Abstract
Pterostilbene, a methoxylated analogue of resveratrol, is a natural compound primarily found in blueberries and several types of grapes. However, little is known about the effect of pterostilbene on the proliferation of hepatoma cells and its modes of actions. This study was undertaken to characterize its ability to suppress the proliferation of hepatoma AH109A cells and the possible mechanism(s) involved. Pterostilbene showed a significant and dose-dependent effect on the anti-proliferative activity against AH109A cells. Pterostilbene exerted little or no effect on the proliferation of rat L6 myoblasts and rat skin fibroblasts. Pterostilbene-loaded rat sera could significantly inhibit the proliferation of AH109A cells, which suggests that pterostilbene could be absorbed through gastrointestinal tract and retain its anti-proliferative activity. Pterostilbene arrested the cell cycle of AH109A cells at G0/G1 phase and reduced the protein expression of cyclin-dependent kinase 4 and cyclin-dependent kinase 6 dose-dependently. We also found that pterostilbene could significantly increase the intracellular peroxide level of AH109A cells, which may be involved in its anti-proliferative activity.
Keywords: Cell cycle, Hepatoma, Proliferation, Pterostilbene, Reactive oxygen species
Introduction
Cancer is a leading cause of death worldwide. The potential use of phytochemicals for preventing cancer is one of the most hopeful means and extensively investigated worldwide. We have been investigating the effects of phytochemicals on the prevention of cancer, using in vitro and ex vivo assay systems and have already reported that some food components, such as carotenoids, tea catechins, and resveratrol, have anti-proliferative and/or anti-invasive properties on hepatoma cells (Kozuki et al. 2000; Zhang et al. 2000a; Kozuki et al. 2001). Pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene) is a naturally occurring stilbenoid compound isolated from several natural plant sources including several types of grapes (Adrian et al. 2000), blueberries (Rimando et al. 2002), and the stem bark of several trees used in folk medicine such as Pterocarpus marsupium (Manickam et al. 1997) and Guibourtia tessmanii (Fuendjiep et al. 2002). Pterostilbene is an analogue of a well-studied stilbene, resveratrol. The difference in chemical structures causes pterostilbene to be more lipophilic, increases oral absorption (Lin et al. 2009), and renders a higher potential for cellular uptake than resveratrol.
Previous study demonstrated the effect of pterostilbene on several types of cancer such as breast (Chakraborty et al. 2012), skin (Ferrer et al. 2005), and gastric cancer (Pan et al. 2007). Alosi et al. (2010) showed that pterostilbene induced a significant concentration- and time-dependent decrease in the breast cancer cell viability. Other studies showed that pterostilbene suppressed multiple signals associated with TPA-induced metastasis (Pan et al. 2009) and induced apoptosis (Hasiah et al. 2011) on hepatocellular carcinoma cells. The molecular mechanisms of cell cycle arrest by naturally occurring phenolic compounds, such as stilbenes, remain largely unclear but appear to involve modulation of multiple cell cycle regulatory proteins. Resveratrol was found to inhibit the proliferation of malignant natural killer (NK) cells through a significant accumulation of cells in the G0/G1 phase and down-regulation of cell-cycle regulator proteins (Trung et al. 2013). Furthermore, resveratrol also induced apoptosis in a dose- and time-dependent manner by down-regulating anti-apoptotic proteins. Pterostilbene treatment inhibits the growth of breast cancer MCF-7 and MDA-MB-231 cells in vitro through caspase-dependent apoptosis and alteration of cell cycle. Mitochondrial membrane depolarization and increased superoxide anion may contribute to the activation of downstream effector caspases (Alosi et al. 2010). However, little is known about the effects of pterostilbene on the proliferation of hepatoma cells and its mechanisms. This study is aimed to investigate the effect of pterostilbene on hepatoma cells and its modes of action.
Materials and methods
Materials
Pterostilbene was purchased from Tokyo Chemical Industries, Ltd. (Tokyo, Japan). Pterostilbene was dissolved in dimethyl sulfoxide (DMSO, Sigma Chemical Co., St. Louis, MO, USA), and added to the medium at the final DMSO concentration of 0.1 %. The control medium contained 0.1 % DMSO alone. Anti-rat γ-tubulin mouse monoclonal antibody was purchased from Sigma Chemical Co. (St. Louis, MO, USA), whereas anti-rat CDK4 rabbit monoclonal antibody (anti-CDK4 antibody) and anti-rat CDK6 mouse monoclonal antibody (anti-CDK6 antibody) were purchased form Cell Signalling Technology (Beverly, MA, USA). All other chemicals were of the best grade commercially available.
Culture of cell lines
A rat ascites hepatoma cell line of AH109A cells was provided by the Institute of Development, Aging, and Cancer, Tohoku University (Sendai, Japan). AH109A cells were maintained in peritoneal cavities of male Donryu rats, isolated from accumulated ascites and then cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Nissui Pharmaceutical Co., Tokyo, Japan) containing 10 % fetal bovine serum (FBS, obtained from Nichirei Biosciences Inc, Tokyo, Japan), streptomycin (100 μg/mL) and penicillin G (100 U/mL) (Nacalai Tesque, Inc., Kyoto, Japan) (10 % FBS/DMEM). These cells were cultured for at least 2 weeks after isolation to eliminate contaminating macrophages and neutrophils, and used for the experiment described below.
