Abstract
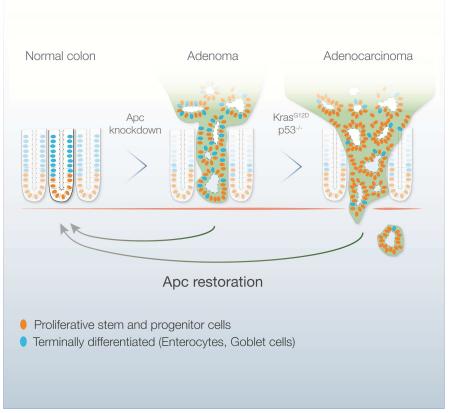
The Adenomatous Polyposis Coli (APC) tumor suppressor is mutated in the vast majority of human colorectal cancers (CRC) and leads to deregulated Wnt signaling. To determine whether Apc disruption is required for tumor maintenance, we developed a mouse model of CRC whereby Apc can be conditionally suppressed using a doxycycline-regulated shRNA. Apc suppression produces adenomas in both the small intestine and colon that, in the presence of Kras and p53 mutations, can progress to invasive carcinoma. In established tumors, Apc restoration drives rapid and widespread tumor-cell differentiation and sustained regression without relapse. Tumor regression is accompanied by the re-establishment of normal crypt-villus homeostasis, such that once aberrantly proliferating cells reacquire self-renewal and multi-lineage differentiation capability. Our study reveals that CRC cells can revert to functioning normal cells given appropriate signals, and provide compelling in vivo validation of the Wnt pathway as a therapeutic target for treatment of CRC.
Keywords: APC, polyposis, shRNA, Wnt, tumor regression, FAP
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in developed countries and almost half of the population will develop at least one benign intestinal tumor during their lifetime (Jemal et al., 2011). Treatment regimes for advanced CRC involve combination chemotherapies that are toxic and largely ineffective, yet have remained the backbone of therapy over the last decade. Molecularly, the vast majority (~80-90%) of colorectal tumors contain inactivating mutations in the Adenomatous Polyposis Coli (APC) tumor suppressor (Brannon et al., 2014), and individuals with specific germline mutations in APC (Familial Adenomatous Polyposis or FAP) invariably develop colon cancer before the age of 35. Collectively, APC mutant CRC accounts for more than 600,000 deaths annually worldwide, a number greater than KRAS mutant lung or pancreas cancer. Hence, strategies to exploit APC alterations in CRC have broad clinical potential.
APC regulates a number of cellular functions, including mitosis, migration and the maintenance of genome stability (Nelson and Nathke, 2013). Most importantly, APC, along with AXIN1 and GSK3β, is part of a multi-protein complex that controls output of the Wnt signalling pathway by regulating the sub-cellular localization and stability of CTNNB1 (β-catenin), a key transcriptional regulator that drives Wnt signalling output. APC inactivation is considered the initiating event in most CRCs, and Apc loss is sufficient to induce benign and dysplastic adenomas in the small and large mouse intestine (Cheung et al., 2010; Su et al., 1992). ApcMin (Multiple Intestinal Neoplasia) mice carry a single mutant Apc allele and develop 50-100 benign adenomas in the small intestine by 4-6 months of age, invariably associated with loss of the remaining wild-type gene (Su et al., 1992). Conditional Apc truncation drives a similar neoplastic phenotype, associated with hyperproliferation, reduced multi-lineage differentiation, disrupted tissue structure, and an expansion of intestinal stem cells outside the crypt domain (Barker et al., 2008; Sansom et al., 2004).
Besides APC disruption, CRCs show a high incidence of mutations in KRAS (45%) and TP53 (54%) that cooperate to drive tumor progression (2012). Additionally, recent large-scale sequencing efforts have catalogued additional genetic changes that likely influence disease progression (2012). Still, little is known about which, if any, of these alterations are required for maintenance of established disease and continued malignant progression. Indeed, it remains unclear whether APC disruption, the predominant CRC-associated event, is required for maintenance of CRC and thus whether hyperactivated Wnt signalling is a viable therapeutic target.
To address this question, we generated shRNA transgenic mice that enable conditional and reversible control of Apc expression by TRE-regulated, GFP-linked short-hairpin RNAs (TG-shRNAs)(Dow et al., 2012; Premsrirut et al., 2011). In mice that also express a reverse tet-transactivator (rtTA), doxycycline (dox) administration drives GFP expression and Apc silencing, and subsequent dox withdrawal results in restoration of endogenous Apc expression. Our work demonstrates a crucial role for Apc loss in CRC maintenance, and reveals an unexpected ability of Apc to re-establish control of crypt homeostasis in animals with hyperproliferative polyps or cancer. Collectively, our results validate the APC/WNT pathway is an attractive target for the treatment of CRC.
Results
Potent Apc silencing blocks differentiation and drives hyperproliferation in the intestine
Acute genetic disruption of Apc in the intestine drives hyperproliferation and expansion of undifferentiated progenitor cells (Sansom et al., 2004). This results in the disruption of the crypt-villus axis, whereby stem and progenitor cells, normally restricted to the crypt base, expand and fail to differentiate as they move up the villus (Sansom et al., 2004). To assess how shRNA-mediated Apc suppression affects crypt-villus homeostasis we examined Wnt pathway activation in the intestinal epithelium in multiple Apc shRNA strains (TG-Apc.3374 and TG-Apc.9365) (Premsrirut et al., 2011). We observed that only the most potent suppression of Apc (TG-Apc.3374) caused an increase in non-phosphorylated β-catenin in the intestine (Figure S1A). This response was further amplified with a CAGs-rtTA3 transgene, driving greater Apc knockdown and more than 20-fold transcriptional induction of the canonical Wnt target Axin2 (Figure S1A,B).
