Abstract
Hyperkalemia can present with a spectrum of clinical manifestations with progressive EKG changes and life-threatening arrhythmias. Although no formal guidelines exist as to when to initiate treatment for hyperkalemia, it is generally recommended in clinically symptomatic patients with or without EKG changes. Timely diagnosis and reversal can relieve symptoms and prevent life-threatening arrhythmias. We review the EKG changes associated with hyperkalemia and management principles along with an example of a case of severe hyperkalemia resulting in arrhythmia and flaccid paralysis.
Keywords: hyperkalemia, arrhythmia, paralysis
Hyperkalemia can present with a spectrum of clinical manifestations ranging from asymptomatic to life-threatening arrhythmias and secondary hyperkalemic paralysis (1) depending on the serum concentration and the underlying comorbidities, such as concomitant renal failure. Prompt diagnosis and treatment can lead to complete resolution of symptoms and termination of arrhythmias and paralysis, whereas delays can lead to death. We report a case of severe hyperkalemia resulting in arrhythmia and flaccid paralysis as a springboard to review the Electrocardiogram (EKG) changes associated with hyperkalemia and management principles.
Case
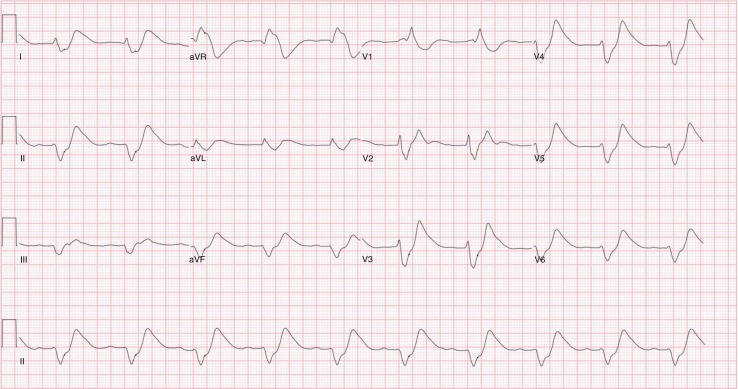
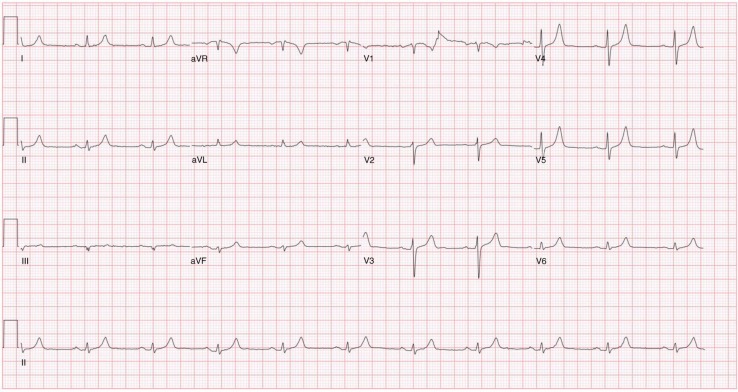
A 68-year-old female presented to the emergency department with progressive weakness involving the upper and lower extremities and subsequent difficulty with ambulation for 1 week. She was unable to get out of bed and walk on the day of presentation. Past medical history was significant for Gitelman's syndrome, with very labile, but generally very low, potassium levels being maintained on potassium sparing diuretics (eplerenone) and supplemental potassium 300 mEq/day. On presentation, she was afebrile with blood pressure, 106/51 mm of Hg; heart rate, 96 beats/min; respiratory rate, 16/min; and normal oxygen saturation on room air. Motor exam was significant for reduced power of 60 percent in both upper and lower extremities with flaccidity and diminished reflexes bilaterally. Sensation and cranial nerves were intact. Initial labs were as follows: sodium 131 mEq/L (range 135–153 mEq/L), potassium 9.9 mEq/L (range 3.5–5.3 mEq/L), blood urea nitrogen 41 mg/dL (range 5–26 mg/dL), and creatinine 2.1 mg/dL with baseline of 1.0 mg/dL (range 0.50–1.50 mg/dL). EKG showed dramatically widened QRS complex and tall broad T waves (Fig. 1). Emergent therapy for hyperkalemia was started with 1 g of intravenous calcium gluconate, 10 units of regular insulin with 50 g of dextrose, and 100 mEq of sodium bicarbonate. She also received albuterol nebulization and intravenous furosemide 120 mg. A dialysis line was placed and emergent dialysis was done with improvement of her potassium to 4.8 mEq/L post-dialysis. Neurological symptoms and EKG returned to baseline (Fig. 2). She subsequently became hypokalemic requiring potassium repletion. Diuretics (torsemide, zaroxolyn, and eplerenone) were stopped and she was discharged on potassium chloride 60 mEq three times daily for maintenance. She shortly thereafter became profoundly hypokalemic and her maintenance dose of potassium was increased to 300 mEq/day.
