Abstract
Maldevelopment of the collecting system resulting in urinary tract obstruction (UTO) is the leading identifiable cause of CKD in children. Specific etiologies are unknown; most cases are suspected by discovering hydronephrosis on prenatal ultrasonography. Congenital UTO can reduce nephron number and cause bladder dysfunction, which contribute to ongoing injury. Severe UTO can impair kidney growth in utero, and animal models of unilateral ureteral obstruction show that ischemia and oxidative stress cause proximal tubular cell death, with later development of interstitial fibrosis. Congenital obstructive nephropathy therefore results from combined developmental and obstructive renal injury. Due to inadequacy of available biomarkers, criteria for surgical correction of upper tract obstruction are poorly established. Lower tract obstruction requires fetal or immediate postnatal intervention, and the rate of progression of CKD is highly variable. New biomarkers based on proteomics and determination of glomerular number by MRI should improve future care. Angiotensin inhibitors have not been effective in slowing progression, although avoidance of nephrotoxins and timely treatment of hypertension are important. Because congenital UTO begins in fetal life, smooth transfer of care from perinatologist to pediatric and adult urology and nephrology teams should optimize quality of life and ultimate outcomes for these patients.
Keywords: urinary tract obstruction, child, chronic kidney disease, progression, biomarkers
Congenital anomalies of the kidneys and urinary tract account for the majority of CKD in children, and congenital urinary tract obstruction (UTO) is the leading cause of pediatric end-stage kidney disease.1 Although complications of diabetes and hypertension are the dominant causes of kidney failure in adults, it is now recognized that in most children requiring renal replacement therapy for congenital urinary tract anomalies, the onset of kidney failure is delayed until adulthood.2,3 It is therefore appropriate that perinatologists along with pediatric and adult nephrologists and urologists develop an understanding of the natural history of these disorders. This is particularly important as specialty care is transferred from obstetrics to pediatric and then adult nephrologists and urologists. To optimize outcomes, such transitions require close communication and coordination of services throughout the life of the patient
Pathogenesis of congenital urinary tract obstruction
Factors contributing to maldevelopment of kidneys and urinary tract are poorly understood. Candidate genes have been identified in murine spontaneous congenital hydronephrosis, and knockout mice with a hydronephrotic phenotype have been studied to determine underlying mechanisms.4 These include abnormalities of ureteral or bladder development, and dysfunctional ureteral peristalsis leading to functional (not mechanical) UTO.5
Significant advances have been made in understanding the cell and molecular biology of nephrogenesis, and it is now recognized that the number of nephrons per kidney can vary by over ten-fold in normal individuals.6 Maturing nephrons adapt to the number of nephrons formed: glomeruli and tubules exhibit compensatory growth when nephrogenesis is terminated before the normal range of nephron number is reached.7 Human nephrogenesis is complete by 36 weeks gestation, and additional nephrons are not formed after term birth. Preterm, particularly very low birth weight infants, are born with low nephron number, and preliminary reports suggest that normal nephrogenesis does not continue postnatally.8
Multiple animal models have been developed to unravel the pathophysiology of congenital obstructive nephropathy, which results from the superposition of obstructive renal injury and developmental injury.9 Surgical obstruction of the ureter has become the most widely-employed animal model of CKD, with renal interstitial fibrosis serving as the primary end-point.10 We have recently reported that complete unilateral ureteral obstruction (UUO) in the adult mouse results in rapid loss of renal parenchyma due to a 65% reduction in proximal tubular mass, the result of cell death by necrosis, apoptosis, and autophagy (Fig. 1).11 Progressive tubular atrophy leads to the formation of numerous atubular glomeruli.12 Complete UUO results in tubular oxidative stress and reduced renal metabolism and oxygen consumption (largely contributed by proximal tubular cells) (Fig. 1).11 It is likely that this is attributable to mitochondrial damage and decreased generation of ATP.13,14
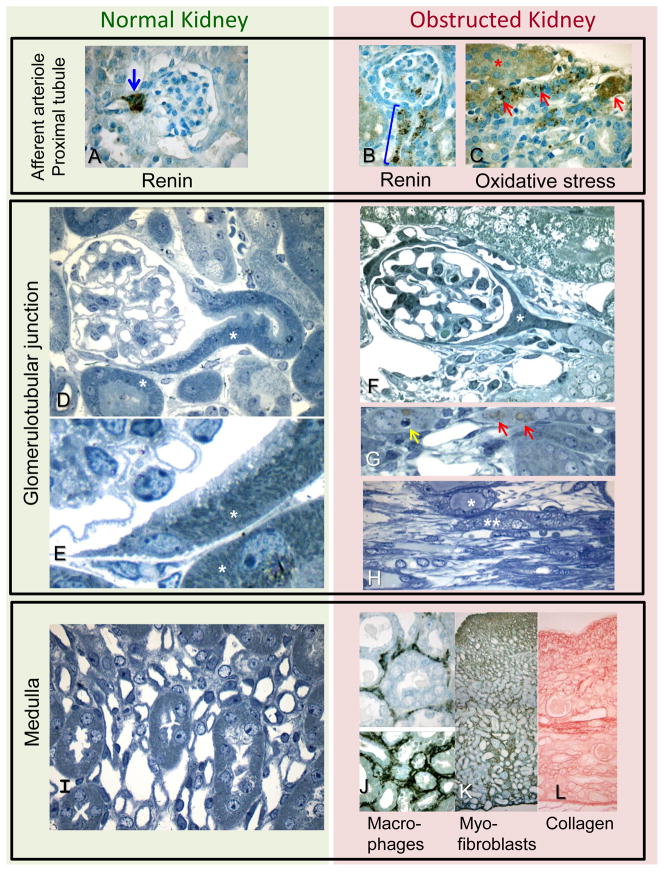
Figure 1.
