Summary
Motor behaviors recruit task-specific neuronal ensembles in motor cortices, which are consolidated over subsequent learning. However, little is known about the molecules that can identify the participating neurons and predict the outcomes of the consolidation process. Using a mouse rotarod-learning task, we showed that lesion or inactivation of the secondary motor (M2) cortex disrupts learning of skilled movements. We tracked the endogenous promoter activity of the neuronal activity-regulated gene Arc in individual M2 neurons during rotarod learning by in vivo two-photon imaging of a knock-in reporter. We found that task training initially recruits Arc-promoter-activated neurons and then consolidates them into a specific ensemble exhibiting persistent reactivation of Arc-promoter. The intensity of a neuron’s initial Arc-promoter activation predicts its reactivation probability and neurons with weak initial Arc-promoter activation are dismissed from the ensemble during subsequent training. Our findings demonstrate a task-specific Arc-dependent cellular consolidation process in M2 cortex during motor learning.
INTRODUCTION
Motor learning involves coordinated activities in motor cortical neuronal ensembles. Activities in these ensembles are recruited by specific motor behaviors and consolidated over the course of motor learning (Dayan and Cohen, 2011; Nicolelis and Lebedev, 2009; Shmuelof and Krakauer, 2011). This consolidation process is characterized by the dismissal or retention of task-related activities in neurons and is correlated with the organism’s ability to acquire and retain new motor skills (Costa et al., 2004; Huber et al., 2012; Peters et al., 2014). However, little is known about the molecules that can identify the participating neurons in the task-specific ensembles and predict the outcomes of the cellular consolidation process during motor learning.
The activity-regulated cytoskeletal-associated protein Arc/Arg3.1 (Bramham et al., 2008; Shepherd and Bear, 2011) is a strong candidate molecule to study in the motor learning-induced cellular consolidation process. Arc expression is induced by motor learning in motor cortical neurons (Hosp et al., 2013; Ren et al., 2014), and application of protein synthesis inhibitors to motor cortex (Dayan and Cohen, 2011; Kleim et al., 2003; Luft et al., 2004) or genetic knockout of Arc (Ren et al., 2014) disrupts long-term motor learning behavior. However, the cellular process by which Arc is involved in motor learning remains unknown. The Arc-GFP knock-in mouse line, in which a destabilized GFP-coding region replaces the endogenous Arc-coding region (Wang et al., 2006), offers an opportunity to track endogenous Arc-promoter activation in individual neurons over multiple days by in vivo two-photon imaging. This approach will allow us to assess whether neurons with Arc-promoter activation are specifically recruited and consolidated during motor learning.
The accelerating rotarod task is a commonly used rodent motor learning paradigm (Costa et al., 2004; Rothwell et al., 2014; Yang et al., 2009), in which animals learn skilled stepping movements on a rotating rod over the course of multiple training days (Buitrago et al., 2004; Farr et al., 2006; Rothwell et al., 2014). This task robustly activates neurons in motor cortical areas and increases the level of Arc expression (Costa et al., 2004; Ren et al., 2014). Therefore, this rotarod training task is well suited for the study of neuronal recruitment and consolidation in motor learning.
Here, we used heterozygous Arc-GFP mice to examine Arc-promoter activation patterns in motor cortex following rotarod training. We found that rotarod training recruits more Arc-promoter-activated neurons in the secondary motor (M2) cortex compared to the primary motor (M1) cortex, and that M2 function is needed for learning skilled stepping movements on the rotarod. We then tracked Arc-promoter activation-defined M2 neuronal ensembles by in vivo two-photon imaging over multiple days of motor behaviors. Our findings demonstrate a cellular process by which motor learning consolidates task-specific neuronal ensembles and identify Arc as a critical molecule in this process.
