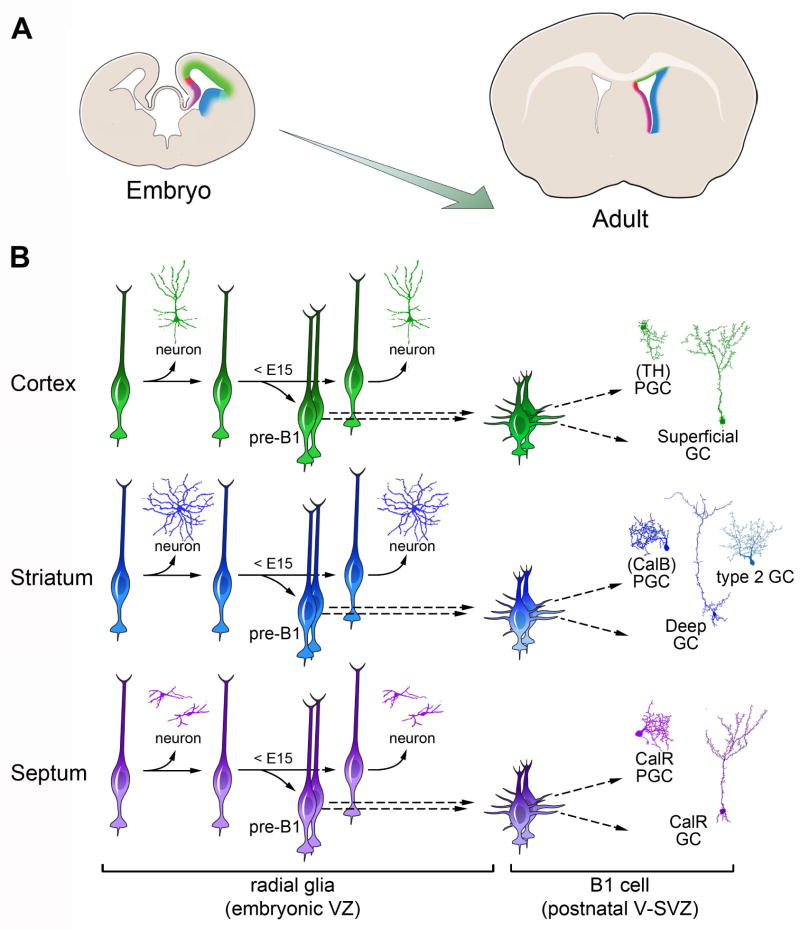
Abstract
Adult neural stem/progenitor (B1) cells within the walls of the lateral ventricles generate different types of neurons for the olfactory bulb (OB). The location of B1 cells determines the types of OB neurons they generate. Here we show that the majority of mouse B1 cell precursors are produced between embryonic days (E) 13.5 and 15.5, and remain largely quiescent until they become reactivated postnatally. Using a retroviral library carrying over 100,000 genetic tags, we found that B1 cells share a common progenitor with embryonic cells of the cortex, striatum and septum, but this lineage relationship is lost before E15.5. The regional specification of B1 cells is evident as early as E11.5 and is spatially linked to the production of neurons that populate different areas of the forebrain. This study reveals an early embryonic regional specification of postnatal neural stem cells and the lineage relationship between them and embryonic progenitor cells.
Graphical abstract
Introduction
Somatic stem cells are retained throughout life in germinal niches where they maintain some of the cellular and molecular characteristics of their embryonic counterparts. Although the origin of adult stem cells is unclear, these similarities have prompted the hypothesis that postnatal somatic stem cells could correspond to embryonic progenitors that persist into postnatal and adult life (Alvarez-Buylla et al., 2001; Eckfeldt et al., 2005; Benitah and Frye, 2012; Costa et al., 2012). An understanding of the origin of adult stem cells may shed light on how they have retained or acquired their potential.
Neural stem cells (NSCs), known as B1 cells, are retained into adulthood in the ventricular-subventricular zone (V-SVZ) (Doetsch et al., 1999; Zhao et al., 2008; Ming and Song, 2011). These NSCs have been best studied in rodents and lie within the walls of the lateral ventricles, next to the cortex, hippocampus, striatum and septum (Cx, Hp, St, and Sp). B1 cells have many features of astrocytes (Doetsch et al., 1999), and retain expression of Nestin, BLBP, GLAST, and Sox2 (Lagace et al., 2007; Giachino et al., 2014), which are also expressed in radial glia cells (RGs), the NSCs in the developing brain. Indeed, B1 cells are derived from RGs (Merkle et al., 2004) and display epithelial apico-basal organization reminiscent of RG morphology (Mirzadeh et al., 2008). These observations have suggested a linear NSC lineage from neuroepithelial cells to RGs to adult B1 cells (Alvarez-Buylla et al., 2001; Temple, 2001; Kriegstein and Alvarez-Buylla, 2009).
B1 cells give rise to neuroblasts that migrate a long distance to the olfactory bulb (OB) (Lois and Alvarez-Buylla 1994) where they differentiate into multiple types of inhibitory interneurons (Carleton et al., 2003). Importantly, different types of OB interneurons are derived from different locations in the V-SVZ (Merkle et al., 2007; Ventura and Goldman, 2007). NSCs in the dorsal V-SVZ of the lateral wall generate mostly superficial granule cells (GCs) and dopaminergic periglomerular cells (PGCs), while ventral NSCs produce deep GCs and calbindin (CalB+) PGCs. In contrast, calretinin (CalR+) GCs and CalR+ PGCs are derived from medial V-SVZ NSCs. The embryonic origin of this regional specification remains unknown, but it has been suggested that it is associated to the early subdivision of the embryonic forebrain into territories with the expression of a specific set of transcription factors (Alvarez-Buylla et al., 2008). The adult V-SVZ exhibits the expression of transcription factors present in different forebrain domains during development such as Gsx1&2, Nkx6.2, Dbx1, Emx1, Pax6, SP8, and Zic1/2/3 (Hack et al., 2005; Waclaw et al., 2006; Kohwi et al., 2007; Young et al., 2007; López-Juárez et al., 2013; Merkle et al., 2014). Mice null for some of these transcription factors are deficient in the production of specific subtypes of OB interneurons in adult mice (Alvarez-Buylla et al., 2008). This raises the interesting question of whether adult B1 cells share a lineage with and inherit regional specification from RGs that earlier in development produced the different types of forebrain neurons, e.g. cortical pyramidal cells, striatal medium spiny neurons or septal neurons.
In this study we investigated the origin of B1 cells from dividing embryonic progenitors and their clonal relationship to neurons and glial cells in Cx, Hp, St, and Sp. Our results indicate that the embryonic progenitors of B1 cells (pre-B1 cells) were produced during mid-fetal development (E13.5–E15.5) and remained relatively quiescent until they were reactivated in postnatal life. We found that the regional specification of B1 cells took place as early as E11.5. Interestingly, some of these adult progenitors were related to RGs that generated neurons in Cx, St, and Sp, but this relationship was lost before E15.5. This work indicates that: 1) adult NSCs were allocated and specified early in embryonic development, and 2) adult and embryonic NSC cell lineages diverge during mid embryonic development.
