Abstract
Objective
To comprehensively assess the pharmacogenomic evidence of routinely-used drugs for clinical utility.
Methods
From January 2, 2011 to May 31, 2013, we assessed 71 drugs by identifying all drug/genetic variant combinations with published clinical pharmacogenomic evidence. Literature supporting each drug/variant pair was assessed for study design and methodology, outcomes, statistical significance, and clinical relevance. Proposed clinical summaries were formally scored using a modified AGREE (Appraisal of Guidelines for Research and Evaluation) II instrument, including recommendation for or against guideline implementation.
Results
Positive pharmacogenomic findings were identified for 51 of 71 cardiovascular drugs (71.8%) representing 884 unique drug/variant pairs from 597 publications. After analysis for quality and clinical relevance, 92 drug/variant pairs were proposed for translation into clinical summaries, encompassing 23 drugs (32.4% of drugs reviewed). All were found recommended for clinical implementation using AGREE, with average overall quality scores of 5.18 (out of 7.0; range 3.67 to 7.0; SD 0.91). Drug guidelines had highest scores in AGREE domain 1 (Scope) (average 91.9 out of 100; SD 6.1), and moderate but still robust scores in domain 3 (Rigour) (average 73.1; SD 11.1), domain 4 (Clarity) (average 67.8; SD 12.5), and domain 5 (Applicability) (average 65.8; SD 10). The drugs clopidogrel (CYP2C19), metoprolol (CYP2D6), simvastatin (rs4149056), dabigatran (rs2244613), hydralazine (rs1799983, rs1799998), and warfarin (CYP2C9/VKORC1) were distinguished by the highest scores. Eight of the 10 most commonly-prescribed drugs warranted translation guidelines summarizing clinical pharmacogenomic information.
Conclusions
Considerable clinically actionable pharmacogenomic information for cardiovascular drugs exists, supporting the idea that consideration of such information when prescribing is warranted.
Introduction
Each year, over 2 million patients experience adverse drug reactions (ADRs), the fifth leading cause of death in the United States1. In particular, cardiovascular drugs are a common cause of ADRs2,3. It is estimated that 53,457 individuals of all ages are treated annually in emergency rooms for adverse reactions to cardiovascular agents2. In adults over age 65, cardiovascular drugs are implicated in a sizeable fraction of hospitalizations for ADRs, most notably warfarin (33.3%) and antiplatelet agents (13.3%), among others3.
In addition to the harm caused by drug-related toxicities, the healthcare system wastes resources when medications are ineffective. Intolerance and suboptimal response rates to cardiovascular drugs have been widely reported4–7. For example, the response rate to any given hypertension medication is approximately 50%, regardless of the class of medication4,8. In general, drugs are developed based on their effectiveness in large, carefully selected populations; a drug’s performance in that setting is less informative when treating individual patients5,9. Thus, there is a need to better identify therapies that are both more likely to be beneficial and less likely to cause harm to individual patients, who show remarkable variability in their response to medications10,11.
Pharmacogenomics, the study of genetic variation in drug response, has enabled the identification of genetic variants that impact response or toxicity to several prominent cardiovascular drugs5,9,12–16. While the effective clinical translation of this information has the potential to guide the selection and dosing of medications4,17 few cardiovascular drug pharmacogenomic findings have been translated into clinical practice18,19. This gap in translation exists for numerous reasons, including lack of knowledge and cost-effectiveness concerns19. Foremost among these, however, is the need to establish clinical utility16,20. Yet, as exemplified by the cases of clopidogrel and warfarin, even when a Food and Drug Administration (FDA) label is changed in recognition of the potential clinical impact of pharmacogenomic evidence, controversy concerning the implementation of this information persists16,18,21–29. In light of these challenges, there is considerable disagreement concerning the overall strength of pharmacogenomic evidence, with some30,31 arguing the evidence is considerable and others32,33 refuting its overall usefulness.
Since cardiovascular drugs are widely prescribed34, our study aimed to rigorously assess the state of potential clinical utility for the pharmacogenomic evidence surrounding cardiovascular drugs—a necessary foundation for clinical implementation. We systematically assessed the quality and quantity of pharmacogenomic data to permit and inform clinical implementation projects that will ultimately determine utility on clinical outcomes. We sought to critically appraise the pharmacogenomic literature and propose translation-enabling clinical summaries on a drug-by-drug basis. We hypothesized that the composite amount of clinically relevant pharmacogenomic information for cardiovascular drugs would provide considerable evidence for a major contribution to drug prescribing decisions.
Methods
Data collection
From publicly available sources, including all FDA-approved drugs and the Pharmacogenomics Knowledge Database (PharmGKB)35, a list of commonly prescribed cardiovascular drugs was selected (Supplementary Material, Appendix A). For each drug, a manual literature search of PubMed was performed. The formal search began in January 2011, but inclusion of papers was not restricted to this interval; rather, any publication from any month and year until May 2013 that met search criteria was included for subsequent review. The search criteria used was “[Drug name] polymorphism.” Only articles that assessed a link between a germline genetic variant and a pharmacologic or clinical outcome were included. Non-English language articles, articles concerning in vitro studies, pediatric studies, manuscripts simply describing literature searches, and reviews were excluded. All articles meeting these inclusion and exclusion criteria were then formally reviewed using the below process. The complete date range of the study was January 2, 2011 to May 31, 2013.
Data assessment
The unit of study, the drug/variant pair, refers to a specific drug and genetic variant (e.g., hydrochlorothiazide and rs1799752). The drug/variant pairs reported within each article were cataloged with supporting PMID(s) in a database built to support a larger clinical pharmacogenomics implementation project, The 1200 Patients Project36. This database catalogs a list of pharmacogenomic publications and reported drug/variant pairs for over 650 drugs36. The publication concerning each pair in the database is classified as “Positive PGx” if the authors reported a positive genotype-phenotype association, or “Negative PGx” if the association was not reported as significant; these designations were verified during literature review and were corrected if necessary after reviewing the paper. Drug/variant pairs were then first stratified regarding their supporting evidence using four criteria: (1) a drug/variant pair with three or more positive supporting publications in The 1200 Patients Project database, (“3+ Studies”); (2) a drug/variant pair independently clinically annotated (publicly available pharmacogenomic “Clinical Annotation” on the PharmGKB webpage35) by PharmGKB, (“PharmGKB”); (3) both of (1) and (2), (“3+ Studies & PharmGKB”); or (4) none of the above (1) through (3) (“Other”). The PharmGKB data used for these analyses was captured between January 2012 and May 2013.
Each positive publication supporting a cardiovascular drug/variant pair was then comprehensively assessed. Pharmacogenomic associations were assessed for study cohort size, whether statistical significance was reported using a correction for multiple testing, consideration of Hardy-Weinberg equilibrium, and, importantly, the clinical relevance of the phenotypes being reported. If, for each drug/variant pair, the data was determined by two independent members of the research team to provide clinically relevant evidence that could influence a physician’s drug prescribing decision, a clinical translation summary was proposed and written. For each proposed clinical summary, a level of evidence was assigned:
Level 1: from a well-performed large study including replication, or replicated by two or more large, well-performed studies; published dosing guidelines or FDA label information likely exists; or
Level 2: from at least one well-performed study of at least 100 patients; or from several small or moderately-sized studies which show consistent results; or
Level 3: from a relatively small single study (<100 patients); or several similarly executed contradictory studies exist.