RESULTS
Initial rotarod training preferentially recruits Arc-promoter-activated neurons in M2 cortex
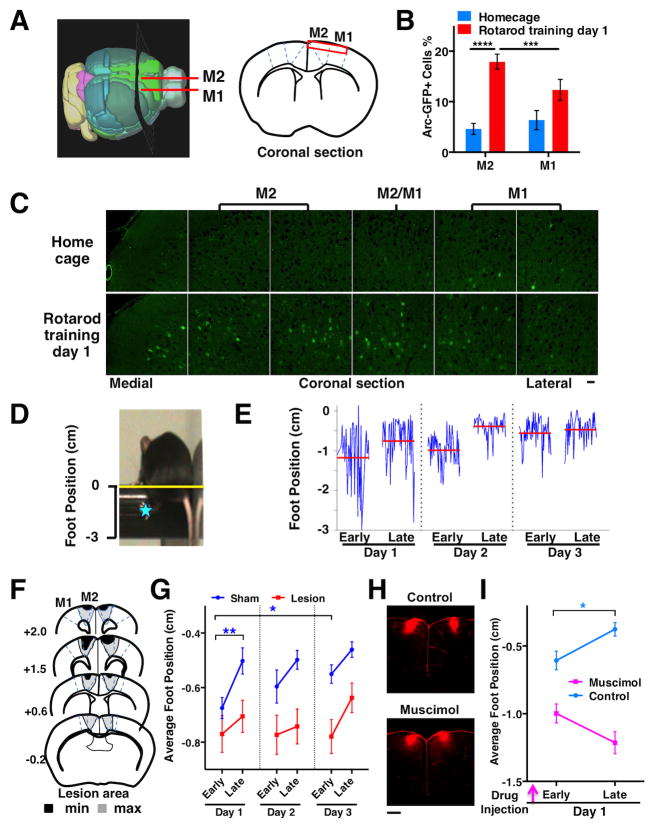
We first investigated whether initial rotarod training will activate Arc-promoter in neurons of motor cortices. We compared Arc-GFP expression in fixed brain sections from Arc-GFP heterozygous mice in the homecage to that after one session of rotarod training (Figure 1A–C). Under the homecage condition, the percentage of neurons showing Arc-promoter activation is low and comparable between M1 and M2 (P=0.25, N=6 mice). After rotarod training (N=6 mice), the percentage of M2 neurons with Arc-promoter activation increases significantly compared to the home cage condition (P<0.0001), whereas the change in M1 is less significant (P=0.042). An interaction effect analysis further supports this finding (two-way RM ANOVA, region-by-behavior interaction, F(1,10)=23.36, P=0.0007; rotarod M2 vs. M1, P=0.0008), suggesting that initial rotarod training recruits Arc-promoter-activated neurons preferentially in M2 cortex.
Figure 1. Rotarod training recruits neurons in M2 cortex and M2 function is required for learning skilled stepping movements.
(A) A 3D mouse brain model (Allen Brain Atlas) and coronal section schematic. The red rectangle indicates superficial layers of the motor cortical regions imaged in B and C. (B) Preferential activation of Arc-GFP by rotarod training in M2 compared to M1 (two-way RM-ANOVA, region-by-behavior, F(1,10)=23.36, P=0.0007; Rotarod M2 vs. M1, P=0.0008; M2 Rotarod vs. Homecage, P<0.0001; N=6 mice per condition). The percentage of GFP+ cells was estimated from the total number of GFP+ (green) and GFP− (dark) cells. (C) Confocal image montage of coronal sections from heterozygous Arc-GFP mice. Scale bars, 30 μm. (D) A video frame showing mouse performing the accelerating rotarod task. The distance from the left rear paw of the mouse (blue star) to the apex of the rod (yellow line) was measured. (E) Foot position traces (blue) of a normal mouse during Early and Late trials from 3 training days. The red lines indicate the average foot position in each trial. (F) Illustration of minimum and maximum extents of M2 surgical lesions: N=23 mice. (G) Average foot positions during Early and Late trials from sham and M2 lesion groups. Two-way RM ANOVA, lesion effect, F(1,44)=8.86, P=0.0047, N=23 mice per group. Short-term learning, Early vs. Late on Day 1: Sham P=0.0057, Lesion P=0.46. Long-term learning, Day 1 vs. Day 3 Early: Sham P=0.014, Lesion P=0.80. (H) Confocal images of coronal sections showing injection sites for muscimol inactivation of M2 cortex. Dextran-TRITC (red) was mixed in ACSF and injected into the M2 cortex in the presence or absence of muscimol (0.5μl, 1μg/μl) 45 min before rotarod training. Scale bar, 500 μm. (I) Average foot positions during Early and Late trials on training Day 1 with muscimol inactivation. Two-way RM ANOVA, muscimol effect, F(1,15)=61.07, P<0.0001, N=11 mice for control and 10 mice for muscimol group. Early vs. Late: Control P=0.013, Muscimol P=0.11. Error bars indicate SEM.