Rat L6 myoblast was obtained from American Type Culture Collection (Manassas, VA, USA; ATCC 123 number CRL-1458), and rat skin fibroblast was a generous gift from Prof. Yoshihiro Nomura of Tokyo University of Agriculture and Technology. Both cells were maintained in DMEM supplemented with 10 % FBS, streptomycin (100 μg/mL), and penicillin G (100 U/mL) (10 % FBS/DMEM).
In vitro proliferation assay
The effect of pterostilbene on the proliferation of AH109A cells was examined by measuring the incorporation of [methyl-3H] thymidine (0.15 μCi/well, specific radioactivity; 20 Ci/mmol, New England Nuclear, Boston, MA) into acid insoluble fraction of the cells. The proliferation of other cell lines was determined using the WST-8 Cell Counting Kit (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instruction. This assay is based on the cleavage of the tetrazolium salt to formazan by cellular mitochondrial dehydrogenase. The amount of the dye generated by activity of dehydrogenase is directly proportional to the number of living cells as described previously (Ishiyama et al. 1997). Briefly, the cells were seeded in the medium containing pterostilbene at the indicated concentration and cultured for 20 h. Then [methyl-3H] thymidine or WST-8 reagent were added and the incubation was continued for another 4 h. After a total of 24 h incubation, the incorporated radioactivity into DNA fraction of the cells or the absorbances of formazan dyes produced by the viable cells were measured with a liquid scintillation counter (LS6500, Beckman-Coulter, Hialeah, FL, USA) or microplate reader (Bio-Rad, Richmond, CA, USA), respectively.
Ex vivo proliferation assay
The animal experiment was conducted in accordance with the guidelines established by the Animal Care and Use Committee of Tokyo University of Agriculture and Technology and were approved by this committee (No. 25–20). Male Donryu rats obtained from Japan SLC Inc. (Hamamatsu, Japan) were fasted overnight prior to the experiment. Pterostilbene was suspended on 0.3 % carboxy methyl cellulose (CMC) solution (Wako Pure Chemical Industries Ltd., Osaka, Japan), intubated into rats at a dose of 40 mg pterostilbene/100 g body weight and blood was collected at 0, 30, 60, 120, 180, and 360 min after the oral intubation. Sera were prepared, sterilized by filtration, and added to the culture medium at a concentration of 10 % instead of FBS. The proliferative activity of AH109A cells in the presence of these sera was then measured by the WST-8 Cell Counting Kit.
Cell cycle analysis
To elucidate the mechanism of anti-proliferative effect of pterostilbene on AH109A cells, the effect on cell cycle was examined with a flow cytometer. Briefly, 2.5 × 105 cells of AH109A were seeded in a 6-well plate (Nunc A/S, Roskilde, Denmark) and serum starved for 24 h. After serum starvation, these cells were treated with medium containing 0, 50, 100, and 200 μM pterostilbene and cultured for another 24 h. After 24 h incubation, cells were collected and washed twice with sterilized phosphate buffered saline without Ca2+ and Mg2+ (PBS(-)). The pellet was stained with 25 μg/ml propidium iodide solution containing 0.1 % Triton X-100 (Sigma Chemical Co., St. Louis, MO, USA), 0.1 % sodium citrate (Wako Pure Chemical Industries Ltd., Osaka, Japan) and 5 μg/ml RNase (Sigma Chemical Co., St. Louis, MO, USA), and the cells were incubated for 30 min on ice. Cells were then analyzed with a flow cytometer (EPICS ELITE EPS, Beckman-Coulter, Hialeah, FL, USA) as previously described (Zhang et al. 2000b).
Western blot analysis
AH109A cells were cultured for 24 h on 10 % FBS/DMEM with various concentration of pterostilbene. The cells were lysed with RIPA buffer (50 mM Tris–HCl, 150 mM NaCl, 1 % NP-40, 0.5 % sodium deoxy cholate and 0.1 % sodium dodecyl sulfate) (Nacalai Tesque Inc., Kyoto, Japan), 10 μL/mL protease inhibitor cocktail (Nacalai Tesque Inc., Kyoto, Japan) and 10 mM sodium orthovanadate (Sigma Chemical Co., St. Louis, MO, USA). Samples were placed on ice for 30 min to allow lysis and then centrifuged at 18,000×g and 4 °C for 20 min. The aliquots of the lysates (50 μg of total protein) were mixed with Laemmli’s buffer (62.5 mM Tris–HCL, 25 % glycerol, 2 % sodium dodecyl sulphate, and 0.01 % bromophenol blue), boiled for 10 min, electrophoresed on 10 % SDS–polyacrylamide gel, and transferred to PVDF membranes (Millipore Corp, Billerica, MA, USA). After transferring, the membranes were blocked with 5 % BSA (Sigma Chemical Co., St Louis, MO, USA) in Tris–HCl buffered saline (TBS) containing 0.05 % Tween 20 (TBST) for 2 h. After the incubation, the membranes were washed three times with TBST and incubated with primary antibodies overnight at 4 °C. The membranes were then washed three times with TBST and incubated with horseradish peroxidise-conjugated anti-rabbit or mouse IgG antibodies (GE Healthcare UK, Little Chalfont, Buckinghamshire, UK) for 2 h at room temperature. Immunoreactive bands were detected using Super Signal West pico chemiluminescent substrate (Pierce Chemical Co., Rockford, IL, USA) according to the manufacturer’s instruction. The intensity of each band was visualized with a lumino image analyzer (Model LAS-4000 Mini, Fujifilm, Tokyo, Japan) and image analysis software (Multi Gauge Ver. 3.0; Fujifilm).
