Abstract
Obesity increases susceptibility for numerous diseases and neurological disorders including cardiovascular disease, metabolic syndrome, and dementia. One factor that may contribute to the increased risk for these conditions is the development of chronic inflammation. The current study evaluated whether diet-induced obesity (DIO) affects cognitive performance by increasing neuroinflammation and prolonging the behavioral and inflammatory response to an immune challenge. Adult male C57BL/6J mice were fed a high-fat (60% fat) or control diet (10% fat) for 2 or 5 months. After consuming their respective diets for two months, sickness associated behaviors were assessed 4 and 24 hours after a lipopolysaccharide (LPS) or saline injection. In a separate experiment, DIO and control mice were tested for spatial learning in the water maze and challenged with LPS one month later. Peripheral cytokine production was assessed in adipose and spleen samples and the neuroinflammatory response was assessed in hippocampal, cortical, and brain samples. DIO impaired acquisition of a spatial learning task relative to control mice. However, these deficits are unlikely to be related to inflammation as DIO showed no changes in basal cytokine levels within the periphery or brain. Further, in response to LPS DIO mice showed comparable or attenuated levels of the proinflammatory cytokines interleukin-1β and interleukin-6 relative to control mice. DIO also reduced hippocampal expression of brain-derived neurotrophic factor and the pre-synaptic marker synaptophysin. Presently, the data indicate that DIO suppresses aspects of the immune response and that cognitive deficits associated with DIO may be related to reduced neurotrophic support rather than inflammation.
Keywords: High-fat diet, LPS, CD74, spatial learning, BDNF, synaptophysin
1. INTRODUCTION
The prevalence of obesity continues to rise in industrial nations, particularly within the United States of America which has shown a doubling of obese adults over the last 40 years [1]. The implications for health care and an individual’s overall health are vast as obesity is a risk factor for several life threatening and debilitating diseases including type II diabetes, cardiovascular disease, stroke, several types of cancer and Alzheimer’s disease among other forms of dementia [1–3]. The precise mechanism through which obesity promotes these diseases is yet unknown, but substantial evidence has indicated the obesity-induced changes in immune function may contribute to the onset and/or progression of several diseases. Assessment of immune activity in obese individuals has shown deficits in the ability to defend against an infection as well as recover following an injury [4, 5]. For instance, obesity is associated with slower wound healing time following surgery or a burn [4, 6]. Additionally, obesity is associated with an increase in susceptibility to infection after surgery [5]. Collectively, findings indicate that obesity is associated with compromised immune function that increases susceptibility to disease and infection.
Animal models of diet-induced obesity (DIO) have confirmed that the immune system is affected by an organism’s diet. There is evidence that DIO leads to a basal increase in proinflammatory cytokines within the brain and periphery, particularly interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) that were found to be elevated in the cortex and hippocampus [7, 8]. However, this is not always observed as others report no changes in basal levels of proinflammatory cytokines following high-fat diet consumption [9, 10]. Similar variation has been observed in response to an immune challenge with the bacterial endotoxin lipopolysaccharide (LPS), the data indicate that rats fed a high-fat diet show increased plasma levels of IL-6 and TNF-α and increased expression of IL-6 and interleukin-1β (IL-1β) within the hypothalamus [11]. In contrast a recent report found that macrophages isolated from mice fed a high-fat diet showed lower IL-1β expression compared to cell from control mice [9]. Additionally, LPS-induced expression of IL-6 and TNF-α as well as toll-like receptor-4 (TLR-4), the primary receptor for LPS, was attenuated in peripheral macrophages from rabbits fed a high-fat diet compared to those on a control diet [12]. Clearly, the data support an altered immune profile as a consequence of DIO, however, whether the resulting response is suppression or enhancement of the inflammatory response, as well as the variables that may contribute to developing one over the other has not been fully delineated.
Activation of the immune system leads to a host of behavioral changes, collectively termed sickness behaviors, as well as deficits in cognitive function [13, 14]. Sickness behaviors are considered an adaptive behavioral response that reflects an altered motivational state that facilitate recovery from an infection [13]. Transient expression of these behaviors is generally beneficial whereas enhancing the degree or duration of these behaviors can be aversive and potentially indicative of an overactive inflammatory response. Current evidence indicates that DIO increases the fever response following an immune challenge as prior work has shown a higher and/or prolonged fever response in DIO animals following LPS administration [9, 11]. Additionally, reports indicate that DIO increases sensitivity to reductions in social behavior and anorexia following an immune challenge [9, 11, 15]. Interestingly, the prolonged anorexic response in the DIO mice was not associated with an increase in peripheral cytokine levels though changes within the brain were not assessed. Further the anorexic response was induced at a lower dose of LPS than what was required to induce a fever response indicating a dissociation of the reactions [9]. Though additional work is needed to clarify the DIO associated changes in the behavioral response to an immune challenge the existing data indicate the behavioral reaction is altered, but whether the changes reflect an exaggerated cytokine response is unknown.
Several reports have demonstrated that DIO results in cognitive deficits including spatial learning and working memory impairments [7, 8, 16]. Though limited, a few studies suggest a potential connection between DIO associated cognitive deficits and the altered immune prolife [7, 8]. For instance, Pistell et al. [7] report that middle-aged mice fed a high-fat diet made more errors during the acquisition phase of the Stone T-maze compared to control diet mice. Further, consuming the high-fat diet increased expression of the microglial cell marker Iba-1 and expression of IL-1β, IL-6 and TNF-α in the cortex. These data indicate that DIO-induced changes in the immune system may have relevance to cognitive function even in the absence of an immune challenge. However, further work is needed to determine whether enhancement of the inflammatory response is the underlying cause of the DIO-associated cognitive deficits.
The objective of the current study was to elucidate the potential role of inflammation in the development of cognitive deficits in a DIO model. Additionally, we assessed whether DIO exaggerates and/or prolongs the duration of sickness behavior following an immune challenge and whether the corresponding inflammatory response as measured by changes in central and peripheral cytokine levels basally and following immune activation are associated with enhanced behavioral and cognitive deficits.