Photomicrographs of normal kidney (left column) and kidney from adult mouse with complete ipsilateral unilateral ureteral obstruction (UUO) of 14 days duration (right column): original images not previously published; representative of morphometric studies.11,12 Similar cellular responses are exhibited following partial or complete UUO in the neonate.15,23
Top Panel. Afferent arteriole and proximal tubule. A. Immunostaining renin is normally restricted to the juxtaglomerular region of the afferent arteriole (arrow); B. Following UUO, renin is produced by cells extending down the afferent arteriole (bracket). C. Antibody to 4-hydroxy-nonenal (HNE), a marker of oxidative stress, binds to cells of intact (*) and atrophic (arrows) proximal tubular cells of the obstructed kidney.
Middle Panel. Glomerulotubular junction. D-H. Semithin plastic sections reveal details of cellular morphology in normal (D, E) and obstructed (F, G, H) kidneys. D and E. The glomerulotubular junction of a normal kidney is patent, with a continuous lining of tall epithelial cells packed with mitochondria (*). F and G. Chronic UUO leads to proximal tubular atrophy and collapse (*), due to cell death by apoptosis (yellow arrow), autophagy (red arrows), and necrosis (not shown). Tubular mitochondrial content is markedly diminished. H. Chronic obstruction leads to medullary atrophy with tubular dilatation (*) and collapse (**), and interstitial spindle-shaped fibroblasts (below tubules).
Bottom Panel. Medulla. The normal medulla (I), contains proximal and distal nephron segments and microvasculature with minimal interstitial space. J-L. Late expansion of interstitial space of the obstructed kidney is characterized by macrophage infiltration revealed by F4/80 antibody (J), accumulation of myofibroblasts localized by α-smooth muscle actin staining (K), and accumulation of collagen (Sirius red stain) (L). All kidney tissue processing and photomicrographs were provided by Michael S. Forbes.
Complete UUO in the neonatal mouse also results in tubular oxidative stress, but cell death is delayed until mitochondrial maturation is complete and tubular energy generation has switched from glycolytic to oxidative metabolism.15 Renin production by the obstructed kidney is markedly increased, and is the result of recruitment of renin-producing cells along the afferent arteriole (Fig. 1).12,16 Activation of the intrarenal renin-angiotensin system contributes to tubular and interstitial injury, as demonstrated by a close correlation between injury and angiotensinogen gene copy number in transgenic mice.17 Increased production of angiotensin stimulates the production of transforming growth factor-β, which also contributes to tubular and interstitial injury.18 With progression of injury, macrophages are attracted to the peributular interstitium, and fibroblasts become activated by releasing cytokines, thus transforming into myofibroblasts, which increase deposition of intracellular matrix.19,20 Compared to the extent of tubular damage, there is only a modest increase (15%) in interstitial collagen and fibronectin (Fig. 1).11 These findings are consistent with those in kidneys from children with ureteropelvic junction obstruction: proximal tubular mass is often reduced, whereas interstitial injury is limited to 10–25% of cases.21
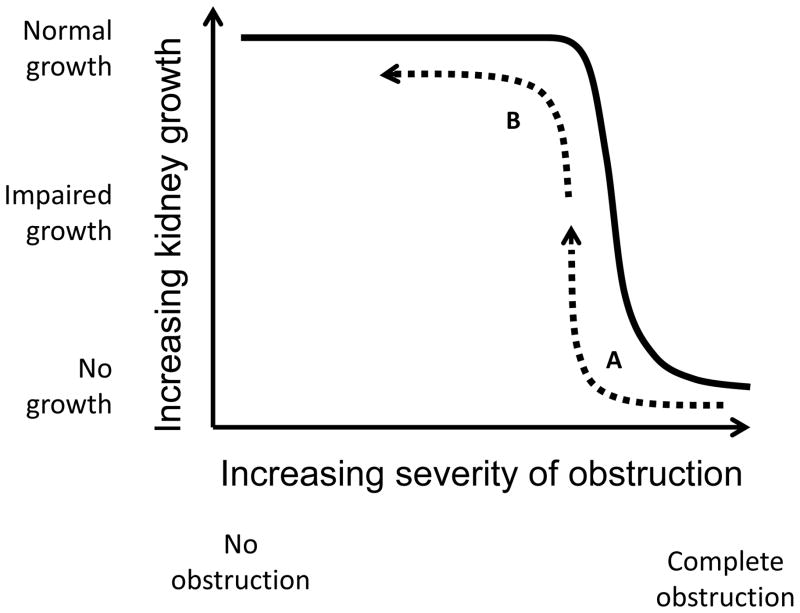
Congenital obstructive nephropathy is almost always due to partial, rather than complete UTO, and the development of renal cellular responses is gradual, rather than acute. We have therefore developed a model of partial UUO in the neonatal mouse or rat, species in which nephrogenesis is not completed until after the third postnatal day.22,23 This model permits varying the severity, timing, and duration of obstruction; release of the obstruction also permits the study of recovery.23 Adaptation by remaining nephrons takes place in both obstructed and intact contralateral kidneys, thereby revealing the impact of persistent or transient obstruction on individual nephrons. Impairment of kidney growth is dependent on the severity of obstruction, but this is not a linear relationship: there is a critical luminal diameter below which kidney growth is reduced (Fig. 2).22 This likely explains the poor correlation of renal pelvic diameter with kidney function in infants with hydronephrosis. It is obviously desirable to ensure that the luminal diameter of the ureter be kept well to the left of the inflection, which provides a rationale for pyeloplasty (Fig. 2). Following surgical intervention, however, ureteral peristaltic dysfunction and persistently reduced ureteral compliance may limit postoperative functional recovery.
