children with ASD (and the parental reports of the same) cannot be due to a general pointing impairment.
However, clear differences in pointing behavior emerged between ASD children when grouped by language level. Those with lower language scores tended to point mostly to objects, with very little reference to self or other. Those with higher language scores produced pronouns (especially first-person pronouns) at a much higher rate. This finding could reflect the proto-declarative pointing impairment that has been documented in numerous studies of hearing children with ASD (e.g., Baron-Cohen, 1989); pointing at objects can be done either imperatively or declaratively whereas pointing at people is usually done to refer rather than to request. First-person pronouns accounted for the majority of pronominal occurrences in both groups. This could be a result of the tendency of first-person forms to emerge before second- or third-person forms (Petitto, 1983). Alternatively, the relative absence of second- and third-person forms could be due to the general lack of social reciprocity that is characteristic of ASD.
We did not find evidence of children spontaneously referring to themselves by name during the ADOS. Our data thus suggest that children with ASD tend to refer to themselves by name under certain circumstances (such as the picture identification task), but do not invariably do so. There was also scant evidence of pronoun reversals in these data. The only examples occurred in echoed utterances, and even then the child (ASD-M4) did not appear to have any communicative intent. This nine-year-old child had a language-age equivalent of a three-year-old, which is consistent with the interpretation that pronoun reversals are produced very early in language development.
In sum, the analysis of spontaneous pointing clarified that signing children with ASD can point, but that this pointing behavior varies greatly as a function of language ability, with children with higher language levels producing more personal pronouns and children with lower language producing mostly referential points.
Parental questionnaire
Several findings emerged from the parental questionnaire. First, parents reported that their children sometimes referred to themselves and to their parents with sign names rather than pronouns. Importantly, some parents reported that their children had previously used sign names but had learned to use pronouns over time, suggesting a developmental trajectory. Parental reports supported the findings of the pronoun elicitation tasks: seven of the eight children (87.5%) whose parents reported the use of sign names by the child produced their sign name in the pronoun elicitation tasks. These results suggest that the children's production of sign names was not just an artifact of these tasks.
Parents also reported using sign names, both for themselves as well as for their children. Therefore, input may have some role in the referential behavior of these children. However, parental input is unlikely to be the sole factor responsible for this phenomenon, since some parents of pronoun-avoidant children did not report using sign names with their children.
The parents’ comments indicated that they gauged children's understanding in their choice to use sign names rather than pronouns. Sign names are opaque and often arbitrary linguistic symbols, whereas sign pronouns are transparent and indexical. Yet parents intuited that their children could more easily understand sign names.
General Discussion
Taken together, these three tasks paint a complex and nuanced picture of pronoun use by signing children with ASD. In our view, there are two issues in the use (or non-use) of pronouns by these children. The first is whether the child chooses to use a pronoun or a name sign to refer to oneself or to others. The second issue is, if the child elects to use a pronoun, does he produce the correct form or does he reverse the pronoun? We address each of these issues in turn.
We find strong evidence that signing children with ASD are less likely than TD children to produce a pronoun on a picture-identification task, closely replicating the findings of two previous studies that tested hearing children with ASD (Jordan, 1989; Lee, et al., 1994). The children with ASD in this study were of comparable average language age (M = 6;0) to the children in the prior studies (Jordan: M = 5;7, Lee, et al.: M = 4;7), although the children in those studies were somewhat older (Jordan: M = 10;5; Lee, et al.: M = 14;9) than the children in our study (M = 9;8). It thus appears that this pattern of behavior is consistent in both deaf and hearing children with ASD at a certain stage in their linguistic development, and also occurs in some non-ASD children, particularly those with less well-developed language. From this result we now know that the opacity of spoken language pronouns cannot account for the findings from prior research with hearing children. Children with ASD are less likely to use pronouns, even when those pronouns are the highly transparent pointing signs of ASL.
We can be less certain of the unique contribution of autism spectrum disorder to the results we report. Are our results ascribable entirely to the language delay that is typically associated with ASD (and that is also experienced by other children), or is ASD an important contributor over and beyond the effects of language delay? Elicited and spontaneous pronoun production were strongly correlated with ASL comprehension, suggesting that the use of sign pronouns requires fairly sophisticated linguistic competence. Since the TD and ASD groups could not be matched for overall language ability, it appears likely that language level is responsible in part for the difference observed between the two groups. However, it is telling that both Lee et al. (1994) and Jordan (1989) matched children with ASD to children with intellectual disability, and found that language-matched, non-autistic children showed significantly less pronoun avoidance than children with ASD. We were able to match a subsample of children with ASD and TD for language and found that language level accounted for some, but not all, of the difference observed. In particular, only children with ASD used a name or noun on the second-person task, while none of the TD children did.