2. MATERIAL AND METHODS
2.1. Animals
Subjects were 72 male C57BL/6J mice. For Experiment 1, mice (N=34) bred in the University of North Carolina Wilmington (UNCW) animal facility were used, with breeding stock obtained from The Jackson Laboratory (Bar Harbor, Maine). In Experiment 2, nineteen Diet-Induced Obese (DIO) C57BL/6J male mice and nineteen C57BL/6J control male mice were purchased from The Jackson Laboratory at 14 weeks old. These mice were fed the same high-fat or control diets employed in the current study (see below) at Jackson Laboratory and were started on the diets at 6 weeks of age. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the UNCW.
2.2. Diets and housing
Mice were assigned to the high-fat diet (HF; Open source diets, D12492) or control diet (Open source diets, D12450B). The HF diet contained 60% of calories from fat and the control diet contained 10% of calories from fat. Mice were group housed with two to four mice per cage. All mice in Experiment 2 were group housed for one month, but had to be individually housed for the remainder of the study due to fighting within cages. All cages were given a wooden chew toy. In Experiment 1 mice were fed the HF or control diet for 2 months. In Experiment 2 mice were fed the HF or control diet for 5.5 months (2 months prior to arrival and an additional 3.5 months at UNCW).
2.3. Experiment 1: Effect of DIO on the duration of LPS-induced sickness behavior
Thirty-four male mice were divided into the DIO or control diet group. After consuming the high-fat or control diet for two months mice received an intraperitoneal (ip) injection of LPS (250 μg/kg) or saline. To ensure no differences in body weight within a dietary condition prior to LPS or saline treatment mice were assigned to their treatment condition based on body weight (heaviest to lightest). The dose of LPS administered is a commonly used dose in this strain of mouse that is more resistant to the effects of LPS than other strains [17]. Further, the 250 μg/kg dose of LPS reliably induces sickness behavior and cognitive deficits in variety of behavioral task [18–22]. Three hours after an LPS or saline injection mice were tested in the open field test (described below) for changes in locomotor activity. Twenty-four hours after the LPS or saline injection a portion of the mice (n=18) were retested in the open field to assess any enduring changes in locomotor activity. Tissue collection was conducted after behavioral testing either four hours (n=16) or twenty-four hours (n=18) after LPS or saline treatment. Assessment of LPS-induced changes in total body weight (i.e., grams lost or gained) was determined by calculating a difference score for each mouse by subtracting the animal’s body weight on the day of the injection from the animal’s body weight twenty-four hours after an LPS or saline injection.
2.3.1. Open field testing
Three-hours after an injection of LPS or saline, subjects were tested for locomotor activity in an open field area (28 cm × 28 cm) for 5 min. The mice in the twenty-four hour group were placed back in the area the following day for an additional 5 min test. Dependent measures included total distance travelled (mm) and velocity (mm/sec). Behavior was recorded and analyzed by a computerized animal tracking system (Topscan, CleverSystems, Reston, VA).
2.3.2. Tissue collection
Mice were sacrificed by rapid decapitation. Cortex samples were dissected on a chilled glass dish and immediately placed into RNAlater solution (Qiagen, Valencia, CA) and stored at −20°C until RNA isolation. Spleens were collected and snap frozen on dry ice and stored at −80 °C until analysis with ELISA.
2.3.3. qRT-PCR
Cortex samples were sonicated and RNA purified by the RNeasy Mini kit (Qiagen, Valencia, CA), then quantified and assessed for purity using a Gen5 Epoch spectrophotometer (BioTek Instruments, Highland Park, VT). All samples had a 260/280 ratio of 1.99 or above. Conversion of RNA into cDNA was completed by following the instructions of the High-capacity cDNA reverse transcription kit that included an RNase inhibitor step (Life Technologies) using the following cycling conditions: 10 min at 25°C, 120 min at 37°C and 5 min at 85°C. After reverse transcription, cDNA was held at 2–8° C in a refrigerator overnight before conducting two-step quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) to determine the amount of specific mRNA transcript present in each sample. Cortex samples were analyzed for expression levels of IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), TNF-α (Mm00443260_g1) and BDNF (Mm04230607_s1). β-actin (Mm00607939_s1) served as the endogenous control gene. Levels of β-actin expression did not differ across groups. Each sample was run in triplicate for each gene in a 10 μl reaction that contained 80 ng of cDNA and 0.5 μl of a 20X probe/primer mix in one 384 well plate. The amount of specific mRNA present was determined by utilizing TaqMan™ probe and primer chemistry (Applied Biosystems, Foster City, CA) specifically designed to bind to reverse-transcribed cDNA of the genes of interest using an Applied Biosystems Viia7 PCR instrument (Applied Biosystems, Foster City, CA) using the following cycle parameters: 2 min at 50°C, 10 min at 95°, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. The amplification efficiency was equal across all wells for a given gene. Florescence data (ΔRn) was exported from the Applied Biosystems SDS software and analyzed by DART (Data Analysis for RT-PCR) [23]. Gene expression data were normalized by dividing the Ro (florescent intensity) values of the target genes by the Ro values of the endogenous control gene β-actin.
2.3.4. ELISA
Spleen samples were homogenized in the proteinase inhibitor phenylmethylsulfonyl fluoride (PMSF), centrifuged for 30 min at 3500 rpm at 4° C, and assayed for IL-1β with an ELISA kit (BD Biosciences, San Diego, CA; Detection limits 15.6 to 2000 pg/ml). Total protein levels were determined in each sample by a protein assay (Pierce Thermo Scientific, Rockford, IL). ELISA results are expressed as picograms of cytokine protein per mg of total protein.