Finally, each proposed summary was formally assessed using a modified AGREE II scoring instrument to determine whether each clinical summary warranted clinical implementation37. After the writing of each draft summary, three independent appraisers applied a modified AGREE II scoring system to the summaries for each drug/variant pair. The modified AGREE II scoring system encompassed all domains of AGREE II with the exceptions of domain 2 (Stakeholder Involvement) and domain 6 (Editorial Independence), which were removed from our modified instrument since these domains were not applicable to any of the accumulated drug/variant evidence summaries in our project. The resulting modified AGREE II instrument included the specific AGREE II instrument items encompassing the domains of Scope and Purpose, Rigor of Development, Clarity of Presentation, and Applicability, as shown in full in Appendix B. The AGREE instrument has been previously validated as a tool for guideline assessment, and the items comprising the tool have been found to be useful, easy-to-use, and transparent38–41.
In addition to applying the modified AGREE II instrument to obtain scores across the AGREE domains for the proposed summaries from our evidence assessment, each summary was given an overall score and was rated as to whether it deserved standing as a clinical guideline. Domain scores were calculated as per the methods outlined in the AGREE II user manual37 and the overall guideline score was obtained by averaging scores from the three appraisers. The AGREE appraisal of whether to recommend or not was used as the final determination of worthiness for implementation.
Results
Drugs with High-Level Pharmacogenomic Evidence
Among the included 71 cardiovascular drugs, 51 (71.8%) had positive pharmacogenomic (“Positive PGx”) findings reported in the literature (Table 1). The literature supporting these 51 drugs encompassed 597 unique publications, 611 unique genetic variants, and 884 unique drug/variant pairs (some drugs were associated with the same genetic variants) (Supplementary Material, Appendix C).
Table 1.
Comprehensive evaluation of the scope, depth, and quality of pharmacogenomic evidence for cardiovascular drugs.
| Cardiovascular drugs, N=71 | |||||
|---|---|---|---|---|---|
| Positive PGxa | 51 (71.8%) | ||||
| Clinical summary warranted | 23 (32.4%) | ||||
|
| |||||
| Drug/variant pairs, N=884 | |||||
|
| |||||
| Classification
|
Clinical summaries warranted
|
||||
| N | N | Level 1 | Level 2 | Level 3 | |
| 3+ Studies & PharmGKB | 33 | 25 (75.8%) | 4 | 6 | 15 |
| PharmGKB only | 43 | 10 (23.3%) | 0 | 5 | 5 |
| 3+ Studies only | 37 | 9 (24.3%) | 0 | 4 | 5 |
| Other (None of above) | 771 | 48 (6.2%) | 0 | 32 | 16 |
|
|
|
||||
| 884 | 92 (10.4%) | 4 | 47 | 41 | |
71 cardiovascular drugs with pharmacogenomic information were identified and critically reviewed, encompassing analysis of 884 unique drug/variant pairs; of the 71 drugs, 51 had positive pharmacogenomic findings in the literature (“Positive PGx”). The existence of 3 or more positive supporting publications for any given drug/variant pair (“3+ Studies”) and/or existence of a PharmGKB clinical annotation (“PharmGKB”) were the criteria used to stratify drug/variant pairs into one of four groups.
The 884 unique drug/variant pairs were divided into four categories (Table 1). The first category, “3+ Studies & PharmGKB,” comprised 33 drug/variant pairs that were supported by 3 or more studies and had a publicly available clinical annotation in the external PharmGKB database. Per our inclusion criteria, 25 (75.8%) of the pairs in this group warranted proposed clinical summaries, of which 4 were rated as Level 1 evidence, and 6 were Level 2 evidence (Table 1). The second category, pairs with only a pharmacogenomic clinical annotation (“PharmGKB only”), consisted of 43 drug/variant pairs annotated by PharmGKB, but which, in our comprehensive search, did not have at least 3 identified, published studies. Of these, 10 pairs (23.3%) warranted proposed clinical summaries, of which 5 had Level 2 evidence ratings and 5 had Level 3 (Table 1). The third category, “3+ Studies only,” comprised 37 drug/variant pairs supported by 3 or more published studies but for which no public annotation was found. Of these, 9 (24.3%) warranted proposed clinical summaries, of which 4 had Level 2 evidence ratings and 5 had Level 3 (Table 1). The final category, “Other,” consisted of the 771 drug/variant pairs that did not have 3 or more studies nor a public annotation. Of these, 48 (6.2%) warranted proposed clinical summaries, the majority of which 32 pairs had Level 2 evidence ratings while 16 had Level 3 (Table 1).
In total, 92 (10.4%) of the 884 drug/variant pairs warranted proposed clinical implementation summaries, of which 25 were supported by both 3 or more publications and a PharmGKB annotation and 9 were supported by 3 or more positive publications alone (Table 2). A few key genes, CYP2C9, CYP2C19, and VKORC1, comprise half (17 of 34) of the summaries supported by three or more positive publications (Table 2). In sum, 23 of the 71 cardiovascular drugs (32.4%) warranted at least one proposed clinical summary (Table 3).
Table 2.