Results
The majority of adult NSCs are generated at E13.5–E15.5
The neuroepithelial-RG-B1 cell lineage has been suggested to contain the central NSC continuum from which differentiated neurons and glia are derived (Kriegstein and Alvarez-Buylla, 2009). However, whether B1 cells are the end product of this lineage or whether they become separated from other RG during development, has not been investigated. To address the contribution of dividing RGs at different stages of development to the postnatal day 21 (P21) B1 cell population, we injected timed pregnant hGFAP::GFP mice at different embryonic stages with the thymidine analog bromodeoxyuridine (BrdU) (Figure 1A–C and Figure S1A–B). We found that the majority of double-labeled BrdU+/GFP+ B1 cells in the P21 V-SVZ were derived from animals injected with BrdU at E14.5 and E16.5. This suggests that the majority of B1 cells were derived from RGs that divided during this period, with smaller contributions at E12.5 and E17.5–E18.5. Surprisingly, many of these B1 cells were brightly labeled, suggesting that they did not divide repeatedly in the intervening period between the time of BrdU injection in the embryo and P21.
Figure 1. The majority of pre-B1 cells are derived at E13.5–E15.5.
A) Timed-pregnant hGFAP::GFP female mice received two injections of BrdU at E12.5, E14.5, E16.5, or combined E17.5/E18.5, and whole-mounts of the V-SVZ in the offspring were analyzed 21d after birth. B) The number of GFP+/BrdU+ cells was significantly higher in mice injected at E14.5 compared to other ages. Many of the ependymal cells were also BrdU-labeled at E14.5, as previously shown (Spassky et al., 2005). C) Quantification of V-SVZ GFP+/BrdU+ cells from animals injected at different timepoints. Data are expressed as mean ± s.e.m., n=6/stage, three independent experiments each (*** p<0.0001). D) NIT-GFP retroviruses were intraventricularly delivered into CD1 embryos at E12.5, E13.5, E15.5, E17.5 or P0. Injected animals received BrdU for one week at P28 and their OB were analyzed 3 weeks later. E) The majority of GFP+/BrdU+ OB neurons were derived from retroviral injections at E15.5. F) Quantification of GFP+/BrdU+ cells in the OB of animals injected at different stages. Data are expressed as mean ± s.e.m., n=12 (E12.5), n=6 (E13.5), n=16 (E15.5), n=11 (E17.5), n=4 (P0), three independent experiments each stage (*** p<0.0001). Scale bars represent 50 μm in B and E. See also Figure S1.
We could not rule out the possibility that dilution of BrdU, by intervening cell cycles, could account for the lower numbers of BrdU+/GFP+ cells observed at E12.5 or E17.5–E18.5. Moreover, although B1 cells can be distinguished from parenchymal astrocytes based on their morphology (Ponti et al., 2013), it is not clear whether all GFP+ cells in the V-SVZ correspond to B1 cells that actually function as postnatal neuronal progenitors. We therefore used GFP-expressing retroviruses to transduce dividing embryonic VZ progenitors at different ages to permanently label their progeny in the postnatal brain (Cepko et al., 1998). We then labeled a cohort of postnatal (P28) dividing V-SVZ cells with BrdU for 7 days and quantified the number of GFP+/BrdU+ interneurons in the OB three weeks later (Figure 1D–F). The majority of GFP+/BrdU+ OB interneurons were derived from progenitor cells that divided between E13.5–E15.5. A higher production of pre-B1 cells at E14.5 compared to E11.5 was also observed in Nestin::CreER mice; Ai14. Injection of tamoxifen (Tmx) at E14.5 resulted in higher number of OB interneurons generated at P28 compared to mice that receive the Tmx at E11.5 (Figure S1C–F).
The above experiments suggest that postnatal B1 cells are derived from embryonic cells that divide during mid-fetal development and then remained relatively quiescent until they become reactivated to generate progeny in the postnatal brain (see below). Interestingly, the period when pre-B1 cells became quiescent coincided with increased numbers of cells expressing VCAM-1, a recently described apical marker for quiescent B1 cells (Figure S1G–K) (Kokovay et al., 2012; Codega et al., 2014).
Postnatal B1 cells are clonally related to early NSCs that generate forebrain cells in the embryo
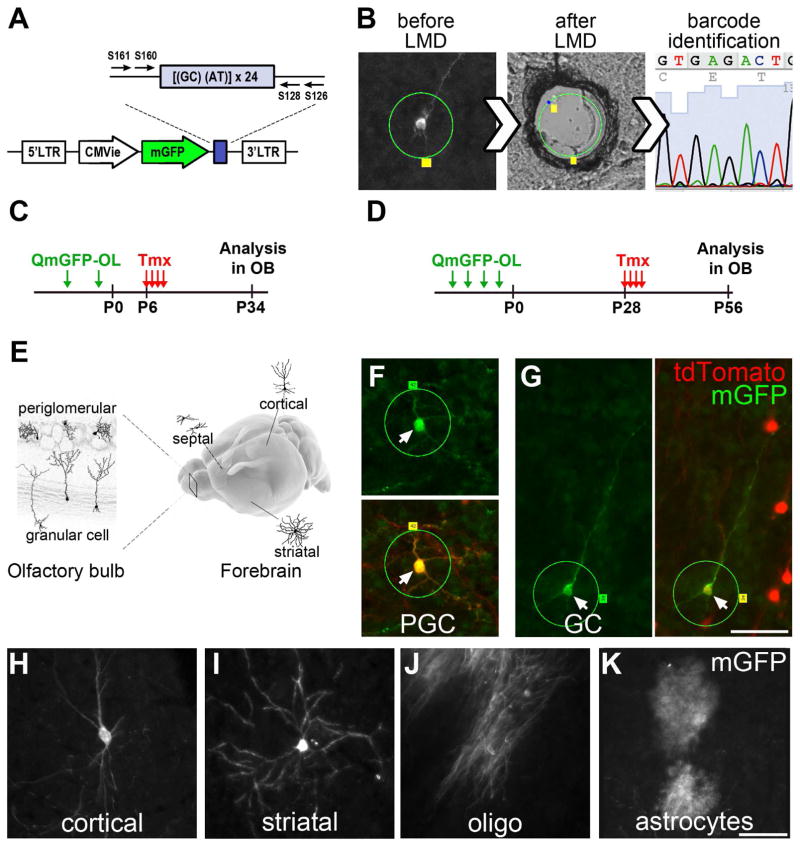
B1 cells are found in the dorsal, lateral and medial walls of the postnatal lateral ventricle - far from the OB and next to Cx, Hp, St, and Sp. It is unclear whether adult NSCs are derived from multipotent RGs, which earlier in development generate different subpopulations of forebrain neurons and glial cells. An alternative possibility is that a reserved subpopulation of RGs, separate from those producing neurons during embryogenesis, retains NSC properties for later use in postnatal V-SVZ neurogenesis (see Table S1 for representations of these two alternatives). We asked if early embryonic progenitor cells, before turning into pre-B1 cells, also generate neurons and glia for Cx, Hp, St and Sp. Since OB neurons migrate far from their progenitor cells in walls of the lateral ventricle, a lineage tracing method that determines clonal relationships among cells that are widely distributed is required. A barcoded retroviral library containing a degenerate 24bp oligonucleotide with a complexity of ~105 (QmGFP-OL) (Walsh and Cepko, 1992; Golden et al., 1995) was modified to include membrane-bound GFP (mGFP). The retroviral library was injected at different embryonic stages and individually labeled cells were identified as neurons, astrocytes and oligodendrocytes by their morphology, revealed by mGFP, and by the expression of NeuN, GFAP, and Olig2, respectively (Figure 2 and Figure S2A–G). Their locations were mapped and individual labeled cells were collected using laser capture. Each barcode was then identified by PCR amplification and sequencing. In order to label different cohorts of OB neurons born during postnatal development, the QmGFP-OL retroviral library was injected into Nestin::CreER;Ai14 embryos and Tmx was administered at P6 or P28 (hereafter identified as P6- or P28-group, respectively). Four weeks later mGFP+/TdT+ OB cells and mGFP+ cells in Cx, Hp, St, and Sp were analyzed in five and six hemispheres of the P6- and P28-group, respectively. If OB neurons share their origin with embryonically-born neurons and glia in Cx, Hp, St and Sp, they should have the same barcode, i.e. belong to the same clone.
