Abstract
Placebo and nocebo play an important role in clinical practice and medical research. Modulating placebo/nocebo responses using noninvasive brain stimulation methods, such as transcranial direct current stimulation (tDCS), has the potential to harness these effects to therapeutic benefit in a clinical setting. In the present study we assessed the effect of anodal and cathodal tDCS over the right DLPFC on conditioned placebo/nocebo cue response to heat pain. Two matched groups of healthy volunteers were subjected to an identical session of conditioning, during which low and high cues (abstract images) were associated with low and high pain levels, respectively. Twenty-minute 2mA tDCS (either anodal or cathodal) over the right DLPFC was applied. The influence of tDCS current polarity (anodal vs. cathodal) on placebo and nocebo was assessed, using subjects’ pain ratings in response to identical pain preceded by the conditioned high or low cues. The duration of cue presentation varied to allow either fully conscious or subliminal processing. Significant placebo and nocebo effects in the anodal but not the cathodal group were elicited with the conditioning paradigm. This study provides evidence of a possibility to modulate the conditioned placebo and nocebo effect by changing the excitability of the right DLPFC using tDCS.
Keywords: tDCS, placebo, nocebo, right dorsolateral prefrontal cortex (rDLPFC), conditioning, pain
Introduction
Placebo and nocebo effects are salubrious benefits or negative outcomes attributable to the non-specific components of health care and have profound implications for basic and clinical research and for medical practice [3,17,18,41,42]. It is well accepted that placebo/nocebo effects are related to cognition and are, to some extent, learning phenomena, involving expectation, conditioning and cognitive anticipation [1,2,11–14,32,39,44,51,53,55,57]. Conditioned placebo and nocebo have been directly related to associative learning [11] subserved by the prefrontal cortex during cue learning, cue anticipation, pain perception and subjective pain reports [1,32,37].
Substantial evidence [18,46] suggests that the cognitive modulation of pain observed during placebo/nocebo effects may initiate from (or at least be crucially influenced by) the dorsolateral prefrontal cortex (DLPFC), a cognitive-executive control brain region [38]. Current models suggest that the DLPFC can influence the pain descending modulation system [30,58] to diminish pain, as well as activate the fear-anxiety network [5,29,54] to intensify pain, thus producing placebo analgesia and nocebo hyperalgesia respectively [53]. Several studies specifically point to the role of the right DLPFC in both placebo and nocebo effects [6,35–37].
The causal role of DLPFC in mediating the placebo effect has been demonstrated using noninvasive neuromodulation techniques, specifically Transcranial Magnetic Stimulation (TMS). Disrupting DLPFC function with inhibitory 1Hz rTMS, has been shown to block placebo analgesia [33]. This study is important from a mechanistic perspective, as it provides evidence for the possibility to manipulate (in this case reduce) the placebo effect with noninvasive brain stimulation. Although reducing the placebo effect may be beneficial in the context of clinical trials, in medical practice placebo effects are often desirable and their interaction with treatment produces better results compared to treatment only [26]. At the same time, nocebo effects are mostly harmful and related to anxiety [3,21,47]. Therefore, the possibility of modulating the nocebo effect is also important to explore. Furthermore, tDCS offers a number of advantages compared to TMS: it has an even better safety and tolerability profile, it is portable, cost-effective and therefore holds great potential from a public health perspective [40]. Improvements in many aspects of learning have been demonstrated using tDCS [9,10,19,22,43,45]. Neuroimaging studies have shown that tDCS to the rDLPFC can significantly change the activity and functional connectivity of the DLPFC [27,50], thus making it appealing to use for manipulation of cognitive placebo/nocebo effects [49].
In the current study we investigated the possibility of modulating placebo and nocebo effects by changing the excitability of the right DLPFC using tDCS in a conditioning learning paradigm involving healthy volunteers. We hypothesized that anodal tDCS to F4 would significantly enhance and cathodal tDCS over F4 would significantly inhibit the excitability of the rDLPFC, which would result in larger placebo and nocebo effects after anodal compared to cathodal stimulation, as decreasing rDLPFC excitability with TMS has been implicated in placebo inhibition [33]. We focused on the effects of conditioning with consciously perceived stimuli and also explored subliminal effects which have been previously reported with this experimental paradigm [24,25] in a secondary analysis.