Drug/variant pairs with 3 or more positive publications warranting a proposed clinical summary
| A. | ||||
|---|---|---|---|---|
| Drug
|
Variant
|
Gene
|
Positive PMIDs N
|
PharmGKB Clinical Annotation
|
| Warfarin | rs1057910 | CYP2C9 | 75 | Yes |
| Warfarin | rs1799853 | CYP2C9 | 57 | Yes |
| Clopidogrel | rs4244285 | CYP2C19 | 42 | Yes |
| Warfarin | rs9923231 | VKORC1 | 42 | Yes |
| Warfarin | rs9934438 | VKORC1 | 27 | Yes |
| Clopidogrel | rs4986893 | CYP2C19 | 15 | Yes |
| Warfarin | rs7294 | VKORC1 | 12 | Yes |
| Warfarin | rs2359612 | VKORC1 | 10 | Yes |
| Warfarin | rs28371686 | CYP2C9 | 10 | Yes |
| Warfarin | rs8050894 | VKORC1 | 9 | Yes |
| Metoprolol | rs1801253 | ADRB1 | 8 | Yes |
| Pravastatin | rs4149056 | SLCO1B1 | 8 | Yes |
| Warfarin | rs2108622 | CYP4F2 | 6 | Yes |
| Warfarin | rs28371685 | CYP2C9 | 6 | Yes |
| Warfarin | rs2884737 | VKORC1 | 6 | No |
| Carvedilol | rs1042714 | ADRB2 | 5 | Yes |
| Clopidogrel | rs12248560 | CYP2C19 | 5 | Yes |
| Rosuvastatin | rs2231142 | ABCG2 | 5 | Yes |
| Clopidogrel | rs1045642 | ABCB1 | 4 | Yes |
| Hydrochlorothiazide | rs4961 | ADD1 | 4 | No |
| Simvastatin | rs2032582 | ABCB1 | 4 | Yes |
| Warfarin | rs56165452 | CYP2C9 | 4 | No |
| Warfarin | rs9332131 | CYP2C9 | 4 | Yes |
| Aspirin | rs730012 | LTC4S | 3 | Yes |
| Atorvastatin | rs4149056 | SLCO1B1 | 3 | Yes |
| Atorvastatin | rs20455 | KIF6 | 3 | No |
| Benazepril | rs1801133 | MTHFR | 3 | No |
| Carvedilol | rs1801253 | ADRB1 | 3 | Yes |
| Clopidogrel | rs28399504 | CYP2C19 | 3 | No |
| Metoprolol | rs1065852 | CYP2D6 | 3 | No |
| Perindopril | rs1799752 | ACE | 3 | No |
| Pravastatin | rs17238540 | HMGCR | 3 | Yes |
| Simvastatin | rs4149056 | SLCO1B1 | 3 | Yes |
| Warfarin | rs17880887 | VKORC1 | 3 | No |
| B. | ||||
|---|---|---|---|---|
| Drug
|
Variants
|
Gene
|
Positive PMIDs N
|
Clinical summary warranted Y/N
|
| Warfarin | All | CYP2C9 | 183 | Y |
| Warfarin | All | VKORC1 | 130 | Y |
| Clopidogrel | All | CYP2C19 | 73 | Y |
| Metoprolol | All | CYP2D6 | 20 | Y |
| Aspirin | All | FSIP1 | 20 | N |
| Digoxin | All | ABCB1 | 16 | N |
| Pravastatin | All | SLCO1B1 | 16 | Y |
| Atorvastatin | All | APOE | 13 | N |
| Metoprolol | All | ADRB1 | 12 | Y |
| Losartan | All | CYP2C9 | 12 | N |
| Phenprocoumon | All | CYP2C9 | 12 | N |
| Lovastatin | All | LPL | 12 | N |
| Simvastatin | All | ABCB1 | 11 | Y |
| Aspirin | All | GPIIIa | 10 | N |
Part A. The associated drug, variant, and corresponding gene are shown for the 34 drug/variant pairs with 3 or more positive pharmacogenomic publications that warranted a proposed clinical summary. Of these, 25 had a publicly available Clinical Annotation in PharmGKB at the close of data capture.
Part B. Results are displayed at the level of drug/gene pairs, encompassing all positivelyassociated variants within the corresponding gene, for each of the drug/gene pairs with 10 or more positive publications. For each drug, yes (“Y”) or no (“N”) indicates whether at least one variant warranted a proposed clinical summary.
Table 3.
Drugs warranting clinical summaries for implementation per modified AGREE guideline assessment
| Drug | Variant | Method of identification1 | Modified AGREE II Scores2 | |||||
|---|---|---|---|---|---|---|---|---|
| Domain 1 Scope | Domain 3 Rigour | Domain 4 Clarity | Domain 5 Applicability | Overall Quality Score, Averaged | Recommended for Implementation | |||
| Amlodipine | rs2238032 | Candidate gene | 90.7 | 64.8 | 72.2 | 61.1 | 5.33 | Yes |
| rs2239050 | Candidate gene | 90.7 | 64.8 | 72.2 | 61.1 | 5.33 | Yes | |
| rs2239128 | Candidate gene | 90.7 | 63.0 | 66.7 | 61.1 | 5.33 | Yes | |
| rs2246709 | Candidate gene | 85.2 | 70.4 | 50.0 | 50.0 | 4.33 | Yes | |
| Aspirin | rs730012 | Candidate gene | 87.0 | 74.1 | 57.4 | 61.1 | 5.33 | Yes |
| Atenolol | rs688 | Candidate gene | 74.1 | 66.7 | 57.4 | 63.9 | 3.67 | Yes |
| rs699 | Candidate gene | 87.0 | 72.2 | 63.0 | 63.9 | 4.33 | Yes | |
| rs5051 | Candidate gene | 87.0 | 72.2 | 63.0 | 63.9 | 4.33 | Yes | |
| rs5443 | Candidate gene | 85.2 | 70.4 | 63.0 | 63.9 | 5.33 | Yes | |
| rs1801252/rs1801253 | Candidate gene | 88.9 | 68.5 | 70.4 | 61.1 | 5.33 | Yes | |
| rs2301339 | Candidate gene | 85.2 | 70.4 | 63.0 | 63.9 | 5.33 | Yes | |
| rs11064426 | Candidate gene | 85.2 | 70.4 | 63.0 | 63.9 | 5.33 | Yes | |
| Atorvastatin | rs5443 | Candidate gene | 88.9 | 66.7 | 51.9 | 52.8 | 4.67 | Yes |
| rs20455 | Candidate gene | 98.1 | 74.1 | 79.6 | 69.4 | 6.00 | Yes | |
| rs4149056 | Candidate gene | 87.0 | 63.0 | 44.4 | 58.3 | 3.67 | Yes | |
| Benazepril | rs7079 | Candidate gene | 87.0 | 66.7 | 63.0 | 58.3 | 5.00 | Yes |
| rs1799752 | Candidate gene | 94.4 | 81.5 | 68.5 | 63.9 | 5.33 | Yes | |
| rs1799998 | Candidate gene | 88.9 | 70.4 | 63.0 | 61.1 | 5.00 | Yes | |
| rs1801133 | Candidate gene | 85.2 | 51.9 | 46.3 | 50.0 | 3.67 | Yes | |
| Candesartan | rs1799998 | Candidate gene | 87.0 | 59.3 | 55.6 | 63.9 | 4.00 | Yes |
| Carvedilol | rs1042714 | Candidate gene | 98.1 | 81.5 | 70.4 | 63.9 | 6.00 | Yes |