2.4. Experiment 2: Effect of DIO on spatial learning and LPS-induced changes in BDNF, synaptic makers and cytokines expression in adipose and brain tissue
Thirty-eight male mice were maintained on the DIO or control diet for 5.5 months, as they had been fed the diets for two months at Jackson Laboratory prior to arriving in our facility. To assess the effects of DIO on cognitive function mice were tested in the water maze after consuming their respective diets for 4.5 months. One month following behavioral testing mice were divided by weight within each diet condition to receive a single ip LPS (250 μg/kg) or saline injection. Four or twenty-four hours after LPS or saline treatment mice were sacrificed and tissue collected to assess changes in gene expression via qRT-PCR and protein levels of proinflammatory cytokines via ELISA.
2.4.1. Spatial learning: water maze
Four and a half months after starting the DIO or control diet, mice were tested in the water maze to assess spatial learning. The maze consisted of a circular tub (121 cm diameter) and a white circular platform (12.7 cm). The platform was submerged 1 cm under the surface of the water. The water was made opaque with white tempera paint to conceal the platform. Water temperature was maintained at 19° ± 1° C throughout testing. Extra-maze cues were located around the maze. Mice received three trials (up to 60 sec) per day from different start locations for five consecutive days. If a mouse failed to locate the platform within the 60 sec they were gently guided to the platform. All mice remained on the platform for 10 seconds at the end of each trial. A video tracking system (Topscan, CleverSystems, Reston, VA) was used to measure distance swam (mm), latency to locate the platform (sec), and swim speed (mm/sec). A single 60 s probe trial was conducted approximately two hours after the subject’s last trial on day 5. The platform was removed and the number of times the animal crossed the original location of the platform was recorded by the tracking system.
2.4.2. Tissue collection
Mice were sacrificed by rapid decapitation. Hippocampal samples were dissected on a chilled glass dish and immediately placed into RNAlater solution (Qiagen, Valencia, CA) and stored at -20°C until RNA isolation. The remaining brain (i.e., everything but the hippocampus was placed in a separate tube and snap frozen. Additionally, the spleen and gonadal adipose pads from the abdominal cavity were collected and snap frozen for analysis with ELISA and qRT-PCR, respectively.
2.4.3. qRT-PCR
RNA from hippocampal samples were processed as described above in section 2.3.3 and analyzed for expression levels of IL-1β, IL-6, BDNF, synaptophysin, and PSD 95 which were normalized to the endogenous control gene β-actin. RNA was also extracted from adipose samples and analyzed for expression levels of IL-6, TNF-α and IL-1β normalized against β-actin levels. qRT-PCR procedure was identical to the produced described above with the exception that RNA from adipose samples was isolated using TRIzol (Life Technologies, Grand Island, NY) according to the manufactures instruction.
2.4.4. ELISA
Splenic levels of IL-1β and IL-6 and brain levels of IL-6 were measured by ELISA (BD Biosciences, San Diego, CA; Detection limits 3.8 to 1000 pg/ml), as described in 2.3.4.
2.5. Statistical analyses
Body weight data and water maze data were analyzed by repeated measures ANOVA with diet (DIO or control diet) as the between-subject factor and week or test day as the within-subject factor. Distance traveled in the open field was analyzed by a two-way ANOVA with diet and treatment as the between-subject variables. Splenic cytokine data, brain ELISA data, and gene expression data were analyzed by three-way ANOVA with diet (DIO or control diet), treatment (LPS or saline), and collection time (4 or 24 hours) as the between-subject variables. When appropriate, Fisher’s least significant difference (LSD) was used as a post hoc test to determine significant differences between groups. A p<0.05 was considered statistically significant.
3. RESULTS
3.1. Experiment 1: Effect of DIO on the duration of LPS-induced sickness behavior
3.1.1 Body weight
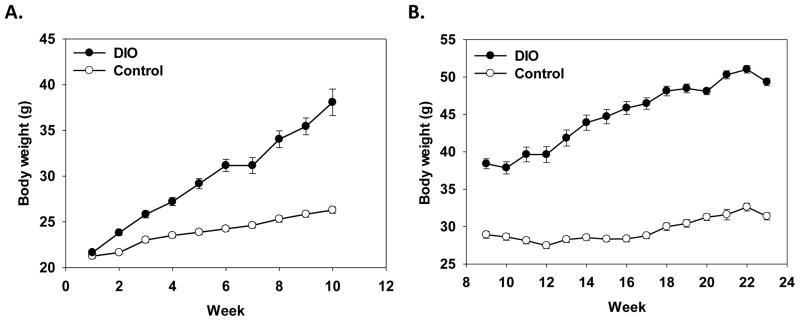
In experiment 1, there was a significant main effect of diet and a diet x week interaction (F(1, 24)=92.56, p<.0001; F(9, 252)=78.22, p<.0001, respectively) that showed DIO mice weighed (average weight = 38.34g) more than mice in the control diet condition (average weight = 26.25g) and the DIO mice gained more weight across the 2 months than control mice (see Figure 1A). Both the DIO and control mice showed a significant decrease in body weight twenty-four hours after LPS administration as compared to saline-treated mice (F(1, 13)=89.78, p<.0001, data not shown). There was no difference in LPS-induced weight loss between the DIO and control mice as both DIO and control mice lost on average two grams following LPS treatment.
Figure 1.
Data are shown as mean body weight in grams across the experiment in weeks ± standard error of the means (SEMs). In Experiment 1, initially starting weights were equal, but DIO mice showed greater weight gain than mice fed the control diet (A). In Experiment 2, DIO mice weighed more than control mice (B).
3.1.2. Locomotor activity in an open field
As expected, LPS administration significantly reduced distance traveled in an open field 3 hours after treatment as shown by a main effect of treatment (F(1, 24)=126.06, p<.0001, Figure 2A). The effects of LPS administration were similar in DIO and control mice as there were no significant effects or interactions with diet. A portion of the mice (N=18) were tested in the open field 24 hours after LPS or saline treatment. There were no significant effects of treatment or diet, indicating that 24 hours after administration LPS was no longer suppressing locomotor activity in either the control or DIO mice (Figure 2B).
Figure 2.