M2 Cortex has been reported to receive multiple inputs that may provide somatosensory and spatial information for movement planning (Hoover and Vertes, 2007; Reep and Corwin, 2009; Uylings et al., 2003) (Figure S1A–D). The strong activation of Arc-promoter in M2 cortex, along with the fact that little is known about the function and plasticity of this brain region during rotarod training, led us to focus our subsequent experiments in M2 cortex.
Functional involvement of M2 cortex in rotarod training
To examine the role of M2 cortex during rotarod training, we compared the behavioral outcomes in the presence or absence of a functioning M2. Video-based movement analyses were used to characterize the behavioral outcomes in rotarod training (Buitrago et al., 2004; Farr et al., 2006; Rothwell et al., 2014). The motor performance level is indicated by the average position of the left hind foot relative to the rotarod apex during a trial (Figure 1D). Short-term motor learning is indicated by performance improvement (upward shift of average foot position towards rod apex) between early and late trials in a single day; long-term motor learning is indicated by performance improvement between early trials conducted on multiple days (Figure 1E).
To assess whether M2 is required for rotarod motor performance and learning, we performed surgical excision of M2 in wild-type mice (Figure 1F). After a two-week post-surgery recovery, we conducted three days of rotarod training on the lesioned and sham mice (N=23 mice per group). During early trials on day 1, the average foot position of the lesioned mice is not different from that of the sham mice (Figure 1G, P=0.22), suggesting that their initial motor performance levels are comparable. However, unlike the sham mice, the lesioned mice do not show upward shifts of their foot positions between early and late trials on day 1 (P=0.46) or between early trials on day 1 and 3 (P=0.80), suggesting that both short-term and long-term learning are impaired in the chronic M2-lesioned mice.
In addition, we assessed whether acute inactivation of M2 may affect rotarod motor performance and short-term learning. We injected the GABA receptor agonist muscimol into M2 of wild-type mice (Figure 1H). Approximately 45 minutes after drug injection, we tested rotarod motor performance and learning in the muscimol-injected mice (N=10) and vehicle control mice (N=11). During early trials, the foot position of the muscimol-injected mice is lower than that of the control mice (Figure 1I, P=0.01), suggesting that the initial motor performance level is reduced in the muscimol-injected mice. However, the muscimol-injected mice also do not show any upward shifts of their foot positions between early and late trials (Figure 1G, P=0.11), suggesting that short-term motor learning is disrupted in these mice.
The initial motor performance is reduced in the acute M2-inactivated mice compared to the control mice, yet it is comparable between the chronic M2-lesioned mice and the sham mice. These findings suggest that M2 cortex function may be involved in the motor performance of normal mice, but its role is not essential and may be compensated for after a chronic lesion (see additional analyses in Figure S1E–H). In contrast, both acute inactivation and chronic lesion of M2 cortex disrupt the learning of skilled stepping movements, suggesting that M2 cortex function is required for motor skill learning during rotarod training.
M2 neuronal ensembles identified by Arc-promoter activation are consolidated in subsequent days of rotarod training
We next studied how the initially recruited Arc-promoter-activated neurons in M2 cortex change during subsequent motor learning. We used in vivo two-photon microscopy to track Arc-GFP fluorescence (Cao et al., 2013) in the M2 cortical region where peak Arc-GFP activation had been previously observed (Figures 1C, S1C). A 3D image stack was first acquired under the baseline homecage condition for all Arc-GFP heterozygous mice. On each of the three following days, approximately half of the animals were exposed to the rotarod training (R) condition, whereas the other animals remained under the homecage (H) condition. A 3D image stack was acquired daily at the same coordinates either after 2-hours of rotarod training or under the homecage condition (Figure 2A).