Materials and methods
Subjects
Thirty-four healthy participants naïve to tDCS and our conditioning paradigm with no psychiatric or neurological conditions and not taking any psychotropic medication were enrolled. Four subjects did not complete all the experimental sessions and were excluded from the study. The final sample consisted of thirty participants randomized into two tDCS groups (anodal and cathodal rDLPFC excitation), 15 subjects per group. The groups were matched on gender (anodal: F=9, M=6; cathodal: F=9, M=6) and age (anodal: 24.1±4.9 (Mean±SD); cathodal: 23.7±2.7, p=0.78, 2-tailed).
The Institutional Review Board at Massachusetts General Hospital approved all study procedures. Methods were carried out in accordance with the approved guidelines. All enrolled subjects provided written informed consent before beginning any study procedures, were debriefed at the end of the study and agreed to allow their data to be used after learning about the purpose of the study.
Stimuli and equipment
Visual Stimuli
A set of three fractal images was used as cues. Two of the three cues were first used during the conditioning phase: the ‘high’ cue was associated with a high pain level and the ‘low’ cue was coupled with a low pain level. During the test phase, both high and low cues appeared with moderate pain stimuli, together with the third ‘neutral’ cue that had not been shown during conditioning and first appeared during the test phase (also with a moderate level of pain). The specific assignment of fractal stimuli to a given trial type was fully counterbalanced across participants. All stimuli were presented using the Presentation® software (Version 16.3, www.neurobs.com).
Heat pain administration
Noxious heat stimuli were delivered using a PATHWAY system (Medoc Advanced Medical Systems, Israel). All stimuli were initiated from a baseline temperature of 32 °C and increased to a target temperature. Each stimulus was presented for 11 seconds, including 2.5 seconds to ramp up to the target temperature, 4 seconds maintaining the same temperature, and 2.5 seconds to ramp down to the baseline again. Heat stimuli were applied to the right volar forearm, the position of the probe was changed for every session. Mean moderate temperature (used during the test phase) for cathodal and anodal groups did not differ significantly between groups (anodal: M=46.6 SD=±1.0; cathodal: M=45.8±1.1, two-samples 2-tailed t-test, p=0.07), although on average slightly lower temperature was used for the subjects in the cathodal group. These temperatures were calibrated individually based on subjects’ ratings as described below.
In order to determine the individually calibrated pain stimuli, we used an ascending heat pain sequence, starting at 38°C and slowly increasing by 1°C up to 50°C (or the subject’s maximum tolerance). Temperatures that elicited subjective intensity ratings of low=5, moderate=10, and high=15 on a scale from 0 to 20 were selected for each subject. Once the low, moderate and high heat pain levels for each subject were determined, subjects were tested for rating response consistency. A random sequence of 3 low and 3 high intensity noxious stimuli was administered. If the participants could reliably rate the high stimuli as more intense than the low stimuli, they proceeded to the conditioning phase of the experiment.
Transcranial direct current stimulation (tDCS) administration
For both groups, bipolar tDCS was administered using two saline-soaked surface sponge electrodes (area: 5 × 5 cm each), using a NeuroConn tDCS-PLUS stimulator (NeuroConn Inc., Ilmenau, Germany). We used the 10–20 International EEG System to identify the targets of stimulation and position the electrodes. For cathodal stimulation of the right DLPFC the cathode was placed over the F4 position and the anode above the left orbit. For the anodal stimulation of the right DLPFC the same configuration was used with opposite polarity (the anode was placed over F4 and the cathode above the left orbit). For both groups, 2mA tDCS was administered for 20 minutes. Stimulation started and finished with a 15 second gradual current ramp up and ramp down, to minimize local tingling and subjects’ discomfort. Subjects were stimulated during rest and were asked to remain seated and awake (with their eyes open). Subjects in either group did not report any discomfort or changed mood.