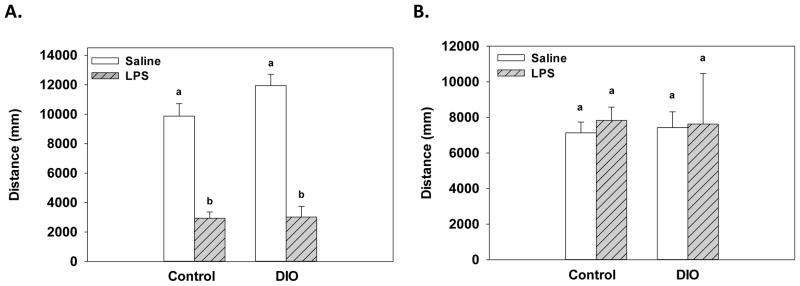
In response to LPS administration, both DIO and control mice showed a reduction in locomotor activity as compared to saline-treated mice three hours after treatment (A). Twenty-four hours after treatment there was no significant differences found between the LPS- and saline-treated mice for either the DIO or control mice (B). Bars represent mean distance traveled ±SEMs. Means marked with different letters (a or b) are significantly different from each other.
3.1.3. Splenic levels of IL-1β
A significant treatment x collection time interaction for splenic IL-1β levels (F(1, 24)=7.87, p<0.05, data not shown) showed that LPS administration increased IL-1β levels 4, but not 24, hours after treatment relative to time matched saline controls. No significant effects or interactions with diet were found for IL-1β levels. No difference were observed between the DIO or control diet saline-treatment mice.
3.1.4. Cortex RNA data
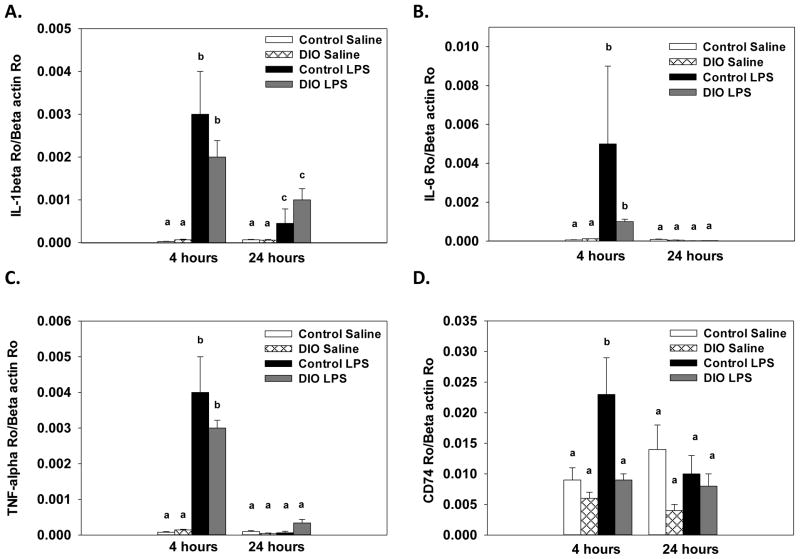
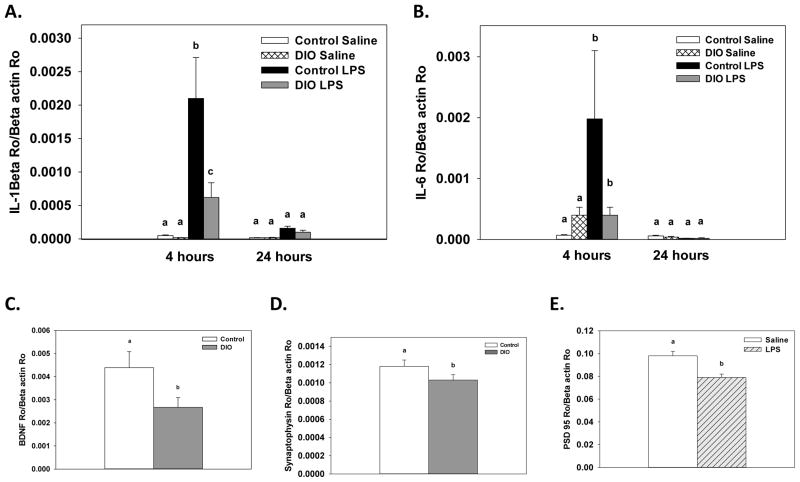
Consumption of the high-fat diet significantly reduced BDNF expression in the cortex compared to the control diet (F(1, 24)=5.12, p<.05, Figure 3). Additionally, there was a significant treatment x collection time interaction that showed LPS administration decreased BDNF expression at 4, but not 24, hours after treatment (F(1, 24)=14.02, p<.001). For expression of the proinflammatory cytokines IL-1β, IL-6 and TNF-α there were significant treatment x collection time interactions that showed LPS increased expression at 4 hours compared to saline-treated mice (F(1, 24)=27.93, p<.0001; F(1, 24)=5.81, p<.05; F(1, 24)=116.79, p<.0001, respectively, Figure 4A–C). For IL-1β, levels were still elevated 24 hours after LPS compared to saline-treated mice, but they were significantly lower relative to the 4 hour time point (p<.01). At the 24 hour time point, expression of IL-6 and TNF-α in the LPS-treated mice were not different than saline-treated mice. Diet did not affect IL-β, IL-6, or TNF-α expression, as saline-treated DIO and control diet mice showed similar levels of cytokine expression. Assessment of CD74 expression revealed there was significant diet x treatment x collection time interaction (F(1, 24)=6.56, p<.01, Figure 4D). LPS administration significantly increased CD74 expression in control diet mice at 4 hours, whereas no increase was seen in the DIO mice on the high-fat diet following LPS administration.
Figure 3.