| rs1801253 | Candidate gene | 92.6 | 77.8 | 74.1 | 66.7 | 5.67 | Yes | |
| Clopidogrel | CYP2C19 | GWAS + Candidate gene | 100 | 96.3 | 100 | 91.7 | 7.00 | Yes |
| rs1045642 | Candidate gene | 98.1 | 87.0 | 79.6 | 72.2 | 6.00 | Yes | |
| Dabigatran | rs2244613 | GWAS | 98.1 | 98.1 | 85.2 | 83.3 | 6.33 | Yes |
| Felodipine | rs2238032 | Candidate gene | 90.7 | 64.8 | 72.2 | 61.1 | 5.33 | Yes |
| rs2239050 | Candidate gene | 90.7 | 64.8 | 66.7 | 61.1 | 5.33 | Yes | |
| rs2239128 | Candidate gene | 90.7 | 64.8 | 66.7 | 61.1 | 5.33 | Yes | |
| Fenofibrate | rs320 | Candidate gene | 92.6 | 57.4 | 63.0 | 55.6 | 4.00 | Yes |
| rs676210 | Candidate gene | 92.6 | 72.2 | 66.7 | 61.1 | 5.33 | Yes | |
| rs2727786 | Candidate gene | 90.7 | 53.7 | 59.3 | 58.3 | 3.67 | Yes | |
| Fluvastatin | rs1799752 | Candidate gene | 96.3 | 66.7 | 63.0 | 58.3 | 5.33 | Yes |
| rs11045819 | Candidate gene | 96.3 | 72.2 | 72.2 | 66.7 | 5.33 | Yes | |
| Hydralazine | rs1799983 | Candidate gene | 100 | 79.6 | 85.2 | 80.6 | 6.33 | Yes |
| rs1799998 | Candidate gene | 100 | 79.6 | 85.2 | 80.6 | 6.33 | Yes | |
| HCTZ | rs4961 | Candidate gene | 96.3 | 72.2 | 57.4 | 58.3 | 4.67 | Yes |
| rs11240688 | Candidate gene | 96.3 | 75.9 | 63.0 | 61.1 | 4.67 | Yes | |
| Irbsartan | rs1367117 | Candidate gene | 74.1 | 66.7 | 57.4 | 63.9 | 3.67 | Yes |
| rs1799752 | Candidate gene | 90.7 | 72.2 | 64.8 | 63.9 | 4.33 | Yes | |
| Metoprolol | CYP2D6 | Candidate gene | 100 | 100 | 98.1 | 97.2 | 7.00 | Yes |
| rs1024323 | Candidate gene | 92.6 | 75.9 | 57.4 | 61.1 | 4.33 | Yes | |
| rs1801253 | Candidate gene | 83.3 | 55.6 | 53.7 | 58.3 | 4.33 | Yes | |
| Nifedipine | rs2238032 | Candidate gene | 92.6 | 64.8 | 66.7 | 61.1 | 5.33 | Yes |
| Perindopril | rs1799752 | Candidate gene | 96.3 | 77.8 | 70.4 | 66.7 | 5.00 | Yes |
| Pravastatin | rs4149056 | Candidate gene | 96.3 | 74.1 | 51.9 | 61.1 | 4.00 | Yes |
| rs17238540 | Candidate gene | 96.3 | 81.5 | 74.1 | 72.2 | 6.00 | Yes | |
| rs17244841 | Candidate gene | 96.3 | 81.5 | 68.5 | 72.2 | 5.67 | Yes | |
| rs4986790 | Candidate gene | 96.3 | 83.3 | 74.1 | 72.2 | 6.00 | Yes | |
| Rosuvastatin | rs2231142 | Candidate gene | 98.1 | 77.8 | 74.1 | 69.4 | 6.00 | Yes |
| Simvastatin | rs2032582 | Candidate gene | 88.0 | 63.0 | 51.9 | 61.1 | 4.00 | Yes |
| rs4149056 | GWAS | 98.1 | 92.6 | 92.6 | 77.8 | 6.33 | Yes | |
| Verapamil | rs1801252/rs1801253 | Candidate gene | 98.1 | 81.5 | 81.5 | 72.2 | 6.00 | Yes |
| Warfarin | All variants | GWAS + Candidate gene | 100 | 100 | 92.6 | 97.2 | 7.00 | Yes |
| Average | 91.9 | 73.1 | 67.8 | 65.8 | 5.18 | |||
| SD | 6.1 | 11.1 | 12.5 | 10.0 | 0.91 | |||
| Range | 74.1–100 | 51.9–100 | 44.4–100 | 50.0–97.2 | 3.67–7.00 | |||
Genome-wide association study (GWAS), Candidate gene
Brouwers M, et al. AGREE II: Advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 2010.
AGREE Assessment of Clinical Translation Summaries for Clinical Implementation
The clinical summaries were assessed in the four domains (Scope & Purpose, Rigour of Development, Clarity of Presentation, and Applicability) and given an overall score (on a scale of 1 to 7) (Table 3). For the overall guideline assessment, all proposed clinical summaries were recommended for implementation. The average overall quality score was 5.18, with a standard deviation (SD) of 0.91 and range of 3.67 to 7 (Table 3). For domain 1 (Scope & Purpose) scores averaged 91.9, with a SD of 6.1 and range of 74.1 to 100. For domain 3 (Rigor of Development), scores averaged 73.1, with a SD of 11.1 and range of 51.9 to 100. For domain 4 (Clarity of Presentation), scores averaged 67.8, with a SD of 12.5 and range of 44.4 to 100. For domain 5 (Applicability), scores averaged 65.8, with a SD of 10 and range of 50 to 97.2. Per the AGREE scores, the summaries for clopidogrel (CYP2C19), metoprolol (CYP2D6), warfarin (CYP2C9/VKORC1), simvastatin (rs4149056), dabigatran (rs2244613), and hydralazine (rs1799983 and rs1799998) performed the highest, with average quality scores greater than 6 on a 7-point scale (Table 3).
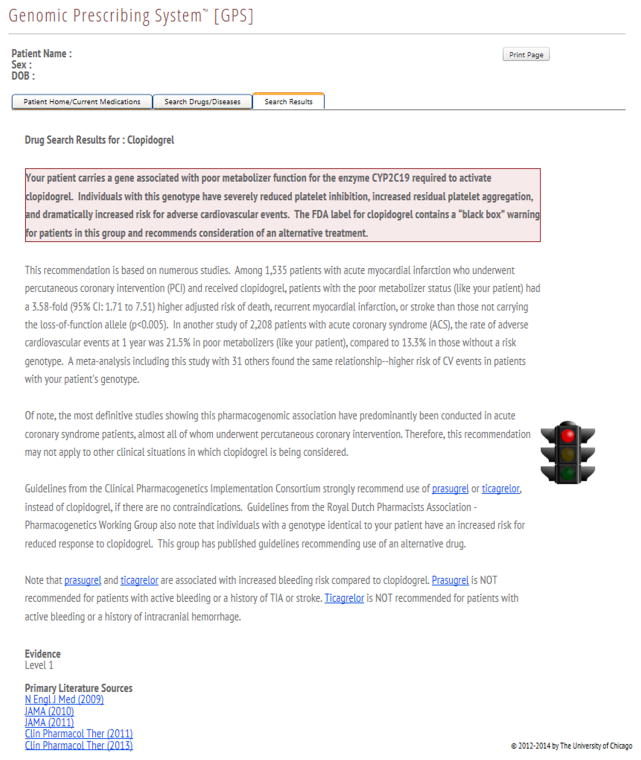
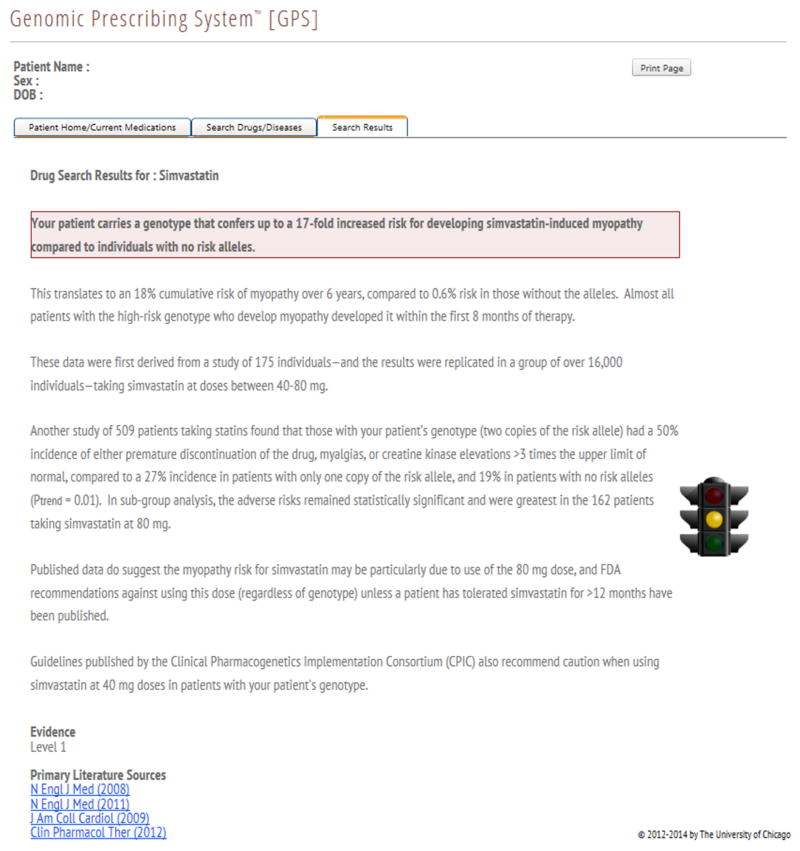
The AGREE-rated summaries that were appraised as worthy of clinical implementation are currently being delivered to physicians through a pharmacogenomic results delivery interface in an institutional pharmacogenomic clinical implementation project36. These clinical summaries provide key information about the implications of a pharmacogenomic genotype result on clinical outcomes (such as adverse drug reactions, response to treatment, or drug vs. drug comparisons) and represent a synthesis of the key published studies including information on effect size and statistical significance, with an emphasis on replication whenever possible. The clinical summaries for two different cardiovascular drugs are shown in Figure 1.
Figure 1.
Clinical implementation summaries / clinical decision supports for given genotypes of (A) clopidogrel and (B) simvastatin.