Figure 2.
Impact of severity of obstruction (ureteral luminal diameter) on interval kidney growth in neonatal rats subjected to ipsilateral unilateral ureteral obstruction (UUO) within the first 2 days of life. Growth restriction of the obstructed kidney is dependent on the severity of obstruction, but the relationship is not linear. Fetal urinary tract obstruction causes combined obstructive injury and interference with nephron development: surgical correction of severe obstruction results in partial recovery of kidney growth (dashed arrow A), whereas timely correction of less severe obstruction permits normal growth (dashed arrow B).
It should be noted that most of the increase in renal parenchymal mass in infancy is a reflection of proximal tubular growth (both proliferation and hypertrophy).24 Nephron damage following neonatal partial UUO, although progressing more slowly than when following complete obstruction, is also characterized by proximal tubular apoptosis and necrosis, leading to glomerulotubular disconnection and the formation of atubular glomeruli.23 Although release of obstruction arrests progression of the proximal tubular lesion and is followed by remodeling of the renal architecture, glomerular growth remains impaired.23 In neonatal rats with variable partial UUO, the rate of compensatory growth of the contralateral (nonobstructed) kidney is dependent on the severity and duration of obstruction, and takes place at the single nephron level.25 In neonatal mice, there is enhanced growth of the proximal tubule and a reduction of interstitial collagen in the nonobstructed contralateral kidney following release of partial UUO.26 These responses are likely the result of oxidative stress, which increases in both kidneys following UUO, and decrease following release of obstruction.15 Although fine-tuning of compensatory growth of the contralateral kidney is detectable in inbred rodents, biologic variation in man, along with limitations in measurement of kidney size, diminish the utility of such measurements in predicting the function of the obstructed kidney as proposed previously.27,28
In addition to impaired nephrogenesis resulting from (or associated with) UTO, the risk for reduced nephron number is compounded in affected preterm and intrauterine growth-restricted infants. This concern has grown from epidemiologic studies by Barker and his group, which linked low birth weight to adult cardiovascular disease, an association that has been extended to CKD. Low nephron number is also associated with low birth weight, and it is now becoming clear that nephron endowment at birth is a strong determinant of renal health throughout the life cycle.6,29,30 To determine the role of nephron number in the progression of congenital obstructive nephropathy, mutant mice with 50% reduction in nephron number were compared to wild-type mice subjected to transient partial UUO.26 In contrast to mice with normal nephron number, nephron growth in mice with congenital nephron deficiency was not restored after release of UUO, and there was additional nephron loss.26 This suggests that low birth weight infants with congenital UTO are likely to be at increased risk for progression, and to have limited recovery following surgical correction of obstruction.
Clinical predictors of progression in congenital urinary tract obstruction
It is ironic that at the present time, there is no generally accepted definition of congenital UTO. Craig Peters has proposed: “Obstruction is a condition of impaired urinary drainage which, if uncorrected, will limit the ultimate functional potential of a developing kidney”.31 While this definition rightly emphasizes the importance of kidney function over the life of the individual, there remains considerable disagreement regarding the definition of “impaired urinary drainage”. Arbitrary cut-offs for acceptable washout time in a diuretic renogram are poor predictors of outcome, although no satisfactory alternative imaging studies are generally available.32 The other challenge with the definition is the prediction of “ultimate functional potential”—this presupposes knowledge of variables that would individually or collectively interfere with achieving this potential. There is even greater urgency in discovering a measure of kidney function that has predictive value. Measurements of plasma creatinine concentration are imprecise, particularly in the range of normal for infants.33 Moreover, estimation or calculation of GFR, regardless of the marker (e.g. cystatin C, inulin, iothalamate), represents a summation of a heterogeneous population of nephrons, comprising hypertrophied and atrophied glomeruli and tubules, and even atubular glomeruli.34 The well-established adaptation by remaining nephrons to undergo hypertrophy serves to mask underlying pathology. This has been demonstrated experimentally in the rat subjected to temporary UUO in the first week of life, followed by release of obstruction. At one month of age, GFR of the postobstructed kidney is normal, but there is a significant decrease in number of nephrons, along with tubular atrophy and interstitial fibroblast and collagen accumulation.35 At one year of age, however, GFR has decreased by 80%, and both postobstructed and contralateral kidneys reveal severe progressive injury, including glomerular sclerosis.36 A measure of “renal functional reserve” has been proposed, based on the latent capacity of a kidney to acutely increase blood flow and GFR in response to a protein stimulus.37 While theoretically appealing, permutations of such stimuli have not been shown to correlate reliably with long-term outcome.37
Compared to postnatal life, measurement of kidney function is far more difficult in fetal life, during which time developmental injury is initiated: ischemic, hypoxic, and oxidative nephron injury is superimposed on abnormal nephrogenesis. Kidney ultrasonography has been used to gauge the severity of renal pelvic dilatation, with the generation of normative parameters adjusted for gestational age.38 Unfortunately, the correlation of pelvic dilatation with renal scintigraphy is predictive only in the most severe cases, and not in the majority of “intermediate” cases.39 In suspected cases of lower tract obstruction, estimates of fetal renal function based on fetal urine sampling also lack predictive power.32 This dilemma has prompted a search for reliable biomarkers based on known pathophysiology of congenital UTO. These include markers of pathogenic stimuli (e.g. hypoxic or oxidative stress), markers of cellular response to injury (e.g. transforming growth factor-β1 [TGF-β1] or kidney injury molecule-1 [KIM-1]), and “unbiased’ markers associated with severity or progression of obstructive injury (e.g. proteomics, metabolomics).40–42 It will be necessary to correlate the biomarkers with clinically useful outcomes (such as kidney or somatic growth in children, appetite, or urinary concentrating capacity) and to account for age-related changes.