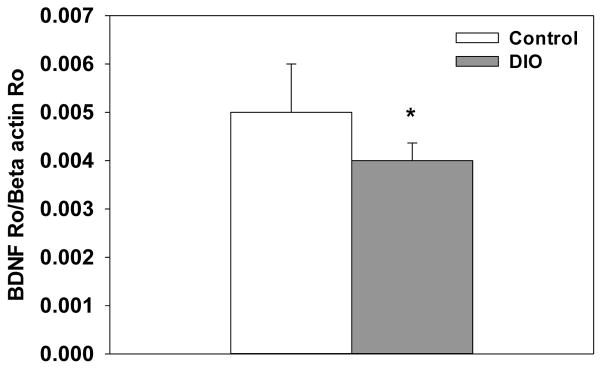
DIO mice fed showed a significant decrease in cortical BDNF expression. Bars represent means ±SEMs. * Indicates a significant difference from control diet mice.
Figure 4.
Expression levels of IL-1β (A), IL-6 (B) and TNF-α (C) were significantly increased fours after LPS administration in cortex samples, but diet did not influence this response. Expression of CD74 (D) showed that LPS increased expression four hours after treatment in the mice fed the control diet, but not in DIO mice. Bars represent means ±SEMs. Means marked with different letters (a, b, or c) are significantly different from each other.
3.2. Experiment 2: Effect of DIO on spatial learning and LPS-induced changes in BDNF, synaptic makers and cytokine expression in adipose and brain tissue
3.2.1. Body weight
As expected there was a significant main effect of diet and a diet x week interaction (F(1, 36)=318.92, p<0.0001; F(14, 504)=70.88, p<0.0001, respectively) that showed mice in the DIO condition weighed more than mice in the control diet condition and the DIO mice gained more weight across the 23 weeks than control mice (see Figure 1B).
3.2.2. Water maze
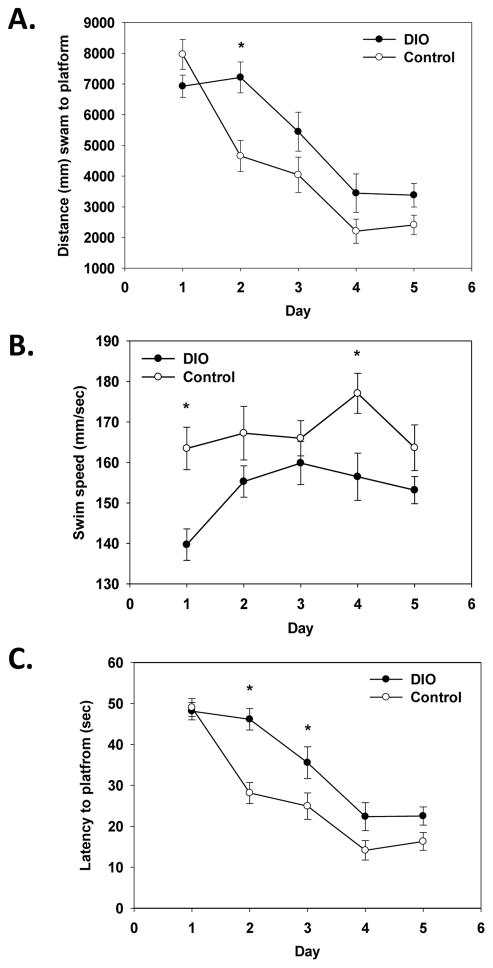
A significant main effect of diet and a diet x test day interaction (F(1, 36)=5.07, p<0.05; F(4, 144)=4.80, p<0.01, respectively) for distance swam (path length) showed that DIO mice swam a longer distance to locate the submerged platform than mice on the control diet (see Figure 5A). Post hoc testing showed that DIO mice swam a longer path on test day 2 compared to control mice (p<0.05). In agreement, DIO mice had significantly longer latencies to locate the platform than control mice on test days 2 and 3 as shown by a significant diet x test day interaction F(4, 144)=4.06, p<0.01; see Figure 5C). A significant main effect of diet showed that DIO mice overall swam slower than mice on the control diet (F(1, 36)=13.60, p<0.001; see Figure 5B). Post hoc testing showed DIO mice swam slower on days 1 and 4 of testing compared to control mice (p<0.05). Analysis of the probe trial data showed no significant differences between DIO and control mice (data not shown).
Figure 5.
DIO mice swam a longer distance to locate the submerged platform on Day 2 of testing (A). Analysis of swim speed showed that DIO mice swam slower on Days 1 and 4 of testing compared to control mice (B). DIO mice had longer latencies to locate the platform on Days 2 and 3 of testing (C). Lines represent group averages of three trials per test day ±SEMs. * Indicates a significant difference from control diet mice.
3.2.3. Adipose RNA data
A significant treatment x collection time interaction showed that LPS administration significantly increased expression levels of IL-1β, IL-6, and TNF-α in adipose tissue relative to saline-treated mice when samples were collected 4, but not 24, hours after treatment (F(1, 30)=19.03, p<0.0001; F(1, 30)= 16.56, p<0.001; F(1, 30)=13.17, p<0.001, respectively, Figure 6A–C). There were no significant effects or interactions with diet. Saline-treated DIO and control diet mice showed similar expression of IL-1β, IL-6, and TNF-α.
Figure 6.
Expression of IL-1β (A), IL-6 (B) and TNF-α (C) in adipose tissue was increased four hours after LPS administration as compared to saline treated mice. No differences or interactions were found with diet. Means marked with different letters (a or b) are significantly different from each other.