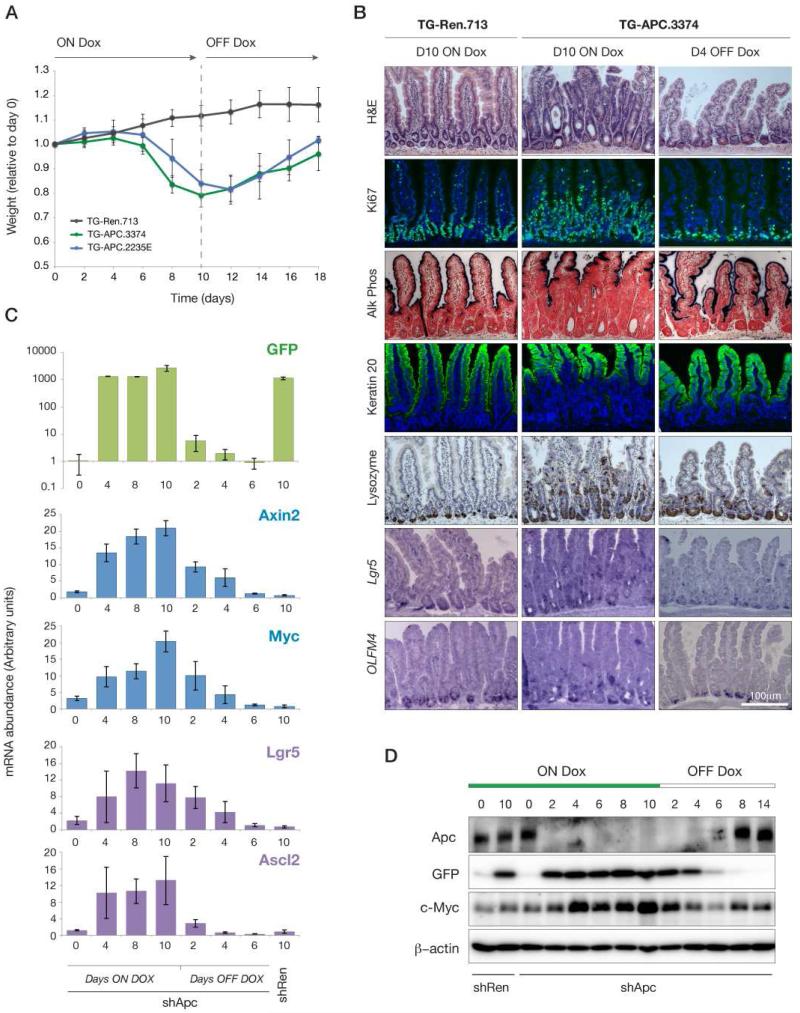
To examine the intestinal response to Apc silencing, we treated TG-Apc.3374 (hereafter, shApc) animals with dox and monitored their weight as a surrogate measure of intestinal function. After six days of dox treatment shApc animals began to show signs of weight loss relative to neutral TG-shRen.713 (shRen) controls (Zuber et al., 2011), and by day 10, shApc mice were lethargic and moribund (Figure 1A). Depletion of Apc caused a marked expansion of Alkaline Phosphatase (AP) and Keratin 20 (Krt20) negative progenitors and hyperproliferation throughout the crypt-villus axis (Figure 1B). The block in differentiation and expansion of stem and progenitor cells was further confirmed by the identification of Lysozyme-positive Paneth cells and Lgr5 and Olfm4-positive stem cells outside their normal position at the base of the crypt (Figure 1B). Apc silencing induced a progressive increase in the expression of canonical Wnt targets, including Axin2 and cMyc, as well as markers of intestinal stem cells Lgr5 and Ascl2 (Figure 1C,D). Importantly, a second transgenic strain harboring an independent and potent Apc shRNA (TG-Apc.2235E) produced identical phenotypes (Figure 1A, Figure S1C).
Figure 1. Acute depletion of Apc in the mouse.
A. Animal weight during dox treatment and following dox withdrawal at 10 days, normalized to Day 0 within each cohort. Lines represent TG-Ren.713 (black), TG-Apc.3374 (green) and TG-Apc.2235E (blue) mice. B. Immunohistochemical (H&E, Alkaline phosphatase and Lysozyme), immunofluorescent (Ki67 and Keratin 20) stains and in situ hybridizations (Lgr5 and Olfm4) from shRen (control) and shApc intestine following dox treatment for 10 days (left two panels) and withdrawn from dox for 4 days (right panel). C. Quantitative RT-PCR analysis of gene expression in intestinal villi following dox treatment and withdrawal for TG-Apc.3374 mice as indicated. Markers of transgene induction (GFP), Wnt activation (Axin2, Myc) and stem cells (Lgr5, Ascl2) are shown for each time point. Gene expression in day 10-treated TG-Ren.713 is indicated to the right on each plot. D. Western blot of whole cell lysates from intestinal villi following dox treatment and withdrawal at time points indicated, for TG-Ren.713 and TG-APC.3374 mice, probed for Apc, GFP, cMyc and β-Actin, as indicated.
These data demonstrate that shRNA-driven Apc silencing can recapitulate the conditional knockout phenotype (Sansom et al., 2004). However, in contrast to genetic deletions, shRNAs suppress gene function in trans and, as such, the endogenous protein can be restored simply by eliminating shRNA expression. To assess whether Apc restoration could rescue the intestinal dysfunction, we treated shApc mice for 10 days and then withdrew dox from the diet. Apc restoration induced a rapid and dramatic phenotypic response, and by 8 days the mice recovered to their original weight (Figure 1A). Within 2 days of dox withdrawal, GFP-shRNA transcription from the TRE promoter was reduced more than 100-fold and by 4 days had returned almost to baseline levels (Figure 1C). Remarkably, 4 days of dox withdrawal was sufficient to restore crypt-villus homeostasis as evidenced by basal localization of proliferative stem and progenitor cells and Lysozyme+ Paneth cells, while differentiated markers (AP, Krt20) were strongly expressed throughout the villus (Figure 1B). Accordingly, a decrease in levels of Wnt-responsive mRNAs, as well as Myc protein, were apparent as early as 2 days after dox withdrawal, and returned to baseline by 4 days (Figure 1C,D). Together these data show that acute Apc suppression recapitulates the phenotypes of Apc deletion and, importantly, that restoration of endogenous Apc expression can recover normal intestinal function.
A mosaic model of shRNA driven tumor development in the small and large intestine
The above results are striking and highlight the utility of inducible shRNA transgenic mice to study the reversibility of loss of function phenotypes. However, these models are not ideal for studying cancer, as tissue-wide Apc suppression produces lethality well before mice develop intestinal tumors. Moreover, tumorigenesis in humans initiates from individual mutated cells surrounded by otherwise normal tissue. To address this, we modeled mosaic Apc loss by combining a stem cell restricted 4-hydroxytamoxifen (4OHT)-inducible CreER strain (Lgr5-GFP-IRES-CreER, hereafter Lgr5-CreER)(Barker et al., 2007) with a Cre-dependent R26-CAGs-LSL-rtTA3 (LSL-rtTA3) strain that we recently generated (Dow et al., 2014). In these mice, a single treatment with 4OHT initiates LoxP-recombination in stem cells of the small and large intestine, inducing long-lived, rtTA3 expression in a small percentage of individual crypts and villi (Figure S2A,B). In LSL-rtTA3/Lgr5-CreER/TG-Ren.713 (shRen/Lgr5) control mice treated with 4OHT and dox we observed GFP induction in individual crypts and villi throughout the length of the small intestine and colon for up to 40 weeks (Figure S2C, not shown). Of note, the shRNA linked GFP reporter is significantly more abundant than GFP expressed from the Lgr5-GFP-IRES-CreER knock-in allele and can be used as a surrogate marker of shRNA expression and Apc knockdown.
Next, we generated LSL-rtTA3/Lgr5-CreER/shApc (shApc/Lgr5) mice and monitored tumor development longitudinally by small animal colonoscopy. Four-to-six weeks following 4OHT/dox treatment we noted the development of macroscopic polyps in the small intestine and colon (Figure 2A, Figure S3). By 12-16 weeks, shApc/Lgr5 mice developed large colonic polyps that appeared histologically as well-differentiated tubular adenomas. As is characteristic of tumors initiated by mutations in Apc, polyps arising in shApc/Lgr5 animals showed a massive increase in proliferation, marked by BrdU incorporation (Figure 2B). These lesions also carried reduced numbers of Krt20+ enterocytes and Alcian blue+ goblet cells, and showed a significant increase in Lgr5+ stem cells outside of their normal position at the crypt base (Figure 2B, Figure S3). Together these data highlight a block in the production of differentiated cell types and an expansion of the stem and progenitor compartment.