Experimental procedures
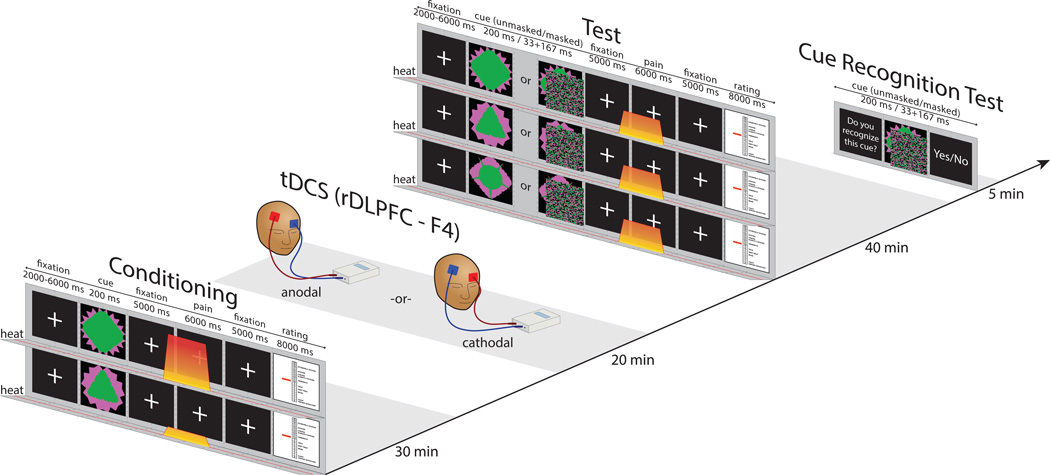
The experiment consisted of four phases: conditioning, tDCS, test and cue recognition task (Figure 1).
Figure 1. Experimental procedure.
Conditioning phase, tDCS application, test phase, cue recognition test.
Prior to undergoing the conditioning phase, all subjects were informed that they would see images on the computer screen and they would experience periodic heat pain stimulation on their arm. Subjects were told, “there will be a link between the cue that you see and the pain stimulus that you feel shortly thereafter”.
During the conditioning phase, subjects saw two images, consistently associated with high or low pain level. Each cue was presented for 200 ms and appeared 22 times in a randomized order (Figure 1). After each pain stimulus, subjects rated the pain they felt on a 0–20 scale [20]. Following the conditioning phase subjects in Group 1 received 20 minutes of anodal tDCS and subjects in Group 2 received 20 minutes of cathodal tDCS. There was no task during the stimulation. Immediately after the tDCS session, subjects underwent the test phase, during which 3 visual cues (the cue previously associated with low pain (low), tje cue previously associated with high pain (high) and a new (neutral) cue) were presented, followed by identical moderate pain (see Figure 1 and Stimuli and equipment section for details). During the test, half of the stimuli were presented supraliminally (200 ms) and half were presented subliminally (33 ms image followed by a mask for 167 ms). After the test, subjects underwent a cue recognition test, during which they were presented with masked and unmasked familiar and unfamiliar (new) images to determine if they could see and recognize masked stimuli. A similar conditioning paradigm (not involving tDCS) has been previously used [24].
Statistical analysis
In accordance with our main hypothesis, we started by investigating only the effects of consciously presented stimuli. First, we compared subjects’ low and high pain ratings during the conditioning phase by applying two-samples t-tests (2-tailed) to determine if there was any difference between the groups prior to the tDCS manipulation.
Then, we checked if applying anodal or cathodal tDCS (irrespective of the conditioning manipulation) produced an analgesic effect compared to each other, as tDCS has been shown to have a direct analgesic effect, although other brain regions were usually targeted [28]. We compared subjects’ ratings to moderate heat pain in response to neutral cues (not presented during the conditioning phase) by performing a two-samples t-test (2-tailed) for the two tDCS groups.