Although the children who used pronouns during the elicitation tasks tended on average to have better-developed language than those who did not, the pattern was not straightforward. Of the nine children who produced names instead of pronouns on the first-person task and for whom an ADOS-2 was available, seven fell into the low-language group (Subjects ASD-M1, ASD-M2, ASD-F3, ASD-M5, ASD-M7, ASD-M8, and ASD-M11) whereas two children fell into the high-language group (Subjects ASD-F4 and ASD-M10). Additionally, one child in the lower-language group produced both pronouns, and two others produced the second-person pronoun only. Therefore, the results of the pronoun elicitation task are not perfectly predicted by language level.
The interpretation of Lee et al. (1994) that the use of names rather than pronouns may be evidence of a difference in self-concept resonates with our experience. When shown a picture of themselves, the TD children in our study often reacted to the question “Who is this?” with a smile or laugh and an emphatic point to their own bodies. The children with ASD had no such emotional reaction. They were in all cases able to identify the picture of themselves, but did not appear to have feelings about the picture in the same way as the TD children.
Nouns (names) and pronouns can each designate the same person (e.g., oneself). TD children show a strong preference for using a pronoun to refer to themselves, perhaps because the pronoun is more strongly linked to a self-representation. For example, children in the second year of life who show self-recognition in a mirror use more pronouns than children who do not, indicating that the two abilities are related and interdependent (Lewis & Ramsay, 2004). For children with ASD, however, pronouns may not be as strongly linked to a notion of selfhood, and indeed, in some contexts (such as the picture-identification task), a noun appears to be preferred by these children. Our data suggest that the use of sign pronouns is related to overall language development, but that some children may still choose to use sign names under certain circumstances, even if they demonstrate the ability to use pronouns in other contexts.
In our sample, children with better-developed sign language skills showed the ability to produce sign pronouns, especially first-person pronouns, in spontaneous discourse. The question now becomes: when children with ASD do select a pronominal form, which form do they choose – the correct form or a reversed form? For some hearing children with ASD, the selection of the correct form is problematic and results in pronoun reversals. Pronoun reversals have also been reported early in the acquisition of ASL by TD deaf children (Jackson, 1989; Petitto, 1987; Pizzuto, 1990). In our own data we find just two pronoun reversals in a corpus of 393 spontaneous sign pronoun tokens, a rate of less than one percent. We cannot be certain whether this low rate is representative of all signing children with ASD, or is an artifact of our still relatively small sample. However, we would like to raise the possibility that the differing modalities of sign and speech could account for the relative absence of reversals in sign.
In order to motivate this hypothesis, it is useful to look to the literature on gesture imitation by hearing children with ASD. Some young hearing children with ASD have been found to make reversal errors when copying the gestures of others, such that a gestured hand wave with the palm facing outward would be copied by the ASD child with an inward-facing palm (Ohta, 1987; Smith, 1998; Whiten & Brown, 1998). This type of error has been theorized to be a reflection of a deficit in “self-other mapping”, that is, a difficulty with translating the body movements of others into one's own body movements (Rogers & Pennington, 1991). In other words, children with ASD sometimes imitate gestures as they appear from their own perspective, leading to the reversed palm in the example of a wave gesture described above. Building on this work, we have shown that native-signing children with ASD also produce similar palm reversal errors in their productions of sign language (Shield & Meier, 2012).
The crucial difference between sign and speech is that an understanding of self in relation to other is necessary for selecting the correct pronominal form in speech, but not in sign. In speech, a child must understand that when a parent says you it refers to the child, but when the child says you it does not. By contrast, sign pronouns point in the direction of their referents. Thus, a child who is addressed by her parent with the sign you could merely reproduce the sign as it appears from her perspective (i.e., with the tip of the index finger directed to the child), and the result would be the sign me. In this way, pronominal reference can be maintained in sign even without a fully-developed understanding of the relation between self and other, or of the related self-other mapping processes entailed in gesture imitation and sign learning.