Figure 2. Apc knockdown drives the development of colonic polyps.
A. Colon endoscopic images from LSL-rtTA3/shRen/Lgr5-CreER (shRen) or LSL-rtTA3/shApc/Lgr5-CreER (shApc) mice following dox treatment for times indicated, showing gradual polyp development in shApc/Lgr5 animals. B. Immunohistochemical (H&E, Alcian blue), immunofluorescent (GFP/BrdU and Krt20) stains and in situ hybridization (Lgr5) from shRen/Lgr5 and shApc/Lgr5 colons following dox treatment for 10 weeks. C. Hematocrit in peripheral blood of shRen/Lgr5 and shApc/Lgr5 mice over time following 4OHT and dox treatment. D. Kaplan-Meier plot showing survival of dox-treated ApcMin/+ (red line), Apcflox/flox/Lgr5 (blue line), shRen/Lgr5 (black line), and shApc/Lgr5 (green line). Lgr5-CreER containing mice were treated with 4OHT at 5-6 weeks of age and all animals (including ApcMin/+) were treated continually with doxycycline from this time onward. E. Quantification of tumor burden from H&E stained paraffin embedded sections of Apcflox/flox/Lgr5 and shApc/Lgr5 mice. Tumor area was calculated as a percentage of total tumor burden for each of five intestinal regions representing the duodenum, proximal and distal jejunum, ileum and colon, as indicated. F. Dot plot showing number of macroscopic polyps counted in whole mount colon tissue in ApcMin/+, Apcflox/flox/Lgr5, and shApc/Lgr5 mice at necropsy. G. Heatmap representing the top 50 up (red) and down regulated (blue) transcripts in shApc colonic adenomas relative to shRen mucosa (OFF Dox). Transcript abundance (log2 fold change) for each gene is also shown for shApc/Kras tumors, shRen mucosa (ON Dox) and in human Stage I-IV colorectal cancers (Ongen et al., 2014). Genes listed to the right are those among the top 50 deregulated in both the mouse shApc and Human CRC datasets, and those genes in bold are validated by quantitative RT-PCR.
In line with previous observations in ApcMin animals (Moser et al., 1990), shApc/Lgr5 mice treated with 4OHT displayed a progressive decrease in hematocrit, associated with blood loss following polyp development (Figure 2C), and a median survival of 115 days (Figure 2D). However, in contrast to Apcfl/fl/Lgr5 (and ApcMin) animals that developed disease almost exclusively in the small intestine, concentrated in the distal jejunum and ileum (Figure 2E), tumors in shApc/Lgr5 mice arose in the colon and duodenum/proximal jejunum (Figure 2E,2F), which more closely reflects the anatomy of disease presentation in FAP patients (Sarre et al., 1987). This regional difference in tumor distribution was not due to the restricted activation of Cre or doxycycline treatment, as we observed Cre recombination (GFP induction) throughout the small intestine and colon (Figure S2C) and both ApcMin and Apcfl/fl/Lgr5-CreER mice were treated with dox from 5-6 weeks of age. Regardless of the precise mechanism, the appearance of colorectal disease enabled longitudinal studies of disease progression and/or regression in live mice using small animal endoscopy.
To examine the molecular characteristics of colonic adenomas formed in shApc/Lgr5 mice, we performed gene expression analysis by RNAseq and identified 3225 (1339 up, 1886 down) genes that were significantly deregulated (2-fold or greater) relative to shRen/Lgr5 control mucosa. This expression signature was enriched for both up and down regulated transcripts identified in human colorectal cancers (Stages I-IV, FDR <.001) (Figure 2G, Figure S2D) (Ongen et al., 2014) as well as human colon adenomas (FDR <.001) (Figure S2D)(Sabates-Bellver et al., 2007). shApc/Lgr5 tumors also contained an overrepresentation of transcripts found in purified Lgr5+ stem cells and enterocyte progenitors (FDR<.001) and an underrepresentation of genes exclusively expressed in secretory progenitors or enterocytes (Figure S2D and Table S1) (Kim et al., 2014). Further, shApc/Lgr5 colon tumors showed a decrease in Krt20 (Figure 2B) and Slc26a3 expression (Figure 2G, bottom), which together, define a two-gene classifier that indicates poor prognosis in human CRC (Dalerba et al., 2011). Thus, the gene expression profile of shApc/Lgr5 tumors is consistent with our histological analyses and indicates that Apc suppression impairs differentiation and drives the expansion of progenitor cells. In all, the histological, anatomical, and molecular characteristics of colon adenomas formed in shApc/Lgr5 mice suggest they accurately reflect the early stages of human colorectal cancer.
Apc restoration promotes disease regression in the small and large intestine
We next asked whether sustained Apc loss was essential for disease maintenance by restoring endogenous Apc protein expression in established polyps of shApc/Lgr5 mice. The tumor response to dox withdrawal was striking: in the colon, longitudinal endoscopic imaging of dox-withdrawn animals showed rapid regression of polyps over many weeks (Figure 3A, Figure S3A). Small intestinal polyps regressed even more rapidly, and indeed, no macroscopic polyps were visible in the small intestine after two weeks of Apc restoration (Figure S3C). In some cases, we noted the persistence of small polyp-like masses visible in the colon for weeks following dox withdrawal (Figure S3A, arrows). While abnormal in structure, these masses were composed of histologically normal, differentiated epithelium (Figure S3A, right), perhaps retained due to irreversible changes in tissue structure or fibrosis during tumor growth.
Figure 3. Apc restoration drives adenoma regression.
A. Longitudinal colonoscopic images of a dox-treated shApc/Lgr5 mouse at 8 and 15 weeks, and following 2, 8 and 15 weeks of dox withdrawal. B. Immunofluorescent stains for BrdU (green) in colonic adenomas either ON Dox or 1, 2 or 4 days following dox withdrawal, showing progressive decrease in cells incorporating nucleotide. C. Immunohistochemical (H&E), immunofluorescent (Keratin 20 and Cleaved Caspase 3) stains and in situ hybridization (Lgr5) from shRen/Lgr5 and shApc/Lgr5 colon/adenomas following dox treatment for 12-15 weeks (left two panels) and withdrawal from dox for 4 days and 20 weeks (right two panels). D. Quantitative RT-PCR analysis of Wnt target genes (Axin2, Myc) and stem cell markers (Lgr5, Ascl2) in isolated colonic adenomas (shApc/Lgr5) and normal mucosa (shRen/Lgr5) 15 weeks on dox and 4 days following dox withdrawal, as indicated. Values are normalized to shRen (on dox) for each target. E. Kaplan-Meier plot showing survival of dox-treated shApc/Lgr5 mice (dotted green line, also represented in Figure 2D) and shApc/Lgr5 mice removed from dox treatment between 12-16 weeks (blue line).
