Abstract
Introduction
Approximately 25 million people in the United States visit their primary care physician each year for acute respiratory infections (ARI). They are a common cause of unnecessary prescription of antibiotics; despite well-validated national treatment guidelines, around 73% of adults with ARI are prescribed antibiotics in the United States. Inappropriate use of antibiotics has profound implications.
Methods
Our aim was to increase adherence to antibiotic guidelines for treatment of ARI in an internal medicine outpatient practice. We used a package of active and passive interventions to improve physician awareness of treatment guidelines; these included short sessions of didactic teaching, antibiotic guidelines posters in patient examination rooms and staff areas, clinical decision support (CDS) tools integrated into the electronic medical record system, guideline adherence report cards for providers, and reiteration of CDS tool use and guideline adherence at monthly group meetings. Process measures were the rate of use of CDS tools for the management of ARI and patient callbacks within 72 h for the same issue. Outcome measures were compliance with antibiotic prescribing guidelines.
Results
Our low-cost interventions led to a significant improvement in ARI treatment guideline adherence. There was improvement in compliance with treatment guidelines for sinusitis (90.90% vs. 57.58%, p<0.001), pharyngitis (64.28% vs. 25.00%, p=0.003), upper respiratory infection (96.18% vs. 73.68%, p=0.008), and the aggregated measure of ARI (91.25% vs. 78.6%, p<0.001). Rate of CDS tool usage was 40.5% with a 72-h callback rate of 0.05%.
Conclusion
Simple, low-cost interventions can improve appropriate antibiotic use for ARI and change the prescribing habits of providers in an outpatient setting. Provider and patient education is a vital component of antibiotic stewardship. Simple interventions for common outpatient conditions can have a positive impact on patient outcomes and reduce unnecessary healthcare costs.
Keywords: antibiotics, guidelines, upper respiratory tract infection, sinusitis, pharyngitis, acute respiratory tract infections
Inappropriate usage of antibiotics is the key driver of antibiotic resistance worldwide (1, 2). Over a 3-year period, antibiotics were prescribed in more than 101 million ambulatory visits across the United States, representing fully 10% of all outpatient visits (3). Of these, 41% of antibiotic prescriptions were written for a respiratory tract infection, in which the clinical benefit is known to be, at best, limited, and at worst, detrimental to the patient's health through their exposure to side effects, medication interactions, and complications, including Clostridium difficile colitis (3–5). Another contributor to antibiotic resistance is patient non-adherence to antibiotics, with up to 40% of patients failing to appropriately complete their antibiotic regimen (6, 7). To address these concerns national educational campaigns were launched in the 1990s, including the United States Centers for Disease Control and Prevention (CDC) ‘Get Smart: Know When Antibiotics Work’ campaign launched in 1995 (8). These awareness efforts, as well as the use of routine childhood immunizations with pneumococcal conjugate vaccine, are believed to have decreased antibiotic prescription rates in child and adolescent populations. However, in the adult population there was no change in overall antibiotic prescribing patterns from the year 2000 to 2010. In addition, there was an overall increase in broad spectrum antibiotic prescribing during this time (9).
Given the existing unacceptably high antibiotic prescription rates in our patient population, this quality improvement initiative aimed to build on previously published work to reduce rates of inappropriate antibiotic usage for the diagnoses of upper respiratory infection (URI), sinusitis, and pharyngitis, collectively called acute respiratory infections (ARI) (10, 11). A needs assessment was performed after the first Plan-Do-Study-Act (PDSA) cycle. A PDSA cycle is a commonly used tool in continuous quality improvement; where a problem and the required change are identified, changes are then implemented with the subsequent results being measured and analyzed to determine next steps (12). In this way, small tests of change are performed successively in a real work setting to ensure changes result in improvement (13). The results of our initial PDSA cycle indicated that the largest areas of perceived need were a point of care clinical decision support (CDS) tool and recurring, active educational interventions with real-time feedback. To address these issues, CDS tools were created to calculate a Modified Centor Score and to determine whether a sinus infection met the criteria for a bacterial sinusitis and antibiotic therapy. Active educational interventions were instituted including provider report cards, academic detailing, and inclusion into the residency quality improvement curriculum (10, 11). The purpose of this study was to examine the effects of the CDS tool and educational support on appropriate antibiotic guideline adherence for ARIs.