It is plausible, therefore, that the same underlying deficit (in the understanding of the relationship between self and other) results in two different modality-dependent linguistic phenomena: pronoun reversals in speech, and reversals of the palm in sign. This hypothesis should be tested in future work, particularly on younger signing children with ASD. If this turns out to be the case, then there are important implications for clinical practice. Clinicians who work with signing children with ASD should be on the lookout for palm reversals rather than pronoun reversals, and current screening and diagnostic instruments that focus on pronoun reversals as a criterion for ASD risk must be rethought when used with signing children. Regardless, however, of whether or not signing children produce frequent pronoun reversals, clinicians can expect that they will sometimes avoid sign pronouns in favor of name signs.
Conclusion
We have reported the results of a novel study on an under-examined research population, deaf children with ASD who have been exposed to a sign language from birth by their Deaf parents. Research with these children provides an opportunity to study how language deficits in ASD manifest in the visual-gestural modality. Note that although sign has often been employed as an augmentative communication system with hearing children with severe ASD (Schlosser & Wendt, 2008), six sign-exposed children with ASD could not participate in our tasks because of a lack of expressive language. Clinicians should expect to encounter minimally-verbal children with ASD, even when those children are being reared by parents who use a sign language as their primary language.
Pronouns in sign are qualitatively different from pronouns in speech in that they transparently pick out their referents. Despite such transparency, deaf children with ASD performed identically to hearing children with ASD using a very similar task. The deaf children with ASD in our study tended to produce sign names instead of sign pronouns when they were asked to identify a photograph of themselves or of their interlocutor (the experimenter). This finding helps us understand that it cannot be the arbitrary nature of spoken language pronouns that impedes their use by hearing children with ASD. We believe that a difference in self-representation in combination with language delay may be at the root of this phenomenon. Future work with larger samples of deaf children with ASD will help us to identify the separate contributions of these two factors to the use and non-use of pronouns by these children. Studies of deaf, native-signing children with ASD will shed new light on the nature of cognitive and linguistic abilities in ASD.
Acknowledgments
The authors thank J. Pyers and R. Hoffmeister for help with the study design, S. Butler and D. Mood for conducting the ADOS evaluations, B. Makofske for evaluating clinical impression, T. Sampson and M. Gandhi for coding data, A. Marks for taking photos, F. Ramont for modeling signs, and the schools, parents, administrators, teachers, and children who made this research possible. This study was supported by a postdoctoral fellowship to the first author from the National Institute on Deafness and Other Communication Disorders (grant number #F32-DC011219) and a research enhancement grant from the Autism Science Foundation to the first author.
Appendix
Modifications to the ADOS-2 administered to deaf participants
In order to administer the ADOS-2 to children who are deaf and communicate using ASL, several modifications were made. The test authors (Lord et al., 2012) clearly indicate that the ADOS-2 is not intended to be used with children with hearing loss. However, the ADOS-2 is commonly used in clinical practice among professionals trained in working with children who are deaf and hard of hearing, in order to gather information regarding social communication and behavior in a semi-structured format. Currently, there are no published best-practice guidelines regarding appropriate modifications to the ADOS-2 when used with this population. For the purposes of this study, modifications were made based on the examiner's clinical experience. Where possible, every effort was made to adhere as closely as possible to standardized test procedures. Otherwise, modifications that were made were intended to be consistent across subjects. The following describes the modifications that were made regarding 1) module selection; 2) task selection and administration; and 3) scoring.
Module selection
In order to administer the ADOS-2, a module based on the child's language level must be chosen. It is standard practice in choosing a module to count signs as gestures rather than words. However, doing so would underestimate the language ability of deaf, signing children. Therefore, for the purposes of this study, children's use of ASL signs was considered equivalent to spoken language and were not scored as gestures (e.g., two to three signs paired together were considered equivalent to spoken “phrase speech”; combining two thoughts through complex signed phrases was considered equivalent to “fluent speech”). Module selection was therefore based on the examinee's fluency in sign language (e.g., an examinee communicating only in sign language using complex signed phrases was administered a Module 3, rather than a Module 1 as would be indicated if signs were considered equivalent to gestures).