As expected, the marked response to Apc reactivation was associated with a corresponding decrease in Wnt target gene expression (Figure 3D). However, in contrast to studies showing forced overexpression of APC in tumor cells can promote apoptosis (Morin et al., 1996), endogenous Apc produced no increase in apoptotic cell death as measured by histology and lack of cleaved-Caspase 3 staining (Figure 3C, Figure S3C). Instead, Apc restoration was associated with rapid cell cycle arrest and massive differentiation. Specifically, within 4 days following dox withdrawal, almost all tumor cells had ceased proliferation (Figure 3B, Figure S3B), showed a dramatic increase in Krt20 expression, and a decrease in Lgr5+ stem cells within the polyp (Figure 3C, Figure S3C). The tumor suppressive effect of Apc was potent, and indeed, we have never observed relapsed disease in the small intestine or colon in mice monitored up to 6 months following Apc restoration (n = 13, Figure 3E).
Apc re-expression restores homeostasis in the intestinal crypt
The rapid differentiation response of cells within adenomas prompted us to explore if any Apc-restored cells remained at the site of the regressed adenoma or if they were eliminated as differentiated epithelial tissue. In our model, bright GFP expression marks Cre-recombined cells that express a dox-dependent shRNA, however, following dox withdrawal, GFP expression is lost. To identify any remaining tumor cells 2 weeks following Apc restoration, we pulsed mice with dox for two days (Figure 4A). Importantly, 2 days is sufficient time to induce GFP expression and thus ‘lineage trace’ Cre-recombined cells, but not long enough to produce a hyperproliferative response to Apc silencing (Figure 4B, ‘shApc - naïve label’). GFP re-labeling in control (shRen/Lgr5) mice revealed entirely GFP positive crypts, indicating the presence of long-lived, Cre-recombined stem cells that contribute continually to the normal mucosa (Figure 4B, ‘shRen re-label’). In regressing adenomas, we noted similar GFP “ribbons” extending from the crypt base to the outer differentiated epithelium (Figure 4B, ‘shApc re-label’). GFP positive regions appeared histologically similar to GFP negative neighboring crypts, containing markers of differentiated enterocytes (Krt20 and Villin) and Goblet cells (Muc2 and Alcian blue) (Figure 4B, Figure S4).
Figure 4. Apc restorations re-establishes crypt homeostasis.
A. Schematic representation of GFP relabeling experiment. 4OHT treated mice that had been on dox for 20 weeks (green bars) were removed from dox treatment for 2 weeks (grey bar) to promote Apc-driven tumor regression, then refed dox food for 2 days to induce GFP expression. As a control, naïve shApc/Lgr5 mice were treated with 4OHT, and then fed dox food for 2 days to indicate the outcome of Apc shRNA induction for 2 days. B. Immunofluorescent (Krt20/GFP, Muc2/GFP and Ki67/Villin) stains showing naïve shApc/Lgr5 mice treated with 4OHT/dox for 2 days (upper left), and shRen/Lgr5 (left) and shApc/Lgr5 mice (right) pulsed with dox for 2 days after 2 weeks of dox withdrawal. Arrows highlight GFP positive (green) and GFP negative (white) crypts present in the regressing polyp. C. Longitudinal colonoscopic images of a dox-treated shApc/Lgr5 mouse withdrawn and retreated with dox twice to induce tumor regression and regrowth, for times indicated.
Apc-restored cells retained within regressed tumors maintained the potential for tumor growth and 10 weeks following re-silencing of Apc, regressed polyps had regrown to original size (Figure 4C). We repeated the cycle, this time maintaining the animals off dox for 15 weeks, and again all tumors showed regression and subsequent regrowth at their original sites (Figure 4C). Thus, Apc restoration in colorectal polyps not only triggers differentiation, but also restores normal homeostasis in crypt based columnar cells, even after multiple rounds of tumorigenic growth and regression.
Reacquisition of tissue homeostasis is cell intrinsic
The in vivo experiments described above provide a clear demonstration that Apc restored cells can re-establish normal behavior, however closely following cell fate and differentiation capacity longitudinally in a complex tissue is challenging. Ex vivo intestinal crypt culture (Sato et al., 2009) provides a means to directly interrogate cellular response to Apc in the absence of a complex microenvironment. To ask whether Apc-restored cells could reestablish the balance of self-renewal and multi-lineage differentiation without cues from surrounding normal tissue, we derived small intestinal crypt cultures from shApc mice and examined their behavior following the addition and withdrawal of dox. Consistent with studies using Apc mutant organoids (Sato et al., 2011) (Dow et al., 2015), shRNA-mediated gene silencing of Apc triggered a transition to hyperproliferative ‘spheroids’ (Figure 5A,B). Like shApc-driven adenomas, spheroids showed a significant increase in proliferation, a block in differentiation (marked by loss of Krt20, Alk Phos and Muc2 positive cells) and loss of crypt-like projections containing Paneth cells (Figure 5A). Restoration of Apc expression in this setting (Figure 5B), induced a rapid phenotypic reversion and by 4 days following dox withdrawal greater than 90% of the culture showed morphological evidence of normal differentiation and a dramatic increase in Krt20 staining (Figure 5A,C). Apc-restored organoids could be maintained indefinitely in culture (Figure S5) and contained all differentiated cell types present in untreated (dox naïve) cultures, including Alkaline phosphatase positive enterocytes, Muc2 positive Goblet cells, and showed relocalization of Paneth cells to the base of crypt-like projections (Figure 5A). Thus, Apc re-expression engages a cell intrinsic mechanism to restore tissue homeostasis, such that once aberrantly proliferating cells can return to normal crypt architecture capable of self-renewal and differentiation.
Figure 5. Apc restores self-renewal and multi-lineage differentiation.
A. Brightfield, epifluorescent, and immunofluorescent images of shApc intestinal organoid cultures treated and withdrawn from dox (0.5μg/ml) as indicated. GFP signal (second row) shows induction of the TRE-regulated GFP-shRNA transgene. Panels below show the induction of markers of differentiation (Krt20, Alk Phos and Muc2) and localization of proliferative cells (EdU) and Paneth cells (Lysozyme) at the base of crypts following Apc restoration (or in No Dox controls). Scale bars represent 50μm. High magnification images of Muc2 positive cells in the villus-like domain and Lysozyme positive cells in the crypt are shown on the lower right. Scale bars are 10μm. B. Western blot of whole cell lysates from shRen and shApc intestinal organoid cultures following dox treatment and withdrawal at time points indicated. C. Graph represents the percentage (%) of undifferentiated spheroids (green) and differentiated, crypt-containing organoids (blue) in dox-treated and dox-withdrawn shRen and shApc cultures.
Kras and p53 mutant CRCs remain dependent on Apc disruption
Although the response to Apc restoration in polyps was dramatic, these lesions are benign and may not recapitulate the response of malignant CRCs to Wnt pathway agonists. As adenomas develop into carcinomas they acquire additional genetic lesions, often activating mutations in KRAS (43% of human CRC) or disruption of TP53 (54%) (Figure 6A), that could modify the response to Apc restoration. Consistent with this notion, mutational activation of Kras or loss of p53 in Wnt1-driven mammary tumors is sufficient to bypass the requirement for sustained Wnt activation in tumor maintenance, leading to eventual relapse upon oncogene withdrawal (Debies et al., 2008; Jang et al., 2006). Thus, we asked whether activation of Kras or loss of p53 would drive disease progression and/or render colon tumors insensitive to Apc-mediated tumor suppression.