3.2.4. Brain IL-6 protein data
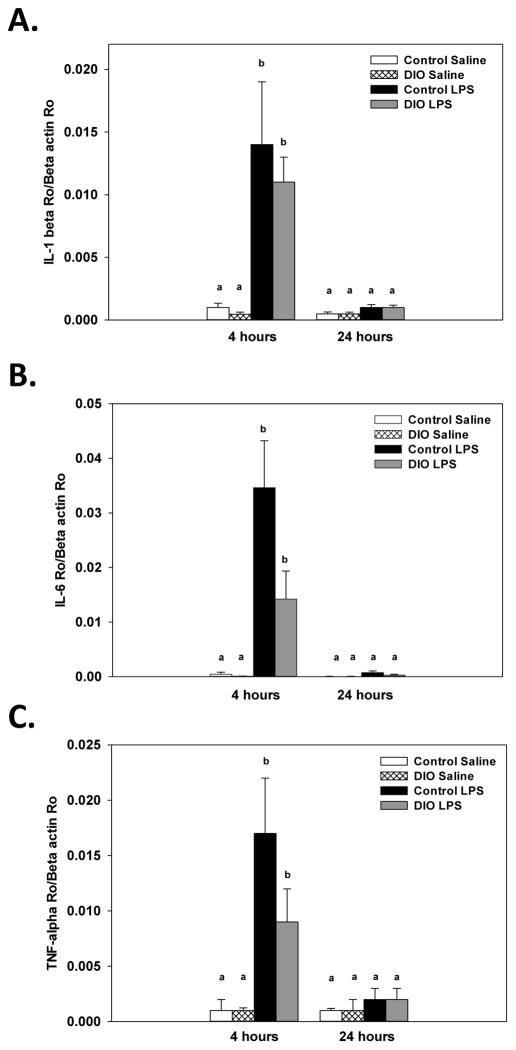
A significant diet x treatment x collection time interaction for IL-6 levels (F(1, 30)=7.28, p<0.05, Figure 7A) showed that LPS administration increased levels of IL-6 relative to saline-treated mice at 4, but not 24, hours after administration. The LPS-induced increase in IL-6 at 4 hours was significantly higher in the mice fed the control diet compared to DIO mice.
Figure 7.
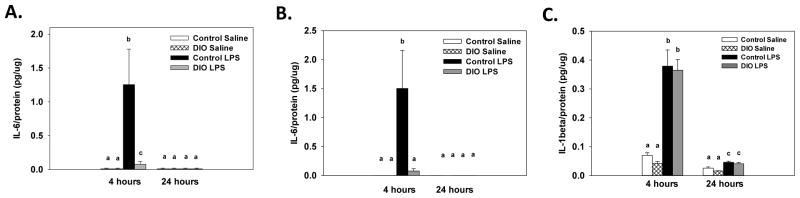
Four hours after LPS administration brain protein levels of IL-6 were increased compared to saline and this response was higher in the control diet mice (A). LPS increased splenic IL-6 levels four hours after treatment in the control diet mice, but not in the DIO mice (B). LPS administration increased splenic levels of IL-1β four and twenty-four hours after administration in both the control and DIO mice, but IL-1β levels were at higher four hours (C). Bars represent means ±SEMs. Means marked with different letters (a, b or c) are significantly different from each other.
3.2.5. Spleen protein data
For IL-6, a significant diet x treatment x collection time interaction (F(1, 30)=13.48, p<0.001, Figure 7B) showed that in the control diet mice LPS administration increased splenic IL-6 levels 4, but not 24, hours after treatment. For the DIO mice, no significant differences were found between the DIO mice given LPS or saline at either collection time point. A significant treatment x collection time interaction for splenic IL-1β levels (F(1, 30)=64.09, p<0.001, Figure 7C) showed that LPS administration increased IL-1β levels 4 and 24 hours after treatment relative to time matched saline controls. However, IL-1β levels were significantly higher at 4 hours than at 24 hours. No significant effects or interactions with diet were found for IL-1β levels.
3.2.6. Hippocampus RNA data
A significant diet x treatment x collection time interaction for hippocampal expression of IL-1β (F(1, 30)=6.23, p<0.01, Figure 8A) showed that LPS administration increased expression of IL-1β at 4, but not 24, hours after treatment relative to saline-treated mice. Mice fed the control diet showed higher expression levels of IL-1β four hour after LPS than DIO mice. A significant treatment x collection time interaction for hippocampal expression levels of IL-6 (F(1, 30)=6.15, p<0.05, Figure 8B) showed that LPS administration increased expression of IL-6 at 4, but not 24, hours after treatment relative to saline-treated mice. No significant effects or interactions with diet were found for IL-6. For BDNF expression, a significant main effect of diet (F(1, 30)= 4.66, p<0.05) showed that DIO mice had lower expression of BDNF in the hippocampus relative to mice on the control diet (Figure 8C). A separate assay was run on hippocampal samples to assess changes in synaptophysin and PSD 95. Two samples showed significant differences in β-actin levels and were removed from the analysis. For synaptophysin a significant main effect of diet showed that DIO mice had lower expression of synaptophysin compared to control diet mice (F(1, 28)= 4.67, p<0.05, Figure 8D). For PSD 95 expression, a significant main effect of treatment showed that LPS-treated mice had lower levels of PSD 95 relative to saline-treated mice (F(1, 28)= 11.75, p<0.05, Figure 8E).
Figure 8.
LPS administration significantly increased hippocampal expression of IL-1β (A) and IL-6 (B) four hours after treatment compared to saline-treated mice. LPS-induced IL-1β expression was higher in control mice relative to DIO mice (A). Consuming the high-fat diet decreased hippocampal expression of BDNF (C) and synaptophysin (D) as compared to control mice. LPS administration decreased PSD95 (E) expression compared to saline treated mice. Means marked with different letters (a, b or c) are significantly different from each other.
4. DISCUSSION
The present data indicate that DIO modulates the inflammatory response following an immune challenge, as DIO reduced expression of IL-6 in the periphery and the brain and lowered IL-1β expression in the hippocampus after LPS treatment relative to mice on the control diet. This attenuated inflammatory response in the DIO mice was not sufficient to alter the LPS-induced reductions in locomotor behavior, as the DIO and control mice showed a similar locomotor depression and recovery time following LPS administration. Whether DIO mice would show differential behavioral response to a sub-threshold dose of LPS that does not induce a sickness behavior response has yet to be investigated [24, 25]. Lastly, our data indicate that the cognitive deficits in the DIO mice are unlikely to results from changes in the immune system, but more likely derive from changes in neurotrophic support and potentially synaptic activity.