Intracellular peroxide level analysis
Briefly, AH109A cells were seeded in the medium containing 0, 50, 100, and 200 μM of pterostilbene for 24 h for dose-dependent effect of pterostilbene, or in the medium containing 100 μM of pterostilbene for 0, 2, 4, and 24 h for time-dependent effect of pterostilbene. Intracellular peroxide levels in AH109A cells were investigated using a peroxide-dependent fluorescent dye, 2′, 7′-dichlorofluorescin diacetate (DCFH-DA, Molecular Probes, Inc., Eugene, OP, USA) (Bass et al. 1983) by a flow cytometer (EPICS ELITE EPS, Beckman-Coulter, Hialeah, FL, USA) as described previously (Hirakawa et al. 2005).
Statistical analysis
Data were expressed as mean ± SEM. Multiple comparison was performed by one-way analysis of variance (ANOVA), followed by Tukey–Kramer multiple comparisons test, and p < 0.05 was considered statistically significant.
Results and discussion
In the present study, we first examined the in vitro effect of pterostilbene on the proliferation of hepatoma cells AH109A as well as on normal cells, L6 myoblasts and skin fibroblasts. We clarified that pterostilbene commenced to suppress the proliferative activity of AH109A cells at 25 μM by 21 % and continued the inhibition dose-dependently up to 200 μM by more than 97 % as compared to the corresponding control (Fig. 1a). We have confirmed that pterostilbene had no cytotoxic effect at 0–100 μM on AH109A cells as evaluated using trypan blue dye exclusion method (data not shown). Previous studies also demonstrated that 65 μM pterostilbene treatment for 24 h could inhibit the cell growth of MCF-7 breast cancer up to 50 % (Chakraborty et al. 2012) as well as on HCT-116 colon cancer at 45.3 μM and A-375 melanoma at 421 μM (Remsberg et al. 2007). These data showed that the effect of pterostilbene was varying depending on the cancer types and sites. On normal cells, pterostilbene had no effect on proliferation of L6 myoblasts and skin fibroblasts at 0, 12.5, 25, 50, and 100 μM as shown in Fig. 1b, c. However, at high concentration (200 μM), pterostilbene significantly inhibited the proliferation of both L6 myoblasts and skin fibroblasts. These data were supported by the work of Pan et al. (2008) which found that pterostilbene was not cytotoxic at 25–100 μM in human lymphocyte cells. Our data, along with previous reports, clearly indicated that pterostilbene significantly suppressed the proliferation of cancer cells without any effect on the proliferation of normal cells, suggesting that pterostilbene could be a potential candidate for tumor prevention.
Fig. 1.
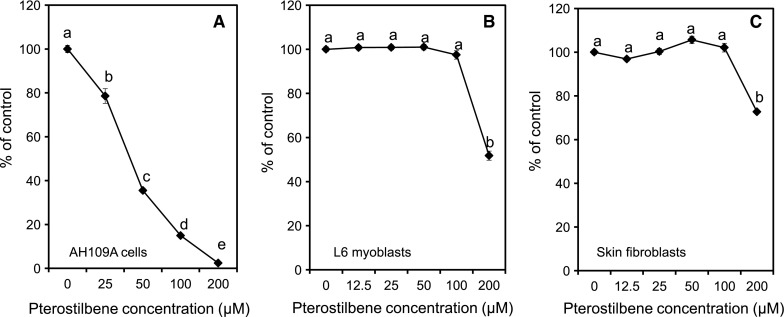
Effects of pterostilbene on the proliferation of AH109A cells (a), L6 myoblasts (b), and skin fibroblasts (c). Pterostilbene was dissolved in DMSO and added to the medium at the final DMSO concentration of 0.1 %. Cells were cultured with various concentration of pterostilbene for 24 h. The proliferation of the cell lines was studied by measuring the incorporation of [methyl-3H] thymidine into DNA fraction of the cells (a) and by measuring the formazan dyes produced by viable cells using WST-8 assay (b, c). Data are mean ± SEM of 5 wells (a) and 8 wells (b, c). Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test
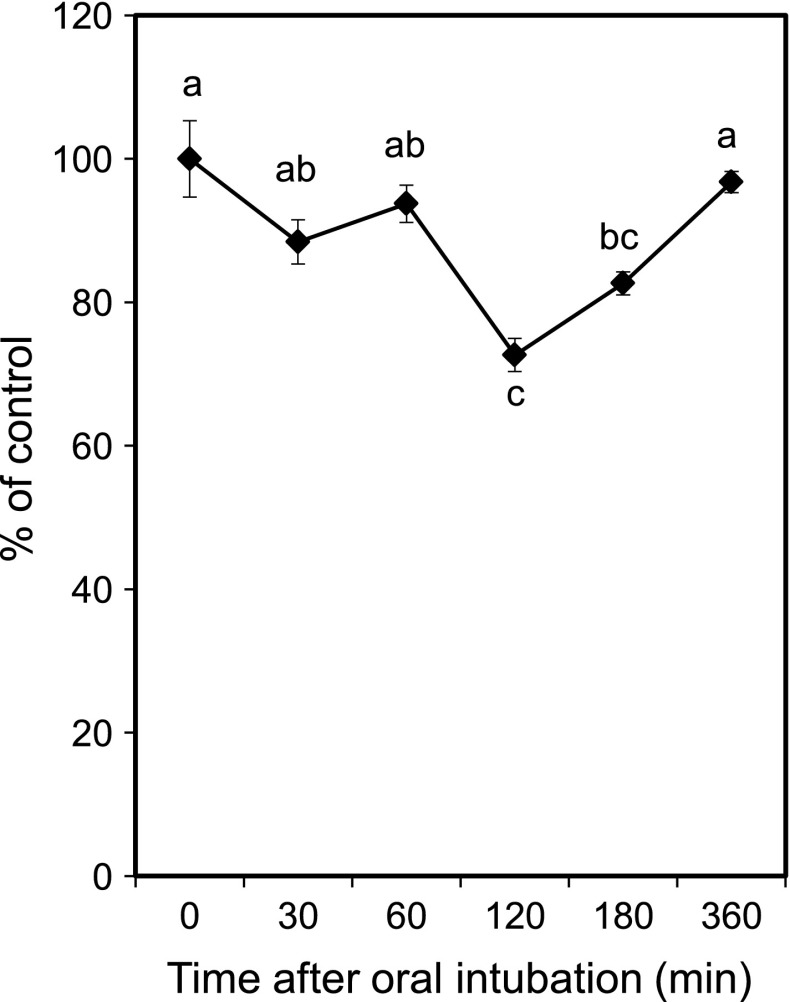