We proceeded to investigate the effect of anodal or cathodal tDCS on subjective pain ratings in response to moderate heat pain preceded by conditioned low and high cues. We applied a mixed model regression analysis with tDCS (anodal vs. cathodal) and cue (low, neutral, high) as fixed effects, using R software [48] packages lme4 [4] and lmerTest [34]. A linear relationship was assumed for the factor cue, with the ‘neutral’ condition at the midpoint between ‘low’ and ‘high’, based on a previous study [25]. In addition, we also performed a repeated measures ANOVA with tDCS as a between subject effect and cue as a within-subject effect to further confirm the results.
Planned pairwise comparisons - high vs. neutral and low vs. neutral cues within each tDCS group were performed (2-tailed paired t-tests adjusted for multiple comparisons at p=0.05 FDR-corrected level) to determine whether there was a significant placebo/nocebo response in each of the groups.
In addition, we also compared the size of the difference between high and neutral (nocebo) cues and low and neutral (placebo) cues between the tDCS groups (2-sample t-test, 1-tailed), expecting anodal placebo and nocebo to be greater than cathodal placebo and nocebo.
Finally, we explored conditioning and tDCS effects of subliminally presented cues by applying the same tests to the ratings of moderate pain following subliminally presented conditioned cues.
Results
Pain ratings during conditioning
During the conditioning phase (before application of tDCS), no significant difference was observed between the two groups in ratings of either high pain (anodal: 13.5±2.9; cathodal: 12.6±2.6, p=0.40) or low pain (anodal: 5.2±3.3; cathodal: 4.9±3.6, p=0.82).
Pain ratings during conscious cue test
A comparison of the effect of tDCS on the ratings of moderate pain following neutral cues revealed no difference between the groups (anodal: 8.0±3.4; cathodal: 6.0±4.1, p=0.16), suggesting that changing tDCS polarity (anodal vs. cathodal) did not affect general perception of the pain level in this study (Figure 2A), and reflected statistically insignificant but quantitatively slightly different moderate temperatures used during the test.
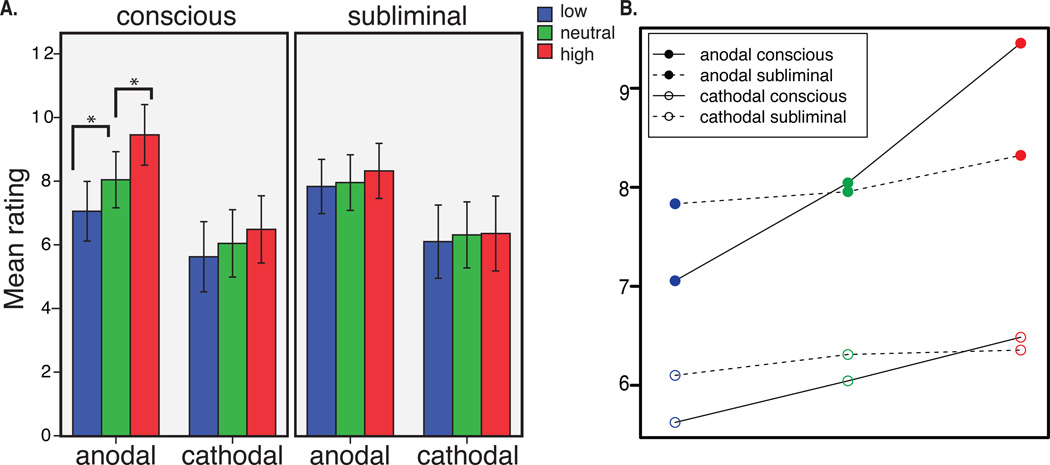
Figure 2. Main results.
A. Subjective pain ratings by perception level (conscious/subliminal) by tDCS group (anodal vs. cathodal). Mean and standard error of mean (SEM) values are reported. B. Mixed linear regression analysis: mean values and slopes for pain ratings by tDCS group by perception level.