The creation of a clinical implementation summary (genotype-specific pharmacogenomic result with clinical decision support), which informs physicians of important pharmacogenomic information, was our measure of the ultimate clinical applicability of a specific drug/variant pair. Each summary contains an interpretation of the evidence, the assigned Level of Evidence rating, and hyperlinks to the primary literature evidence forming the basis for the summary.
External Comparison of Implementation Guidelines with FDA Labels
Using data from the FDA’s published Table of Pharmacogenomic Biomarkers in Drug Labeling, three drugs have key pharmacogenetic information incorporated into their FDA labels concerning the same genetic variants that we report on (warfarin, clopidogrel, and metoprolol)42. We agree with the labeling for clopidogrel and CYP2C19, metoprolol and CYP2D6, and warfarin and CYP2C9 and VKORC1. However, our analyses for these drugs also contain additional information on variants beyond those listed on FDA labels (Tables 2 and 3).
Additional drugs, including atorvastatin, pravastatin, and carvedilol also have pharmacogenomic relationships incorporated into their FDA labels, but our findings cover different subgroups, variants, and clinical phenotypes than those on the label42. The remaining drugs we report on do not presently contain pharmacogenomic markers within their labeling.
Special Considerations
The AGREE instrument identified four very high scoring drug/variant pairs that have received particular attention in multiple prior publications, and therefore we consider those drugs specially here.
Clopidogrel
We identified 30 drug/variant pairs with positive pharmacogenomic associations for the antiplatelet agent clopidogrel, of which 10 pairs warranted proposed clinical summaries. Of the 10 clinical summaries, 3 were designated as Level 1 evidence. All 3 of these variants are located in the gene CYP2C19: rs12248560, rs4244285, and rs4986893. The clopidogrel/CYP2C19 pair was among the highest AGREE scoring summaries in both overall quality score and by domain scores. Scores for clopidogrel across almost all items in the modified instrument were considerably above published cut-point values for “high quality”40.
Metoprolol
Metoprolol and CYP2D6 was determined to warrant clinical implementation as a drug/gene pair with a very high AGREE score. The metoprolol/CYP2D6 pair received an average overall quality score of 7 with domain 1, 3, 4, and 5 scores of 100, 100, 98.1, and 97.2 respectively (Table 3). Similar to clopidogrel and CYP2C19, scores for almost all individual items assessing the metoprolol/CYP2D6 pair were above published cut-points defining “high quality.”
Simvastatin
For simvastatin, 2 of the 61 identified drug/variant pairs with positive pharmacogenomic information were determined to warrant clinical summaries (Table 3). Of these, one variant (rs4149056) in the gene SLCO1B1 concerning myopathy risk was assigned Level 1 evidence and was among the highest scoring pairs when the modified AGREE instrument was applied (Figure 1B, Table 3). This pair was identified by a GWAS and had an average overall quality score of 6.33, with domain 1, 3, and 4 scores of 98.1, 92.6, and 92.6 respectively. Additionally, almost all item scores in the modified AGREE evaluation of this pair were well above published values for “high quality”.
Warfarin
The proposed warfarin clinical summary is unique in that it provides a recommended starting dose based on several factors, including genetic variants. According to the modified AGREE instrument, the warfarin summary was among the highest performing; it had an average overall quality score of 7 and domain scores over 90 in all four scored domains. The average scores for the items comprising domains 1, 3, and 4 are all above published values for “high quality.” Despite these high scores on AGREE assessment, the level of evidence designation for this summary in our strata is only Level 3. This is because, subsequent to the primary literature capture period, the level of evidence designation for warfarin was lowered to reflect the data from several high impact studies that were published during data analysis that brought into question the clinical value of genomically-guided warfarin dosing compared to simply dosing based on patient-specific clinical factors24,28,29.
Blockbuster Drugs
Finally, we examined the published pharmacogenomic evidence for the 10 most commonly prescribed cardiovascular drugs in the United States in 201134 (Table 4). Of these 10 blockbuster drugs, 8 warranted at least one clinical pharmacogenomic summary per assessment by the modified AGREE instrument. The average overall quality score for these 8 drugs was 5.03, with a SD of 0.94. For these 8 drugs, the domain 1 scores averaged 89.9 (SD 6.3), the domain 3 scores averaged 72.4 (SD 11.4), the domain 4 scores averaged 65.8 (SD 14.3), and the domain 5 scores averaged 65.0 (SD 11.7).
Table 4.