In our hands, mice that consumed a high-fat diet showed selective impairments in their inflammatory response in both the periphery and brain following endotoxin administration. Of particularly interest is the lack of an increase in CD74 expression in the cortex of DIO mice following LPS administration. CD74 plays a role in trafficking major histocompatibility complex type II (MHC II) proteins to the cell surface [26]. The MHC II molecule is the primary mechanism though which antigen presenting cells signal the presence of a foreign antigen and initiate an immune response. Our data shows that following an immune challenge the control diet mice show the expected increase in CD74 expression whereas the DIO mice did not differ from saline-treated mice, potentially indicating that DIO impairs the ability of brain to mount a normal response to an immune challenge. In addition, our data demonstrate the production of the proinflammatory cytokines IL-1β and IL-6 were reduced within the CNS. These data are the first to demonstrate a reduction in the LPS-induced inflammatory response within brain. However, related work by Lawrence et al. [9] revealed that DIO mice show blunted neural activation, as measured by expression of the immediate early gene c-Fos, to an LPS challenge. In contrast to our findings prior reports have shown an exaggerated expression of IL-6, TNF-α, and IL-1 receptor antagonist in the hypothalamus of DIO rats following LPS administration [11]. Several factors including the species employed, the age of the animals, as well as the duration and type of diet consumed are likely to influence the nature of the immune alterations induced by high-fat diet consumption. Though further work is needed to identify mediating factors that determine the direction of the change, the present data in combination with prior reports indicate that DIO modifies the neuroinflammatory response to an immune challenge.
In agreement with our data from the CNS as well as prior work [12], we found that DIO mice show attenuated production of IL-6 in the spleen following LPS administration. The reduction in IL-6 levels is likely related to altered macrophage function in DIO mice. Prior research has shown that macrophages from genetic models of obesity have reduced phagocytic capacity as well as oxidative bursts [27, 28]. In agreement, macrophages from DIO mice show reduced production of inducible NO synthase (iNOS) and cytokines following a bacterial infection when compared to control mice [29, 30]. In response to an infection, the ability to up regulate surface expression of TLR-4 and TLR-2 is impaired in macrophages from DIO mice [30]. Further, DIO has also been shown to decrease activation of the transcription factor nuclear factor kappa B (NF-κB), which initiates the production of proinflammatory cytokines [12, 29, 30]. The DIO-associated impairments in the immune response have been shown to slow the clearance of bacteria and increase mortality following infection [29, 31]. The blunted release of IL-6 may result from impairments in the ability of macrophages to detect and mount an adequate response following an immune challenge that may subsequently reduce production of mediators of the innate immune response [30].
In accordance with prior reports [16, 32] we found that DIO impairs spatial learning, as acquisition of the task was disrupted in the DIO mice as compared to control diet mice. Recent reports have implicated inflammation as the potential underlying mechanism of DIO associated cognitive deficits [7, 8]. For instance, Pistell et al. [7] report that mice fed a high-fat diet showed impaired cognitive performance, reduced cortical BDNF levels and increased basal levels of proinflammatory cytokines IL-6 and TNF-α potentially indicating a link between inflammation and DIO-induced cognitive deficits. However, we observed dissociation between the cognitive deficits and inflammation as DIO mice showed impaired spatial learning, but no changes in basal cytokine levels in the hippocampus, cortex, or brain samples analyzed. One potential reason for the difference between the current data and the Pistell et al. [7] study is the age of the mice employed; Pistell et al. used middle-aged mice whereas the current study used adults. Given that middle-aged animals show age-related changes in immune function [33–36] they may be more prone to develop an exaggerated inflammatory response, but additional work is needed to confirm this possibility. Our data indicate that the cognitive deficits associated with consuming a high-fat diet may result from reductions in BDNF levels as well as alterations in synapses. The DIO mice showed reduced cortical and hippocampal expression of BDNF, a neurotropic factor that supports neuron survival, growth, and synaptic communication [37]. Further DIO decreased expression of synaptophysin within the hippocampus. Synaptophysin is a presynaptic vesicle protein suggested to participate in synapse formation, stabilization as well as neurotransmitter packaging and release [38]. In accordance, prior work has shown that consuming a high-fat/glucose diet decreases hippocampal dendritic spine density as well as synaptic protein levels, collectively indicating that DIO may impair synaptic plasticity [39, 40]. Taken together, the data indicate that while DIO can alter immune function these changes are unlikely to account for the associated cognitive deficits, but rather alteration in neurotrophic levels and synaptic activity appears to be contributing factors.
Consuming a high-fat diet and the subsequent development of obesity has a negative impact on cognitive performance, as impairments in learning and memory have been observed across different species, ages, and durations of diet consumption [7, 16, 41–43]. However, the mechanisms of these deficits are still unknown. The current data bolsters the contention that diminished neurotropic and synaptic support contributes to the resultant deficits. While elevated cytokine levels within the brain can disrupt cognitive performance [14, 21, 33] this does not seem to account for the DIO associated cognitive deficits we observed as the impairments in spatial learning occurred independent of changes central cytokine levels as no differences were observed between the saline-treated DIO and control mice. Further the present data indicate that DIO alters immune function. We report that DIO blunts aspects of the peripheral and neural inflammatory response to an immune challenge, as evidenced by the attenuated cytokine response and the failure to increase expression of an immune cell signaling molecule following endotoxin exposure. Our findings in conjunction with prior reports [9, 12, 30, 31] indicate that DIO can suppress immune function and impairs an organism’s ability to mount an immune response. Such deficits have substantial implications for an individual’s susceptibility to as well as their ability to recover from an infection.
Highlights.
Diet-induced obesity (DIO) blunts the immune response to endotoxin.
DIO impairs spatial learning that appears unrelated to changes in inflammation.
Hippocampal BDNF and synaptophysin levels are reduced in diet-induced obese mice.
Acknowledgments
Funding source: This work was supported by grants from National Institute on Aging R00AG040194 and UNCW Charles Cahill awarded to R.A.K. Funding sources had no involvement in the experimental design or interpretation of the results.
Footnotes
Conflict of interests: All authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katherine M. Baumgarner, Email: KBAUMGARNER@USCUPSTATE.EDU.