We then applied a mixed regression analysis with 2 fixed effects (tDCS and cue). The results showed a significant interaction between the two factors (b=0.707, t(58) = 2.67, p<0.01), suggesting that changing the excitibility of rDLPFC modulates conscious conditioning effects (Figure 2B). This result was also observed in the repeated measures ANOVA analysis, also showing a significant interaction of tDCS and cue [F(2,56)=3.529, p=0.048 (Huynh-Feldt corrected for the violation of the sphericity assumption)]. The analysis of the conditioning effects within each of the groups showed that, in the anodal group, pain ratings (low cue: 7±3.6, high cue: 9.5±3.7) were significantly different between the low vs. neutral cues (punc.=0.016, pFDR=0.032), and high vs. neutral cues (punc.=0.009, pFDR=0.032), confirming significant conditioned placebo and nocebo effects. In the cathodal group (low cue: 5.6±4.3, high cue: 6.5±4.1) neither placebo (punc.=0.09, pFDR=0.16) nor nocebo (punc.=0.16, pFDR=0.16) effects were significant (Figure 2A), however the trend was in the same direction as following the anodal stimulation.
The difference between the tDCS groups was significant for the conscious nocebo t(1,28) = 2.41, p=0.022, as well as conscious placebo t(1,28) = 2.05, p=0.049; the difference in the nocebo effect thus seems to be more robust, compared to placebo.
Pain ratings during subliminal cue test
The analysis of the effect of tDCS on pain ratings following subliminal cues (anodal low: 7.8±3.3, neutral: 7.9±3.4, high: 8.3±3.3; cathodal low: 6.1±4.4, neutral: 6.3±4.5, high: 6.3±4) performed using a mixed regression analysis with fixed effects tDCS and cue did not reveal any significant main effects or interactions, p=0.55 (Figure 2B).
Cue recognition test
Accuracy of recognizing the cue presented consciously was high (79.2±13.4% correct), while for the subliminal presentation it was at chance levels (55.8±13.8% correct). No significant differences between the anodal and cathodal tDCS groups in subliminal (p=0.28) or supraliminal (p=0.22) cue recognition were observed.
Discussion
In this study we manipulated the excitability of the right DLPFC with tDCS and demonstrated that both placebo and nocebo effects can be elicited with a cue conditioning paradigm following anodal but not cathodal stimulation. The comparison of the regression slopes showed that the conditioning cue effect in the anodal group was larger than that in the cathodal group. In fact, neither a significant placebo nor a significant nocebo effect in the cathodal group was observed. These results support previous reports that rDLPFC is causally involved in producing the placebo effect [33] and shed new light on the principal role of rDLPFC in the nocebo effect as well. These findings demonstrate a great potential for clinical application of tDCS in combination with conditioning learning paradigms for pain treatment, allowing the possibility to reduce both placebo and nocebo effects.
tDCS modulation of pain and placebo/nocebo responses
The use of tDCS to treat pain has been extensively studied in the past decade [28]. A number of studies with various tDCS montages, e.g., anodal and cathodal motor cortex (M1) or anodal left DLPFC stimulation, were used to test the effects of tDCS on sensory-discriminative pain processing. These studies attempted to reduce pain sensation by stimulating the brain with anodal tDCS during pain administration. They found that anodal tDCS to M1 decreased pain perception [15,52]. Most of these studies targeted M1 but in the study by Boggio and colleagues they additionally stimulated the left DLPFC with anodal tDCS and reported a similar pain decrease [7]. These results, however, were not fully supported by a more recent study [23], showing non-significant pain reduction in the post- minus pre- M1 stimulation in the anodal tDCS group and no differences in pain ratings between the tDCS groups, albeit with a lower intensity (1mA) and duration (15 minutes) than used in other clinical studies [15,52]. Despite the lack of an analgesic effect, the authors demonstrated differences in BOLD signal within pain-processing regions between the anodal and the cathodal groups.