Considerable pharmacogenomic evidence exists for the Top 10 most commonly prescribed cardiovascular drugs in the U.S.
| Drug | Modified AGREE Overall Guideline Assessment | Number of Positive Publications | Number of Subjects Studied | Clinical Outcome(s) Studied | Reported Effect Size | Annual U.S. Prescriptions, in millions34 | |
|---|---|---|---|---|---|---|---|
| Decision to Recommend | Average Quality Score | ||||||
| Simvastatin (toxicity) | Y | 6.33 | 3 | 17,323 | Myopathy | Odds ratio of myopathy up to 4.5× greater for carrying 1 risk allele, and 17× greater for carrying 2 risk alleles | 96.8 |
| Simvastatin (response) | Y | 4.0 | 4 | 2,943 | Cholesterol lowering | Approximately 6% greater total cholesterol lowering in patients with favorable allele | 96.8 |
| Lisinopril | Not proposed | N/A | 5 | 17,472 | N/A | N/A | 88.8 |
| Metoprololi | Y | 4.33 – 7.0 | 12 | 916 | Blood pressure lowering | Odds ratio of reaching target MAPii ~2× higher with favorable allele | 72.3 |
| Amlodipine | Y | 5.33 | 2 | 263 | Blood pressure lowering | Patients with favorable allele are 2× as likely to reach target MAP | 62.5 |
| Hydrochlorothiazide | Y | 4.67 | 5 | 530 | Blood pressure lowering | 1.5× greater SBPiii decline and 2× greater MAP decline with favorable allele | 48.1 |
| Atorvastatin (toxicity) | Y | 3.67 | 3 | 500 | Myopathy | Risk of adverse event 1.4× higher with 1 risk allele and 2.5× higher with 2 risk alleles | 43.3 |
| Atorvastatin (response) | Y | 4.67 – 6.0 | 4 | 8,078 | Risk of MIiv or major cardiovascular event | ~10% reduction in risk with favorable alleles | 43.3 |
| Furosemide | Not proposed | N/A | 2 | 192 | N/A | N/A | 42.3 |
| Warfarin | Y | 7.0 | 87 | 28,050 | Prediction of required warfarin dose | Identifies the required stable dose in approximately 16% of patients not accurately predicted by a clinical algorithm who needed extreme warfarin dosesv | 33.9 |
| Atenolol (blood pressure response) | Y | 3.67 – 5.33 | 3 | 1,413 | Blood pressure lowering | ~4× greater SBP decline and 1.75× greater DBPvi decline with two favorable alleles | 33.4 |
| Atenolol (risk of death) | Y | 5.33 | 1 | 5,895 | Mortality risk | Differential risk of all-cause mortality depending on haplotype makeup; hazard ratio of 8.58 for certain genotypes | 33.4 |
Includes metoprolol succinate and metoprolol tartrate
Mean arterial pressure (MAP)
Systolic blood pressure (SBP)
Myocardial infarction (MI)
Prospective randomized trials examining attainment of therapeutic INRs showed no improvement of pharmacogenomic-guided algorithm dosing except when compared to an empiric dosing strategy. Therefore, in our clinical implementation project, the warfarin summary is supplied as a Level 3 dosing algorithm that includes clinical factors along with pharmacogenomic factors with the understanding that an algorithm containing such clinical information will promote among providers the use of the algorithm-based approach rather than an empiric dosing strategy.
Diastolic blood pressure (DBP)
Among these blockbuster drugs, the potential for clinical impact is large. As one consideration, three of the drugs—simvastatin, atorvastatin and atenolol—have two different clinical outcomes that can be considered pharmacogenomically: treatment response and adverse effect. Additionally, for some drugs, the potential impact could touch millions of patients: simvastatin (and the genetic variant for myopathy risk) is illustrative here, as our analysis identified that the evidence base is large (encompassing data from over 17,000 patients) but the pool of patients potentially impacted by this information is even orders of magnitude larger (as simvastatin is the most commonly prescribed cardiovascular drug with 96.8 million annual prescriptions). Some drugs have clinical outcomes of very high interest: for atorvastatin (with 43.3 million annual prescriptions), we identified 4 studies representing 8,078 patients that show an approximate 10% reduction in the risk of myocardial infarction or major cardiovascular event for patients with a favorable genotype. In total, for these top 10 cardiovascular blockbuster drugs alone, pharmacogenomic information having a published clinical impact on outcomes has been gathered from studies including over 83,575 total patients and is available to inform potentially 390 million current prescriptions per year.
Discussion
In this study, we comprehensively assessed the literature concerning the pharmacogenomics of cardiovascular drugs for clinical relevance, leading to the creation of pharmacogenomic composite summaries that could impact drug prescribing as part of pharmacogenomic clinical implementation efforts. We identified 51 cardiovascular drugs with positive published pharmacogenomic evidence, including 23 higher-evidence drugs warranting clinical summaries worthy of consideration for clinical implementation. These data, and the development of these clinical translation summaries for each of the highest drug/gene pairs, impact 8 of the 10 most commonly prescribed cardiovascular agents in the United States.
Given these results, our key finding is that the breadth and depth of clinically relevant pharmacogenomic information for cardiovascular therapeutics is both considerable and potentially meaningful. Several projects utilizing summarized pharmacogenomic information for clinical implementation are already underway36,43–48, including use of the above developed summaries for clinical delivery at our institution. Aggregate, longer-term outcomes research from these and other projects will be necessary to ultimately evaluate clinical utility.
The determination of clinical relevance always depends on the specific patient in question being cared for by an individual physician, who may have his or her own interpretation of the data. We assigned relevance by assessing which clinical outcomes would be potentially important to clinical decision-making in two domains: treatment response and adverse events. For treatment response, we assessed studies that examined a drug’s ability to achieve its desired clinical outcome based on its indication for use and we focused on results that physicians commonly measure as evidence of treatment response. Conversely, for genetic variants that govern risk of adverse reactions, our requirement was that the adverse effect be clinically important and have a clinically important effect size. In doing so, we tried to focus on those adverse effects a physician would factor into a decision to stop or change a medication. Whenever possible, published guidelines or dosing algorithms were referenced, and summaries were compared with such publicly available sources.
We applied Level of Evidence designations in addition to evaluating the strength of our summaries based on a modified AGREE II instrument. Our criteria for Level 1 designations were rigorous, and accordingly, only the best-studied drug/variant pairs were classified as such. Our stratified designations were consistent with Level of Evidence stratification systems developed similarly by PharmGKB49, though small differences exist. Use of this system revealed that those drug/variant pairs not meeting either classifying criterion (“Other”) yielded the lowest percent of pairs translated to summaries, as expected. Yet, this category still produced 48 summaries, highlighting the importance of a broad examination.
When applying the AGREE instrument, the average overall quality scores suggested very good quality to our summaries (Table 3). Our clinical summaries perform best in domain 1 (Scope & Purpose), averaging 91.9 out of 100, suggesting that our clinical summaries are clear in their objective, health question, and target population. The summaries perform relatively less well in the other domains, with the lowest scores in domain 5 (Applicability) with an average of 65.8. This is likely due to the fact that our guideline summaries were not initially designed to describe facilitators and barriers to application (the specific focus of domain 5 item 18) and only a few drug guidelines have strong enough evidence for specific tools for implementation (domain 5 item 19). Scores in domains 3 (Rigor of Development) and 4 (Clarity of Presentation), while still robust, are likely lower than those of domain 1 due to the difficulty in making unambiguous and specific recommendations when supporting studies are contradictory and/or small.