Figure 6. Kras and p53 mutation drive disease progression in shApc tumors.
A. Oncoprint map representing frequency of gene loss (blue bars), mutation (green) and amplification (red) in TCGA analysis of human CRC (www.cbioportal.org). B. Endoscopic images from shRen/Kras/Lgr5 and shApc/Kras/Lgr5 mice, 1 year and 6 weeks post-4OHT, respectively. C. Immunohistochemical (H&E) and immunofluorescent (Ki67, Krt20 and Cleaved-Caspase 3) stains from shRen/Kras, shApc/Kras, shRen/Kras/p53fl/fl and shApc/Kras/p53fl/fl colons following dox treatment for 6 weeks (shApc mice) or 20 weeks (shRen mice). Arrows indicate regions of hyperproliferation driven by KrasG12D, as previously described (Haigis et al., 2008). D. Kaplan-Meier plot showing survival of dox-treated shApc (dotted green line, also represented in Figure 2D), shApc/Kras/Lgr5 (purple line) and shApc/Kras/p53fl/fl/Lgr5 (orange line), following 4OHT treatment at 5-6 weeks of age. E. Blind-scored histological analysis of intestinal sections from shApc/Lgr5, shApc/Kras/Lgr5 and shApc/Kras/p53fl/fl/Lgr5 mice, as indicated. Animals were scored based on most advanced disease evident in an H&E section through the entire intestine. F. H&E stains section of tissue obtained sub-cutaneous transplanted shApc and shApc/Kras organoids/spheroids, 7 weeks following transplant.
To test this, we crossed a conditional Kras mutant allele (LSL-KrasG12D, hereafter KrasG12D) and a conditional null p53 allele (Trp53fl/fl, hereafter p53fl/fl) to our shApc/Lgr5 mice (Jackson et al., 2001; Marino et al., 2000). Consistent with previous work (Haigis et al., 2008; Hung et al., 2010; Janssen et al., 2006; Onuma et al., 2013), mutation of Kras (G12D) strongly synergized with Apc suppression to promote rapid and widespread tumor growth in both the small and large intestine of shApc/KrasG12D/Lgr5 mice (Figure 6B, not shown), reducing median survival to 63 days (n=15) (Figure 6D). Tumors were highly proliferative and showed no evidence of differentiation or cell death (Figure 6C). Similar to shApc/Lgr5 animals, lesions were most apparent in the duodenum and colon, although were also observed in more distal regions of the small intestine (not shown). Histologically the tumors were composed of low-grade and high-grade tubular adenomas, and intramucosal adenocarcinoma that sometimes showed local invasion into the submucosa (Figure 6E, S6A). Additional loss of p53 in shApc/KrasG12D/p53fl/fl/Lgr5 mice did not significantly alter median survival (59 days, n=5), but drove more widespread features of intramucosal adenocarcinoma (Figure 6E). Accordingly, while shApc organoids formed benign sphere-like structures that did not progress as sub-cutaneous transplants, shApc/KrasG12D organoids produced lesions that resembled adenocarcinomas, showing nuclear atypia, loss of epithelial polarity and a dramatic recruitment of host stromal cells (Figure 6F, S6B,C). Kras mutation induced further upregulation in some key Wnt targets, relative to shApc/Lgr5 tumors (Figure S7A), including Myc, perhaps in part accounting for the enhanced tumor growth.
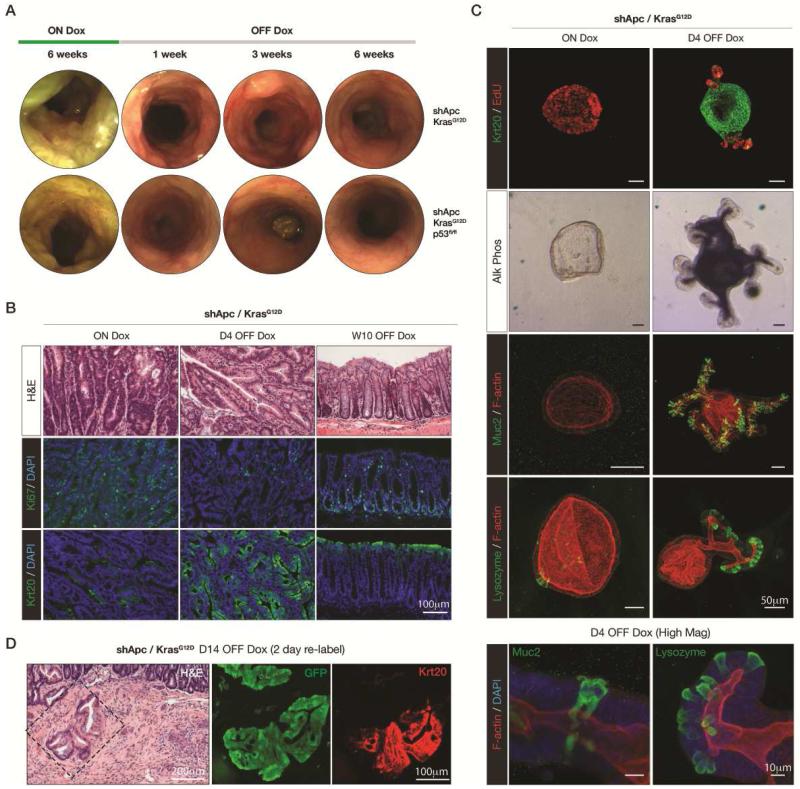
Despite the dramatic acceleration of tumor initiation and progression in KrasG12D and KrasG12D/p53 mutant CRCs, restoration of Apc still induced rapid and sustained tumor regression (Figure 7A). Indeed, even in the context of these potent oncogenic insults, we have not observed any relapse for over 15 weeks [n = 5 (Kras), n = 3 (Kras/p53)] (Figure S7A), with the exception of small regions of hyperproliferation and microadenomas also seen in shRen control animals that were presumably due to Kras and/or p53 alteration (Figure 6, Figure S7B, arrows). Tumor cells showed rapid cell cycle arrest and differentiation, identical to the response observed in shApc/Lgr5 adenomas (Figure 7B, Figure S7B) and elevated Wnt target genes, including those further upregulated following Kras mutation (i.e. cMyc), returned to baseline levels only 4 days following dox withdrawal (Figure S7C).
Figure 7. Apc restoration induces sustained regression of colorectal adenocarcinoma.
A. Longitudinal colonoscopic images of dox-treated shApc/Kras and shApc/Kras/p53fl/fl mice, as indicated, and following 1, 3 and 6 weeks of dox withdrawal. B. Immunohistochemical (H&E) and immunofluorescent stains for Ki67 and Krt20 in shApc/Kras colon tumors, ON Dox (left) or 4 days and 10 weeks following dox withdrawal. Scale bars represent 100μm C. Immunofluorescent images of shApc/Kras intestinal organoid cultures (ON Dox and D4 OFF dox, as indicated) showing markers of proliferation (EdU), differentiation (Krt20, Alk Phos and Muc2) and crypt integrity (Lysozyme). Scale bars represent 50μm. High magnification images of Muc2 and Lysozyme staining in Apc-restored/KrasG12D organoids are shown below, scale bars represent 10μm. D. Immunohistochemical (H&E) and immunofluorescent stains (GFP/Krt20) of an invasive lesion in an shApc/KrasG12D/Lgr5 tumor where Apc was restored 14 days before pulsing with dox for 2 days to relabel GFP positive cells sacrifice.