To determine whether pterostilbene could be absorbed into the body and retained its anti-proliferative activity when orally given to rats, the ex vivo proliferation assay was conducted. Rats were orally given pterostilbene at a dose of 40 mg pterostilbene/100 g of body weight, and blood was obtained at 0, 30, 60, 120, 180, and 360 min after oral intubation. The sera were prepared and added to the culture medium instead of FBS at a concentration of 10 %. As shown in Fig. 2, we found that pterostilbene-loaded rat sera could significantly inhibit the proliferation of AH109A cells. Thirty minutes after the oral intubation, pterostilbene only slightly inhibited the proliferation of the cells by 12 %. The inhibition reached its peak at 120 min after oral intubation by 23 % and the effect has disappeared after 360 min. These data suggest that pterostilbene was absorbed across the gastrointestinal tract, and pterostilbene and/or its metabolites retained its anti-proliferative activity against AH109A cells. Remsberg et al. (2008) investigated the pharmacokinetics and metabolism of pterostilbene, and showed that pterostilbene was absorbed and underwent a glucuronidation process in the liver. Ferrer et al. (2005) evaluated the plasma level of pterostilbene on mice administered with 2 mg pterostilbene/100 g body weight and found out that the plasma level of pterostilbene reached its peak at 60 min after oral administration. Total pterostilbene concentration in the plasma was around 10 μM between 30 and 240 min and it was still detected 720 min after administration. By making a regression from Fig 1a, the anti-proliferative activities of pterostilbene-loaded rat sera at 30 min (12 % inhibition) and 120 min (23 % inhibition) after oral intubation were estimated to equal to 3.9 and 8.4 μM of pterostilbene treatment. These data suggest that oral administration of 40 mg pterostilbene/100 g body weight on rats resulted in the highest concentration of pterostilbene in the blood of around 8.4 μM after 120 min. However, since pterostilbene may be metabolised in the liver, the possibility that anti-proliferative activity of pterostilbene-loaded rat sera was the effect of pterostilbene itself and/or synergistic effects of its metabolite(s) needs to be clarified.
Fig. 2.
The effect of pterostilbene-loaded rat sera on the proliferation of AH109A cells. Pterostilbene was suspended in 0.3 % CMC aqueous solution and intubated at a dose of 40 mg/100 g body weight in male Donryu rats who had fasted overnight. Blood was collected at 0, 30, 60, 120, 180, and 360 min after oral intubation. After centrifugation, experimental medium containing 10 % of each serum were prepared and subjected to the proliferation assay using WST-8 cell counting kit. Data are mean ± SEM of 8 wells. Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test
To elucidate the mechanism of pterostilbene action, its effect on cell cycle was examined by flow cytometry. AH109A cells were cultured on pterostilbene for 24 h and the distribution of cells among cell cycles was analyzed using FACS. As shown in Fig. 3a, pterostilbene treatment led to a dose-dependent increase in the fraction of AH109A cells in the G0/G1 phase; the fraction of G0/G1 phase increased from 37.3 % at 0 μM to 46.8 % at 200 μM. Even though the sub-G1 phase of the cell cycle was slightly increased after 100 and 200 μM pterostilbene treatment, there were no apoptotic cells as confirmed using the detection of DNA ladders and Annexin V/PI double staining method (data not shown).
Fig. 3.