Methods and analysis
This was the second PDSA cycle in a quality improvement process carried out at an internal medicine outpatient practice at a university affiliated community hospital internal medicine residency program (10, 11). The initial PDSA cycle involved a package of passive education-based small-scale interventions that demonstrated a small improvement in adherence to appropriate antibiotic prescribing guidelines (10). Following these initial efforts, further analysis was done to identify an additional seven interventions for the second PDSA cycle (Table 1). The first intervention occurred in October 2013 and was an educational session regarding appropriate usage of antibiotics in ARI conducted for internal medicine residents and faculty. In November 2013, summaries of CDC antimicrobial guidelines for treatment of URIs, sinusitis, and pharyngitis were placed in patient examination rooms, staff conference areas, and patient and employee restrooms (14–16). A CDS for appropriate antimicrobial usage integrated into the Epic electronic health record (Epic Systems Corporation, Verona, Wisconsin) was initiated in December 2013. Regular monthly reminders to adhere to antibiotic adherence guidelines and use CDS were sent via email to all practice providers (22 residents, 1 nurse practitioner, and 5 attending physicians). Further reinforcement regarding antibiotic guideline adherence and use of the CDS were included via academic detailing by team members in monthly practice meetings. In addition, visual prompts to use the CDS for appropriate diagnoses were placed at each computer workstation in the office. In March 2014, the final intervention, provider report cards, were sent to the clinicians outlining their adherence to guidelines from October 2013 to February 2014 (Table 1).
Table 1.
Timeline for interventions in PDSA cycles 1 and 2
| Three simultaneous interventions in October 2012 | ||
|---|---|---|
| First PDSA cycle | Date | Intervention |
| Second PDSA cycle | October 2013 | Education session for attendings and residents |
| November 2013 | CDC antibiotic guideline summaries in patient examination rooms, conference room, staff and patient restrooms | |
| Monthly email reminders regarding antibiotic guideline adherence and CDS tool usage | ||
| Reinforcement in monthly practice meetings | ||
| December 2013 | CDS tool available in EPIC | |
| February 2014 | Visual reminders for CDS tool on every computer in the practice | |
| March 2014 | Individual provider report cards regarding guideline adherence | |
A physician author (RH) performed a chart review to determine rates of appropriate antibiotic guideline adherence, CDS tool usage rate, and patient callback within 72 h based on predefined criteria for inclusion and exclusion (10, 11). A second physician author (RA) independently reviewed the first 10% of encounters and then subsequently every 10th encounter to assess inter-rater variability. There was 100% consensus between the two independent reviewers.
Data were pooled by year, individual diagnosis (URI, sinusitis, pharyngitis), aggregate diagnosis ARI, and rate of guideline adherence. Analysis was performed by chi-square test of association using the statistical software SPSS version 20.0.0 (International Business Machines, Armonk, NY) with significance of results determined by p<0.05.
Results
Patient characteristics
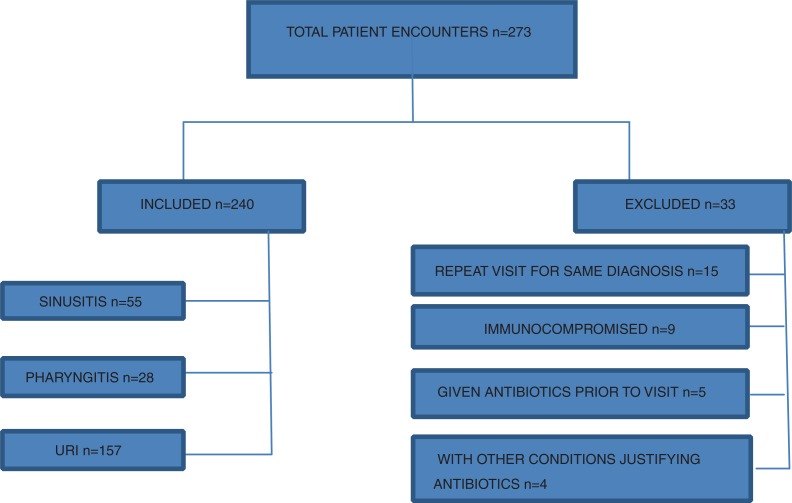
From 24 October 2013 to 15 July 2014, a total of 273 patients were identified using the Epic electronic health record by reviewing acute patient visits for diagnoses of URI, sinusitis, or pharyngitis. Of these encounters, 240 patients met the inclusion criteria of acute visits that were identified in the EPIC (EPIC Systems Corporation, Verona, WI) medical health records system using visit diagnosis of URI, pharyngitis, and sinusitis. The remainder met the exclusion criteria of active malignancy, immunocompromised state, including HIV infection, more than one visit for the same presentation during the study period, and concurrent use of antibiotics for another etiology, for example, urinary tract infection (Fig. 1).