As described for shApc/Lgr5 driven adenomas, we noted areas of shApc/KrasG12D/Lgr5 regressed tumors (D14 off dox) that showed ribbons of GFP positive crypts following re-labeling (Figure S7D). Re-labeled crypts contained differentiated enterocytes (Krt20 and Villin) and Goblet cells (Muc2) and showed proliferation restricted to the base of the crypt, indicating that even in the presence of a Kras mutation, Apc-restored cells can re-establish normal tissue behavior. Similarly, Apc restoration in KrasG12D and KrasG12D/p53−/− mutant spheroids induced a rapid reversion to normal organoid structures, containing proliferative crypt-like domains, a differentiated central lumen (Figure 7C, Figure S7E). Detailed staining revealed functional enterocytes (Alk Phos) and Goblet cells (Muc2), as well as relocalization of Paneth cells (Lysozyme) to the base of crypts (Figure 7C). Apc restored KrasG12D mutant organoids could be maintained indefinitely in culture following Apc re-expression, implying they retained long-term self renewal and differentiation capability (Figure S5).
In addition to differentiation within the tumor mass, we identified regions of sub-mucosal invasion in Apc-restored animals that showed strong expression of Krt20 (Figure 7D), implying the differentiation response induced by Apc is maintained even when cells have migrated beyond the borders of the primary tumor. Consistent with this notion, subcutaneously transplanted shApc/KrasG12D organoids showed sustained regression in response to Apc restoration, forming sheets of differentiated, monolayered epithelium (Figure S6B,D). Together, these data show that mutational activation of Kras and loss of p53 can drive CRC progression in Apc-depleted intestinal epithelium, but that the resulting carcinomas remain strictly dependent on Apc disruption for sustained tumor growth. Remarkably, even in the presence of potent oncogenic mutations, cells within these tumors can re-establish the homeostatic balance of self-renewal and differentiation in response to endogenous Apc protein.
Discussion
The major genetic events that contribute to CRC have been clearly delineated over the past 20 years, and more recently elaborated using large-scale sequencing approaches. The high percentage of APC loss-of-function mutations in CRC (~80-90%) and their identification and functional characterization in early, pre-malignant disease has supported the notion that this is the initiating and driving event in tumor progression. However, experimentally, the requirement for APC loss in tumor maintenance has been challenging to address because restoring normal gene function in tumors, without ectopic overexpression, is difficult. To circumvent this technical challenge we used a tissue specific and mosaic shRNA-based transgenic model of Apc loss that accurately recapitulates the histological and molecular features of human CRC. This model revealed that Apc loss is strictly required for disease maintenance, even in the context of additional CRC-associated oncogenic insults that drive progression to invasive cancer.
The vast majority of Apc-based CRC model systems generated to date develop tumors primarily in small intestine (Zeineldin and Neufeld, 2013), or require the use of specific Cre strains to effectively restrict disease development to the large intestine (Hinoi et al., 2007). Thus, due to their bias toward the development of small intestinal disease, these mouse models have been considered inadequate surrogates of human CRC. In contrast, the shRNA-driven, mosaic model described here develops adenomas in both the duodenum and colon, consistent with common sites of polyp development in FAP patients (Sarre et al., 1987). The reason behind this difference remains unclear, but may reflect differences in the mechanism of Apc disruption and/or the level of Wnt signaling induction. In agreement, analysis of human CRCs reveals selection for particular types of alterations in WNT pathway modulators in different regions of the colon (Albuquerque et al., 2010; Christie et al., 2013), while detailed comparisons between the mouse and human intestine show distinct differences in the expression of Wnt pathway genes (Leedham et al., 2013). Alternatively, the mouse-human differences in previous models may be due to species-specific changes in stem cell behavior (Tomasetti and Vogelstein, 2015). However, differences in stem cell behavior alone cannot account for the regional bias of Apc-driven intestinal tumors because simply changing the mode of Apc disruption (Apcfl/fl vs. shApc) causes a ‘humanization’ of tumor distribution (Figure 2E). Importantly, shApc-driven adenomas show both the histological and molecular characteristics of familial and sporadic APC mutant human adenomas and CRCs. In addition to increasing its human relevance, the presence of colorectal tumorigenesis in the shApc model enables longitudinal colonoscopic imaging as a powerful means to study the initiation and progression of mammalian colorectal cancers.
The tumor response to the restoration of endogenous Apc protein was rapid and striking. Within days of dox withdrawal, tumor cells suppressed Wnt target gene expression, ceased proliferation, and showed signs of intestinal differentiation. In fact, the phenotypic response to Apc restoration was measurable days before full Apc restoration was established (Figure 1D), implying that only a very small amount of Apc is sufficient for tumor suppression. These data also support our earlier observations that potent Apc silencing is critical to induce adenomas in mice. Previous work has inferred the importance of sustained APC disruption and/or Wnt activity in maintenance of CRCs, though these studies have largely relied on overexpression of APC that induces changes in adhesion, migration, exosome secretion and/or trigger apoptosis, depending on the cell line used and level of APC expression enforced (Faux et al., 2004; Groden et al., 1995; Lim et al., 2012; Morin et al., 1996). In some contexts, full length APC re-expression induces molecular and cell morphological changes reminiscent of terminal differentiation (Faux et al., 2004) and this parallels observations that RNAi-mediated β-catenin silencing can induce markers of differentiation in certain human CRC cell lines (Scholer-Dahirel et al., 2011). However, loss of β-catenin also drives ectopic differentiation of normal intestinal epithelium (Fevr et al., 2007), suggesting that completely blocking Wnt signaling would likely be severely toxic to normal intestine. By contrast, our data show that re-establishing normal control of this pathway through endogenous Apc restoration is sufficient to induce rapid and sustained tumor regression without deleterious effect on normal crypt function.
Previous work using conditional transgenes has helped establish the concept of ‘oncogene addiction’, whereby tumors undergo sustained regression following withdrawal of the driving genetic event (Bellovin et al., 2013). In particular, disruption of the MYC oncogene, a key factor supporting Apc-driven intestinal disease (Sansom et al., 2007), can induce tumor regression via multiple mechanisms: apoptosis, senescence and/or terminal differentiation, depending on cellular context (Felsher, 2008). In one example, Mycm withdrawal leads to differentiation of hepatocellular carcinoma, and while tumor cells are retained in the tissue, they lie dormant until Myc is re-induced (Shachaf et al., 2004). In contrast, at least some Apc-restored tumor cells were capable of reverting to functional intestinal units, composed of proliferative crypt bases and differentiated enterocytes and Goblet cells. Remarkably, while reverted crypts maintain a high proliferative rate, self-renewal capability and a predisposition for tumorigenesis, we observed no relapse of disease in mice following dox withdrawal over more than 30 weeks.