Figure 2. Lineage tracing of individual embryonic progenitors with QmGFP-OL.
A) The QmGFP-OL retroviral library: Each retrovirus expresses mGFP and contains a unique 24 bp sequence. B) M-GFP+ cell tagged for laser microdissection (LMD, green circle), laser-cut and captured, followed by barcode identification. C) QmGFP-OL retroviruses were injected intraventricularly into Nestin::CreER;Ai14 embryos (at E12.5 or E15.5); 6 days after birth, injected mice received Tmx for 4–5d; neurons that were mGFP+/TdT+ in OB and mGFP+ Cx, Hp, St and Sp cells were laser-captured at P34. D) QmGFP-OL retroviruses were injected into Nestin::CreER;Ai14 embryos (at E11.5, E12.5, E13.5 or E15.5); 28 days after birth, injected mice received Tmx; mGFP+/TdT+ OB neurons and mGFP+ Cx, Hp, St and Sp cells were laser-captured at P56. E) Schematics showing regions analyzed and examples of dissected cells in the OB and in Cx, Hp, St and Sp. F–G) Examples of mGFP+/TdT+ PGC (F) and GC (G) before LMD. H–K). Examples of mGFP+ cells found in the telencephalon: neurons (H, cortex; I, striatum), oligodendrocyte (J, cortex) and an astrocyte (K, cortex). Scale bars represent 50 μm in G and K. See also Figure S2A–G.
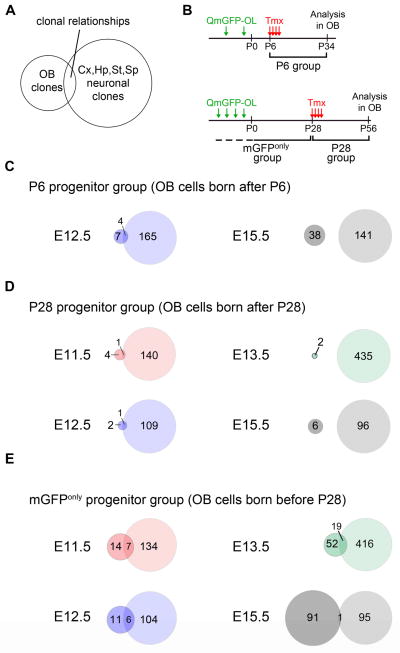
In the P6-group, we identified 49 mGFP+/Tdt+ clones in the OB and 694 mGFP+ clones of neurons and glia in the rest of the forebrain (from 335 mGFP+/Tdt+ cells in the OB and 2637 mGFP+ cells in Cx, Hp, St and Sp; retroviral library injected at E12.5 (n=3) and E15.5 (n=2)) (Figure 3A–C, Table S1). Surprisingly, clonal relationships between P6 OB neurons and Cx, Hp, St or Sp neurons were only found in E12.5 injected animals (4 out of 11 clones at E12.5 vs 0 out of 38 clones at E15.5). In mice injected at E15.5, approximately 1/4 of OB clones contained only glia in Cx, Hp, St and Sp (astrocytes and/or oligodendrocytes) (Table S2). One third of these clones contained mGFP+/TdT+ oligodendrocytes, indicating that they were also produced postnatally. This is consistent with previous studies showing that, in addition to neurons, the postnatal V-SVZ also generates oligodendrocytes (Rowitch and Kriegstein, 2010). In the P28-group, we identified 14 mGFP+/Tdt+ OB and 1128 mGFP+ Cx, Hp, St and Sp clones (from 60 mGFP+/Tdt+ cells in OB and 3683 mGFP+ cells in Cx, Hp, St and Sp; retroviral library injected at E11.5 (n=1), E12.5 (n=1), E13.5 (n=2), and E15.5 (n=2)). Out of the 14 double-labeled OB clones, two were clonally related to mGFP+ Cx neurons from animals injected at E11.5 and E12.5, respectively (Figure 3D, Table S1).
Figure 3. Clonal relationships between postnatal OB interneurons and Cx, Hp, St and Sp neurons.
A) Venn diagrams throughout this figure represent clonal relationships between OB and neurons found in the Cx, Hp, St or Sp (number of clones indicated in the center of each circle). The intersection shows the number of clones that contains both OB and Cx, Hp, St or Sp neurons. For the total number of amplified vs. microdissected cells including all OB and all Cx, Hp, St, Sp cells, see Table S1. B–E) Three experimental groups were analyzed: P6- (n=5, Venn diagrams in C), P28- (n=6, in D) and mGFPonly- (n=6, in E) progenitor groups. See also Table S2.
Next, we identified the barcodes of mGFP+ but TdT-negative OB neurons (mGFPonly) in the P28-group. This allowed us to expand our analysis to include OB neurons produced before P28, a period when many OB interneurons are born (Bayer, 1983; Batista-Brito et al., 2008). Of 201 mGFPonly OB clones (828 dissected cells) and 1228 Cx, Hp, St and Sp clones, 40 shared barcodes (Figure 3E, Table S1) and were, therefore, clonally related. Of these, 33 clones had mGFPonly OB cells as well as sibling neurons in Cx, Hp, St and Sp. Interestingly, the percentage of clones containing both OB interneurons and Cx, Hp, St, Sp neurons decreased significantly with embryonic age; from 33% at E11.5 to < 2% in brains injected at E15.5 (p value < 0.0001, Table S1).
In addition to mGFP+/TdT+ neurons in the OB of the P6- and P28-group, we also found 8 double-labeled cells in the subgranular zone (SGZ) of the Hp, a second germinal zone that continues receiving new neurons postnatally (Zhao et al., 2008; Ming and Song, 2011). None of these postnatal SGZ clones carried the same barcode as mGFP+/TdT+ OB neurons, suggesting that embryonic progenitors for postnatal neurogenesis in the OB and Hp are already separate by E11.5.
Taken together, these results demonstrate a direct clonal relationship between progenitor cells that generate OB interneurons postnatally and those that produce neurons for other regions of the telencephalon during embryonic development. The progenitor cells that produce these clones decrease rapidly between E11.5 and E15.5, suggesting that the common progenitor cells of pre-B1 cells and embryonic Cx, Hp, St or Sp progenitors are present at early stages of development. Since many neurons destined for Cx, Hp, St or Sp are still produced at, and after, E15.5 (Takahashi et al., 1999), the above results suggest that pre-B1 cells become separate from forebrain RGs before this age.