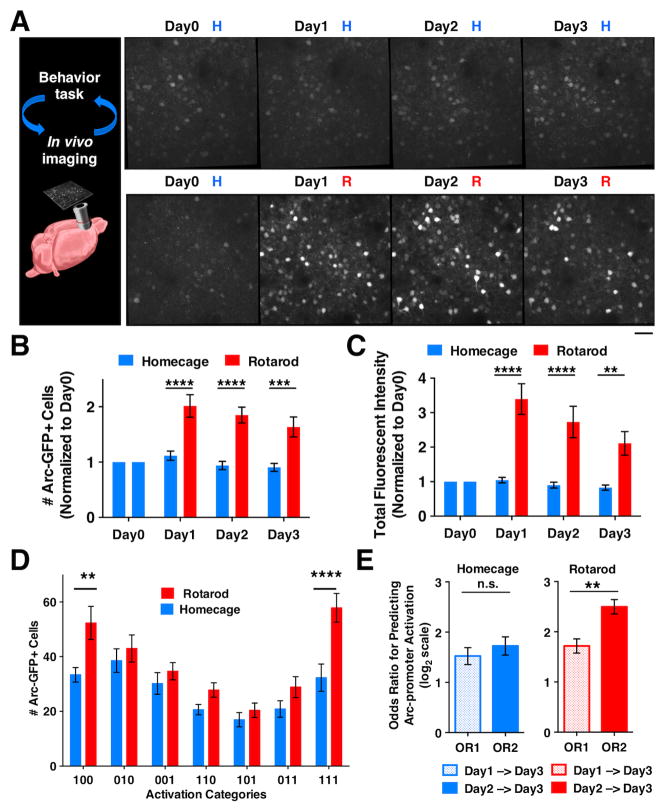
Figure 2. M2 neuronal ensembles identified by Arc-promoter activation are consolidated in subsequent days of rotarod training.
(A) In vivo two-photon images showing Arc-GFP expression in superficial layer (~180 μm from pia) M2 neurons of a heterozygous Arc-GFP mouse under the repeated homecage (H) condition, and another mouse under the repeated rotarod (R) training condition. Scale bar 30μm. (B) The number of Arc-GFP+ neurons (normalized to the level on Day 0) in homecage (N=11) and rotarod trained (N=10) mice. Two-way RM ANOVA, rotarod effect, F(1,19)=28.92, P<0.0001; H vs R: Day 1 P<0.0001; Day 2 P<0.0001, Day 3 P=0.0002. (C) Total fluorescence intensity of matched cell regions (normalized to the level on Day 0) in homecage and rotarod trained mice. Two-way RM ANOVA, rotarod effect, F(1,19)=25.66, P<0.0001; H vs R: Day 1 P<0.0001, Day 2 P<0.0001, Day 3 P=0.0025. (D) The number of Arc-GFP+ neurons in each of the 3-day activation categories under the Homecage (HHH) and Rotarod (RRR) conditions. Two-way RM ANOVA, condition-by-category, F(6,174)=2.985, P=0.0084; H vs. R: “100”, P=0.004; “111”, P<0.0001). (E) Odds ratios (OR) for predicting Arc-promoter activation. Under the homecage condition, day-1 (OR1) and day-2 (OR2) Arc-promoter activation have similar effectiveness in predicting the reactivation on day 3 (paired t-test, P=0.280, N=16 mice). Under the rotarod condition, day-2 Arc-promoter activation is more effective than day-1 activation in predicting the reactivation on day 3 (paired t-test, P=0.0063, N=15 mice). Error bars indicate SEM.
Under the homecage condition, the number of Arc-GFP+ neurons remained low and stable on all four testing days; whereas on all three days under the rotarod training condition, the number of Arc-GFP+ neurons increased significantly in comparison to the homecage condition (Figure 2B, two-way RM ANOVA, rotarod effect, F(1,19)=28.92, P<0.0001). We also determined the intensity of Arc-promoter activation in M2 neurons by measuring the green fluorescent intensity of individual neurons (normalized by the value of tissue autofluorescence, Figure S2A–B), and found a consistent increase on each of the three days under the rotarod condition (Figure 2C, two-way RM ANOVA, rotarod effect, F(1,19)=25.66, P<0.0001). This continued increase is not due to long-lasting Arc-promoter activity after initial activation, as the half-life for Arc-promoter activation is much shorter than a day (Shepherd and Bear, 2011; Wang et al., 2006) and Arc-GFP expression returns to the baseline homecage level in the absence of daily rotarod training (Figure S2C–D).
Next, we examined whether the initial rotarod-training-recruited neurons may become reactivated on subsequent training days. The activation pattern of an Arc-GFP+ neuron over three days can be represented by a 3-digit string composed of either 1’s (active) or 0’s (inactive) (Figure 2D). The neurons activated by the initial rotarod training on day 1 theoretically have four subsequent activation categories: “111”, “100”, “101”, and “110”. We found that the number of Arc-GFP+ neurons in the “111” and “100” categories under the rotarod training condition is significantly greater than that under the homecage condition, whereas the numbers of neurons in the other categories are comparable between the two behavioral conditions (two-way RM ANOVA, condition-by-category interaction, F(6,174)=2.985, P=0.0084; H vs. R: “111”, P<0.0001; “100”, P=0.004). This selective increase of neurons suggests that the initial rotarod-training-recruited neurons are predominantly consolidated into the persistently reactivated (“111”) category and the subsequently dismissed (“100”) category (see additional analyses in Figure S2E–F).