Diagnosis and surgical management of congenital urinary tract obstruction
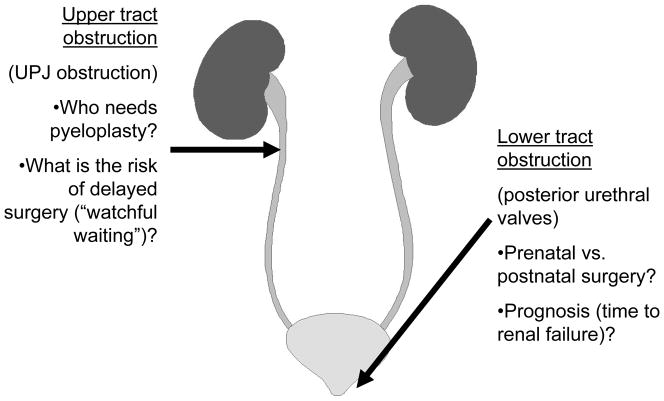
Fetal hydronephrosis without other anomalies is identified by prenatal ultrasonography in approximately 5% of pregnancies, but following birth most of these infants do not have functional or mechanical obstruction.43 In one report, as many as 1.5% of the initial screened population were determined to qualify for long-term care.44 There are important considerations that distinguish the diagnosis and management of upper tract vs. lower UTO (Fig. 3). The most common cause of congenital ureteral obstruction, ureteropelvic junction obstruction, is fortunately usually unilateral, and may not impair long-term function of the affected kidney. This has led to substantial disagreement regarding timing and indications for pyeloplasty: there are risks to “watchful waiting,” including loss to follow up and further nephron loss due to obstructive injury.45 One of the most promising approaches to biomarker discovery has been the “unbiased” proteome analysis by mass spectrometry of urinary protein fragments from infants with ureteropelvic junction obstruction.46 Using the criteria for pyeloplasty defined by this team, a prospective study to validate the predictive value of initial correlations yielded 94% correct prediction at 9 months, and 97% at 15 months.46 Longer follow up will be required to determine the ultimate value of the proteome. Notably, a subsequent study of ureteropelvic junction obstruction in children older than one year of age did not reveal any predictive value of urinary proteome analysis, suggesting that greater variation in dietary intake of older children may account for greater proteomic variability.47 However, the urinary proteome after a 5-year follow up of children not undergoing pyeloplasty remained abnormal despite a lack of symptoms or signs of obstructive injury—the clinical significance of this remains to be determined.48
Figure 3.
Major considerations in the management of upper tract and lower tract obstruction. For upper tract obstruction, most commonly unilateral ureteropelvic junction (UPJ) obstruction, the severity of functional obstruction is a major determinant for timing and selection of candidates for pyeloplasty. For lower tract obstruction, most commonly due to posterior urethral valves, surgical intervention is generally in the immediate postnatal period, although fetal intervention may be attempted in specialized centers. Long-term prognosis will be dependent on the degree of nephron maldevelopment and on the severity of bladder as well as kidney dysfunction.
Lower tract (bladder outlet) obstruction, usually the consequence of posterior urethral valves, is suspected when the fetal renal ultrasound reveals bilateral hydronephrosis, echogenic renal parenchyma, bladder dilatation, and oligohydramnios (Fig. 3). Prenatal surgical intervention continues to be attempted in a few specialized pediatric centers, but overall outcomes of vesicoamniotic shunting are disappointing.49 For those infants with poor kidney function after several weeks of stabilization following bladder drainage (and/or valve ablation), there is a significant risk of progression to kidney failure, often not until reaching adulthood.2 Careful measurement of renal parenchymal area has been shown to reliably predict kidney failure in these patients.50 We have recently reported that in murine models of polycystic kidney disease, enlarging cyst size is tightly correlated with obstruction of cystic as well as adjacent noncystic nephrons, which undergo cellular destruction and formation of atubular glomeruli similar to the process following UUO.51 Thus, polycystic kidney disease appears to be a form of obstructive nephropathy, with obstruction at the level of collecting ducts rather than the ureter. Notably, measurement of total cyst volume by magnetic resonance imaging of patients with polycystic kidney disease has emerged as a better predictor of progression than any other biomarker tested to date.52 These observations suggest that we turn our attention to morphometry and imaging studies to complement available functional assays.53 The development of a reliable technique to count functioning nephrons in vivo, and to track this parameter from birth to adulthood, would provide valuable information regarding long-term prognosis. Early feasibility studies have allowed resolution of individual glomeruli by magnetic resonance imaging (MRI) in the intact kidney following intravascular injection of cationic ferritin, which is localized to the glomerular basement membrane.54
Medical management and long-term outcome of congenital obstructive nephropathy
The question for the practicing clinician becomes, “what can be done for my patient with congenital UTO to optimize long-term outcome”? This must be considered in the context of the life-cycle (Fig. 4). For the perinatologist, optimizing prenatal care (including monitoring amniotic fluid volume, detecting prenatal hydronephrosis), as well as preventing premature birth, may reduce developmental injury (due to maldevelopment of the kidneys or urinary tract).9 For the neonatologist, optimizing cardiopulmonary and general supportive care (including minimizing nephrotoxic drugs) in the critical early postnatal period may reduce injury to the obstructed kidney.55 For the pediatric nephrologist and urologist, timely correction of the obstruction, adjustment of dietary intake, avoidance of nephrotoxins, and tracking of blood pressure and kidney growth may preserve functioning nephrons.55 Recent reports suggest that progression of chronic kidney disease is induced or accelerated by episodes of acute kidney injury, even following apparent clinical recovery (Fig. 4).56 As discussed above, the impact of obstructive injury on either developing or mature nephrons may not be reversed by release of the obstruction: patients undergoing surgical correction of upper or lower tract obstruction should be followed into adulthood. The transition of care from pediatric to adult health care providers can be critical, as suggested by the majority of children with significant congenital urinary tract anomalies who develop kidney failure in adulthood.57,58 Because of the importance of good bladder function on kidney growth and function, close communication between nephrologist and urologist can slow the rate of progression of patients with lower tract obstruction.59