Importantly, restoration of the Wnt pathway by Apc induced disease regression and reestablished tissue homeostasis even in tumors carrying KrasG12D and Trp53 mutations. This observation stands in stark contrast to results obtained using conditional Wnt or Myc-driven mammary tumor models, which showed that Kras or p53 mutations can relieve Wnt oncogene dependence (Debies et al., 2008; Jang et al., 2006). Indeed, in many tumor contexts Kras and Trp53 alterations are sufficient to initiate and sustain malignant disease (Hingorani et al., 2005; Jackson et al., 2005). For example, in lung, Kras mutation drives disease initiation, while p53 and Wnt alterations enable progression (Feldser et al., 2010; Juan et al., 2014), whereas in colon, our data suggest that Apc/Wnt deregulation is the master cancer driver, while Kras and p53 play an important, but supporting role. What regulates this remarkable tissue-specific oncogene dependence is not clear, but perhaps reflects the lineage-based requirement for high Wnt activity in normal colonic tissue that is retained as cells transform. Of course, while our data suggest that combined Kras and p53 mutations are not sufficient to alleviate the dependence on Wnt signaling, it is possible that other lesions or specific environmental signals could confer such independence. Regardless, the observation that Apc-restored tumor cells are capable of multi-lineage differentiation is reminiscent of prior work showing that nuclei derived from murine melanoma cells can be reprogrammed in wild-type oocytes, and in rare instances, produce viable mice (Hochedlinger et al., 2004). To our knowledge, our results provide the first demonstration that neoplastic cells can reacquire their ability to balance self-renewal and terminal differentiation upon re-establishing control of a single tumor suppressor pathway.
Our findings have important implications for the clinical utility of strategies targeting the Wnt pathway, particularly those aimed at re-engaging the endogenous tumor suppression mechanisms. First, although it is currently impractical to directly restore wild-type APC function to CRCs, our results imply that small molecules that aimed at modulating, but not blocking, the Wnt pathway, might achieve similar effects. In this regard, the recent development of small molecule Tankyrase (Tnk) inhibitors, which modulate Wnt activity by regulating the activity of the β-catenin destruction complex, represent a positive step in this direction (Huang et al., 2009; Waaler et al., 2011). Second, we note that the induction of differentiation upon Apc restoration is extremely rapid, and occurs well before full Apc expression is restored. This observation implies that only partial inhibition of hyperactive Wnt signaling will be required for potent anti-tumor effects, while sparing surrounding normal tissue. Third, our data suggest that strategies to mimic Apc reactivation will drive the conversion of some tumor cells to long-lived ‘normal’ proliferative crypts and thus require sustained suppression of the pathway to prevent relapse. Still, given the high level of genomic instability associated with CRC, it is difficult to imagine many tumor cells reintegrating into normal tissue and, indeed, our lineage tracing studies suggest that most tumor cells are eliminated upon differentiation. Further work will be critical to determine whether Wnt inhibition (or similar approaches) would provide long-term therapeutic value in the clinic, but it is clear that Apc disruption plays a central role in driving and maintaining tumorigenesis, and that CRCs carrying other potent oncogenic drivers likely remain exquisitely sensitive to APC-mediated tumor suppression.
Experimental Procedures
Animals
Animals were maintained on a mixed C57B6/129 background unless otherwise stated. Production of mice and all treatments described were approved by the Institutional Animal Care and Use Committee (IACUC) at Memorial Sloan Kettering Cancer Center (NY), under protocol number 11-06-012. Doxycycline was administered via food pellets (625mg/kg) (Harlan Teklad). 4-hydroxytamoxifen (4OHT, Sigma Aldrich, 70% Z-isomer) was delivered by a single intraperitoneal injection (0.5mg/mouse) at 5-6 weeks of age. For DNA labelling experiments, animals were injected with 1mg BrdU 2 hours prior to tissue harvest.
Immunohistochemistry and immunofluorescence
Full staining and antibody detail provided as Supplemental Material. Briefly, 4% paraformaldehyde-fixed paraffin sections were rehydrated, unmasked and blocked in TBS/0.1% Triton X-100 containing 1% BSA. Primary antibodies were incubated at 4C overnight in blocking buffer. For immunohistochemistry, sections were incubated with anti-rabbit ImmPRESS HRP-conjugated secondary antibodies (Vector Laboratories, #MP7401) and chromagen development performed using ImmPact DAB (Vector Laboratories, #SK4105). Stained slides were counterstained with Harris’ hematoxylin. For immunofluorescent stains, secondary antibodies were applied in TBS for 1 hour at room temp and sections counterstained with DAPI. In situ hybridization for Olfm4 and Lgr5 were performed essentially as previously described (Gregorieff et al., 2005) (van der Flier et al., 2009).
Isolation, culture and staining of small intestinal and colon organoids
Full detail for isolation and maintenance of organoids provided as Supplemental Material. Briefly, the intestine was removed, flushed, cut into 5 mm pieces and placed into 10 ml cold 5mM EDTA-PBS and vigorously resuspended using a 10ml pipette. After multiple washes, the supernatant was aspirated and crypt-containing fraction was collected twice following repeated trituration. Crypts were filtered through 100μm and 70μm filters, collected by centrifugation, resuspended in basal media and mixed 1:10 with Matrigel (BD, 354230). Growth media was changed on organoids every two days and they were passaged by mechanical disruption every 5-7 days. For subcutaneous transplantation experiments, organoids were harvested and mechanically disrupted as usual and then resuspended in 100μl of 100% Matrigel, and then injected into the flank of nude Foxn1nu mice. A sample of the resuspended fragments were plated, allowed to reform organoid spheres over 24 hours, and then counted to determine the total number of viable organoids transplanted. On average, 40-60 ‘organoid forming units’ were transplanted per injection.
Supplementary Material
Acknowledgements
We thank Danielle Grace, Sha Tian and Meredith Taylor for technical assistance with animal colonies, and other members of the Lowe laboratory for advice and discussions. Microscopy was performed at the MSKCC Molecular Cytology Core Facility with help from Yevgeniy Romin and Sho Fujisawa. This work was supported by a program project grant from the NIH/NCI (CA-013106). LED was supported by a National Health and Medical Research Council (NHMRC) Overseas Biomedical Fellowship and a K22 Career Development Award from the NCI/NIH (CA 181280-01). KPO was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM07739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. SWL is the Geoffrey Beene Chair of Cancer Biology and an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
LED and SWL conceived of the project. LED, KPO, DFT, JHvE, HC and SWL designed and analyzed experiments. LED, KPO, JS, DFT and JHvE performed experiments. LED, KPO and SWL wrote the paper.