Task administration
Due to differences between the modalities of sign and speech, it was also necessary to modify several tasks. Directions for all tasks were translated into ASL. The Response to Name task was administered with modifications. The examiner first presented the examinee's name sign within their peripheral vision (three times). If the examinee did not respond, attention-getting procedures not involving touch typically used within Deaf culture were administered (e.g., tapping on the ground, waving within the individual's line of sight). If the examinee did not respond, standardized directions for attempting to get the examinee's attention first by implying they would be tickled and then by tickling them were administered (either by the examiner or a parent, if available).
An effort was made to adhere to standardized procedures for administering Joint Attention. Modifications to standardized statements included using the sign SEE or LOOK + a head turn, without the directional element of that sign, during initial presses. The sign SEE was then paired with a point on the last press.
Demonstration Task was modified by fingerspelling elements of directions rather than using signs which were iconic in nature and/or providing an alternative task such as making a bowl of cereal. When it was necessary to administer Anticipation of Social Routine to older children with limited language, the peekaboo task was modified to be more age-appropriate. Signed instructions for Functional Symbolic Imitation were modified incorporating appropriate ASL classifiers to maintain the task's intention (e.g., airplane + 5 handshape in a forward, flying motion).
Scoring
Modifications to codes and scoring algorithms were also necessary. For the purpose of this paper, only modified codes are reported and only modifications affecting scoring algorithms are reported in the chart below:
Table A1.
Modifications to scoring on the ADOS-2
| Item | Modification |
|---|---|
| Frequency of spontaneous vocalization directed toward others | Interpreted as frequency of spontaneous signing or vocalizations (if any) directed toward others. |
| Gestures | Pointing was considered a conventional gesture; descriptive gestures were scored if ASL classifiers or other hand shapes/movements were used in a descriptive way (e.g., to describe the shape of a mirror); lexical signs were not counted as gestures (e.g., blue, dog) even when there was a descriptive quality about them (e.g., the generic sign big would not be considered a descriptive gesture necessarily, but using other classifiers to more precisely indicate the size of an object may be counted as descriptive gesture use). |
| Facial Expressions Directed Toward Others | Emotional facial expressions were scored based only on communication of affect rather than ASL linguistic features (e.g., furrowing brow when asking a question, use of facial features to denote topic identification, etc. were not scored as affective expression unless also paired with emotion signs such as puzzled + puzzled expression). |
| Spontaneous Initiation of Joint Attention | Followed general scoring procedures but also took into consideration cultural norms for initiating joint attention such as signing within the examiner/parent's line of sight between the parent and object of interest. Signing within parent's visual space without eye contact was scored as 1. |
| Intonation of Vocalizations/Verbalizations | Scored as 8 with notations made to assist in interpretation regarding ASL fluency attending to difficulties with rate, position/location of signs, mixed hand dominance. |
| Idiosyncratic/Stereotyped language | Scored for frequency of the occurrence of any of the following: scripted/overly formal language, palm rotation errors, persistent use of a gesture when a known sign was within the examinee's repertoire or exposure (based on parent/teacher report), unusual pronoun use, presence of non-meaningful signs/fingerspelling. |
Footnotes
Exceptions are the ASL signs we and our (Meier, 1990).
As is conventional, we denote ASL signs with their English translations in small caps.
The linguistic status of personal pronouns in ASL and other signed languages has been a matter of continuing controversy (Cormier, Schembri, & Woll, 2013; McBurney, 2002; Meier, 1990; Meier & Lillo-Martin, 2010). However, ASL unequivocally has points to self and points to addressee; for the purpose of this paper we label these points as first- and second-person pronouns.
Signed English is a system of manual signs that follows English grammar and thus is not considered an independent language.
We use non-parametric tests throughout this paper because assumptions of normality are violated.
We use Cohen's d as a measure of effect size when comparing two means; a value greater than 0.8 typically represents a large effect.
A sign that functions as a unique name for a person, often invented by Deaf parents (Supalla, 1992).
We also analyzed whether the group differences were significant in terms of who responded with names and who did not (rather than who produced pronouns and who did not), since some children produced both in their answer. The group difference was again significant under this criterion for both the first-person task (Fisher's Exact Test, p <.05, one-tailed) and the second-person task (Fisher's Exact Test, p <.0001, one-tailed).