The field of pharmacogenomics suffers from a relative lack of large trials50 and replicated findings which are required for the highest quality guidelines. As highlighted in Table 3, it is notable that most of the high-evidence variants were identified through candidate gene studies. Indeed, data from prospective, randomized controlled trials is not available for most pharmacogenomic drug/variant pairs. It has been argued that the lack of these studies presents a strong barrier to wider clinical implementation of pharmacogenomics. However, high quality drug/variant associations from other types of trials, many of which are reproduced across multiple studies and/or have strong supporting pharmacokinetic or pharmacodynamic evidence, can provide valuable and worthy information. We would argue that our formal analysis here has in fact shown that. Therefore, we posit that implementation of pharmacogenomic evidence can occur even in the absence of a randomized trial, if high quality data standards are met, and especially for situations of prescribing equipoise. For example, the clinical summaries recommended in our manuscript highlight the range of information that can be provided to clinicians, from predicting the chance a patient will respond clinically in terms of cholesterol lowering (Supplemental Figure 1A) or heart failure improvement (Supplemental Figure 1B), to providing a drug vs. drug comparison (Supplemental Figure 1C). Likewise, the clinical summaries of atenolol and verapamil (Supplemental Figure 1D–E) inform the physician about mortality in coronary artery disease patients and specifically provide a treatment recommendation choice between these two drugs for an individual patient.
The real life clinical decision of which drug to prescribe is multi-faceted. Yet, if a physician is faced with the choice of which beta-blocker among several to prescribe, this information may provide additional, additive dimensions to the clinical calculus, while invoking little to no additional risk of harm19,30,36. It may be the case that in situations of clinical equipoise, guidelines with lower AGREE scores may still influence the decision, whereas in other scenarios, only the highest AGREE scoring pairs or only through an assessment of absolute risk could a clinician justify changing a prescribing decision. Additionally, a physician may require a different level of confidence or certainty in the findings to alter his recommendation based on the patient population or the drug itself. In this way, the role of pharmacogenomics in informing therapeutic decisions will necessarily depend on consideration of a number of clinical, environmental, and potentially even economic factors to achieve the most beneficial choice. Genetic relationships are just one of many factors that influence treatment response and will likely be best incorporated in the larger context of individualizing care.
There are limitations to this study. This work focused on a subset of all FDAapproved drugs indicated for the treatment of cardiovascular diseases, so other cardiovascular pharmacogenomic information could exist for drugs that were not included in the analysis. Also, by focusing on individual drugs, our analysis does not consider potential drug class effects (e.g., a specific variant for all statins).
Considering the hundreds of millions of annual cardiovascular drug prescriptions34, frequency of adverse drug events2,3, and variable levels of drug response6,7,11, the impact of this knowledge for improving patient care is potentially prodigious17,51. Key to facilitating clinical implementation appears to be the production of guidelines46, such as the work done by the Clinical Pharmacogenetics Implementation Consortium (CPIC)52 and PharmGKB35, which synthesize basic and clinical science research into tools for clinical use. Our work has attempted to do this as a comprehensive effort for cardiovascular drugs, with the output being clinically usable translations of genomic information into their practice meaning. The establishment of such clinical translations for routine use is essential to—and will permit—subsequent, necessary, larger, and potentially prospective, randomized and/or pragmatic clinical outcomes analyses.
Conclusion
There is substantial pharmacogenomic information on cardiovascular drugs that could potentially be applied to patient care. Given the burden of cardiovascular disease and the potential of personalized medicine, this information merits being made available for clinical implementation in a research context to determine if it impacts physician decision-making and patient outcomes.
Supplementary Material
Supplementary Figure 1. Clinical summaries for results delivery for given genotypes of (A) pravastatin, (B) carvedilol, (C) atorvastatin, (D) atenolol, and (E) verapamil.
The creation of a clinical implementation summary (genotype-specific pharmacogenomic result with clinical decision support), which informs physicians of important pharmacogenomic information, was our measure of the ultimate clinical applicability of a specific drug/variant pair. Each summary contains an interpretation of the evidence, the assigned Level of Evidence rating, and hyperlinks to the primary literature evidence forming the basis for the summary.
Acknowledgments
Financial support
- Supported by The University of Chicago Pritzker Summer Research Program (ALK), NIH K12 CA139160 and K23 GM100288-01A1 (PHO), NIH/NHLBI 5 U01 HL105198-09 (MJR and PHO), The Conquer Cancer Foundation of the American Society for Clinical Oncology (MJR), and The William F. O’Connor Foundation (MJR).
List of Abbreviations
- ADR
adverse drug reaction
- CPIC
Clinical Pharmacogenetics Implementation Consortium
- FDA
Food and Drug Administration
- PharmGKB
Pharmacogenomics Knowledge Database
- PGx
pharmacogenomic information
- PMID
PubMed identifier
Footnotes
Pharmacogenomic information (PGx)
Disclosure:
- MJR is a coinventor holding patents related to pharmacogenetic diagnostics and receives royalties related to UGT1A1 genotyping.