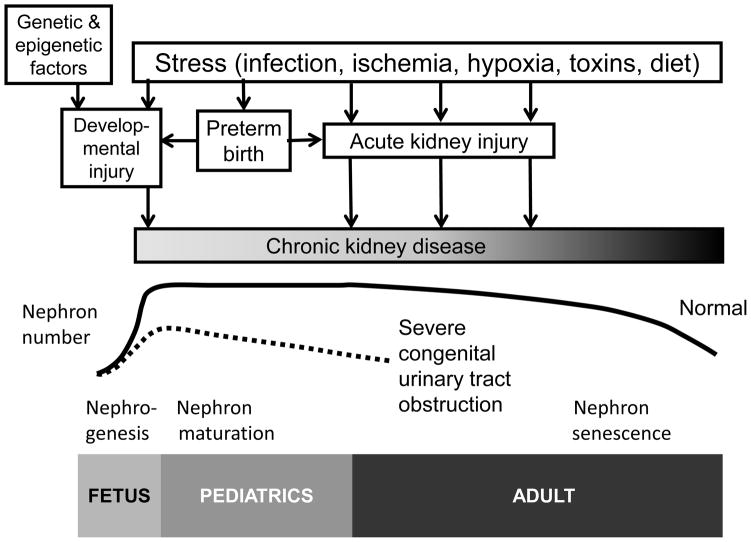
Figure 4.
Evolution of congenital obstructive nephropathy over the life-cycle. Nephrogenesis is complete by 36 weeks gestational age, and nephron number is maintained through young adulthood, decreasing with normal aging. Genetic and epigenetic factors determine nephron number at birth. Maldevelopment of the urinary tract causing obstruction to urine flow induces “developmental” injury in fetal life, which is often combined with abnormal nephrogenesis (prematurity further impairs nephrogenesis). This developmental injury is compounded by “obstructive” injury, which results from hemodynamic and cellular adaptive responses leading to the pathologic changes shown in Fig. 1. Perinatal insults can add acute kidney injury (AKI) through infection, ischemia, hypoxia, toxins, or impaired nutrition, which can also accumulate with additional episodes of AKI throughout life. Optimal outcomes for children with obstructive nephropathy depend on smooth transition of care from obstetrician/perinatologist to pediatric urologist/nephrologist, then on to adult care.
There are few therapeutic agents with demonstrated effectiveness in slowing or arresting the progression of CKD. Virtually all medical intervention to reduce proteinuria or otherwise slow progression of CKD is based on inhibition of endogenous formation of angiotensin using either angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB).60 Since captopril was first approved for treatment of hypertension by the Food and Drug Administration in 1961, angiotensin inhibitors have been approved for the treatment of only one kidney disorder: diabetic nephropathy.61 Despite this, ACE inhibitors and ARB are widely prescribed for children and adults with a wide variety of kidney disorders. The rationale for their use is based primarily on a reduction of proteinuria in addition to controlling hypertension. However, angiotensin inhibition also exacerbates urinary sodium wasting and causes a functional reduction in GFR, and meta-analysis does not confirm renoprotective effects beyond lowering blood pressure.62 It should be noted that ACE inhibitors were found ineffective in slowing the progression of congenital uropathies.63
Although an ACE inhibitor was found to be effective in reducing proteinuria in 1 month-old rats with UUO (age equivalent to a human child), administration of ACE inhibitors or ARB to rats with partial UUO in the first month of life aggravated interstitial cellular infiltrate and collagen accumulation in the obstructed kidney.64,65 These data indicate that an intact renin-angiotensin system is necessary not only for normal kidney development and function, but also to participate in cellular repair and remodeling in response to concurrent obstructive injury. Angiotensin inhibitors have well-documented teratogenic effects on human kidney development, and are strictly contraindicated in pregnancy.66 Moreover, angiotensin inhibition in the neonate can markedly reduce GFR, and may impair kidney development, especially in the preterm infant or baby with kidney disease.67 Pharmacologic inhibition of angiotensin or genetic inactivation of multiple steps in the renin-angiotensin system in mice causes functional obstructive nephropathy and results in hydronephrosis and papillary atrophy.68
There are numerous reports showing that UUO upregulates TGF-β in the obstructed kidney, contributing to interstitial fibrosis (Fig. 1).69 We demonstrated that inhibition of ALK5 TGF-β receptor inhibitor to adult mice with UUO decreased tubular apoptosis, preserved proximal tubular mass, and decreased interstitial collagen accumulation in the obstructed kidney.18 However, administration of the TGF-β inhibitor to neonatal mice with UUO caused widespread tubular necrosis, revealing age-dependent susceptibility to inhibition of this cytokine as well as angiotensin.18
Conclusions
Congenital UTO is most often suspected in the fetus with hydronephrosis detected by prenatal renal ultrasonography. Diagnosis of functionally significant obstruction is difficult because of the poor predictive value of currently available imaging techniques (ultrasonography and diuretic scintigraphy), and lack of available biomarkers. Nevertheless, tracking kidney growth by ultrasonography provides useful information (an index of proximal tubular growth) that complements measurement of estimated GFR (which can mask adaptation by remaining nephrons). Candidates for surgical correction of upper tract obstruction must be carefully selected, balancing the risks of the procedure with risks to the developing kidney of ongoing obstructive injury. While surgical correction of lower tract obstruction is generally performed in the early postnatal period, fetal intervention may be indicated in selected cases, if performed at experienced centers. Regardless of the level of obstruction, and regardless of surgical intervention or its timing, all children with congenital UTO should be followed well into adulthood, as most ESRD will not develop until the second decade or later.