References
- Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque C, Baltazar C, Filipe B, Penha F, Pereira T, Smits R, Cravo M, Lage P, Fidalgo P, Claro I, et al. Colorectal cancers show distinct mutation spectra in members of the canonical WNT signaling pathway according to their anatomical location and type of genetic instability. Genes, chromosomes & cancer. 2010;49:746–759. doi: 10.1002/gcc.20786. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway R, van Es J, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke A, Sansom O, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2008 doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bellovin DI, Das B, Felsher DW. Tumor dormancy, oncogene addiction, cellular senescence, and self-renewal programs. Advances in experimental medicine and biology. 2013;734:91–107. doi: 10.1007/978-1-4614-1445-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon A, Vakiani E, Sylvester BE, Scott SN, McDermott G, Shah RH, Kania K, Viale A, Oschwald DM, Vacic V, et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014;15:454. doi: 10.1186/s13059-014-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AF, Carter AM, Kostova KK, Woodruff JF, Crowley D, Bronson RT, Haigis KM, Jacks T. Complete deletion of Apc results in severe polyposis in mice. Oncogene. 2010;29:1857–1864. doi: 10.1038/onc.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M, Jorissen RN, Mouradov D, Sakthianandeswaren A, Li S, Day F, Tsui C, Lipton L, Desai J, Jones IT, et al. Different APC genotypes in proximal and distal sporadic colorectal cancers suggest distinct WNT/beta-catenin signalling thresholds for tumourigenesis. Oncogene. 2013;32:4675–4682. doi: 10.1038/onc.2012.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P, Kalisky T, Sahoo D, Rajendran PS, Rothenberg ME, Leyrat AA, Sim S, Okamoto J, Johnston DM, Qian D, et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nature Biotechnology. 2011;29:1120–1127. doi: 10.1038/nbt.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debies MT, Gestl SA, Mathers JL, Mikse OR, Leonard TL, Moody SE, Chodosh LA, Cardiff RD, Gunther EJ. Tumor escape in a Wnt1-dependent mouse breast cancer model is enabled by p19Arf/p53 pathway lesions but not p16 Ink4a loss. The Journal of clinical investigation. 2008;118:51–63. doi: 10.1172/JCI33320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Fisher J, O’Rourke KP, Muley A, Kastenhuber ER, Livshits G, Tschaharganeh DF, Socci ND, Lowe SW. Inducible in vivo genome editing with CRISPR-Cas9. Nat Biotechnol. 2015 doi: 10.1038/nbt.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Nasr Z, Saborowski M, Ebbesen SH, Manchado E, Tasdemir N, Lee T, Pelletier J, Lowe SW. Conditional reverse tet-transactivator mouse strains for the efficient induction of TRE-regulated transgenes in mice. PLoS ONE. 2014;9:e95236. doi: 10.1371/journal.pone.0095236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Premsrirut PK, Zuber J, Fellmann C, McJunkin K, Miething C, Park Y, Dickins RA, Hannon GJ, Lowe SW. A pipeline for the generation of shRNA transgenic mice. Nature Protocols. 2012 doi: 10.1038/nprot.2011.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux MC, Ross JL, Meeker C, Johns T, Ji H, Simpson RJ, Layton MJ, Burgess AW. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J Cell Sci. 2004;117:427–439. doi: 10.1242/jcs.00862. [DOI] [PubMed] [Google Scholar]
- Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA, Sanchez-Rivera FJ, Resnick R, Bronson R, Hemann MT, et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010;468:572–575. doi: 10.1038/nature09535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsher DW. Tumor dormancy and oncogene addiction. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2008;116:629–637. doi: 10.1111/j.1600-0463.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Groden J, Joslyn G, Samowitz W, Jones D, Bhattacharyya N, Spirio L, Thliveris A, Robertson M, Egan S, Meuth M, et al. Response of colon cancer cell lines to the introduction of APC, a colon-specific tumor suppressor gene. Cancer Res. 1995;55:1531–1539. [PubMed] [Google Scholar]
- Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67:9721–9730. doi: 10.1158/0008-5472.CAN-07-2735. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, Chin L, Jaenisch R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004;18:1875–1885. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-MA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Hung KE, Maricevich MA, Richard LG, Chen WY, Richardson MP, Kunin A, Bronson RT, Mahmood U, Kucherlapati R. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1565–1570. doi: 10.1073/pnas.0908682107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JW, Boxer RB, Chodosh LA. Isoform-specific ras activation and oncogene dependence during MYC- and Wnt-induced mammary tumorigenesis. Molecular and Cellular Biology. 2006;26:8109–8121. doi: 10.1128/MCB.00404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, Rosty C, Abal M, El Marjou F, Smits R, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131:1096–1109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Juan J, Muraguchi T, Iezza G, Sears RC, McMahon M. Diminished WNT -> beta-catenin - > c-MYC signaling is a barrier for malignant progression of BRAFV600E-induced lung tumors. Genes Dev. 2014;28:561–575. doi: 10.1101/gad.233627.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Li F, Ferreiro-Neira I, Ho LL, Luyten A, Nalapareddy K, Long H, Verzi M, Shivdasani RA. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature. 2014;506:511–515. doi: 10.1038/nature12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedham SJ, Rodenas-Cuadrado P, Howarth K, Lewis A, Mallappa S, Segditsas S, Davis H, Jeffery R, Rodriguez-Justo M, Keshav S, et al. A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts. Gut. 2013;62:83–93. doi: 10.1136/gutjnl-2011-301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JW, Mathias RA, Kapp EA, Layton MJ, Faux MC, Burgess AW, Ji H, Simpson RJ. Restoration of full-length APC protein in SW480 colon cancer cells induces exosome-mediated secretion of DKK-4. Electrophoresis. 2012;33:1873–1880. doi: 10.1002/elps.201100687. [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci U S A. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Nelson S, Nathke IS. Interactions and functions of the adenomatous polyposis coli (APC) protein at a glance. Journal of cell science. 2013;126:873–877. doi: 10.1242/jcs.100479. [DOI] [PubMed] [Google Scholar]
- Ongen H, Andersen CL, Bramsen JB, Oster B, Rasmussen MH, Ferreira PG, Sandoval J, Vidal E, Whiffin N, Planchon A, et al. Putative cis-regulatory drivers in colorectal cancer. Nature. 2014;512:87–90. doi: 10.1038/nature13602. [DOI] [PubMed] [Google Scholar]
- Onuma K, Ochiai M, Orihashi K, Takahashi M, Imai T, Nakagama H, Hippo Y. Genetic reconstitution of tumorigenesis in primary intestinal cells. Proc Natl Acad Sci U S A. 2013;110:11127–11132. doi: 10.1073/pnas.1221926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premsrirut PK, Dow LE, Kim SY, Camiolo M, Malone CD, Miething C, Scuoppo C, Zuber J, Dickins RA, Kogan SC, et al. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell. 2011;145:145–158. doi: 10.1016/j.cell.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA, Bujnicki JM, Menigatti M, et al. Transcriptome profile of human colorectal adenomas. Molecular cancer research: MCR. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarre RG, Frost AG, Jagelman DG, Petras RE, Sivak MV, McGannon E. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut. 1987;28:306–314. doi: 10.1136/gut.28.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, Woolfenden S, Yu KK, Markovits J, Killary K, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D, Gradl D, Paulsen JE, Machonova O, Dembinski JL, et al. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71:197–205. doi: 10.1158/0008-5472.CAN-10-1282. [DOI] [PubMed] [Google Scholar]
- Zeineldin M, Neufeld KL. Understanding phenotypic variation in rodent models with germline Apc mutations. Cancer Res. 2013;73:2389–2399. doi: 10.1158/0008-5472.CAN-12-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, Lowe SW. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol. 2011;29:79–83. doi: 10.1038/nbt.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.