- RBA is a founder of, stockholder of, and consultant to Personalis, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyer UA. Pharmacogenetics and adverse drug reactions. Lancet. 2000 Nov 11;356(9242):1667–1671. doi: 10.1016/S0140-6736(00)03167-6. [DOI] [PubMed] [Google Scholar]
- 2.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006 Oct 18;296(15):1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 3.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011 Nov 24;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JA. Advancing management of hypertension through pharmacogenomics. Ann Med. 2012 Jun;44(Suppl 1):S17–22. doi: 10.3109/07853890.2011.653399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myburgh R, Hochfeld WE, Dodgen TM, Ker J, Pepper MS. Cardiovascular pharmacogenetics. Pharmacol Ther. 2012 Mar;133(3):280–290. doi: 10.1016/j.pharmthera.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Postmus I, Verschuren JJ, de Craen AJ, et al. Pharmacogenetics of statins: achievements, whole-genome analyses and future perspectives. Pharmacogenomics. 2012 May;13(7):831–840. doi: 10.2217/pgs.12.25. [DOI] [PubMed] [Google Scholar]
- 7.Mangravite LM, Thorn CF, Krauss RM. Clinical implications of pharmacogenomics of statin treatment. Pharmacogenomics J. 2006 Nov-Dec;6(6):360–374. doi: 10.1038/sj.tpj.6500384. [DOI] [PubMed] [Google Scholar]
- 8.Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993 Apr 1;328(13):914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 9.Wilffert B, Swen J, Mulder H, Touw D, Maitland-Van der Zee AH, Deneer V. From evidence based medicine to mechanism based medicine. Reviewing the role of pharmacogenetics. Int J Clin Pharm. 2011 Feb;33(1):3–9. doi: 10.1007/s11096-011-9485-2. [DOI] [PubMed] [Google Scholar]
- 10.Zineh I, Johnson JA. Pharmacogenetics of chronic cardiovascular drugs: applications and implications. Expert Opin Pharmacother. 2006 Aug;7(11):1417–1427. doi: 10.1517/14656566.7.11.1417. [DOI] [PubMed] [Google Scholar]
- 11.Roden DM, Johnson JA, Kimmel SE, et al. Cardiovascular pharmacogenomics. Circ Res. 2011 Sep 16;109(7):807–820. doi: 10.1161/CIRCRESAHA.110.230995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009 Feb 19;360(8):753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008 Aug 21;359(8):789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 14.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009 Jan 22;360(4):354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 15.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. N Engl J Med. 2012 Feb 9;366(6):489–491. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011 Mar 24;364(12):1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsios CS, Ziogas DE, Roukos DH. Pharmacogenomics for tailoring cardiovascular and anticancer drugs: from genotyping to whole-genome sequencing. Pharmacogenomics. 2011 Aug;12(8):1081–1085. doi: 10.2217/pgs.11.58. [DOI] [PubMed] [Google Scholar]
- 18.Pirmohamed M. Pharmacogenetics: past, present and future. Drug Discov Today. 2011 Oct;16(19–20):852–861. doi: 10.1016/j.drudis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Mrazek DA, Lerman C. Facilitating clinical implementation of pharmacogenomics. JAMA. 2011 Jul 20;306(3):304–305. doi: 10.1001/jama.2011.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesko LJ, Zineh I, Huang SM. What is clinical utility and why should we care? Clin Pharmacol Ther. 2010 Dec;88(6):729–733. doi: 10.1038/clpt.2010.229. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JL, Horne BD, Stevens SM, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II) Circulation. 2012 Apr 24;125(16):1997–2005. doi: 10.1161/CIRCULATIONAHA.111.070920. [DOI] [PubMed] [Google Scholar]
- 22.Castelan-Martinez OD, Hoyo-Vadillo C, Sandoval-Garcia E, et al. Allele frequency distribution of CYP2C9 2 and CYP2C9 3 polymorphisms in six Mexican populations. Gene. 2013 Jul 10;523(2):167–172. doi: 10.1016/j.gene.2013.03.128. [DOI] [PubMed] [Google Scholar]
- 23.Fang MC, Go AS, Hylek EM, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006 Aug;54(8):1231–1236. doi: 10.1111/j.1532-5415.2006.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimmel SE, French B, Kasner SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013 Dec 12;369(24):2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane S, Al-Zubiedi S, Hatch E, et al. The population pharmacokinetics of R- and S-warfarin: effect of genetic and clinical factors. Br J Clin Pharmacol. 2012 Jan;73(1):66–76. doi: 10.1111/j.1365-2125.2011.04051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahtani KR, Heneghan CJ, Nunan D, et al. Optimal loading dose of warfarin for the initiation of oral anticoagulation. Cochrane Database Syst Rev. 2012;12:CD008685. doi: 10.1002/14651858.CD008685.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penning-van Beest FJ, Koerselman J, Herings RM. Risk of major bleeding during concomitant use of antibiotic drugs and coumarin anticoagulants. J Thromb Haemost. 2008 Feb;6(2):284–290. doi: 10.1111/j.1538-7836.2008.02844.x. [DOI] [PubMed] [Google Scholar]
- 28.Pirmohamed M, Burnside G, Eriksson N, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013 Dec 12;369(24):2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 29.Verhoef TI, Ragia G, de Boer A, et al. A randomized trial of genotype-guided dosing of acenocoumarol and phenprocoumon. N Engl J Med. 2013 Dec 12;369(24):2304–2312. doi: 10.1056/NEJMoa1311388. [DOI] [PubMed] [Google Scholar]
- 30.Altman RB. Pharmacogenomics: “noninferiority” is sufficient for initial implementation. Clin Pharmacol Ther. 2011 Mar;89(3):348–350. doi: 10.1038/clpt.2010.310. [DOI] [PubMed] [Google Scholar]
- 31.Ashley EA, Butte AJ, Wheeler MT, et al. Clinical assessment incorporating a personal genome. Lancet. 2010 May 1;375(9725):1525–1535. doi: 10.1016/S0140-6736(10)60452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte-- an update of guidelines. Clin Pharmacol Ther. 2011 May;89(5):662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 33.Kitsios GD, Kent DM. Personalised medicine: not just in our genes. BMJ. 2012;344:e2161. doi: 10.1136/bmj.e2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Use of Medicines in the United States: Review of 2011. IMS Institute for Healthcare Informatics; 2012. [Google Scholar]
- 35.PharmGKB. [Accessed 5/17/2013];The Pharmacogenomics Knowledgebase. 2013 http://www.pharmgkb.org.
- 36.O’Donnell PH, Bush A, Spitz J, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012 Oct;92(4):446–449. doi: 10.1038/clpt.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839–842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003 Feb;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010 Jul 13;182(10):1045–1052. doi: 10.1503/cmaj.091714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010 Jul 13;182(10):E472–478. doi: 10.1503/cmaj.091716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlayen J, Aertgeerts B, Hannes K, Sermeus W, Ramaekers D. A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005 Jun;17(3):235–242. doi: 10.1093/intqhc/mzi027. [DOI] [PubMed] [Google Scholar]
- 42.FDA. Table of Pharmacogenomic Biomarkers in Drug Labeling. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm.
- 43.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014 Jan;89(1):25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall-Flavin DK, Winner JG, Allen JD, et al. Utility of integrated pharmacogenomic testing to support the treatment of major depressive disorder in a psychiatric outpatient setting. Pharmacogenet Genomics. 2013 Oct;23(10):535–548. doi: 10.1097/FPC.0b013e3283649b9a. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014 Mar;166C(1):45–55. doi: 10.1002/ajmg.c.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shuldiner AR, Relling MV, Peterson JF, et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: overcoming challenges of real-world implementation. Clin Pharmacol Ther. 2013 Aug;94(2):207–210. doi: 10.1038/clpt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014 Apr;95(4):423–431. doi: 10.1038/clpt.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014 Mar;166C(1):56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012 Oct;92(4):414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmes MV, Shah T, Vickery C, Smeeth L, Hingorani AD, Casas JP. Fulfilling the promise of personalized medicine? Systematic review and field synopsis of pharmacogenetic studies. PloS One. 2009;4(12):e7960. doi: 10.1371/journal.pone.0007960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin J, Kayser SR, Langaee TY. Pharmacogenetics: from discovery to patient care. Am J Health Syst Pharm. 2009 Apr 1;66(7):625–637. doi: 10.2146/ajhp080170. [DOI] [PubMed] [Google Scholar]
- 52.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011 Mar;89(3):464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Clinical summaries for results delivery for given genotypes of (A) pravastatin, (B) carvedilol, (C) atorvastatin, (D) atenolol, and (E) verapamil.
The creation of a clinical implementation summary (genotype-specific pharmacogenomic result with clinical decision support), which informs physicians of important pharmacogenomic information, was our measure of the ultimate clinical applicability of a specific drug/variant pair. Each summary contains an interpretation of the evidence, the assigned Level of Evidence rating, and hyperlinks to the primary literature evidence forming the basis for the summary.