Postnatal NSCs become regionally specified before E15.5
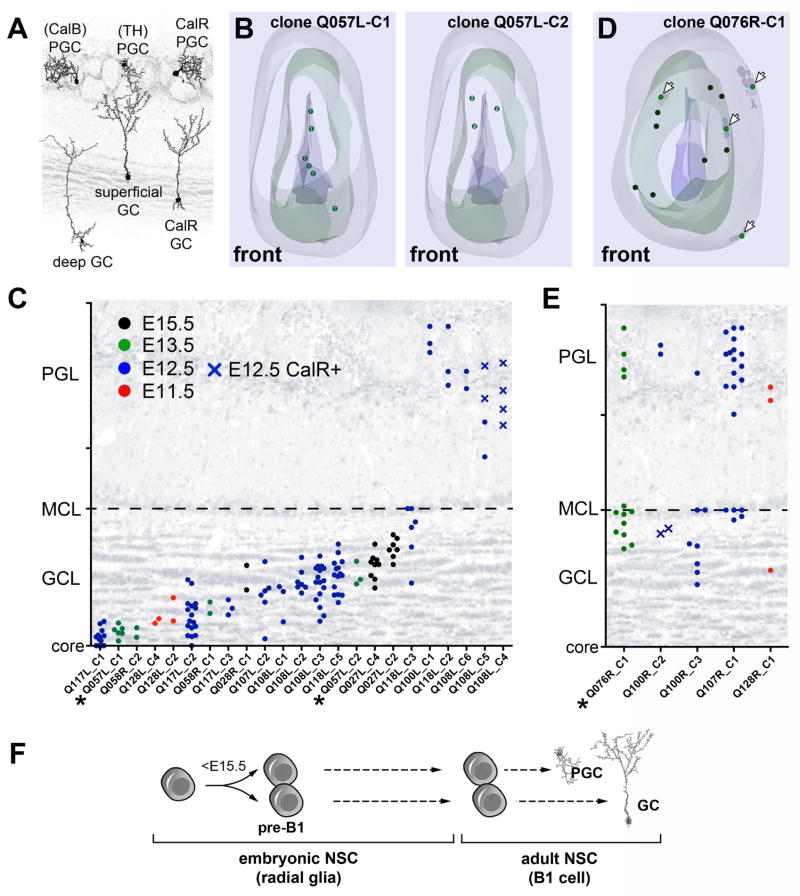
Depending on their location in the V-SVZ, B1 cells produce specific subtypes of OB neurons (see introduction). Stereological 3D reconstructions showed that mGFP+/TdT+ neurons derived from progenitor cells transduced at different stages (E11.5 to E15.5; P6- and P28-group), were widely distributed throughout the rostro-caudal extent of the OB (Movie S1). However, individual clones resided in specific locations in the GC layer, from deep to superficial positions, or in the PGC layer (Figure 4A–C and Figure S2H–I). The majority of mGFP+/TdT+ multi-cell (≥2 cells) clones (89%) were restricted to deep, intermediate or superficial locations in GC layer or were exclusively composed of PGC neurons. Seven clones contained CalR+ cells suggesting an origin in the medial wall.
Figure 4. Adult NSCs become specified before E15.5.
A) Main OB interneuron subtypes and their relative layer-specific positions. PGCs are classified by the expression of CalR, TH, and CalB. GCs are classified as deep, intermediate, or superficial, plus CalR expressing. B) Neurolucida® 3D reconstruction of an OB (Q057L) with two P28-generated mGFP+/TdT+ clones: one containing deep GCs (clone Q057L-C1 (circles in left panel) and one containing intermediate GCs (clone Q057L-C2 (circles in right panel). Note that clones are dispersed rostro-caudally, but occupy specific positions in the GC layer (see Movie S1). The OB surface (gray), mitral cell layer (green) and the OB core (blue) are shown. C) The relative OB distances of individual mGFP+/TdT+ cells within clones show that the majority of P28-generated clones contained either one subtype of GC or PGC (39 of 44 multi-cell (≥2) clones; 23 are shown (remaining clones are plotted in Figure 6). D) Neurolucida® 3D reconstruction of one P28-generated clone containing both superficial GCs and PGCs (clone Q076L-C1). Arrows denote PGCs. E) Relative OB distances of individual cells in mixed GC/PGC clones generated at P28 (5 of 44 multi-cell (≥2) clones); these clones contained one GC subtype (superficial or intermediate) and PGCs. Clones containing deep and superficial GCs were not encountered in any of our embryonic injections. F) Pre-B1 cells are specified for the production of GCs or PGCs subtypes during early embryonic development before E15.5 (see text). Closed circles and crosses denote CalR negative and positive cells, respectively. Asterisks mark Neurolucida® reconstructed clones in C and E. GCL: granule cell layer, MCL: mitral cell layer, PGL: periglomerular cell layer. See also Figure S2H–J and Table S3.
We also observed that 7 (11%) of 66 mGFP+/TdT+ multi-cell clones contained a mixture of GCs and PGCs (from both the P6- and P28 groups) (Figure 4D, Figure S2J). These mixed GC/PGC clones were rare, but more frequent among the brains that received the retroviral library at early embryonic stages (Table S3). To extend our analysis of mixed GC/PGCs clones to a larger number of cells, we also studied all P28-mGFPonly OB neurons (i.e. derived before P28). Of 116 mGFPonly multi-cell clones, 23 were mixed GC/PGC clones (20%) (Table S4); these mixed GC/PGC clones were significantly more common at earlier injection times and became rare by E15.5 (E11.5: 36%; E12.5: 50% E13.5: 24%; E15.5: 3.6% (p value < 0.0001)). Interestingly, these mixed GC/PGC clones showed evidence of GC specification, as they never contained both deep and superficial GCs (Figure 4E, Figure S2J). These observations suggest that the majority of juvenile and adult B1 cells became restricted for the production of specific OB interneuron subtypes very early in embryonic development.
Postnatal NSCs retain the positional information of their embryonic predecessors
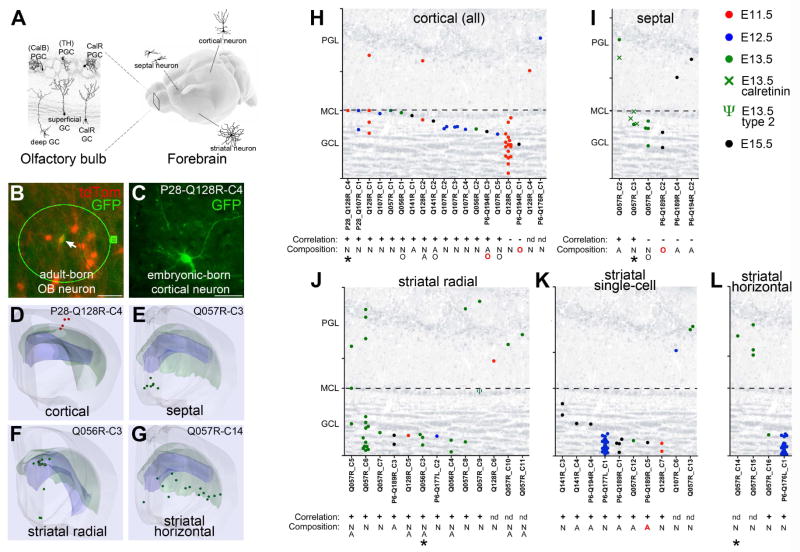
If postnatal B1 cells inherit their positional information from their embryonic progenitor cells, it should be reflected in the positions of clonally related Cx, St, Sp cells. We mapped the locations of all cells within clones that contained both mGFP+/TdT+ and mGFPonly OB interneurons and mGFP+ Cx, St, Sp neurons and glia. We grouped each OB clone according to the location of clonally related cells in Cx, St and Sp (Figure 5A–G, Figure S3), which likely reflects the position of the progenitor cell at the time of transduction. A remarkable relationship emerged: 15 of 17 clones in cortex (which contained radially oriented pyramidal neurons and/or glia (Figure 5H, Movie S2)) were clonally related to superficial GCs, with some containing PGCs.