Fig. 1.
Patient population and subgroups in PDSA cycle 2.
Outcomes measures
Since 2012, adherence to antibiotic guidelines continued to improve with the implementation of the iterative interventions described (10, 11). A total of 240 patients were eligible to be included in this series of interventions. Of these patients, 55 (22.92%) were treated for sinusitis, 28 (11.66%) for pharyngitis, and 157 (65.42%) for URI. The adherence rate for each of these subsets improved to 90.90% for sinusitis (p<0.001), 64.28% for pharyngitis (p=0.003), and 96.18% for URI (p=0.008). The adherence rate for the aggregated measure of ARI was 91.25% (p<0.001), an increase from the ARI adherence rate of 78.68% from the previous PDSA cycle in 2012–2013 (Table 2).
Table 2.
Guideline adherence rates comparing data prior to intervention, PDSA cycle 1, PDSA cycle 2
| Guidelines adherence | 2008–2012 Prior to intervention | 2012–2013 PDSA cycle-1 | 2013–2014 PDSA cycle-2 | p | |
|---|---|---|---|---|---|
| Sinusitis | Yes | 93 | 19 | 50 | <0.001 |
| No | 130 | 14 | 5 | ||
| Total | 223 | 33 | 55 | ||
| Adherence (%) | 41.70 | 57.58 | 90.90 | ||
| Pharyngitis | Yes | 33 | 7 | 18 | 0.0031 |
| No | 104 | 21 | 10 | ||
| Total | 147 | 28 | 28 | ||
| Adherence (%) | 24.09 | 25.00 | 64.28 | ||
| URI | Yes | 287 | 194 | 151 | 0.0083 |
| No | 75 | 25 | 6 | ||
| Total | 362 | 219 | 157 | ||
| Adherence (%) | 79.28 | 88.58 | 96.18 | ||
| ARI | Yes | 413 | 220 | 219 | <0.001 |
| No | 309 | 60 | 21 | ||
| Total | 722 | 280 | 240 | ||
| Adherence (%) | 57.20 | 78.68 | 91.25 | ||
Process measures
Process measures included rate of usage of the CDS tool. The CDS tool was made available for use in December 2013. In the 179 encounters that occurred after the CDS tool rollout, the tool was used in 71 encounters (39.7%).
Balancing measure
Balancing measures were callback rates within 72 h of office visit. There were 11 callbacks within 72 h for the same clinical issue during the entirety of the study period, for a rate of 0.05%, which was unchanged from prior interventions.
Discussion
This quality improvement initiative shows an improvement in overall antibiotic guideline adherence for all process and outcome measures demonstrating that simple, low-cost, iterative interventions can change the prescribing habits of providers. Interventions were chosen on the basis of a needs assessment following the findings of previous PDSA cycles. Previous work indicated that limited-scale passive interventions could generate moderate change but had not yielded the desired levels of antibiotic guideline adherence. By basing our most recent interventions on the needs assessment, we were able to more specifically target areas that would produce the most substantial changes.
The overall antibiotic guideline adherence rate was 91.25%, despite the CDS tool being used in approximately 40% of visits suggesting the other interventions had a positive impact independent of CDS tool usage. The balancing measures suggest high adherence to clinical guidelines with only 0.05% of encounters resulting in a 72-h callback, of which the majority of patients reported symptoms of double worsening which includes worsening cough, nasal discharge and fevers after initial improvement. This rate was unchanged from historical controls, indicating that previously identified fears that antibiotic stewardship would increase condition workload post-visit were unfounded.
This multiyear process was conducted in a combined resident–faculty practice, which is a potential limitation as not all providers were exposed to all interventions. However, even with this variation there was still a statistically significantly improvement in guideline adherence.
Future reassessment of guideline adherence will demonstrate whether our iterative approach to creating an antibiotic stewardship focused cultural change has persisted within this practice in the absence of continued active interventions. The CDS tool remains in EPIC for provider use in the future.