Fig. 1.
Electrocardiogram (EKG) at presentation showing dramatically widened QRS complex and tall broad T waves.
Fig. 2.
EKG showing normalization of the rhythm to baseline after initial treatment of hyperkalemia.
Discussion
Potassium (K + ) is a very tightly regulated cation present ubiquitously inside and outside all living cells. It is responsible for maintaining the cell membrane potential required for physiological functioning. As significant K+ gradient exists between inside and outside of cells, even a slight change in extracellular K+ level has a significant physiologic impact. Muscles, including the heart and nerves, are affected the most.
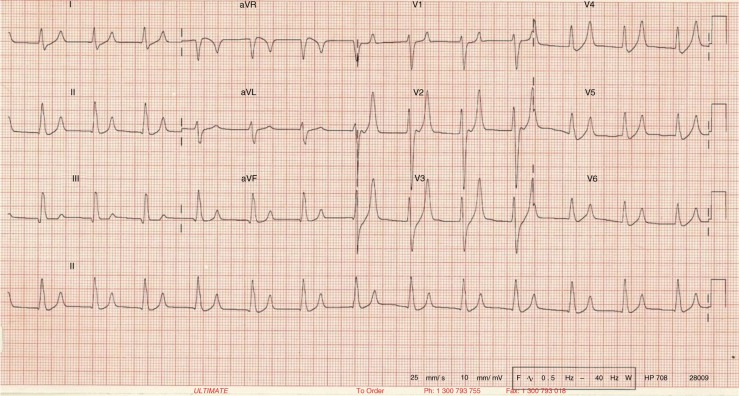
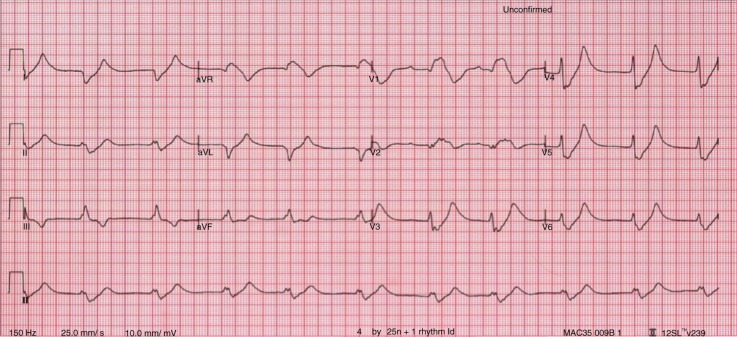
The changes in K+ (hyper- or hypo-) have significant effect on heart muscle and its conduction system. Although absolute serum K+ level usually correlates with clinical symptoms, the rate of change is more important. EKG has an important role in detecting and delineating the effect of change. It is not very sensitive (0.34–0.43) but the changes, if present, are specific (0.85–0.86) (2, 3). The most widely used reference range for normal serum K+ level is 3.5–5.5 mEq/L. Any value >6.5 mEq/L is an important cause of morbidity and mortality (4). EKG changes tend to occur above this level in a typical pattern (Fig. 3) due to differences in sensitivity of cardiac tissues (in decreasing order – atria, ventricle, His cells, atrial nodes, and inter-atrial pathways) to the change in serum K+ level. The earliest change is peaking of symmetric T waves with narrow base in precordial (V2–V4) leads, defined as any T wave taller than the corresponding R wave (Fig. 4). Atria are the earliest to be affected (up to levels of 7.5 mEq/L) manifesting as lengthening of PR interval followed by flattening and subsequent loss of P waves. This transforms the EKG pattern into development of “nodal” rhythm with absent P waves. By this time, the ventricles will also start to get affected manifesting as widening of QRS. If hyperkalemia sustains or worsens, conduction system is also affected. At levels between 7.0 and 8.0 mEq/L, QRS continues to widen and can merge with T wave to form a “sine wave” pattern (Fig. 5), which is an indicator of impending ventricular fibrillation and cardiac arrest (5).
Fig. 3.