Figure 5. Different types of OB interneurons are lineage related to specific subtypes of forebrain neurons in a region-specific manner.
A) We investigated the location and types of cells in Cx, St, Sp that were clonally related to the different subtypes of OB neurons. B–C) Example of a clone (P28-Q128R-C4) containing a mGFP+/TdT+ superficial GC (arrow in B) and a GFP+ neuron found in cortex (C). D–G) Neurolucida® 3D reconstructions of a radial clone in cortex (red symbols from E11.5 retroviral injection; P28-Q128R-C4 in D)), septum (green symbols from E13.5 retroviral injection; Q057-C3 in E), and striatum (green symbols from E13.5 retroviral injection; F and G). Striatal clones can be further distinguished as radial (Q056R-C3 in F) and horizontal (Q057R-C14 in G). Neurons (·), astrocytes (□), and ependymal cells (◆) are shown. The forebrain surface (gray), lateral ventricle (blue), and the corpus callosum (green) are shown. H–L) Relative OB positions of individual cells in the OB were grouped and classified according to their clonal relationships to cortical (H), septal (I), or radial (J), single-cell (K), or horizontal (L) striatal clones. Closed circles and crosses denote CalR negative and positive cells, respectively. ψ denotes a type-2 cell in Q057R-C9 (in J). The expected correlation based on the previous mapping of the postnatal V-SVZ (Merkle et al., 2007; Merkle et al., 2014) is indicated by a + or −. “n.d.” indicates that cell subtype was “not determined” due to technical limitations (see Methods), but these cells were CalR-negative as expected. Asterisks in H, I, J, and L mark Neurolucida® reconstructed clones in D, E, F, and G, respectively. For additional examples, see Figure S3. Scale bars represent 25 and 50 μm in B and C, respectively. See also Figure S4.
In the striatum, we were able to distinguish radially and horizontally distributed clones (Figure 5F–G, Figure S3) (Halliday and Cepko, 1992; Reid and Walsh, 2002). Horizontal striatal clones had cells that were widespread rostro-caudally (Movie S3). Half of these striatal horizontal clones contained deep GCs (Figure 5L), the others were related to PGCs in the OB. Radial striatal clones were confined rostro-caudally (Movie S4) and had a high proportion of astrocytes, which are allocated to radial domains (Tsai et al., 2012). OB cells that were clonally related to these radial striatal clones were found in deep and intermediate GC layers (9 of 10 GC-containing clones (Figure 5J)). The other striatal clone was related to a type-2 OB interneuron, a recently identified neuronal subtype that is derived from B1 cells in the lateral side of the very anterior-ventral V-SVZ (Q057R_c9, ψ in Figure 5J) (Merkle et al., 2014). Clones containing a single striatal cell, which could not be classified as radial or horizontal, also had siblings with deep or intermediate GCs (Figure 5K). Finally, CalR+ OB interneurons were only found in clones containing septal cells (Movie S5) consistent with their production in the medial V-SVZ (Figure 5I) (Merkle et al., 2007).
The above results indicate a remarkable level of regional specification of B1 cells as early as E11.5. We were curious if specification can occur even earlier; however, intraventricular injections of the retroviral library before E11.5 are technically challenging. In order to confirm the early regional specification of the NSC lineage, we selectively labeled E9.5 cortical and dorsal septal progenitor cells with a single dose of Tmx in Emx1::CreER::Ai14 embryos. At P28, a cohort of dividing progenitors cells was labeled by a 7d BrdU treatment and the OB was analyzed three weeks later. In agreement with our clonal findings, the vast majority of TdT+/BrdU+ cells were found in the superficial GC layer (Figure S4). In addition, a significant fraction of TdT+/BrdU+ OB interneurons were TH+ and CalR+, consistent with Emx1 expression in cortical and septal progenitors, respectively (Kohwi et al., 2007, Young et al., 2007). P28 Tmx treatment to label adult Emx1+ progenitors also resulted in the generation of superficial GCs and TH+ PGCs, indicating that Emx1 expression is maintained from early embryonic stages to adulthood (Figure S4).
Taken together with the barcoding clonal analysis, these data demonstrate that positional information within individual embryonic progenitor cells, determining the types of neurons and glia produced for different forebrain regions throughout embryogenesis, is inherited by pre-B1 cells as early as E11.5 (and possibly earlier as suggested by the Emx1 lineage experiments).
B1 cells are mostly quiescent after their embryonic generation
We next asked whether our clonal analyses of the P28-group from QmGFP-OL library injections could also provide insights about the postnatal self-renewing capabilities of B1 cells. If progenitor cells had divided repeatedly to generate different cohorts of OB neurons at different postnatal ages, we would expect that most OB clones containing mGFP+/TdT+ cells should also contain mGFPonly neurons produced before P28. More than half (12 of 23) of the mGFP+/TdT+ OB clones did not have clonal relationship to other mGFPonly OB neurons (262 mGFPonly OB clones) (Table S4, Figure 6G). The remaining 11 clones (containing both mGFP+/TdT+ and mGFPonly neurons) may be derived from either self-renewing postnatal B1 cells (that divided before and after P28) or from progenitor cells that divided symmetrically in the embryo to generate two pre-B1 sister cells; one dividing before and one after P28. Of these 11, four were mixed GC/PGC clones suggesting labeling in the embryo of a more primitive progenitor cell that generated sister pre-B1 cells - one producing GCs and the other PGCs - which are produced before E15.5 (Figure 4 and Reid et al., 1999). In addition, we also investigated whether OB neurons were clonally related to B1 cells in the V-SVZ. Clones containing B1 cells and OB neurons were extremely rare: of all double-labeled mGFP+/TdT+ OB neurons analyzed from all of our embryonic injections, we only found one clone containing a labeled B1 cell (P6-group: 1 of 49; P28-group: 0 of 14) (Table S2). Even when taking into account all clones containing mGFPonly OB neurons, only 7 (3.5%) had B1 cells in the walls of the lateral ventricles (Table S2). As indicated above, we do not know if these correspond to sister pre-B1 cells that diverged in the embryo or to self-renewing B1 cells.
Figure 6. Pre-B1 cells remain quiescent until their reactivation in the postnatal brain.