A potential confounder for this study is the change in the pattern of outpatient encounters for these illnesses. Barnett et al. demonstrated that between 1997 and 2010, visits for pharyngitis in a primary care setting decreased from 7.5 to 4.3% of all visits; however, visits for pharyngitis to emergency departments remained unchanged at around 2.3% (17). In addition to seeking healthcare at the emergency department, there has been an increase in the availability of stand-alone urgent care centers that cater to this patient population (i.e., ‘minute clinics’). Decreased frequency of outpatient encounters for ARI may also be related to improved public awareness of disease processes; availability of advice from other sources, such as the Internet; and medical insurance policy changes where antibiotics may no longer be covered for a certain subset of diagnoses, based on ICD-9 codes used in billing encounters as noted in the operations bulletin for the Geisinger Health Plan-2014. Regardless, it is highly likely that ARI will continue to represent one of the most common diagnoses assessed in the ambulatory setting, and more appropriate antibiotic usage will remain a public health priority.
Simple, low-cost interventions can improve appropriate antibiotic use for ARIs and change the prescribing habits of providers in an outpatient setting. Provider and patient education is a vital component of antibiotic stewardship with a potential for positive impact on patient outcomes and reduction of unnecessary healthcare costs. Physician education including academic detailing and the use of CDS-based interventions are promising areas for improving adherence to antibiotic prescribing guidelines and deserve further study for optimization and avoidance of extinction.
Disclaimer
The authors attest that the materials presented are not under review for publication elsewhere and have not previously been submitted. The authors report no external funding source for this work. The authors have no conflicts of interest to disclose with regards to this work. All authors attest that they made substantial contributions to conception, acquisition of data, or analysis and interpretation of data; drafted the article or revised it critically for important intellectual content; and approved the final version to be published.
Conflicts of interest and funding
The authors have no conflict of interest to disclose with regards to this work. The authors report no external funding source for this work.
References
- 1.United States Department, Health and Human Services, Centers for Disease Control and Publication. Antibiotic resistance threats in the United States, 2013. 2013. Available from: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf#page=6 [cited 17 February 2014]
- 2.Monitoring and management of bacterial resistance to antimicrobial agents: A World Health Organization symposium. Geneva, Switzerland, 29 November-2 December, 1995. Clin Infect Dis Off Publ Infect Dis Soc Am. 1997;24(Suppl 1):S1–176. [PubMed] [Google Scholar]
- 3.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69(1):234–40. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 4.Lipsitch M. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg Infect Dis. 2002;8(4):347–54. doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 6.Kardas P. Patient compliance with antibiotic treatment for respiratory tract infections. J Antimicrob Chemother. 2002;49(6):897–903. doi: 10.1093/jac/dkf046. [DOI] [PubMed] [Google Scholar]
- 7.Kardas P, Devine S, Golembesky A, Roberts C. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–13. doi: 10.1016/j.ijantimicag.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Get smart: Know when antibiotics work. Available from: http://www.cdc.gov/getsmart/campaign-materials/about-campaign.html [cited 17 February 2014]
- 9.Lee GC, Reveles KR, Attridge RT, Lawson KA, Mansi IA, Lewis JS, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014;12:96. doi: 10.1186/1741-7015-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alweis R, Greco M, Wasser T, Wenderoth S. An initiative to improve adherence to evidence-based guidelines in the treatment of URIs, sinusitis, and pharyngitis. J Community Hosp Intern Med Perspect. 2014;4:22958. doi: 10.3402/jchimp.v4.22958. http://dx.doi.org/10.3402/jchimp.v4.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crocker A, Alweis R, Scheirer J, Schamel S, Wasser T, Levingood K. Factors affecting adherence to evidence-based guidelines in the treatment of URI, sinusitis, and pharyngitis. J Community Hosp Intern Med Perspect. 2013;3(2):20744. doi: 10.3402/jchimp.v3i2.20744. http://dx.doi.org/10.3402/jchimp.v3i1.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Best M, Neuhauser D. Walter A Shewhart, 1924, and the Hawthorne factory. Qual Saf Health Care. 2006;15(2):142–3. doi: 10.1136/qshc.2006.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau CY. Quality improvement tools and processes. Neurosurg Clin N Am. 2015;26(2):177–87. doi: 10.1016/j.nec.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales R. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: Background. Ann Intern Med. 2001;134(6):490. doi: 10.7326/0003-4819-134-6-200103200-00015. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales R. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: Background, specific aims, and methods. Ann Intern Med. 2001;134(6):479. doi: 10.7326/0003-4819-134-6-200103200-00013. [DOI] [PubMed] [Google Scholar]
- 16.Hickner JM. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: Background. Ann Intern Med. 2001;134(6):498. doi: 10.7326/0003-4819-134-6-200103200-00017. [DOI] [PubMed] [Google Scholar]
- 17.Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997–2010. JAMA Intern Med. 2014;174(1):138–40. doi: 10.1001/jamainternmed.2013.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]