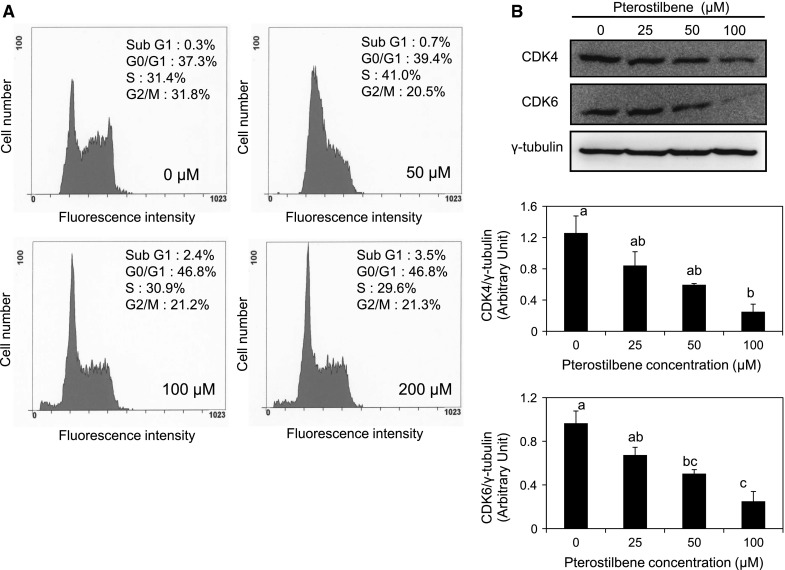
The effect of pterostilbene on cell cycle and cell cycle checkpoints protein expression in AH109A cells. a Pterostilbene was dissolved in DMSO and added to the culture medium at final DMSO concentration of 0.1 %. AH109A cells were cultured with medium containing 0, 50, 100, and 200 μM of pterostilbene for 24 h then subjected to cell cycle analysis using flow cytometer. b Effect of pterostilbene on the cell cycle checkpoints protein expression was assessed using western blot analysis. AH109A cells were cultured on medium containing various concentrations of pterostilbene for 24 h then the protein lysates were prepared. Western blot analysis was performed using antibodies to CDK4 (anti-rat CDK4 rabbit monoclonal antibody), CDK6 (anti-rat CDK6 mouse monoclonal antibody), and γ-tubulin (anti-rat γ-tubulin mouse monoclonal antibody). γ-tubulin was used as an internal control. The representative results are shown in the upper panel. The protein bands were quantified by ImageJ and ratios of CDK4/γ-tubulin (middle panel) and CDK6/γ-tubulin (lower panel) are shown. Data are mean ± SEM of 3 independent experiments. Values not sharing a common letter are significantly different by Tukey–Kramer multiple comparison test at p < 0.05
Cancer progression has been suggested to involve the loss of cell cycle checkpoints controls that regulate the passage through the cell cycle. Checkpoints are control mechanisms that ensure the proper timing of cell cycle events. Cyclin D is one of the major players for G1 phase progression. This protein associates with CDK4 or CDK6 and phosphorylates retinoblastoma protein, Rb. Rb functions as a tumor suppressor, and it is active in its hypophosphorylated form and repressed cell cycle progression by inhibiting E2F transcription factors which are necessary for S phase entry (Blomen and Boonstra 2007). To elucidate the mechanism of cell cycle arrest, the expression of cell cycle checkpoints proteins, CDK4 and CDK6, were determined. As shown in Fig. 3b, the treatment with pterostilbene for 24 h resulted in the dose-dependent decrease of CDK4 and CDK6 protein expression. Up to 50 μM, pterostilbene only slightly inhibited the protein expression of CDK4 in AH109A cells. Pterostilbene has significantly down-regulated the protein expression of CDK4 at 100 μM (Fig. 3b, middle panel). The effect of pterostilbene on the CDK6 protein expression is almost similar to that of CDK4. Pterostilbene induced a dose-dependent down-regulation of CDK6 protein expression in AH109A cells. Starting at 50 μM, the down-regulation of CDK6 protein expression was noticed and pterostilbene continued to reduce it up to 100 μM (Fig. 3b, lower panel).
A study by Notas et al. (2006) showed that resveratrol inhibits cell growth of HepG2 hepatocellular carcinoma cells in a dose- and time-dependent manner by blocking cells in the G1 and G2 phase of the cell cycle and induces apoptosis. This study was supported by Ahmad et al. (2001) which found that resveratrol treatment results in a G1 phase arrest of the cell cycle followed by apoptosis of human epidermoid carcinoma A431 cells. Resveratrol treatment causes an induction of WAF1/p21, a decrease in the protein expression of Cyclin D1, Cyclin D2, and Cyclin A, as well as a significant decrease in the protein expression of CDK2, CDK4, and CDK6. The induction of WAF1/p21 inhibits Cyclin D1/D2-CDK6, Cyclin D1/D2-CDK4, and Cyclin E-CDK2 complexes, thereby arrests the cell cycle at G1 phase, and inhibits the proliferation of A431 cells. Further study by Adhami et al. (2001) showed that resveratrol treatment in human epidermoid carcinoma A431 cells results in a dose-dependent decrease in the hyperphosphorylated form of Rb with a relative increase in hypophosphorylated Rb. This response was accompanied by down-regulation of protein expression of the E2F family member of transcription factors. These data suggest that resveratrol inhibits the proliferation of cancer cells through the modification of Cyclin-CDK complexes, Rb protein expression, E2F transcription factors, led to a cell cycle arrest and the inhibition of proliferative activity.