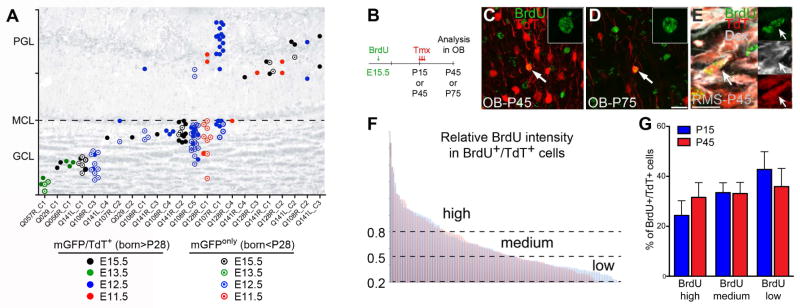
A) Relative OB positions of mGFP+/TdT+ (filled circles, born after P28) and mGFPonly (open circles, born before P28) cells within clones (experiment in Figure 3 D–E). Eleven out of 23 mGFP+/TdT+ OB clones were clonally related to mGFPonly OB neurons and of these 4 were mixed suggesting an origin in the embryo (see also Table S4). B) E15.5 pregnant Nestin::CreER;Ai14 females were BrdU injected; littermates received Tmx at either P15 or P45 and their OBs were analyzed 28d later. C–E) Independent of the time of Tmx administration, brightly labeled BrdU+ nuclei were detected in TdT+ OB neurons (C, D) and TdT+/DCX+ neuroblasts in the RMS (E), indicating limited BrdU dilution. F) Distribution of the relative BrdU intensities in BrdU+/TdT+ OB neurons from both age groups (blue: Tmx P15, n=132 cells; red: Tmx P45, n=126 cells. 7 mice/group, three independent experiments). (G) Percentages of TdT+ OB neurons with high, medium or low BrdU intensities in P15 (blue) or P45 (red) Tmx-treated mice. Data are expressed as mean ± s.e.m., n=7 mice/group, three independent experiments each (n.s.). GCL: granule cell layer, MCL: mitral cell layer, PGL: periglomerular cell layer. See also Table S4.
The above experiments suggest that B1 cells, after their embryonic production, do not divide repeatedly to produce multiple generations of OB neurons in postnatal life, otherwise they should dilute their BrdU labeling (Figure 1A–C). In order to more directly test this possibility, we injected Nestin::CreER;Ai14 timed-pregnant females with BrdU at E15.5 to label dividing pre-B1 cells. Littermates received Tmx at P15 or P45, and BrdU intensity was quantified in BrdU+/TdT+ OB neurons four weeks later (Figure 6A–D). If B1 cells undergo self-renewing divisions to produce multiple cohorts of neurons at different postnatal ages, neurons produced after P45 should contain lower BrdU levels compared to those produced after P15. However, we observed that the BrdU intensity in BrdU+/TdT+ neurons in both groups was indistinguishable, indicating that separate sets of B1 cells became activated at different ages (Figure 6E–F). Some DCX+ neuroblasts in the RMS were also brightly labeled with BrdU, indicating that young neurons were produced from another cohort of brightly labeled B1 cells shortly before sacrifice (P45 and P75). These experiments further suggest that the majority of B1 cells is not dividing repeatedly during postnatal life to produce different generations of young neurons.
The above experiments strongly suggest that after their production in the embryo, pre-B1 cells remain largely quiescent for different periods of time until they become reactivated to generate separate cohorts of neurons postnatally. These observations argue against systematic in vivo self-renewal during postnatal life to generate neurons at different ages.
Discussion
In this study, we uncovered the lineage relationships of embryonic and adult NSCs. Using BrdU and in utero delivery of GFP-expressing retroviruses, we found that the majority of the pre-B1 cells were generated from embryonic cells that divided between E13.5–E15.5. Our results suggest that B1 cells remain relatively quiescent until postnatal life, when they are reactivated to produce OB neurons. Clonal lineage analysis of forebrain progenitor cells demonstrates the presence of a common progenitor for neurons in Cx, Sp, or St and pre-B1 cells that generate OB neurons during postnatal life. Interestingly, the number of clones showing this relationship progressively decreased between E11.5 and E15.5. This suggests that, during this period, the embryonic and postnatal NSC lineages diverge. Clones with both OB cells and embryonically-generated forebrain neurons revealed that the positional information of embryonic progenitor cells is maintained in postnatal NSCs, preserving region-specific production of different OB neuronal subtypes throughout life.
Lineage tracing using retroviral libraries
How adult stem cells are related to embryonic stem cells remains unanswered for many tissues, in part because it is technically challenging to follow the progeny of individual progenitor cells from the embryo into postnatal life. In the V-SVZ, understanding the embryonic origin of postnatal NSCs is further complicated by the long migration that separates the final progeny (interneurons in the OB), from progenitor cells within the walls of the lateral ventricles. Here we used a retroviral library carrying at least 105 tags (barcodes) (Golden et al., 1995) to infer lineage relationships. Other lineage tracing methods, based on the use of fluorescent reporters of different colors (Livet et al., 2007; Schepers et al., 2012; García-Marqués and López-Mascaraque, 2013) do not provide the complexity in tag diversity to convincingly demonstrate that two cells in very different regions are derived from a single progenitor cell.
The retroviral barcode method also has limitations that could affect the interpretation of results. Viral inactivation (Walsh and Cepko, 1992; Golden et al., 1995) may result in an underestimation of clone size and/or complexity. In addition, mGFP that allows identification of transduced cells may not reach detection threshold. Since the rate of viral inactivation may vary among cell types and injection times, we assayed for silent genomes by randomly capturing pieces of tissue containing mGFP-negative cells. In the OB, where the high concentration of PGCs and GCs would make it more likely to capture silenced cells, we did find mGFP-negative microdissected cells carrying retroviral tags. This indicates that a fraction of the cells derived from progenitor cells transduced in the embryo inactivate the reporter gene; the frequency of false negative was low (4 of 252 mGFP-negative microdissected OB tissue yielded a barcode (Table S4)). As indicated above, we could recover the barcode sequence from 51.4% of microdissected mGFP+ cells, which also artificially reduces our clonal sizes. We therefore, do not make inferences about clone size and limit our conclusions to the clonal relationships among cells that were positively identified.
It is also possible that two cells could be derived from independent transductions with viruses carrying the same barcode. Theoretically, given the in vitro tested complexity of the library (105), the probability of false positives is small (Golden et al., 1995). When we compared the frequency of barcodes in six brains with a total of 1446 different tags, we found one tag that was repeated in three brains and 48 tags that were repeated in 2 brains (Table S5). This suggests that some barcodes are overrepresented in the library, increasing the probability of “lumping” errors, i.e. the assumption that cells with the same barcode are siblings when they are actually derived from more than one progenitor cell. From the observed number of repeated tags and the number of clones per brain, less than 2.4% of the barcode tags had a probability to produce “lumping” errors (Table S5). Since our conclusions are based on the analysis of 2186 different tags, we conclude that the vast majority of the cells that share the same barcode were derived from a single progenitor. Furthermore, the close correlation observed between OB subtypes and the location of clonally related cells in the forebrain strongly supports our conclusion that cells presumed to belong to the same clone were indeed derived from single progenitor cells in the embryo.
