Abstract
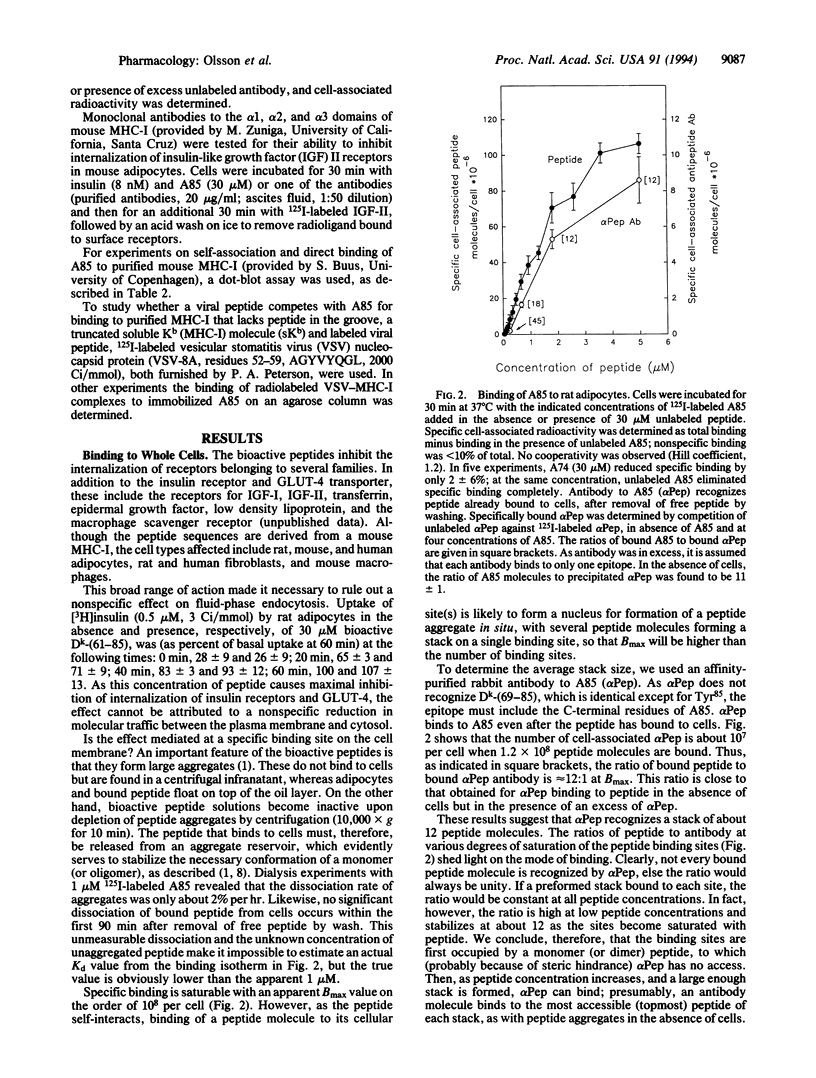
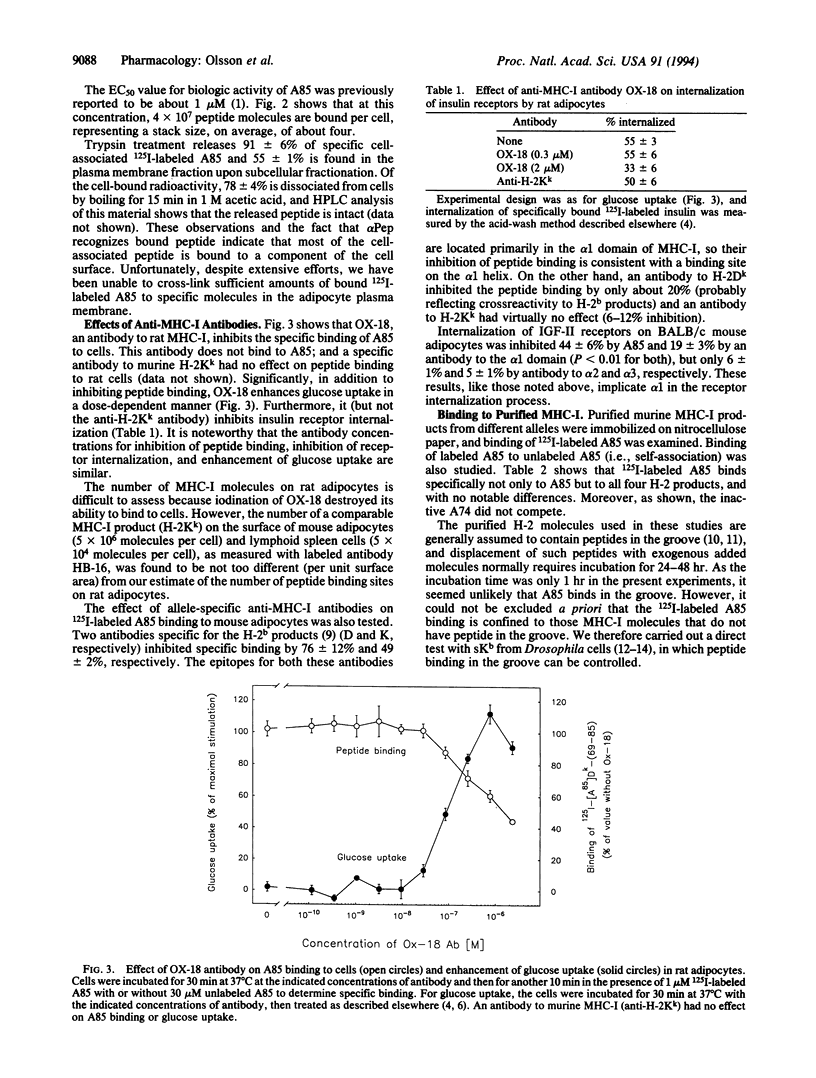
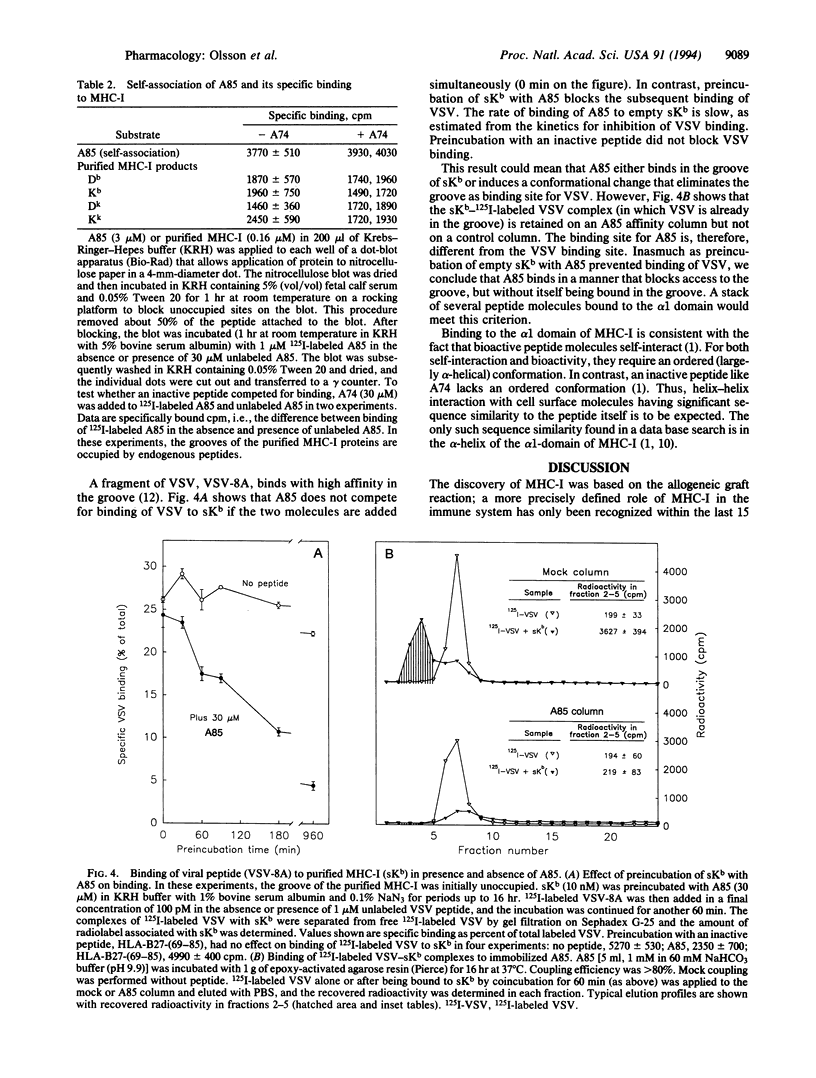
We showed previously that peptides derived from the alpha 1 domain of the major histocompatibility complex class I protein (MHC-I) inhibit internalization of some receptors, thereby increasing the steady-state number of active receptors on the cell surface. In consequence, sensitivity to hormone (e.g., insulin) is enhanced, transport (e.g., of glucose by GLUT-4) is increased, and carrier proteins (e.g., transferrin) operate less efficiently. Now we report that a bioactive peptide (but not closely related inactive ones) binds to MHC-I on the cell surface, not in the groove but apparently to the alpha 1 helix. The binding is saturable, and the number of peptide binding sites on the cell surface approximately equals the number of MHC-I molecules. Antibodies to MHC-I inhibit peptide binding. Most significant, antibodies to MHC-I mimic the effect of a bioactive peptide, inhibiting receptor internalization. These results indicate that MHC-I participates in the regulation of cell surface receptor activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix M., Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J Exp Med. 1992 Sep 1;176(3):829–834. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Constantine K. L., Mapelli C., Meyers C. A., Friedrichs M. S., Krystek S., Mueller L. Micelle-bound conformational preferences of a peptide derived from a murine major histocompatibility complex class I molecule. J Biol Chem. 1993 Oct 25;268(30):22830–22837. [PubMed] [Google Scholar]
- Corr M., Boyd L. F., Frankel S. R., Kozlowski S., Padlan E. A., Margulies D. H. Endogenous peptides of a soluble major histocompatibility complex class I molecule, H-2Lds: sequence motif, quantitative binding, and molecular modeling of the complex. J Exp Med. 1992 Dec 1;176(6):1681–1692. doi: 10.1084/jem.176.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Samson M., Pilch P. F., Fehlmann M. Internalization of insulin receptors and HLA antigens in human hepatoma cells. Biochem J. 1987 Mar 1;242(2):403–410. doi: 10.1042/bj2420403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Peyron J. F., Samson M., Van Obberghen E., Brandenburg D., Brossette N. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8634–8637. doi: 10.1073/pnas.82.24.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Grusby M. J., Auchincloss H., Jr, Lee R., Johnson R. S., Spencer J. P., Zijlstra M., Jaenisch R., Papaioannou V. E., Glimcher L. H. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson S. C., Tope W. D., Tredgett E. M., Windle J. M., Diamond A. G., Howard J. C. Cloning and expression of class I major histocompatibility complex genes of the rat. J Exp Med. 1992 Jun 1;175(6):1749–1757. doi: 10.1084/jem.175.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Brown J. H., Gorga J. C., Stern L. J., Urban R. G., Chi Y. I., Stauffacher C., Strominger J. L., Wiley D. C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994 Apr 21;368(6473):711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Saito Y., Jackson M. R., Song E. S., Peterson P. A. In vitro peptide binding to soluble empty class I major histocompatibility complex molecules isolated from transfected Drosophila melanogaster cells. J Biol Chem. 1992 Nov 25;267(33):23589–23595. [PubMed] [Google Scholar]
- Phillips M. L., Moule M. L., Delovitch T. L., Yip C. C. Class I histocompatibility antigens and insulin receptors: evidence for interactions. Proc Natl Acad Sci U S A. 1986 May;83(10):3474–3478. doi: 10.1073/pnas.83.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Schlessinger J., Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984 Feb;98(2):725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen M., Olsson L. Possible roles of compound membrane receptors in the immune system. Ann Immunol (Paris) 1983 Jul-Aug;134D(1):85–92. doi: 10.1016/s0769-2625(83)80059-2. [DOI] [PubMed] [Google Scholar]
- Solano A. R., Cremaschi G., Sánchez M. L., Borda E., Sterin-Borda L., Podestá E. J. Molecular and biological interaction between major histocompatibility complex class I antigens and luteinizing hormone receptors or beta-adrenergic receptors triggers cellular response in mice. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5087–5091. doi: 10.1073/pnas.85.14.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagsted J., Baase W. A., Goldstein A., Olsson L. A preformed, ordered structure of a 25-residue peptide derived from a major histocompatibility complex class I antigen is required to affect insulin receptor function. J Biol Chem. 1991 Jul 15;266(20):12844–12847. [PubMed] [Google Scholar]