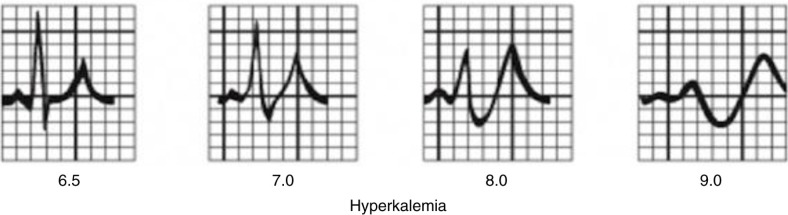
Diagrammatic representation of EKG changes with increasing hyperkalemia (Courtesy of www.lifeinthefastlane.com/hyperkalemia/).
Fig. 4.
EKG showing tall peaked symmetric T waves with narrow base (EKG courtesy, www.lifeinthefastlane.com/hyperkalemia/).
Fig. 5.
EKG showing sine wave pattern (EKG courtesy, www.lifeinthefastlane.com/hyperkalemia/).
Management of hyperkalemia involves addressing three different aspects: (1) preventing immediate complications; (2) correcting the serum K+ level to a physiologically stable level; and (3) elimination of excess potassium from the body. Most immediate complications are due to cardiac arrhythmias. This is controlled with infusion of calcium in the form of calcium gluconate or chloride, which stabilizes the cardiac membrane potential. Immediate correction of serum K+ levels can be achieved with medications that affect the transcellular shift of K+. Insulin and beta-agonists are the major agents used for this purpose. Used together, they have a synergistic effect. Insulin should, however, be used in conjunction with dextrose to prevent hypoglycemia. Bicarbonate infusion, although less effective, may be used if there is co-existing non-gap metabolic acidosis. Next, the elimination of elemental K+ from the body can be achieved via the kidneys or dialysis (2). Chelating agents, such as sodium polystyrene, are not recommended now because of delayed action, questionable efficacy, and a serious side-effect profile, including intestinal necrosis and undesirable sodium load (3, 5, 6). Recent clinical trials have shown promise for two different oral medications with more selective K+ binding. Patiromer in patients with chronic kidney disease (7) and sodium zirconium cyclosilicate in patients with varying causes of hyperkalemia (8) have been shown to be effective in the reduction and maintenance of normokalemia in these patients. However, these medications have only been studied in the outpatient population with mild to moderate hyperkalemia (<6.5 mEq/L) and no EKG changes. Hence, their efficacy in the acute emergency setting as a substitute for sodium polystyrene remains debatable. Similarly, loop diuretics can be used for the elimination of K+ via kidneys if renal function is intact. Dialysis can be used as a last resort for reduction of total body potassium (2).
Hyperkalemia can present with a spectrum of clinical symptoms with progressive EKG changes. Although no formal guidelines exist as to when to initiate treatment for hyperkalemia, it is generally recommended in clinically symptomatic patients with or without EKG changes. Timely diagnosis and reversal can relieve symptoms and prevent life-threatening arrhythmias.
Authors' contributions
Authors PK, DRP, and RP conceived, designed, and participated in literature review and drafting of the initial manuscript. AR and RA provided intellectual content, edited subsequent draft, and approved the final manuscript. PK is responsible as the overall guarantor of the content.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Kimmons LA, Usery JB. Acute ascending muscle weakness secondary to medication-induced hyperkalemia. Case Rep Med. 2014;2014:e789529. doi: 10.1155/2014/789529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2):329–47. doi: 10.1016/j.emc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Wrenn KD, Slovis CM, Slovis BS. The ability of physicians to predict hyperkalemia from the ECG. Ann Emerg Med. 1991;20(11):1229–32. doi: 10.1016/s0196-0644(05)81476-3. [DOI] [PubMed] [Google Scholar]
- 4.Rastegar A. Serum potassium. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: The history, physical, and laboratory examinations. 3rd ed. Boston, MA: Butterworths; 1990. Available from: http://www.ncbi.nlm.nih.gov/books/NBK307/ [cited 7 January 2015] [PubMed] [Google Scholar]
- 5.Alfonzo AVM, Isles C, Geddes C, Deighan C. Potassium disorders—clinical spectrum and emergency management. Resuscitation. 2006;70(1):10–25. doi: 10.1016/j.resuscitation.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Varriale P, Ngai L. Sodium polystyrene sulfonate use revisited. Am J Med. 2014;127(8):e37. doi: 10.1016/j.amjmed.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 8.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–31. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]