Pan et al. (2007) investigated the effect of pterostilbene in human gastric carcinoma cells and found that pterostilbene inhibits the cell proliferation and induce apoptosis by the modification of Cyclin-CDK complexes and modifying cell cycle progress similar to that of resveratrol. Pterostilbene increased the p53, p21, p27, and p16 protein levels and decreased the levels of Cyclin A, Cyclin E, CDK2, CDK4, and CDK6. Furthermore, pterostilbene also decreased the degree of phosphorylation of Rb, leading to an arrest in the G1 phase of the cell cycle. Taking together, our result showed that pterostilbene could significantly and dose-dependently reduced the CDK4 and CDK6 proteins expression in AH109A cells (Fig. 3b) which might lead to the hypophosphorylation of Rb, inhibition of E2F, and the arrest at G0/G1 phase. These data suggest that the down-regulation of CDK4 and CDK6 protein expressions may be at least one of the mechanisms of anti-proliferative activity of pterostilbene against AH109A hepatoma cells.
Mounting evidence suggests that, compared with their normal counterparts, many types of cancer cells have an increased level of reactive oxygen species (ROS). Moreover, the level of ROS scavenging enzymes such as superoxide dismutase, glutathione peroxidise, and peroxiredoxin have been shown to be significantly altered in malignant cells and in primary cancer tissues (Trachootham et al. 2009). Therefore, it is conceivable that these malignant cells would be more dependent on anti-oxidants for cell survival and more vulnerable to further oxidative insults induced by ROS-generating agents or by compounds that abrogate the key antioxidant systems in the cells. Thus, we also examined the effect of pterostilbene on the intracellular peroxide level of AH109A cells. Treatment with 0, 25, 50, and 100 μM of pterostilbene for 24 h led to a significant increase in the ROS level of AH109A cells (Fig. 4a, b). Figure 4a shows a representative data on the increasing ROS level of AH109A cells after treatments with various concentrations of pterostilbene, while Fig. 4b shows the bar graph with the statistical analysis. An increasing level of ROS was noticed at a low concentration (25 μM), reaching its peak at 50 μM, and slightly decreased at 100 μM pterostilbene treatment. The time-dependent effect of pterostilbene on the intracellular ROS level of AH109A cells was also assessed by culturing AH109A cells on medium containing 100 μM pterostilbene for 0, 2, 4, and 24 h (Fig. 4c). Figure 4d shows the changes in the intracellular peroxide level of AH109A cells after pterostilbene treatment at the indicated times in the bar graph. Our data showed that the treatment with pterostilbene could significantly increase the intracellular ROS level of the cells as early as 2 h after treatment. The fluctuations in the intracellular redox state during cell cycle progression could represent a fundamental mechanism linking oxidative metabolic processes to cell cycle regulatory processes (Menon and Goswami 2007).
Fig. 4.
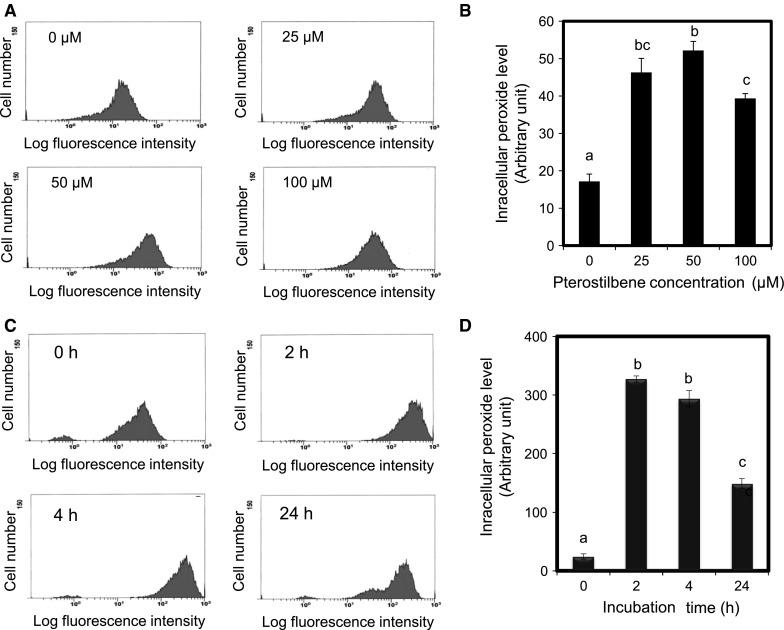
Dose-dependent and time-dependent effect of pterostilbene on the intracellular peroxide level of AH109A cells. AH109A cells were cultured with medium containing 0, 25, 50, and 100 μM pterostilbene for 24 h (a) and with medium containing 100 μM pterostilbene for 0, 2, 4, and 24 h (c). After the treatment, the AH109A cells were subjected to intracellular peroxide level analysis using flow cytometer as described in “Materials and Methods” section. The representative results are shown in a and c. Data are mean ± SEM of 3 wells (b, d). Values not sharing a common letter are significantly different by Tukey–Kramer multiple comparison test at p < 0.05
A recent study by Acharya and Ghaskadbi (2013) showed that pterostilbene exhibited strong anti-oxidant activity against free radicals such as diammonium salt, 1,1′-diphenyl-2-picrylhydrazil (DPPH) and 2,2′-azobis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) in vitro in a dose-dependent manner. The free radical scavenging/inhibiting activity of pterostilbene was able to protect proteins, lipids, and DNA of rat liver mitochondria against tertiary-butyl hydroperoxide (TBHP) and hydroxyl radical generated oxidative damage. The presence of free hydroxyl group in pterostilbene is suggested to be crucial for its anti-oxidant properties. Micstacka et al. (2010) evaluated the anti-oxidant effect of resveratrol, pterostilbene, quercetin, and their combination in human erythrocytes in vitro. Quercetin and pterostilbene protected erythrocyte membranes against H2O2-induced lipid peroxidation. The three compounds synergistically protected the erythrocytes against hemolysis and reduced glutathione (GSH) depletion.