Clinical Summary.
Congenital urinary tract obstruction impairs kidney growth and development, dependent on the severity, duration, and timing of obstruction.
The primary objectives in management of upper tract obstruction are selection of appropriate candidates for surgical correction and optimal timing of the operation.
The primary objective in management of lower tract obstruction is to relieve the obstruction as soon as feasible; generally in the immediate postnatal period (fetal intervention carries significant risk to mother and fetus).
Medical therapy to slow progression of CKD in children with obstructive nephropathy is limited, and angiotensin inhibitors must be used with caution in infants due to their interference with normal kidney development.
Regardless of surgical intervention, long-term outcomes are optimized by smooth transition of care from perinatologist to pediatric and adult nephrologist and urologist
Acknowledgments
Photomicrographs (Figure 1) were contributed by M. S. Forbes. Supported in part by Pediatric Center of Excellence in Nephrology, P50 DK096373 from the National Institutes of Health.
Footnotes
Conflict of Interest: The author has no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22:1999–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanna-Cherchi S, Ravani P, Corbani V, et al. Congenital anomalies of the kidney and urinary tract (CAKUT): longitudinal cohort study on renal outcome. Kidney Int. 2009;76:528–533. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]
- 3.Wuhl E, van Stralen KJ, Verrina E, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of thekidney and urinary tract. Clin J Am Soc Nephrol. 2013;8:67–74. doi: 10.2215/CJN.03310412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bascands JL, Schanstra JP. Obstructive nephropathy: Insights from genetically engineered animals. Kidney Int. 2005;68:925–937. doi: 10.1111/j.1523-1755.2005.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CP, McDill BW, Neilson JR, et al. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram JF, Douglas-Denton RN, Diouf B, et al. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 7.Zalups RK. The Os/+ mouse: A genetic animal model of reduced renal mass. Am J Physiol. 1993;264:F53–F60. doi: 10.1152/ajprenal.1993.264.1.F53. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez MM, Gomez AH, Abitbol CL, et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Devel Pathol. 2004;7:17–25. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 9.Trnka P, Hiatt MJ, Tarantal AF, et al. Congenital urinary tract obstruction: defining markers of developmental kidney injury. Pediatr Res. 2012;72:446–454. doi: 10.1038/pr.2012.113. [DOI] [PubMed] [Google Scholar]
- 10.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 11.Forbes MS, Thornhill BA, Minor JJ, et al. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiol Renal Physiol. 2012;303:F120–F129. doi: 10.1152/ajprenal.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forbes MS, Thornhill BA, Chevalier RL. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am J Physiol Renal Physiol. 2011;301:F110–F117. doi: 10.1152/ajprenal.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blondin J, Purkerson ML, Rolf D, et al. Renal function and metabolism after relief of unilaterl ureteral obstruction. Proc Soc Exp Biol Med. 1975;150:71–76. doi: 10.3181/00379727-150-38976. [DOI] [PubMed] [Google Scholar]
- 14.Middleton GW, Beamon CR, Panko WB, et al. Effects of ureteral obstruction on the renal metabolism of alpha-ketoglutarate and other substrates in vivo. Invest Urol. 1977;14:255–262. [PubMed] [Google Scholar]
- 15.Forbes MS, Thornhill BA, Galarreta CI, et al. Chronic unilateral ureteral obstruction in the neonatal mouse delays maturation of both kidneys and leads to late formation of atubular glomeruli. Am J Physiol Renal Physiol. 2013;305:F1736–F1746. doi: 10.1152/ajprenal.00152.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norwood VF, Carey RM, Geary KM, et al. Neonatal ureteral obstruction stimulates recruitment of renin- secreting renal cortical cells. Kidney Int. 1994;45:1333–1339. doi: 10.1038/ki.1994.174. [DOI] [PubMed] [Google Scholar]
- 17.Fern RJ, Yesko CM, Thornhill BA, et al. Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J Clin Invest. 1999;103:39–46. doi: 10.1172/JCI4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galarreta CI, Thornhill BA, Forbes MS, et al. Transforming growth factor-beta 1 receptor inhibition preserves glomerulotubular integrity during ureteral obstruction in adults but worsens injury in neonatal mice. Am J Physiol Renal Physiol. 2013;304:F481–F490. doi: 10.1152/ajprenal.00496.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange-Sperandio B, Cachat F, Thornhill BA, et al. Selectins mediate macrophage infiltration in obstructive nephropathy in newborn mice. Kidney Int. 2002;61:516–524. doi: 10.1046/j.1523-1755.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- 20.Lange-Sperandio B, Fulda S, Vandewalle A, et al. Macrophages induce apoptosis in proximal tubule cells. Pediatr Nephrol. 2003;18:335–341. doi: 10.1007/s00467-003-1116-2. [DOI] [PubMed] [Google Scholar]