- Stagsted J., Hansen T., Roth R. A., Goldstein A., Olsson L. Correlation between insulin receptor occupancy and tyrosine kinase activity at low insulin concentrations and effect of major histocompatibility complex class I-derived peptide. J Pharmacol Exp Ther. 1993 Nov;267(2):997–1001. [PubMed] [Google Scholar]
- Stagsted J., Mapelli C., Meyers C., Matthews B. W., Anfinsen C. B., Goldstein A., Olsson L. Amino acid residues essential for biological activity of a peptide derived from a major histocompatibility complex class I antigen. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7686–7690. doi: 10.1073/pnas.90.16.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagsted J., Olsson L., Holman G. D., Cushman S. W., Satoh S. Inhibition of internalization of glucose transporters and IGF-II receptors. Mechanism of action of MHC class I-derived peptides which augment the insulin response in rat adipose cells. J Biol Chem. 1993 Oct 25;268(30):22809–22813. [PubMed] [Google Scholar]
- Stagsted J., Reaven G. M., Hansen T., Goldstein A., Olsson L. Regulation of insulin receptor functions by a peptide derived from a major histocompatibility complex class I antigen. Cell. 1990 Jul 27;62(2):297–307. doi: 10.1016/0092-8674(90)90367-n. [DOI] [PubMed] [Google Scholar]
- Stagsted J., Ziebe S., Satoh S., Holman G. D., Cushman S. W., Olsson L. Insulinomimetic effect on glucose transport by epidermal growth factor when combined with a major histocompatibility complex class I-derived peptide. J Biol Chem. 1993 Jan 25;268(3):1770–1774. [PubMed] [Google Scholar]
- Verland S., Simonsen M., Gammeltoft S., Allen H., Flavell R. A., Olsson L. Specific molecular interaction between the insulin receptor and a D product of MHC class I. J Immunol. 1989 Aug 1;143(3):945–951. [PubMed] [Google Scholar]
- Wang C. R., Lindahl K. F. HMT, encoded by H-2M3, is a neoclassical major histocompatibility class I antigen. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2784–2788. doi: 10.1073/pnas.90.7.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]