Temporal progression of region-specific NSC lineages
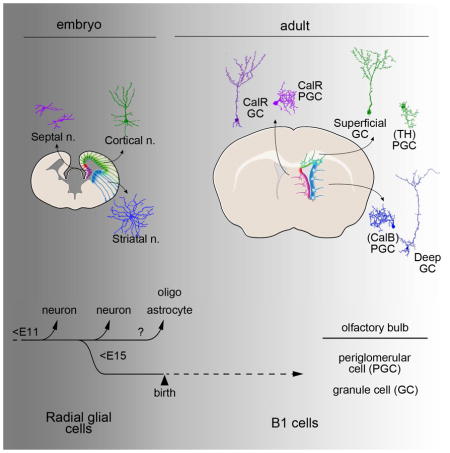
During development, NSCs change in potential and generate different types of neurons and glial cells at different times (Livesey and Cepko, 2001; Kohwi and Doe, 2013). This process, that has been best studied in Drosophila and is referred to as temporal identity specification, depends on transient changes in transcription factor expression that are related to the birth order. In the mammalian brain, progenitors change over time, gradually becoming restricted in potential (McConnell, 1995). For example, a period of neurogenesis in which different types of neurons are sequentially produced, is followed by a period of gliogenesis (Rowitch and Kriegstein, 2010). This raises the question of the origin of adult NSCs, a subset of postnatal progenitor cells that maintains the capacity to generate neurons throughout life. Where do these cells come from? Here we show that subpopulations of embryonic progenitor cells that produce different types of neurons and glial cells in the Cx, St, and Sp, also generate pre-B1 cells. While the embryonic progenitor cells that generate both embryonic neuronal progeny and B1 cells are clearly multipotent, they do not generate all types of forebrain cells. Instead, our findings suggest that, as early as E11.5, the neurogenic potential of embryonic progenitor cells is highly restricted by their location. This regional identity is maintained from embryonic to adult stages (Figure 7). Remarkably, this spatial identity encodes for the production of very different types of neurons in the embryo and in the adult. For example, progenitor cells in the cortex, which in the embryo produce pyramidal cells (glutamatergic), but not local interneurons, also generated B1 cells that during postnatal life give rise to very specific subpopulations of superficial GC and PGC interneurons (GABAergic) in the OB.
Figure 7. Embryonic origin of postnatal NSCs.
A) Left: Coronal section of the embryonic (E12.5) mouse brain illustrating regional organization of cortical (green), striatal/LGE (blue), and septal (purple) VZ progenitor cells. Right: These progenitor domains are retained in the postnatal brain (dorsal (green), ventral (blue), and medial (purple)), and contain NSCs that generate specific subtypes of interneurons that migrate to the OB. B) Individual lineages illustrating the progression from embryonic to adult NSCs. Postnatal NSCs arise from region-specific pre-B1 cells that progressively diverge from other embryonic progenitors that produce specific sets of neurons in cortex (green), striatum (blue), and septum (purple). Later in life, these same regions harbor dorsal (green), ventral (blue), and medial (purple) V-SVZ B1 cells that produce superficial GCs, intermediate/deep GCs, and CalR+ GCs and PGCs, respectively. Pre-B1 cells become restricted for the production of specific subtypes of GCs or PGCs as early as E11.5 and before E15.5.
Temporal identity specification may be orchestrated by a number of cell–intrinsic and –extrinsic factors (Livesey and Cepko, 2001; Kohwi and Doe, 2013). A recent study using time-lapse clonal analysis in vitro showed that individual E11.5 cortical progenitors could generate Tbr1+ projection neurons followed by GAD67+/SP8+ OB interneurons (Cai et al., 2013). These observations suggest that this identity switch may be controlled by intrinsic factors in cortical progenitor cells, as it has been proposed to take place during deep-to-superficial projection neuron and projection neuron-to-glia fate changes (Qian et al., 2000; Shen et al., 2006). Consistent with the concept that spatial specification can have very different outcomes depending on the timing, a recent study shows that conditional ectopic expression of the dorsal LGE transcription factor Gsx2 results in different cell fates depending on the timing (Waclaw et al., 2009). Early (E9.5) Gsx2 mis-expression results in the re-specification of the developing forebrain into an Isl1-expressing striatal cell phenotype, while late (E13.5) Gsx2 mis-expression promoted SP8+ OB fates. Similar temporal changes in cell fate specification could take place in the different regions of the developing telencephalon. Our study follows this switch in progenitor potential from embryonic to adult stages in vivo, revealing that postnatal progenitor cells are related to embryonic progenitor cells that contribute to different areas of the forebrain.
Pre-B1 cells become quiescent until their reactivation in the postnatal brain
Our results indicate that the majority of pre-B1 cells divide around E13.5–E15.5. Several lines of evidence suggest that B1 cells then remain relatively quiescent, until being reactivated at different ages in the postnatal brain: 1) P21 B1 cells show little evidence of BrdU dilution from pre-B1 cells. 2) B1 cell progenies generated at different times had indistinguishable intensities of BrdU when given at E15.5. 3) VCAM1, a marker of quiescent B1 cells (Kokovay et al. 2012; Codega et al., 2014), is expressed in progenitor cells between E13.5 and P0. Finally, 4) a significant fraction (52%) of P28-clones did not contain OB cells born before P28. In these experiments, we used the Nestin::CreER;Ai14 mouse line, which has been shown to label the majority of primary progenitors in the V-SVZ with no evidence of non-specific labeling (Lagace et al., 2007). Clones containing both mGFP+/TdT+ and mGFPonly OB interneurons could result from: 1) Self renewing B1 cells that generated progeny before and after Tmx. 2) Pre-B1 cells that divided symmetrically and each sibling B1 cell then becomes activated at different times (before or after Tmx). 3) Incomplete recombination upon Tmx. Our results cannot distinguish between these possibilities.
Recently, it has been suggested that adult SGZ progenitor cells, which produce granule cells for the hippocampus, are exhausted once activated (disposable stem cell hypothesis) (Encinas et al., 2011). However, another clonal analysis study in the SGZ suggests that some adult progenitor cells self-renew (Bonaguidi et al., 2011). In our study, clones containing B1 cells and OB neurons were rare: only 3.0% had B1 cells in the V-SVZ (Table S2), and as discussed above these could be derived from sister pre-B1 cells that divided in the embryo. While the low numbers of labeled B1 cells associated with neuronal OB clones suggest that B1 cell self-renewal is rare, this hypothesis requires further investigation as B1 cells may inactivate the viral insert and lose their label following division. Several groups, including ours, have previously reported the presence of label-retaining cells in the V-SVZ after thymidine analog labeling (Morshead et al., 1994; Doetsch et al., 1999), yet there is no evidence that these label-retaining cells maintain long-term progenitor properties. The present results raise the interesting possibility that following long periods of quiescence, B1 cells may be exhausted following division, a phenomenon that may explain the progressive decline in V-SVZ neurogenesis with age (Conover and Shook, 2011). We cannot, however, exclude that some B1 cells divide and one or both daughter cells remain quiescent for extended periods.
Conclusion
This study provides a direct link between progenitor cells in the embryo that produce neurons and glial cells for the Cx, St, and Sp, and the postnatal and adult NSCs that are retained in the walls of the lateral ventricle and continue producing neurons for the OB. It is noteworthy how different the clonally related cells in the OB and in the rest of the forebrain are (e.g. superficial GCs compared to pyramidal cells), yet our findings show that these cells have a common progenitor in the embryo. While these early progenitor cells have the potential to make such distinct cells, our data suggest that their multipotency is limited by spatial and temporal cues that are evident as early as E11.5 and are maintained until adult stages. This fundamental information will help determine how the dynamic molecular mechanisms for regional and temporal specification in the embryo -- ranging from secreted factors to transcription factor expression to epigenetic modifications -- are preserved in populations of NSCs for the production of different types of neurons from embryonic to postnatal ages.