- 21.Rosen S, Peters CA, Chevalier RL, et al. The kidney in congenital ureteropelvic junction obstruction: A spectrum from normal to nephrectomy. J Urol. 2008;179:1257–1263. doi: 10.1016/j.juro.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Thornhill BA, Burt LA, Chen C, et al. Variable chronic partial ureteral obstruction in the neonatal rat: A new model of ureteropelvic junction obstruction. Kidney Int. 2005;67:42–52. doi: 10.1111/j.1523-1755.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 23.Thornhill BA, Forbes MS, Marcinko ES, et al. Glomerulotubular disconnection in neonatal mice after relief of partial ureteral obstruction. Kidney Int. 2007;72:1103–1112. doi: 10.1038/sj.ki.5002512. [DOI] [PubMed] [Google Scholar]
- 24.Fetterman GH, Shuplock NA, Philipp FJ, et al. The growth and maturation of human glomeruli and proximal convolutions from term to adulthood. Studies by microdissection. Pediatrics. 1965;35:601–619. [PubMed] [Google Scholar]
- 25.Yoo KH, Thornhill BA, Forbes MS, et al. Compensatory renal growth due to neonatal ureteral obstruction: Implications for clinical studies. Pediatr Nephrol. 2006;21:368–375. doi: 10.1007/s00467-005-2119-y. [DOI] [PubMed] [Google Scholar]
- 26.Sergio M, Galarreta CI, Thornhill BA, et al. The fate of nephrons in congenital obstructive nephropathy: Adult recovery is limited by nephron number. (abstract). Poster presented at: American Society of Nephrology 2014 Kidney Week; November 11–16, 2014; Philadelphia, PA. 2014. [Google Scholar]
- 27.Koff SA, Peller PA, Young DC, et al. The assessment of obstruction in the newborn with unilateral hydronephrosis by measuring the size of the opposite kidney. J Urol. 1994;152:596–599. doi: 10.1016/s0022-5347(17)32659-9. [DOI] [PubMed] [Google Scholar]
- 28.Ferrer FA, McKenna PH, Bauer MB, et al. Accuracy of renal ultrasound measurements for predicting actual kidney size. J Urol. 1997;157:2278–2281. [PubMed] [Google Scholar]
- 29.Barker DJ, Bagby SP. Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol. 2005;16:2537–2544. doi: 10.1681/ASN.2005020160. [DOI] [PubMed] [Google Scholar]
- 30.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 31.Peters CA. Urinary tract obstruction in children. J Urol. 1995;154:1874–1883. doi: 10.1016/s0022-5347(01)66815-0. [DOI] [PubMed] [Google Scholar]
- 32.Chevalier RL. Obstructive Uropathy: Assessment of Renal Function in the Fetus. In: Oh BS, Guignard J-P, Baumgart S, editors. Nephrology and Fluid/Electrolyte Physiology: Neonatology Questions and Controversies. 1. Philadelphia: Saunders-Elsevier; 2008. pp. 225–250. [Google Scholar]
- 33.Clermont MJ, Brion LP, Schwartz GJ. Reliability of plasma creatinine measurement in infants and children. Clin Pediatr (Phila) 1986;25:596–572. doi: 10.1177/000992288602501106. [DOI] [PubMed] [Google Scholar]
- 34.Oliver J, Luey AB. Plastic studies in abnormal renal architecture. Arch Pathol. 1935;19:1–23. [Google Scholar]
- 35.Chevalier RL, Kim A, Thornhill BA, et al. Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int. 1999;55:793–807. doi: 10.1046/j.1523-1755.1999.055003793.x. [DOI] [PubMed] [Google Scholar]
- 36.Chevalier RL, Thornhill BA, Chang AY. Unilateral ureteral obstruction in neonatal rats leads to renal insufficiency in adulthood. Kidney Int. 2000;58:1987–1995. doi: 10.1111/j.1523-1755.2000.00371.x. [DOI] [PubMed] [Google Scholar]
- 37.Thomas DM, Coles GA, Williams JD. What does the renal reserve mean? Kidney Int. 1994;45:411–416. doi: 10.1038/ki.1994.53. [DOI] [PubMed] [Google Scholar]
- 38.Kent A, Cox D, Downey P, et al. A study of mild fetal pyelectasia - outcome and proposed strategy of management. Prenat Diagn. 2000;20:206–209. [PubMed] [Google Scholar]
- 39.Morris RK, Malin GL, Khan KS, et al. Antenatal ultrasound to predict postnatal renal unction in congenital lower urinary tract obstruction: systematic review of test accuracy. BJOG. 2009;116:1290–1299. doi: 10.1111/j.1471-0528.2009.02194.x. [DOI] [PubMed] [Google Scholar]
- 40.Taha MA, Shokeir AA, Osman HG, et al. Pelvi-ureteric junction obstruction in children: the role of urinary transfoming growth factor-beta 1 and epidermal growth factor. BJU Int. 2007;99:899–903. doi: 10.1111/j.1464-410X.2006.06641.x. [DOI] [PubMed] [Google Scholar]
- 41.Wasilewska A, Taranta-Janusz K, Debek W, et al. KIM-1 and NGAL: new markers of obstructive nephropathy. Pediatr Nephrol. 2011;26:579–586. doi: 10.1007/s00467-011-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caubet C, Lacroix C, Decramer S, et al. Advances in urinary proteome analysis and biomarker discovery in pediatric renal disease. Pediatr Nephrol. 2010;25:27–35. doi: 10.1007/s00467-009-1251-5. [DOI] [PubMed] [Google Scholar]
- 43.Mallik M, Watson AR. Antenatally detected urinary tract abnormalities; more detection but less action. Pediatr Nephrol. 2008;23:897–904. doi: 10.1007/s00467-008-0746-9. [DOI] [PubMed] [Google Scholar]
- 44.Ismaili K, Hall M, Donner C, et al. Results of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am J Obstet Gynecol. 2003;188:242–246. doi: 10.1067/mob.2003.81. [DOI] [PubMed] [Google Scholar]