Recently several suggestions were put forward to use tDCS not to change pain sensation but rather to focus on the placebo/nocebo effects. In the context of tDCS clinical trials, it has been suggested that tDCS could be used to modulate the neurobiological mechanisms of the placebo effect not only as it relates to pain but also in other conditions such as major depressive disorder [49]. So far, only an rTMS study has successfully manipulated placebo analgesia [33] but to our knowledge no tDCS studies have investigated direct modulation of the cognitive aspects of pain and placebo/nocebo responses. In the current study, we applied tDCS for 20 minutes after the conditioning phase, during the consolidation period, but before pain administration in the test phase. In other words, we did not manipulate pain sensation but rather the learned association between an a priori neutral cue and pain intensity and tested the effect of tDCS extending beyond the period of stimulation. Similarly to the study of Ihle and colleagues [23], we compared anodal and cathodal stimulation directly and found a significant difference in pain ratings in response to different cues. The conditioning effects (placebo, i.e. low vs. neutral cue, and nocebo, i.e. high vs. neutral cue) were present in the anodal group but were not elicited in the cathodal stimulation group. No difference in pain perception between anodal and cathodal tDCS to the right DLPFC was observed, suggesting stimulating this brain area with the parameters we used does not have a direct analgesic/hyperalgesic effect but rather affects the cognitive-affective network.
Although the present study cannot unequivocally determine the direction of the change (enhancement of the effects in the anodal condition and/or reduction in the cathodal condition), based on previous behavioral reports of conditioned placebo/nocebo induced with similar experimental procedures [25], we speculate that the absence of significant placebo and nocebo effects in the cathodal condition might indicate that cathodal tDCS reduced the effects of conditioning. This is further supported by previous studies, in which inhibitory TMS blocked placebo analgesia [33]. Further studies are needed to determine whether anodal stimulation enhances the effects and how long the effect lasts.
The role of the right DLPFC in placebo and nocebo processing
Previous studies suggested that DLPFC, involving downstream circuits to the anterior insula, ACC, hypothalamus and PAG, is involved in placebo analgesia [2,16,56]. Specifically the right DLPFC has been related to conditioning learning, analgesia anticipation, pain perception and cognitive evaluation, i.e., pain rating [31,37]. A structural-anatomical report suggested that right DLPFC is related to the anterior insula, whereas the left DLPFC is associated with the mid-brain and medial thalamic activity [36]. Despite the wealth of neuroimaging literature on the neural correlates of placebo, it is still not clear whether stimulating the right or left DLPFC with TMS and tDCS would produce a stronger analgesic/hyperalgesic effect or whether the effects on placebo and nocebo would differ, depending on the stimulated site. One study showed that stimulating the left but not the right DLPFC with high frequency rTMS produces a placebo response decreasing the effect of the capsaicin cream [8]. Another study suggested no difference between right and left DLPFC on inhibiting placebo with low frequency rTMS [33]. In our previous study, we reported that although the bilateral DLPFC is engaged in conditioning placebo effect evoked by visual cues, the right fronto-parietal network (including the right DLPFC) may be more involved in producing conditioning effects [31]. Our current results confirm the important role of the right DLPFC for both placebo and nocebo responses.
Conscious and subliminal placebo/nocebo effects
Previous studies raised the possibility that a placebo/nocebo conditioned response could be induced with subliminal cues [24,25]. Here we did not observe any effect with subliminal presentation, nor did we see any interaction of tDCS with the perception (conscious/subliminal) level. The absence of the subliminal conditioned effect in either of the tDCS groups was unexpected and requires further investigation. Two possible explanations that could be tested in the future are whether manipulating rDLPFC with tDCS might have blocked the subliminal conditioning effect and whether the delay between the conditioning and the test phase, which was longer (~30 minutes during the tDCS setup and application) compared to the previous studies reporting the subconsious conditioning effects [24,25], was what caused the subliminal effect to disappear.
In conclusion, placebo and nocebo effects are a critical component of clinical care and efficacy studies. We demonstrated the feasibility of modulating both conditioned placebo and nocebo responses with tDCS, a safe and low cost neural modulation method. The current data seem to suggest that cathodal tDCS to the right DLPFC suppresses placebo and nocebo effects, compared to the anodal stimulation. The possibility of reducing placebo and nocebo effects using tDCS could have a significant and major impact on medical practice. In particular, in randomized clinical trials, where decreasing placebo and nocebo effects can significantly enhance the effect size of the trials and reduce the total sample size required to test the specific effect of treatment, cathodal tDCS could be used to minimize the contribution of non-specific placebo and nocebo.