We next determined to what extent Arc-promoter activation in a neuron on day 1 or day 2 would predict its likelihood of reactivation on day 3 using odds ratio (OR) analysis, where OR2 indicates the predictive effect of day-2 Arc-promoter activation and OR1 indicates the effect of day-1 activation (see Supplemental Experimental Procedures). Under the homecage condition, OR1 is comparable to OR2 (Figure 2E, P=0.28, N=16 mice), indicating that day-1 and day-2 Arc-promoter activation have similar effectiveness in predicting the reactivation on day 3. However, under the rotarod training condition, OR2 is significantly greater than OR1 (Figure 2E, P=0.0063, N=15 mice), indicating that day-2 Arc-promoter activation is more effective than day-1 activation in predicting the reactivation on day 3. The increased predictive effect on the second day of rotarod training provides quantitative evidence of ensemble consolidation under the rotarod training condition but not the homecage condition.
Task-specific recruitment and consolidation of neuronal ensembles defined by Arc-promoter activation
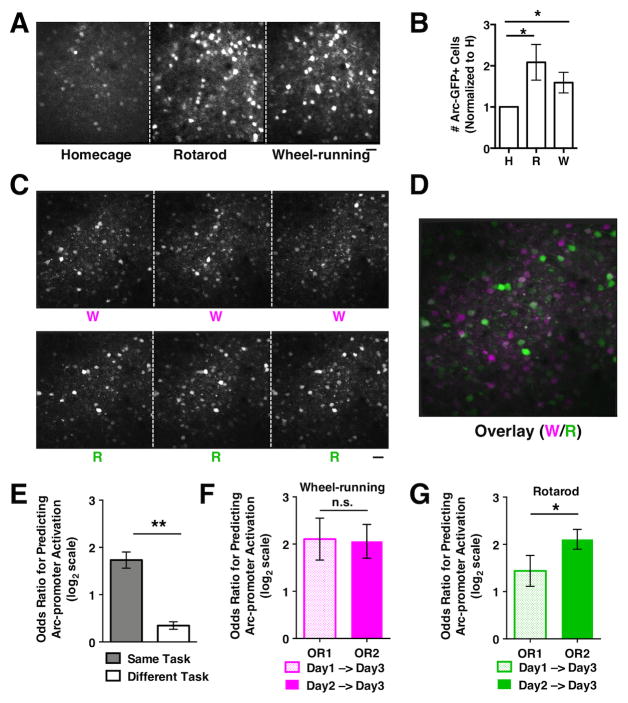
Next, we examined whether a simple motor task such as free wheel running would be sufficient to induce the consolidation of Arc-promoter-activated neurons. We first tested whether free wheel running in a new environment for two hours would induce Arc-promoter activation. Arc-GFP heterozygous mice were exposed to the homecage (H) condition, the rotarod (R) training condition, and the wheel-running (W) condition over three days (Figure 3A). In vivo two-photon imaging shows that both wheel running and rotarod training increase the number of Arc-GFP+ neurons compared to the homecage condition (Figure 3B, R vs. H, P=0.040; W vs. H, P=0.046; N=9 mice), suggesting that both motor tasks recruit neuronal ensembles defined by Arc-promoter activation in M2 cortex.
Figure 3. Task-specific recruitment and consolidation of neuronal ensembles defined by Arc-promoter activation.
(A) In vivo two-photon images showing Arc-GFP expression in superficial layer M2 neurons of a mouse under consecutive days of homecage (H), rotarod (R), and wheel-running (W) conditions. (B) The numbers of Arc-GFP+ cells detected under each condition (normalized to H, paired t-test, R vs. H, P=0.040; W vs. H, P=0.046, N=9 mice). (C) In vivo two-photon images showing Arc-GFP expression in M2 neurons of a mouse under the repeated wheel-running (W) and rotarod-training (R) conditions. (D) Overlay of M2 images under the wheel-running (magenta) and rotarod-training (green) conditions. Cells that are preferentially activated in either condition appear as magenta or green, whereas cells that are equally activated by both conditions appear as white. (E) The odds ratio for predicting Arc-promoter activation under the same motor task (W-W, R-R) is significantly higher than that between the different motor tasks (W-R). Paired t-test, P=0.0012, N=5 mice. (F) Under the wheel-running condition, day-1 (OR1) and day-2 (OR2) Arc-promoter activation has similar effectiveness in predicting the reactivation on day 3 (paired t-test, P=0.924, N=5 mice). (G) Under the rotarod condition, day-2 Arc-promoter activation is more effective than day-1 activation in predicting the reactivation on day 3 (paired t-test P=0.042, N=5 mice). Scale bars, 30μm. Error bars indicate SEM.