- 45.Chertin B, Pollack A, Koulikov D, et al. Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: Lessons learned after 16 years of follow-up. Eur Urol. 2006;49:734–739. doi: 10.1016/j.eururo.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 46.Decramer S, Wittke S, Mischak H, et al. Predicting the clinical outcome of congenital unilateral ureteropelvic junction obstruction in newborn by urinary proteome analysis. Nature Med. 2006;12:398–400. doi: 10.1038/nm1384. [DOI] [PubMed] [Google Scholar]
- 47.Drube J, Zurbig P, Schiffer E, et al. Urinary proteome analysis identifies infants but not older children requiring pyeloplasty. Pediatr Nephrol. 2010;25:1673–1678. doi: 10.1007/s00467-010-1455-8. [DOI] [PubMed] [Google Scholar]
- 48.Bandin F, Siwy J, Breuil B, et al. Urinary proteome analysis at 5-year followup of patients with nonoperated ureteropelvic junction obstruction suggests ongoing kidney remodeling. J Urol. 2012;187:1006–1011. doi: 10.1016/j.juro.2011.10.169. [DOI] [PubMed] [Google Scholar]
- 49.Morris RK, Malin GL, Khan KS, et al. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. [Review] [43 refs] BJOG. 2010;117:382–390. doi: 10.1111/j.1471-0528.2010.02500.x. [DOI] [PubMed] [Google Scholar]
- 50.Pulido JE, Furth SL, Zderic SA, et al. Renal parenchymal area and risk of ESRD in boys with posterior urethral valves. Clin J Am Soc Nephrol. 2014;9:499–505. doi: 10.2215/CJN.08700813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galarreta CI, Grantham JJ, Forbes MS, et al. Tubular obstruction leads to progressive proximal tubular injury and atubular glomeruli in polycystic kidney disease. Am J Pathol. 2014;184:1957–1966. doi: 10.1016/j.ajpath.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Neill WC. Structure, not just function. Kidney Int. 2014;85:503–505. doi: 10.1038/ki.2013.426. [DOI] [PubMed] [Google Scholar]
- 54.Beeman SC, Cullen-McEwen LA, Puelles VG, et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am J Physiol Renal Physiol. 2014;306:F381–F390. doi: 10.1152/ajprenal.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmody JB, Charlton JR. Short-term gestation, long-term risk: Prematurity and chronic kidney disease. Pediatrics. 2013;131:1168–1179. doi: 10.1542/peds.2013-0009. [DOI] [PubMed] [Google Scholar]
- 56.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chevalier RL. When is one kidney not enough? Kidney Int. 2009;76:475–477. doi: 10.1038/ki.2009.244. [DOI] [PubMed] [Google Scholar]
- 58.Little MH, Brown D, Humphreys BD, et al. Defining biology to understand disease. Clin J Am Soc Nephrol. 2014;9:809–811. doi: 10.2215/CJN.10851013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ansari MS, Gulia A, Srivastava A, et al. Risk factors for progression to end-stage renal disease in children with posterior urethral valves. J Pediatr Urol. 2010;6:261–264. doi: 10.1016/j.jpurol.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 61.Lewis EJ, Lewis JB. ACE inhibitors versus angiotensin receptor blockers in diabetic nephropathy: is there a winner? J Am Soc Nephrol. 2004;15:1358–1360. [PubMed] [Google Scholar]
- 62.Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–2033. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- 63.Ardissino G, Vigano S, Testa S, et al. No clear evidence of ACEi efficacy on the progression of chronic kidney disease in children with hypodysplastic nephropathy--report from the ItalKid project database. Nephrol Dial Transplant. 2007;22:2525–2530. doi: 10.1093/ndt/gfm237. [DOI] [PubMed] [Google Scholar]
- 64.Chen CO, Park MH, Forbes MS, et al. Angiotensin converting enzyme inhibition aggravates renal interstitial injury resulting from partial unilateral ureteral obstruction in the neonatal rat. Am J Physiol Renal Physiol. 2007;292:F946–F955. doi: 10.1152/ajprenal.00287.2006. [DOI] [PubMed] [Google Scholar]
- 65.Coleman CM, Minor JJ, Burt LE, et al. Angiotensin AT1 receptor inhibition exacerbates renal injury resulting from partial unilateral ureteral obstruction in the neonatal rat. Am J Physiol Renal Physiol. 2007;293:F262–F268. doi: 10.1152/ajprenal.00071.2007. [DOI] [PubMed] [Google Scholar]
- 66.Shotan A, Widerhorn J, Hurst A, et al. Risks of angiotensin-converting enzyme inhibition during pregnancy: Experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96:451–456. doi: 10.1016/0002-9343(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 67.Tack ED, Perlman JM. Renal failure in sick hypertensive premature infants receiving captopril therapy. J Pediatr. 1988;112:805–810. doi: 10.1016/s0022-3476(88)83213-x. [DOI] [PubMed] [Google Scholar]
- 68.Yosypiv IV, El-Dahr SS. Role of the renin-angiotensin system in the development of the ureteric bud and renal collecting system. Pediatr Nephrol. 2005;20:1219–1229. doi: 10.1007/s00467-005-1944-3. [DOI] [PubMed] [Google Scholar]
- 69.Cheng J, Grande JP. Transforming growth factor-β signal transduction and progressive renal disease. Exp Biol Med. 2002;227:943–956. doi: 10.1177/153537020222701102. [DOI] [PubMed] [Google Scholar]