On the other hand, a study by Chakraborty et al. (2010) showed that pterostilbene was found to cause apoptosis in MCF-7 breast cancer and PC3 prostate cancer cell lines. The apoptosis was due to the production of reactive oxygen species (mainly H2O2 and singlet oxygen) in MCF-7 and nitric oxide overproduction in PC3 cells. Furthermore, the treatment with anti-oxidant enzymes could not nullify the effect of pterostilbene. A further study by Chakraborty et al. (2012) showed that long term induction by pterostilbene results in autophagy and cellular differentiation in MCF-7 cells via ROS dependent pathway. Treatment with 30 μM of pterostilbene for 72 h increased intracellular lipid accumulation of MCF-7 cells. Pterostilbene also caused an increase in the autophagic marker protein Beclin 1 and microtubule-associated protein 1 light chain 3 (LC3) which lead to autophagy. These effects were observed in association with the loss of mitotoxic and metastatic potential of MCF-7 cells, which was abolished in the presence of catalase and autophagic inhibitor. Another study on gastric cancer carcinoma showed that pterostilbene causes increased ROS, which altered mitochondrial transmembrane potential, causing release of cytochrome-c, followed by activation of caspase triggering programmed cell death (Pan et al. 2007).
The chemopreventive effects of anti-oxidants are generally believed due to their ability to scavenge endogenous ROS, however, an anti-oxidant can switch to pro-oxidant under certain conditions. The prooxidant effect of resveratrol and its synthetic analogues on pBR322 plasmid DNA stand breakage and calf thymus DNA damage in the presence of Cu (II) ion have been studied by Zheng et al. (2006). It was found that in the presence of Cu (II) the calf thymus DNA was remarkably damaged by resveratrol and its analogues, and induced cytotoxicity in human leukemia HL-60 and Jurkat cell lines. These data were supported by Ebara et al. (2000) which found that hepatocellular carcinoma have an accumulation of copper than that in the surrounding liver parenchyma. The accumulation of Cu and the present of metallothionein may be related to the carcinogenesis of HCC. Therefore, DNA damage induced by polyphenols in the presence of Cu (II) may be an important pathway through which cancer cells can be killed while normal cells survive. The increased intracellular ROS level on hepatoma AH109A cells after pterostilbene treatment might trigger signal transduction mechanism involved in the regulation of cell growth, disrupt the normal cell cycle progression, and inhibit the proliferation of AH109A cells.
In conclusion, our data indicated that pterostilbene exerted an anti-proliferative effect against AH109A hepatoma cells without any effect on normal cells. Pterostilbene could be absorbed in the gastrointestinal tract and retained its inhibitory effect against the cells. Pterostilbene arrested the AH109A cells at G0/G1 phase by down-regulating the expression of CDK4 and CDK6 proteins. Pterostilbene treatment also resulted in the increasing intracellular peroxide level. Further studies are needed to elucidate the precise mechanisms of the effect of pterostilbene and are now in progress in our laboratory.
Acknowledgments
The authors are grateful for the generous gift of rat skin fibroblasts from Professor Yoshihiro Nomura of Tokyo University of Agriculture and Technology.