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Asendorpf JB. Self-awareness, other-awareness, and secondary representation. In: Meltzoff AN, Prinz W, editors. The imitative mind: Development, evolution, and brain bases. Cambridge studies in cognitive perceptual development. Cambridge University Press; New York: 2002. pp. 63–73. [Google Scholar]
- Baron-Cohen S. Perceptual role taking and protodeclarative pointing in autism. British Journal of Developmental Psychology. 1989;7:113–127. [Google Scholar]
- Bates E, Dick F. Language, gesture, and the developing brain. Developmental Psychobiology. 2002;40:293–310. doi: 10.1002/dev.10034. [DOI] [PubMed] [Google Scholar]
- Beykirch HL, Holcomb TA, Harrington JF. Iconicity and sign vocabulary acquisition. American Annals of the Deaf. 1990;135:306–11. doi: 10.1353/aad.2012.0563. [DOI] [PubMed] [Google Scholar]
- Bischof-Kohler D. Self-objectification and other-oriented emotions: Self-recognition, empathy, and prosocial behavior in the second year. Zeitschrift Fur Psychologie. 1994;202:349–77. [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence. Fourth Edition Pro-Ed; Austin, TX: 2010. [Google Scholar]
- Camaioni L, Perucchini P, Muratori F, Milone A. A longitudinal examination of the communicative gestures deficit in young children with autism. Journal of Autism and Developmental Disorders. 1997;27:715–725. doi: 10.1023/a:1025858917000. [DOI] [PubMed] [Google Scholar]
- Camaioni L, Perucchini P, Muratori F, Parrinini B, Cesari A. The communicative use of pointing in autism: Developmental profile and factors related to change. European Psychiatry. 2003;18:6–12. doi: 10.1016/s0924-9338(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Carmody DP, Lewis M. Self representation in children with and without autism spectrum disorders. Child Psychiatry and Human Development. 2012;43:227–37. doi: 10.1007/s10578-011-0261-2. [DOI] [PubMed] [Google Scholar]
- Casey S. Relationships between gestures and signed languages: Indicating participants in action. In: Baker A, van den Bogaerde B, Crasborn O, editors. Cross-linguistic perspectives in sign language research: Selected papers from TISLR 2000. Signum; Hamburg: 2003. pp. 95–117. [Google Scholar]
- Charney R. Pronoun errors in autistic children: Support for a social explanation. International Journal of Language & Communication Disorders. 1980;15:39–43. doi: 10.3109/13682828009011369. [DOI] [PubMed] [Google Scholar]
- Chiat S. If I were you and you were me: The analysis of pronouns in a pronoun-reversing child. Journal of Child Language. 1982;9:359–379. doi: 10.1017/s0305000900004761. [DOI] [PubMed] [Google Scholar]
- Clark EV. From gesture to word: On the natural history of deixis in language acquisition. In: Bruner JS, Garton A, editors. Human growth and development: Wolfson College lectures 1976. Oxford University Press; Oxford: 1978. pp. 85–120. [Google Scholar]
- Cormier K, Schembri A, Woll B. Pronouns and pointing in sign languages. Lingua. 2013;137:230–47. [Google Scholar]
- Enns CJ, Zimmer K, Boudreault P, Rabu S, Broszeit C. American Sign Language: Receptive Skills Test. Northern Signs Research, Inc.; Winnipeg, MB: 2013. [Google Scholar]
- Evans KE, Demuth K. Individual differences in pronoun reversal: Evidence from two longitudinal case studies. Journal of Child Language. 2012;39:162–91. doi: 10.1017/S0305000911000043. [DOI] [PubMed] [Google Scholar]
- Hatzopoulou M. Acquisition of reference to self and others in Greek Sign Language (Stockholm University, 2008). Sign Language & Linguistics. 2010;13:83–91. [Google Scholar]
- Hoza J. The discourse and politeness functions of HEY and WELL in American Sign Language. In: Roy CB, editor. Discourse in signed languages. Gallaudet University Press; Washington, DC: 2011. pp. 69–95. [Google Scholar]
- Jackson CA. Language acquisition in two modalities: The role of nonlinguistic cues in linguistic mastery. Sign Language Studies. 1989;62:1–22. [Google Scholar]
- Johnston T. Towards a comparative semiotics of pointing actions in signed andspoken languages. Gesture. 2013;13:109–142. [Google Scholar]
- Jordan R. An experimental comparison of the understanding and use of speaker-addressee personal pronouns in autistic children. British Journal of Disorders of Communication. 1989;24:169–179. doi: 10.3109/13682828909011954. [DOI] [PubMed] [Google Scholar]