Acknowledgements
This work was supported by R01AT006364 (NIH/NCCAM) to Jian Kong, R01AT005280 (NIH/NCCAM) to Randy Gollub, and P01 AT006663 to Bruce Rosen. We also thanks Joel Park for technical support and two anonymous reviewers for their suggestions on improving the manuscript.
Footnotes
There is no conflict of interest for any of the authors.
References
- 1.Amanzio M, Benedetti F, Porro Ca, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum. Brain Mapp. 2013;34:738–752. doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas LY, Bolger N, Lindquist Ma, Wager TD. Brain mediators of predictive cue effects on perceived pain. J. Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287:622–627. doi: 10.1001/jama.287.5.622. Available: http://www.ncbi.nlm.nih.gov/pubmed/11829702. [DOI] [PubMed] [Google Scholar]
- 4.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. 2014 Available: http://cran.r-project.org/package=lme4. [Google Scholar]
- 5.Benedetti F. Mechanisms of placebo and placebo related effects across diseases and treatments. Eur. Neuropsychopharmacol. 2010;20:S60–S61. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J-K. Neurobiological mechanisms of the placebo effect. J. Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur. J. Neurol. 2008;15:1124–1130. doi: 10.1111/j.1468-1331.2008.02270.x. [DOI] [PubMed] [Google Scholar]
- 8.Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, Panetta M, Giglia G, Fierro B. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J. Headache Pain. 2011;12:185–191. doi: 10.1007/s10194-011-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark VP, Parasuraman R. Neuroenhancement: enhancing brain and mind in health and in disease. Neuroimage. 2014;85(Pt 3):889–894. doi: 10.1016/j.neuroimage.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 10.Coffman Ba, Clark VP, Parasuraman R. Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage. 2014;85(Pt 3):895–908. doi: 10.1016/j.neuroimage.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 11.Colloca L, Miller FG. How placebo responses are formed: a learning perspective. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:1859–1869. doi: 10.1098/rstb.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson ME. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2008;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DosSantos MF, Love TM, Martikainen IK, Nascimento TD, Fregni F, Cummiford C, Deboer MD, Zubieta J-K, Dasilva AFM. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front. psychiatry. 2012;3:93. doi: 10.3389/fpsyt.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59:195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flöel A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage. 2014;85(Pt 3):934–947. doi: 10.1016/j.neuroimage.2013.05.098. [DOI] [PubMed] [Google Scholar]
- 20.Gracely RH, McGrath P, Dubner R. Ratio Scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 21.Hahn Ra. The nocebo phenomenon: concept, evidence, and implications for public health. Prev. Med. (Baltim) 1997;26:607–611. doi: 10.1006/pmed.1996.0124. [DOI] [PubMed] [Google Scholar]
- 22.Harty S, Robertson IH, Miniussi C, Sheehy OC, Devine Ca, McCreery S, O’Connell RG. Transcranial direct current stimulation over right dorsolateral prefrontal cortex enhances error awareness in older age. J. Neurosci. 2014;34:3646–3652. doi: 10.1523/JNEUROSCI.5308-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ihle K, Rodriguez-Raecke R, Luedtke K, May A. tDCS modulates cortical nociceptive processing but has little to no impact on pain perception. Pain. 2014 doi: 10.1016/j.pain.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J. A Neural Mechanism for Nonconscious Activation of Conditioned Placebo and Nocebo Responses. Cereb. Cortex. 2014:1–8. doi: 10.1093/cercor/bhu275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci. Transl. Med. 2014;6:218ra5. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Brunelin J, Möller H-J, Reiser M, Padberg F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011;31:15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knotkova H, Nitsche Ma, Cruciani Ra. Putative physiological mechanisms underlying tDCS analgesic effects. Front. Hum. Neurosci. 2013;7:628. doi: 10.3389/fnhum.2013.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Rosen B, Kaptchuk TJ. An fMRI study on the neural mechanisms of hyperalgesic nocebo effect. J. Neurosci. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J. Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong J, Jensen K, Loiotile R, Cheetham A, Wey H-Y, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154:459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL. Placebo analgesia: findings from brain imaging studies and emerging hypotheses. Rev. Neurosci. 2007;18:173–190. doi: 10.1515/revneuro.2007.18.3-4.173. Available: http://www.ncbi.nlm.nih.gov/pubmed/18019605. [DOI] [PubMed] [Google Scholar]