Experimental Procedures
Animals
Mice were housed and treated according to the guidelines from the University of California, San Francisco, Institutional Animal Care and Use Committee. Details of procedures for embryonic and adult BrdU administration, embryonic and adult Tmx administration, in utero surgeries, and whole-mount dissections are described in Supplemental Information.
Generation of the QmGFP-OL library and Laser-Microdissection
The Barcode retrovirus was produced by inserting the commercially synthesized oligo TGACAGCTACTAGCGTAGATSWSWSWSWSWSWSWSWSWSWSWSWGTAG ATCACTCGATCTCAGC in the pQXIX retroviral vector (Clontech) encoding membrane-bound GFP (Rompani and Cepko, 2008).
Mice were sacrificed and their brains were processed for immunohistochemistry as previously described (Merkle et al., 2007). Since our microscopic analyses were limited to the detection of only three fluorophores, we were unable to perform co-localization staining for TH and CalB in the PGC layer of the OB. Individual mGFP+ cells were mapped and laser-captured with small amount of surrounding tissue (4000–6000 μm2) using a Zeiss PALM MicroBeam LCM (v4.3.2.13, Carl Zeiss MicroImaging). M-GFP-negative control tissue was dissected every 1–2 mGFP+ dissections. Barcode amplification was done using nested PCR with primers S161 and S126 for the first amplification step and primers S160 and S128 for the second amplification step (Figure 2). Sequence alignments were performed with MAFFT (v7 CBRC, Japan) and MultiAlin (GenoToul bioinformatics). Cells in clones were 3D mapped using Neurolucida® (v10.31 MBF Biosciences-MicroBrightField Inc.), and relative positions of OB cells were measured using ImageJ (v1.45m NIH, USA).
BrdU intensity measurements
Confocal images were taken with Leica SP8 white light laser scanning confocal microscope and BrdU intensities were determined using Leica LAS AF3 as gray scale values per pixel per region of interest (ROI). ROIs were defined as the area encompassing a BrdU+ nucleus with at least three BrdU patches. Normalized intensities were grouped into high, medium and low (above 80%, 50–80% and 50–20% relative to cortex, respectively. See Supplemental Information).
Supplementary Material
Highlights.
Postnatal neural stem cells (NSCs) become regionally specified early in development
Postnatal and fetal forebrain NSCs share common progenitors in the early embryo
Lineages for postnatal and embryonic NSCs diverge during mid-fetal development
After generated in the embryo, postnatal NSCs remain largely quiescent.
Acknowledgments
We would like to thank William Walantus for outstanding technical assistance with in utero procedures; Ernesto Guerra (UC Berkeley) for writing scripts for statistical analyses; Amelia J. Eisch (UT Southwestern Medical Center) and Nicoletta Kessaris (University College London) for generously sharing the Nestin::CreER and Emx1::CreER mice, respectively; Arnold R. Kriegstein for providing the 293gp NIT-GFP cell line. Shawn Sorrells, Corey Harwell, and members of the Alvarez-Buylla laboratory for helpful discussions that improved this study. L.C.F. is a HHMI fellow of the Helen Hay Whitney Foundation. J.I.P is supported by the Chilean Government (Becas Chile). K.O. is supported by the Deutsche Forschungsgemeinschaft. A.A-B is the Heather and Melanie Muss Endowed Chair. This study was supported by grants from the NIH (HD 32116 and NS 28478), the John G. Bowes Research Fund, and HHMI (C.L.C.)
Footnotes
Author Contributions
L.C.F. and A.A-B. designed research; L.C.F., J.I.P., K.O. and R.R. conducted experiments. S.B.R. and C.L.C. developed the QmGFP-OL retroviral library and critically reviewed and edited the manuscript. L.C.F., K.O. and A.A-B. analyzed data. L.C.F. and A.A-B. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Frye M. Stem cells in ectodermal development. J Mol Med (Berl) 2012;90:783–790. doi: 10.1007/s00109-012-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Zhang Y, Shen Q, Rubenstein JL, Yang Z. A subpopulation of individual neural progenitors in the mammalian dorsal pallium generates both projection neurons and interneurons in vitro. Stem Cells. 2013;31:1193–1201. doi: 10.1002/stem.1363. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Fields-Berry S, Ryder E, Austin C, Golden J. Lineage analysis using retroviral vectors. Curr Top Dev Biol. 1998;36:51–74. doi: 10.1016/s0070-2153(08)60495-0. [DOI] [PubMed] [Google Scholar]
- Codega P, Silva-Vargas V, Paul A, Maldonado-Soto AR, Deleo AM, Pastrana E, Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82:545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Shook BA. Aging of the subventricular zone neural stem cell niche. Aging Dis. 2011;2:49–63. [PMC free article] [PubMed] [Google Scholar]
- Costa G, Kouskoff V, Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33:215–223. doi: 10.1016/j.it.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Eckfeldt CE, Mendenhall EM, Verfaillie CM. The molecular repertoire of the ‘almighty’ stem cell. Nat Rev Mol Cell Biol. 2005;6:726–737. doi: 10.1038/nrm1713. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marqués J, López-Mascaraque L. Clonal identity determines astrocyte cortical heterogeneity. Cereb Cortex. 2013;23:1463–1472. doi: 10.1093/cercor/bhs134. [DOI] [PubMed] [Google Scholar]
- Giachino C, Basak O, Lugert S, Knuckles P, Obernier K, Fiorelli R, Frank S, Raineteau O, Alvarez-Buylla A, Taylor V. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells. 2014;32:70–84. doi: 10.1002/stem.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JA, Fields-Berry SC, Cepko CL. Construction and characterization of a highly complex retroviral library for lineage analysis. Proc Natl Acad Sci U S A. 1995;92:5704–5708. doi: 10.1073/pnas.92.12.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday AL, Cepko CL. Generation and migration of cells in the developing striatum. Neuron. 1992;9:15–26. doi: 10.1016/0896-6273(92)90216-z. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Götz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Doe CQ. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci. 2013;14:823–838. doi: 10.1038/nrn3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JL, Alvarez-Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E, Wang Y, Kusek G, Wurster R, Lederman P, Lowry N, Shen Q, Temple S. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell. 2012;11:220–30. doi: 10.1016/j.stem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- López-Juárez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun YY, Yang D, Kuan CY, et al. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes Dev. 2013;27:1272–1287. doi: 10.1101/gad.217539.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 2014;17:207–214. doi: 10.1038/nn.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci U S A. 2013;110:E1045–1054. doi: 10.1073/pnas.1219563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Reid CB, Liang I, Walsh CA. Clonal mixing, clonal restriction, and specification of cell types in the developing rat olfactory bulb. J Comp Neurol. 1999;403:106–118. [PubMed] [Google Scholar]
- Reid CB, Walsh CA. Evidence of common progenitors and patterns of dispersion in rat striatum and cerebral cortex. J Neurosci. 2002;22:4002–4014. doi: 10.1523/JNEUROSCI.22-10-04002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompani SB, Cepko CL. Retinal progenitor cells can produce restricted subsets of horizontal cells. Proc Natl Acad Sci U S A. 2008;105:192–197. doi: 10.1073/pnas.0709979104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS. Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19:10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, Bell SM, Erdélyi F, Szabó G, Potter SS, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 2009;63:451–465. doi: 10.1016/j.neuron.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.