To determine if Arc-promoter activation patterns are different between the rotarod training task and the wheel running task, we subjected the Arc-GFP heterozygous mice to three days of wheel running followed by three days of rotarod training (Figure 3C). An overlay of M2 cortical images acquired daily after the tasks shows distinct ensembles of Arc-GFP+ neurons recruited by rotarod training and wheel running (Figure 3D). The odds ratio for predicting Arc-promoter activation patterns under the same motor task (W-W, R-R) is significantly higher than that between the different motor tasks (W-R) (Figure 3E, P=0.0012, N=5 mice), further supporting the task specificity of Arc-promoter activation-defined neuronal ensembles (see Figure S3 for additional analyses).
To determine if wheel running induces the consolidation of Arc-promoter-activated neurons, we compared the predictive effects of day-1 (OR1) and day-2 (OR2) Arc-promoter activations on day-3 reactivation. We found that OR1 is comparable to OR2 in the wheel-running task (Figure 3F, P=0.924, N=5 mice), in contrast to the increasing trend observed during rotarod training (Figure 3G). These findings indicate that rotarod training, but not free wheel running, induces the consolidation of M2 neuronal ensembles defined by Arc-promoter activation, suggesting that this consolidation is related to motor learning, not just performance of a motor task.
The initial intensity of Arc-promoter activation in a neuron predicts its probability of dismissal or retention in the rotarod ensemble
We further examined whether Arc-GFP fluorescence intensity on rotarod training day 1 carries predictive information for cellular reactivation on subsequent training days. For all the cells activated on day 1, their reactivation patterns are classified into three categories: “Dismissed” if not reactivated on day 2 and 3, “Unstable” if reactivated only once on day 2 or 3, and “Retained” if reactivated on both day 2 and 3 (Figure 4A). These cells are sorted based on their initial fluorescent intensity on day 1 and divided into 5 bins, each containing 20% of the cells. The reactivation category probability is defined as the proportion of cells in each bin that exhibits “Dismissed”, “Unstable”, or “Retained” reactivation patterns. We found that the reactivation category probabilities are dependent on the fluorescent intensity of the initial Arc-GFP expression (Chi-square test, P<0.0001, 2376 neurons from 15 mice). As the cell intensity on day 1 increases, the probability for the “Dismissed” category decreases and the probability for the “Retained” category increases (Figure 4B). The probability for the “Unstable” category is relatively constant and peaks slightly at the medium level of cell intensity. Together, these data indicate that under rotarod training, neurons with relatively weak initial Arc-promoter activation are more likely to be dismissed from the M2 ensemble, whereas neurons with relatively strong initial Arc-promoter activation are more likely to be retained (Figure 4C). Therefore, the initial intensity of Arc-promoter activation in a neuron predicts its probability of dismissal or retention during the cellular consolidation process (Figure 4D).
Figure 4. The initial intensity of Arc-promoter activation in a neuron predicts its probability of dismissal or retention in the rotarod ensemble.
(A) Close-up images of individual M2 neurons showing their fluorescent intensity on rotarod training Day 1 and the classification of their reactivation categories on Day 2 and 3. Red arrowheads indicate additional “Retained” neurons that are near “Dismissed” or “Unstable” neurons. Scale bar, 10 μm. (B) The reactivation category probabilities are dependent on the fluorescent intensity of Day 1 Arc-GFP expression in cells (Chi-square test, P<0.0001, 2376 neurons from 15 mice). (C) Neurons with relatively weak initial Arc-promoter activation are more likely to be dismissed from the rotarod ensemble, whereas neurons with relatively strong initial Arc-promoter activation are more likely to be retained. (D) Diagram showing that the initial intensity of Arc-promoter activation in a neuron predicts its probability of dismissal or retention during the cellular consolidation process.