Sharay Setti, Email: ses8535@uncw.edu.
Carolyn Diaz, Email: diazc@uncw.edu.
Alyssa Littlefield, Email: aml8440@uncw.edu.
Amanda Jones, Email: aj3294@uncw.edu.
Rachel A. Kohman, Email: kohmanr@uncw.edu.
References
- 1.CDC. National Institutes of Health- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obesity Research. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 2.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 4.Vilar-Compte D, Mohar A, Sandoval S, de la Rosa M, Gordillo P, Volkow P. Surgical site infections at the National Cancer Institute in Mexico: a case-control study. American journal of infection control. 2000;28:14–20. doi: 10.1016/s0196-6553(00)90006-3. [DOI] [PubMed] [Google Scholar]
- 5.Falagas ME, Kompoti M. Obesity and infection. The Lancet infectious diseases. 2006;6:438–46. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 6.Gottschlich MM, Mayes T, Khoury JC, Warden GD. Significance of obesity on nutritional, immunologic, hormonal, and clinical outcome parameters in burns. Journal of the American Dietetic Association. 1993;93:1261–8. doi: 10.1016/0002-8223(93)91952-m. [DOI] [PubMed] [Google Scholar]
- 7.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of neuroimmunology. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. Journal of neurochemistry. 2008;106:475–85. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence CB, Brough D, Knight EM. Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Disease models & mechanisms. 2012;5:649–59. doi: 10.1242/dmm.009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, Liu T, Rose JL, Stevens RL, Hoyt DG. Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. Journal of inflammation. 2007;4:22. doi: 10.1186/1476-9255-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohl J, Woodside B, Luheshi GN. Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology. 2009;150:4901–10. doi: 10.1210/en.2009-0526. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Lin G, Lei L, You X, Wu C, Xu W, et al. Hyperlipidemia modifies innate immune responses to lipopolysaccharide via the TLR-NF-kappaB signaling pathway. Inflammation. 2013;36:968–76. doi: 10.1007/s10753-013-9628-9. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. European journal of pharmacology. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Pohl J, Sheppard M, Luheshi GN, Woodside B. Diet-induced weight gain produces a graded increase in behavioral responses to an acute immune challenge. Brain, behavior, and immunity. 2014;35:43–50. doi: 10.1016/j.bbi.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 17.Yang IV, Wade CM, Kang HM, Alper S, Rutledge H, Lackford B, et al. Identification of novel genes that mediate innate immunity using inbred mice. Genetics. 2009;183:1535–44. doi: 10.1534/genetics.109.107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain, behavior, and immunity. 1995;9:129–48. doi: 10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- 19.Gahtan E, Overmier JB. Performance more than working memory disrupted by acute systemic inflammation in rats in appetitive tasks. Physiology & behavior. 2001;73:201–10. doi: 10.1016/s0031-9384(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 20.Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. The European journal of neuroscience. 2000;12:4434–46. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- 21.Sparkman NL, Kohman RA, Garcia AK, Boehm GW. Peripheral lipopolysaccharide administration impairs two-way active avoidance conditioning in C57BL/6J mice. Physiology & behavior. 2005;85:278–88. doi: 10.1016/j.physbeh.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain, behavior, and immunity. 1998;12:212–29. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- 23.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic acids research. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarr AJ, Chen Q, Wang Y, Sheridan JF, Quan N. Neural and behavioral responses to low-grade inflammation. Behavioural brain research. 2012;235:334–41. doi: 10.1016/j.bbr.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain, behavior, and immunity. 2007;21:836–50. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Beswick EJ, Reyes VE. CD74 in antigen presentation, inflammation, and cancers of the gastrointestinal tract. World journal of gastroenterology: WJG. 2009;15:2855–61. doi: 10.3748/wjg.15.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. Journal of immunology. 2002;168:4018–24. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 28.Lee FY, Li Y, Yang EK, Yang SQ, Lin HZ, Trush MA, et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. The American journal of physiology. 1999;276:C386–94. doi: 10.1152/ajpcell.1999.276.2.C386. [DOI] [PubMed] [Google Scholar]
- 29.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20466–71. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q, Leeman SE, Amar S. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10740–5. doi: 10.1073/pnas.0904412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. The Journal of nutrition. 2007;137:1236–43. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 32.Valladolid-Acebes I, Fole A, Martin M, Morales L, Victoria Cano M, Ruiz-Gayo M, et al. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiology of learning and memory. 2013;106:18–25. doi: 10.1016/j.nlm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Kohman RA, Crowell B, Kusnecov AW. Differential sensitivity to endotoxin exposure in young and middle-age mice. Brain, behavior, and immunity. 2010;24:486–92. doi: 10.1016/j.bbi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izgut-Uysal VN, Ozkaya YG, Ozdemir S, Yargicoglu P, Agar A. Effect of L-arginine on age-related changes in macrophage phagocytic activity. Immunological investigations. 2004;33:287–93. doi: 10.1081/imm-120037276. [DOI] [PubMed] [Google Scholar]
- 35.Verbitsky M, Yonan AL, Malleret G, Kandel ER, Gilliam TC, Pavlidis P. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learning & memory. 2004;11:253–60. doi: 10.1101/lm.68204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiology of aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. Journal of pharmacological sciences. 2003;91:267–70. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 38.Clare R, King VG, Wirenfeldt M, Vinters HV. Synapse loss in dementias. Journal of neuroscience research. 2010;88:2083–90. doi: 10.1002/jnr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–8. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. The European journal of neuroscience. 2004;19:1699–707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 41.Soares E, Prediger RD, Nunes S, Castro AA, Viana SD, Lemos C, et al. Spatial memory impairments in a prediabetic rat model. Neuroscience. 2013;250:565–77. doi: 10.1016/j.neuroscience.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 42.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Alzoubi KH, Khabour OF, Salah HA, Hasan Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: the role of oxidative stress. Physiology & behavior. 2013;119:72–8. doi: 10.1016/j.physbeh.2013.06.011. [DOI] [PubMed] [Google Scholar]