- 33.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Kuznetsova A, Bruun Brockhoff P, Haubo Bojesen Christensen R. lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package) 2014 Available: http://cran.r-project.org/package=lmerTest. [Google Scholar]
- 35.Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer Ea. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 37.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro Ca. Neural bases of conditioned placebo analgesia. Pain. 2010;151:816–824. doi: 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Mansouri Fa, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat. Rev. Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Pichora AL, Mankovsky-Arnold TD, Katz J. Implicit versus explicit associative learning and experimentally induced placebo hypoalgesia. J. Pain Res. 2011;4:67–77. doi: 10.2147/JPR.S15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinley RA, Bridges N, Walters CM, Nelson J. Modulating the brain at work using noninvasive transcranial stimulation. Neuroimage. 2012;59:129–137. doi: 10.1016/j.neuroimage.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 41.Miller FG, Colloca L, Kaptchuk TJ. The placebo effect: illness and interpersonal healing. Perspect. Biol. Med. 2009;52:518–539. doi: 10.1353/pbm.0.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller FG, Kaptchuk TJ. The power of context: reconceptualizing the placebo effect. J. R. Soc. Med. 2008;101:222–225. doi: 10.1258/jrsm.2008.070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche Ma, Oliveri M, Pascual-Leone A, Paulus W, Priori A, Walsh V. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008;1:326–336. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Morton DL, El-Deredy W, Watson A, Jones AKP. Placebo analgesia as a case of a cognitive style driven by prior expectation. Brain Res. 2010;1359:137–141. doi: 10.1016/j.brainres.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 45.Nelson JT, McKinley RA, Golob EJ, Warm JS, Parasuraman R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS) Neuroimage. 2014;85(Pt 3):909–917. doi: 10.1016/j.neuroimage.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 46.Petrovic P. Placebo analgesia and nocebo hyperalgesia – Two sides of the same coin? Pain. 2008;136:5–6. doi: 10.1016/j.pain.2008.03.004. doi: http://dx.doi.org/10.1016/j.pain.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Ploghaus a, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J. Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. Available: http://www.ncbi.nlm.nih.gov/pubmed/11739597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Core Team. R: A Language and Environment for Statistical Computing. 2014 Available: http://www.r-project.org/. [Google Scholar]
- 49.Schambra HM, Bikson M, Wager TD, DosSantos MF, DaSilva aF. It’s All in Your Head: Reinforcing the Placebo Response With tDCS. Brain Stimul. 2014:130–131. doi: 10.1016/j.brs.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J. Neurosci. 2013;33:11425–11431. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 52.Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, Boggio PS, Paulo S. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J. Pain Manag. 2010;2:353–361. [PMC free article] [PubMed] [Google Scholar]
- 53.Wager T, Atlas L. How Is Pain Influenced by Cognition? Neuroimaging Weighs In. Perspect. Psychol. Sci. 2014;8:91–97. doi: 10.1177/1745691612469631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wager TD. Expectations and anxiety as mediators of placebo effects in pain. Pain. 2005;115:225–226. doi: 10.1016/j.pain.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 55.Wager TD, Atlas LY, Leotti La, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J. Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 57.Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt Ba, Nadeau V, Jones AKP. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zubieta J-K, Bueller Ja, Jackson LR, Scott DJ, Xu Y, Koeppe Ra, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]