- Jure R, Rapin I, Tuchman R. Hearing-impaired autistic children. Developmental Medicine and Child Neurology. 1991;33:1062–1072. doi: 10.1111/j.1469-8749.1991.tb14828.x. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Klima ES, Bellugi U. The signs of language. Harvard University Press; Cambridge, MA: 1979. [Google Scholar]
- Konstantareas MM, Oxman J, Webster CD. The representational and information-processing foundations of linguistic functioning in autistic children. Neurolinguistics. 1982;11:93–123. [Google Scholar]
- Lee A, Hobson RP, Chiat S. I, you, me, and autism: An experimental study. Journal of Autism and Developmental Disorders. 1994;24:155–176. doi: 10.1007/BF02172094. [DOI] [PubMed] [Google Scholar]
- Lewis M, Brooks-Gunn J. Social cognition and the acquisition of self. Plenum Press; New York: 1979. [Google Scholar]
- Lewis M, Ramsay D. Development of self-recognition, personal pronoun use, and pretend play during the 2nd year. Child Development. 2004;75:1821–31. doi: 10.1111/j.1467-8624.2004.00819.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Western Psychological Services; Torrance, CA: 2012. [Google Scholar]
- Mayberry RI, Eichen EB. The long-lasting advantage of learning sign language in childhood: Another look at the critical period for language acquisition. Journal of Memory and Language. 1991;30:486–512. [Google Scholar]
- Mayberry RI, Lock E, Kazmi H. Development: Linguistic ability and early language exposure. Nature. 2002;417:38. doi: 10.1038/417038a. [DOI] [PubMed] [Google Scholar]
- McBurney S. Pronominal reference in signed and spoken language: Are grammatical categories modality-dependent? In: Meier RP, Cormier K, Quinto-Pozos D, editors. Modality and structure in signed and spoken languages. Cambridge University Press; Cambridge: 2002. pp. 329–69. [Google Scholar]
- Meier RP. Icons, analogues and morphemes: The acquisition of verb agreement in ASL (Doctoral Dissertation) University of California; San Diego: 1982. [Google Scholar]
- Meier RP. Elicited imitation of verb agreement in American Sign Language: Iconically or morphologically determined? Journal of Memory and Language. 1987;26(3):362–376. [Google Scholar]
- Meier RP. Person deixis in American Sign Language. In: Fischer SD, Siple P, editors. Theoretical issues in sign language research, Vol. 1: Linguistics. University of Chicago Press; Chicago: 1990. pp. 175–90. [Google Scholar]
- Meier RP. The acquisition of verb agreement: Pointing out arguments for the linguistic status of agreement in signed languages. In: Morgan G, Woll B, editors. Directions in sign language acquisition. John Benjamins; Amsterdam: 2002. pp. 115–141. [Google Scholar]
- Meier RP, Lillo-Martin D. Does spatial make it special? On the grammar of pointing signs in American Sign Language. In: Gerdts DB, Moore J, Polinsky M, editors. Hypothesis A/Hypothesis B: Linguistic Explorations in Honor of David M. Perlmutter. MIT Press; Cambridge, MA: 2010. pp. 345–360. [Google Scholar]
- Meinzen-Derr J, Wiley S, Bishop S, Manning-Courtney P, Choo DI, Murray D. Autism spectrum disorders in 24 children who are deaf or hard of hearing. International Journal of Pediatric Otorhinolaryngology. 2014;78:112–118. doi: 10.1016/j.ijporl.2013.10.065. [DOI] [PubMed] [Google Scholar]
- Morgan G, Barriere I, Woll B. The Influence of typology and modality on the acquisition of verb agreement morphology in British Sign Language. First Language. 2006;26:19–43. [Google Scholar]
- Newport EL, Meier RP. The acquisition of American Sign Language. In: Slobin DI, editor. The Crosslinguistic Study of Language Acquisition. Volume 1: The Data. Erlbaum; Hillsdale, NJ: 1985. pp. 881–938. [Google Scholar]
- Ohta M. Cognitive disorders of infantile autism: A study employing the WISC, spatial relationship conceptualization, and gesture imitations. Journal of Autism and Developmental Disorders. 1987;17:45–62. doi: 10.1007/BF01487259. [DOI] [PubMed] [Google Scholar]
- Oshima-Takane Y. Analysis of pronominal errors: A case-study. Journal of Child Language. 1992;19:111–131. doi: 10.1017/s0305000900013659. [DOI] [PubMed] [Google Scholar]