We also examined whether Arc protein function is required in the consolidation process, using the homozygous Arc-GFP knock-in mice that completely lack Arc protein production (Ren et al., 2014; Wang et al., 2006) but retain GFP induction under the control of Arc promoter (Figure S4A–C). Two-photon imaging of M2 cortex in the homozygous mice shows that the consolidation of GFP+ neuronal ensembles and the dismissal of weakly activated neurons are impaired (Figure S4D–E). The homozygous Arc-GFP mice also exhibit impairment of long-term learning of skilled stepping movements during rotarod training, in contrast to the heterozygous Arc-GFP and wild type mice (Figure S4F and G). Although these results do not pinpoint where Arc function is required for M2 ensemble consolidation and rotarod learning, they provide additional evidence that the consolidation of Arc-expressing M2 ensembles is associated with the learning of skilled movements during rotarod training.
DISCUSSION
Here we report that motor learning consolidates Arc-expressing neuronal ensembles in the secondary motor cortex. First, we have shown that M2 cortex is required for learning skilled stepping movements in the rotarod task. Second, we have tracked M2 neuronal ensembles defined by Arc-promoter activation during rotarod training and demonstrated task-specific recruitment and consolidation of these ensembles. Third, we have identified that the intensity of the initial Arc-promoter activation in a neuron predicts its probability of dismissal or retention during the cellular consolidation process.
Our results indicate that rotarod training preferentially activates Arc expression in M2 cortex and M2 function is required for learning skilled movements in this task. During rotarod training, animals learn anticipatory stepping movements and whole-body posture adjustments (Buitrago et al., 2004; Farr et al., 2006; Rothwell et al., 2014). Although the precise connectivity and function of M2 cortex in various motor behaviors are areas of continuing research (Brecht, 2011; Schneider et al., 2014), current evidence suggests that task-related somatosensory and spatial information may be relayed to M2 cortex via its inputs from posterior sensory and association cortices (Hoover and Vertes, 2007; Reep and Corwin, 2009; Uylings et al., 2003). In addition, M2 electrical stimulation has been reported to evoke movements of various body parts in anesthetized animals and coordinated whole-body motion in freely moving animals (Neafsey et al., 1986; Tennant et al., 2011; Yeomans and Tehovnik, 1988). Our findings that M2 cortex is preferentially activated by rotarod training and is important for learning coordinated skillful movements provide new evidence in support of a potentially more general role of this brain region in action planning and memory-guided motor behaviors (Erlich et al., 2011; Murakami et al., 2014), and suggest an entry point to dissect the underlying cellular mechanisms and molecular players.
Past behavioral and neurophysiological studies have shown that motor skill learning can be mediated by experience-driven changes in the same motor cortical circuits subserving the performance of a trained task (Dayan and Cohen, 2011). Our M2 lesion and inactivation studies suggest that M2 may be involved in both the performance and the learning of skilled stepping movements on the rotarod task, but is essential mainly for the motor skill learning aspect. When M2 was acutely inactivated, mice still performed the task, but showed lowered initial stepping position and failed to improve their stepping patterns through training. The chronic M2 lesion might have allowed enough time for functional compensation (Farr et al., 2006; Whishaw et al., 2003), such that the initial rotarod performance became comparable between the chronic M2-lesioned mice and the sham mice. However, despite the normal level of initial motor performance, M2-lesioned mice still failed to improve their stepping patterns through training, suggesting that M2 cortex function is essential for learning skilled stepping movements during rotarod training.
Our study further demonstrates a cellular process through which motor learning consolidates neuronal ensembles defined by Arc-promoter activation in M2 cortex. Microelectrode recording and calcium imaging studies have shown that motor behaviors recruit task-specific neuronal activities in motor cortical areas (Costa et al., 2004; Huber et al., 2012; Nicolelis and Lebedev, 2009). The activities of these neurons can change from a more variable to a more stable pattern over subsequent motor learning, suggesting that the task-related neuronal ensembles are consolidated (Costa et al., 2004; Peters et al., 2014). Although previous pharmacological and behavioral studies have shown that new protein synthesis is required in long-term motor learning (Dayan and Cohen, 2011; Kleim et al., 2003; Luft et al., 2004), the specific cellular and molecular processes that may mark the neuronal ensembles undergoing the consolidation process and predict the dismissal or retention of a neuron during motor learning have thus far remained unclear. Our in vivo imaging data provide a direct view of motor training-induced Arc-promoter activation in M2 neuronal ensembles during the course of motor learning, which enables us to begin uncovering the rules underlying the evolution of neuronal ensembles.