References
- Acharya JD, Ghaskadbi SS. Protective effect of pterostilbene against free radical mediated oxidative damage. BMC Complement Altern Med. 2013;13:238–248. doi: 10.1186/1472-6882-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhami VM, Afaq F, Ahmad N. Involvement of the retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) cells. Biochem Biophys Res Commun. 2001;288:579–585. doi: 10.1006/bbrc.2001.5819. [DOI] [PubMed] [Google Scholar]
- Adrian M, Jeandet P, Douillet-Breuil AC, Tesson L, Bessis R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J Agric Food Chem. 2000;48:6103–6105. doi: 10.1021/jf0009910. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- Alosi JA, McDonald DE, Schneider JS, Privette AR, McFadden DW. Pterostilbene inhibits breast cancer in vitro through mitochondrial depolarization and induction of caspase-dependent apoptosis. J Surg Res. 2010;161:195–201. doi: 10.1016/j.jss.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a guarded response to membrane stimulation. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- Blomen VA, Boonstra J. Cell fate determination during G1 phase progression. Cell Mol Life Sci. 2007;64:3084–3104. doi: 10.1007/s00018-007-7271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Gupta N, Ghosh K, Roy P. In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol In Vitro. 2010;24:1215–1228. doi: 10.1016/j.tiv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Bodipati N, Demonacos MK, Peddinti R, Ghosh K, Roy P. Long term induction of pterostilbene results in autophagy and cellular differentiation in MCF-7 cells via ROS dependent pathway. Mol Cell Endocrinol. 2012;355:25–40. doi: 10.1016/j.mce.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Ebara M, Fukuda H, Satano R, Saisho H, Nagato Y, Suzuki K, Nakajima K, Yukawa M, Kondo F, Nakayama A, Sakurai H. Relationship between copper, zinc and methallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J Hepatol. 2000;33:415–422. doi: 10.1016/S0168-8278(00)80277-9. [DOI] [PubMed] [Google Scholar]
- Ferrer P, Asensi M, Segarra R, Ortega A, Benlloch M, Obrador E, Varea MT, Asensio G, Jorda L, Estrela JM. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia. 2005;7:37–47. doi: 10.1593/neo.04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuendjiep V, Wandji J, Tillequin F, et al. Chalconoid and stilbenoid glycosides from Guibourtia tessmanii. Phytochemistry. 2002;60:803–806. doi: 10.1016/S0031-9422(02)00108-5. [DOI] [PubMed] [Google Scholar]
- Hasiah AH, Ghazali AR, Weber JF, Velu S, Thomas NF, Inayat Hussain SH (2011) Cytotoxic and antioxidant effect of methoxilated stilbene analogues on HepG2 hepatoma and Chang liver cells: implications for structure activity relationship. Hum Exp Toxicol 30:138–144 [DOI] [PubMed]
- Hirakawa N, Miura Y, Yagasaki K. Inhibitory effect of ascorbic acid on the proliferation and invasion of hepatoma cells in culture. Cytotechnology. 2005;47:133–138. doi: 10.1007/s10616-005-3750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44:1299–1305. doi: 10.1016/S0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- Kozuki Y, Miura Y, Yagasaki K. Inhibitory effects of carotenoids on the invasion of rat ascites hepatoma cells in culture. Cancer Lett. 2000;151:111–115. doi: 10.1016/S0304-3835(99)00418-8. [DOI] [PubMed] [Google Scholar]
- Kozuki Y, Miura Y, Yagasaki K. Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative action. Cancer Lett. 2001;167:151–156. doi: 10.1016/S0304-3835(01)00476-1. [DOI] [PubMed] [Google Scholar]
- Lin HS, Yue BD, Ho PC. Determination of pterostilbene in rat plasma by a simple HPLC–UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr. 2009;23:1308–1315. doi: 10.1002/bmc.1254. [DOI] [PubMed] [Google Scholar]
- Manickam M, Ramanathan M, Jahromi MA, Chansouria JP, Ray AB. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J Nat Prod. 1997;60:609–610. doi: 10.1021/np9607013. [DOI] [PubMed] [Google Scholar]
- Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26:1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- Micstacka R, Rimando AR, Ignatowicz E. Antioxidant effect of trans-resveratrol, pterostilbene, quercetin, and their combinations in human erythrocytes in vitro. Plant Foods Hum Nutr. 2010;65:57–63. doi: 10.1007/s11130-010-0154-8. [DOI] [PubMed] [Google Scholar]
- Notas G, Nifli AP, Kampa M, Vercauteren J, Kouroumalis E, Castanas E. Resveratrol exerts its antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest, and NOS activation. Biochim Biophys Acta. 2006;1760:1657–1666. doi: 10.1016/j.bbagen.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Pan MH, Chanh YH, Badmaev V, Nagabushanam K, Ho CT. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J Agric Food Chem. 2007;55:7777–7785. doi: 10.1021/jf071520h. [DOI] [PubMed] [Google Scholar]
- Pan Z, Agarwal AK, Xu T, Feng Q, Baerson SR, Duke SO, Rimando AM. Identification of molecular pathways affected by pterostilbene, a natural dimethylether analog of resveratrol. BMC Med Genomics. 2008;1:7–20. doi: 10.1186/1755-8794-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MH, Chiou YS, Chen WJ, Wang JM, Badmaev V, Ho CT. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis. 2009;30:1234–1242. doi: 10.1093/carcin/bgp121. [DOI] [PubMed] [Google Scholar]
- Remsberg CM, Yanez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Pharmacokinetics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflamatory, antioxidant and analgesic activity. Phytother Res. 2008;22:169–179. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO. Cancer chemopreventive and anti-oxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem. 2002;50:3453–3457. doi: 10.1021/jf0116855. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanism: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Trung LQ, Espinoza JL, Takami A, Nakao S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS ONE. 2013 doi: 10.1371/journal.pone.0055183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000;159:169–173. doi: 10.1016/S0304-3835(00)00545-0. [DOI] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Induction of apoptosis and cell cycle arrest in hepatoma cells by in vivo metabolites of teas. Nutr Cancer. 2000;38:265–273. doi: 10.1207/S15327914NC382_16. [DOI] [PubMed] [Google Scholar]
- Zheng LF, Wei QY, Cai YJ, Fang JG, Zhou B, Yang L, Liu ZL. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: mechanism and structure-activity relationship. Free Radic Biol Med. 2006;41:1807–1816. doi: 10.1016/j.freeradbiomed.2006.09.007. [DOI] [PubMed] [Google Scholar]