- Oshima-Takane Y, Benaroya S. An alternative view of pronominal errors in autistic children. Journal of Autism and Developmental Disorders. 1989;19:73–85. doi: 10.1007/BF02212719. [DOI] [PubMed] [Google Scholar]
- Padden C. Interaction of morphology and syntax in American Sign Language (Doctoral Dissertation) University of California; San Diego: 1983. [Google Scholar]
- Petitto LA. From gesture to symbol: The relationship between form and meaning in the acquisition of personal pronouns in American Sign Language. Papers and Reports on Child Language Development. 1983;22:100–07. [Google Scholar]
- Petitto LA. On the autonomy of language and gesture: Evidence from the acquisition of personal pronouns in American Sign Language. Cognition. 1987;27:1–52. doi: 10.1016/0010-0277(87)90034-5. [DOI] [PubMed] [Google Scholar]
- Pizzuto E. The early development of deixis in American Sign Language: What is the point? In: Volterra V, Erting C, editors. From gesture to language in hearing and deaf children. Gallaudet University Press; Washington, DC: 1990. pp. 142–162. [Google Scholar]
- Ritvo ER, Ritvo R, Freeman BJ, Mason-Brothers A. Clinical characteristics of mild autism in adults. Comprehensive Psychiatry. 1994;35:149–56. doi: 10.1016/0010-440x(94)90061-l. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. [Google Scholar]
- Roper L, Arnold P, Monteiro B. Co-occurrence of autism and deafness: Diagnostic considerations. Autism. 2003;7:245–253. doi: 10.1177/1362361303007003002. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Schick B, de Villiers P, de Villiers J, Hoffmeister R. Language and theory of mind: A study of deaf children. Child Development. 2007;78:376–396. doi: 10.1111/j.1467-8624.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Schiff-Myers NB. From pronoun reversals to correct pronoun usage: A case study of a normally developing child. Journal of Speech and Hearing Disorders. 1983;48:394–402. doi: 10.1044/jshd.4804.394. [DOI] [PubMed] [Google Scholar]
- Schlosser RW, Wendt O. Effects of Augmentative and Alternative Communication Intervention on Speech Production in Children With Autism: A Systematic Review. American Journal of Speech-Language Pathology. 2008;17:212. doi: 10.1044/1058-0360(2008/021). [DOI] [PubMed] [Google Scholar]
- Smiley PA, Chang LK, Allhoff AK. Can Toddy give me an orange? Parent input and young children's production of I and You. Language Learning & Development. 2011;7:77–106. [Google Scholar]
- Smith IM. Gesture imitation in autism I: Nonsymbolic postures and sequences. Cognitive Neuropsychology. 1998;15:747–770. doi: 10.1080/026432998381087. [DOI] [PubMed] [Google Scholar]
- Supalla SJ. The book of name signs: Naming in American Sign Language. DawnSignPress; San Diego, CA: 1992. [Google Scholar]
- Shield A, Meier RP. Palm reversal errors in native-signing children with autism. Journal of Communication Disorders. 2012;45:439–54. doi: 10.1016/j.jcomdis.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. Dissociations in form and function in the acquisition of language by autistic children. In: Tager-Flusberg H, editor. Constraints on language acquisition: Studies of atypical children. Lawrence Erlbaum Associates; Hillsdale, NJ: 1994. pp. 175–194. [Google Scholar]
- Tager-Flusberg H, Kasari C. Minimally verbal school-aged children with autism spectrum disorder: The neglected end of the spectrum. Autism Research. 2013;6:468–478. doi: 10.1002/aur.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RL, Vinson DP, Vigliocco G. The link between form and meaning in American Sign Language: Lexical processing effects. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:550–557. doi: 10.1037/a0014547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson DP, Cormier K, Denmark T, Schembri A, Vigliocco G. The British Sign Language (BSL) norms for age of acquisition, familiarity, and iconicity. Behavioral Research Methods. 2008;40:1079–87. doi: 10.3758/BRM.40.4.1079. [DOI] [PubMed] [Google Scholar]
- Whiten A, Brown J. Imitation and the reading of other minds: Perspectives from the study of autism, normal children and non-human primates. In: Bråten S, editor. Intersubjective communication and emotion in early ontogeny. Cambridge University Press; Cambridge: 1998. pp. 260–280. [Google Scholar]