We have identified several principles in the recruitment and consolidation of Arc-expressing neuronal ensembles during motor learning. First, different motor tasks recruit distinctive neuronal ensembles with Arc-promoter activation. Second, motor learning consolidates the initially recruited neurons predominantly into a persistently reactivated category and a subsequently dismissed category. Third, the probability of neuronal dismissal or retention is predicted by the initial intensity of Arc-promoter activation, such that initially weakly activated neurons are dismissed from the subsequent ensembles and initially strongly activated neurons are reactivated in a persistent manner.
Our study identifies Arc as a key molecule that highlights functionally activated neuronal ensembles in the secondary motor cortex and predicts the outcomes of the cellular consolidation process during motor learning. Arc may provide a genetic foothold in those neurons to facilitate mechanistic dissection and prediction of neuronal ensemble functions in action learning and behavioral control (Denny et al., 2014; Guenthner et al., 2013; Silva et al., 2009).
Experimental Procedures
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Detailed methods are in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
The authors thank B. Connors, L. Belluscio, C. McBain, Y. Zuo, C. Gremel, W. Chen and D. Kwon for scientific discussion and technical advice. This work was supported by the Brown-NIH GPP Program (V.C.), NIMH Intramural Research Program (V.C., Y.Y., M.R., S.M., M.C, Q.L, K.W.), and NIAAA (R.C.).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M. Movement, confusion, and orienting in frontal cortices. Neuron. 2011;72:193–196. doi: 10.1016/j.neuron.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem. 2004;81:211–216. doi: 10.1016/j.nlm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cao VY, Ye Y, Mastwal SS, Lovinger DM, Costa RM, Wang KH. In vivo two-photon imaging of experience-dependent molecular changes in cortical neurons. Journal of visualized experiments: JoVE. 2013 doi: 10.3791/50148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron. 2011;72:330–343. doi: 10.1016/j.neuron.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TD, Liu L, Colwell KL, Whishaw IQ, Metz GA. Bilateral alteration in stepping pattern after unilateral motor cortex injury: a new test strategy for analysis of skilled limb movements in neurological mouse models. J Neurosci Methods. 2006;153:104–113. doi: 10.1016/j.jneumeth.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–784. doi: 10.1016/j.neuron.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Mann S, Wegenast-Braun BM, Calhoun ME, Luft AR. Region and task-specific activation of Arc in primary motor cortex of rats following motor skill learning. Neuroscience. 2013;250:557–564. doi: 10.1016/j.neuroscience.2013.06.060. [DOI] [PubMed] [Google Scholar]
- Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Kaelin-Lang A, Dichgans J, Schulz JB. Protein synthesis inhibition blocks consolidation of an acrobatic motor skill. Learn Mem. 2004;11:379–382. doi: 10.1101/lm.72604. [DOI] [PubMed] [Google Scholar]
- Murakami M, Vicente MI, Costa GM, Mainen ZF. Neural antecedents of self-initiated actions in secondary motor cortex. Nat Neurosci. 2014;17:1574–1582. doi: 10.1038/nn.3826. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain research. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain-machine interfaces. Nat Rev Neurosci. 2009;10:530–540. doi: 10.1038/nrn2653. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV. Posterior parietal cortex as part of a neural network for directed attention in rats. Neurobiol Learn Mem. 2009;91:104–113. doi: 10.1016/j.nlm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Ren M, Cao V, Ye Y, Manji HK, Wang KH. Arc regulates experience-dependent persistent firing patterns in frontal cortex. J Neurosci. 2014;34:6583–6595. doi: 10.1523/JNEUROSCI.0167-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, Fowler SC, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron. 2011;72:469–476. doi: 10.1016/j.neuron.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Li K, Whishaw PA, Gorny B, Metz GA. Distinct forelimb and hind limb stepping impairments in unilateral dopamine-depleted rats: use of the rotorod as a method for the qualitative analysis of skilled walking. J Neurosci Methods. 2003;126:13–23. doi: 10.1016/s0165-0270(03)00049-9. [DOI] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Tehovnik EJ. Turning responses evoked by stimulation of visuomotor pathways. Brain research. 1988;472:235–259. doi: 10.1016/0165-0173(88)90008-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.