Abstract
Caffeine adversely affects endochondral ossification during fetal skeletal growth, and results in increased incidence of delayed and abnormal fetal skeletal development. Chronic caffeine intake also decreases growth hormone secretion. Thus, it is conceivable that caffeine may disrupt bone growth during the peripubertal period. This study aimed to investigate the impact of high-caffeine consumption on bone growth throughout puberty. A total of 51 male rats (21 days old) were divided randomly into three groups: a control group and two groups fed caffeine via gavage with 120 and 180 mg kg−1 day−1 for 4 weeks. After death, the final length and weight of leg bones were measured, and the tibia processed for histomorphometric analysis. Caffeine caused a significant decrease in body mass gain. This was accompanied with proportional decreases in lean body mass and body fat. In addition, bone mass and osteogenic activity in vivo were assessed using dual-energy X-ray absorptiometry and 18F-NaF positron emission tomography. The results showed significant decreases of bone mass and in vivo osteogenic activity in the caffeine-fed groups. Rats fed with caffeine showed a significantly shorter and lighter tibia and femur and the vertebral column compared with controls. In addition, caffeine does not increase the width of the growth plates (GPs), it slows the rate at which the GP closes due to a slower rate of growth. These results demonstrated that caffeine altered osteogenic activity, leading to delayed peripubertal longitudinal bone growth and maturation. Given that osteogenic cells undergo dynamic changes in metabolic activity and that the pubertal growth spurt is mainly stimulated by growth hormone/insulin-like growth factor-1 and sex steroids during pubertal development, caffeine could suppress ossification by interfering with both physiological changes in hormonal secretion and osteogenic activity during this critical period. Further study will be needed to investigate the cellular/molecular mechanism by which caffeine affects osteogenesis using in vitro experimental models.
Keywords: 18F-NaF PET, caffeine, DXA, growth spurt, osteogenesis, puberty
Introduction
Caffeine is readily available in energy drinks (ranging from 50 to 505 mg per can or bottle; Reissig et al. 2009). The consumption of high-caffeine-content energy drinks has increased markedly in recent years, and 30–50% of consumers are adolescents and young adults (Oddy & O'Sullivan, 2009; Seifert et al. 2011). As the number of adolescent caffeine consumers increases, it is important to educate the public on the potential dangers of caffeine consumption during development and improve toxicity surveillance. Accurate knowledge on the effects of caffeine could help to regulate the consumption of caffeine by adolescents who may be more vulnerable to the negative effects of caffeine. However, there is still limited knowledge on the effects of high-dose caffeine exposure during puberty.
The effects of caffeine on an individual may vary depending on the age of exposure. Puberty is another vulnerable developmental period in which physical growth accelerates (Gluckman & Hanson, 2006). Although it has been suggested that caffeine does not exert an adverse effect on calcium economy in healthy adult individuals who consume the recommended daily amount of calcium (Heaney, 2002), a number of studies have confirmed that maternal caffeine consumption causes fetal skeletal growth retardation by affecting endochondral ossification and the insulin-like growth factor-1 (IGF-1) signaling pathway (Huang et al. 2012; Tan et al. 2012). Longitudinal bone growth occurs at the growth plate (GP) through a process called endochondral ossification, in which cartilage is formed and then remodeled into bone tissue (Hunziker & Schenk, 1989). Endochondral ossification involves deposition of calcium on the epiphyses (Brighton & Hunt, 1986). Early adolescence is the time of maximal calcium deposition in bone, and caffeine interferes with intestinal calcium absorption (Huang et al. 2002). Thus, it is to be expected that the effects of caffeine on bone growth are likely to be manifested during pubertal development. Currently it is known that high-dose caffeine consumption is associated with bone mineral loss, lower mineral density and lower calcium content in growing and young rats (Huang et al. 2002). However, data on the effects of caffeine on pubertal development are still sparse, and to the authors’ knowledge, there are no interventional studies analyzing the effect of caffeine on in vivo osteogenic activity and endocrine factors related to the growth spurt during puberty. In this study, it was aimed to investigate the impact of high-caffeine exposure on parameters of growth and bone maturation in prepubertal male rats throughout most of their growth spurt. In particular, high-resolution Na18F positron emission tomography (PET) was applied to analyze the effects of caffeine on in vivo osteogenic activity during the pubertal growth spurt, because 18F-NaF is taken up in bone in proportion to blood flow and bone metabolic activity (Schiepers & Hoh, 1998). In addition, the potential of caffeine to affect serum hormone levels, such as IGF-1, estradiol (E2) and testosterone (T4), known important endocrine factors for the pubertal growth spurt, was assessed.
Materials and methods
Animals and treatment
Two-week-old Sprague–Dawley male rats (n = 51) were obtained from Samtako Biokorea (Kyunggi, Korea). The rats were allowed to acclimate until 21 days old before being subjected to experimental conditions. Each animal was housed in a separate plastic cage under controlled conditions (22–24 °C, humidity 40–50%, 12 h light–dark cycle), with free access to food and water. Animal care was consistent with institutional guidelines, and the Hanyang University ACUC committee approved all procedures involving animals (HY-IACUC-2013-0110A).
Experimental design
Previous reports found that male offspring are more susceptible than females to caffeine treatment during either uterine development or suckling (Dorostghoal et al. 2012). Thus, male rats were chosen to evaluate whether pubertal caffeine exposure has effects that are similar to prenatal exposure. The experiment commenced when the rats were 22 days old, because 22–25 days postnatal is considered the beginning of puberty in the rat (Hansson et al. 1972). Groups were assigned by the stratified randomization method, based upon body mass on the day before the start of treatment to eliminate variation in mean body mass among the groups. Rats were 22 days old and weighed approximately 50 ± 2 g on the first day of the experiment. Caffeine (C0750; Sigma-Aldrich, St Louis, MO, USA) was dissolved in distilled water (feeding volume 10 mL kg−1 body mass) at concentrations calculated to deliver 120 and 180 mg kg−1 body mass day−1 (designated as CF1 and CF2, N = 17/group). Dose levels were chosen based on literature and studies to avoid sub-lethal effects at the top dose level. The median lethal dose of caffeine is 192 mg kg−1 through oral administration (Olchowik et al. 2011). The doses chosen here were within the range shown to have no undue systemic toxicity such as renal toxicity in the preliminary study (data not shown; BUN/Cr was within the normal range in both groups), but have been reported to induce delayed ossification in the fetuses of treated pregnant rats (Tan et al. 2012). Using body surface area for dose conversion (Reagan-Shaw et al. 2008), the dosages employed in this study were equivalent to approximately 19.4 and 29.1 mg kg−1 in humans (for a 20 kg child, 28.8 and 43.2 mg kg−1). Most energy drinks provide more than 1 mg mL−1 of caffeine. Therefore, one or two energy drinks can easily lead to the above doses of caffeine. Caffeine was dissolved in water and fed to animals via gavage to ensure complete consumption of the established dose once daily in the morning (09:00–11:00 hours). Seventeen animals in the control group (CT) received the same volume of distilled water as vehicle. Treatment duration of 4 weeks was selected to cover the rapid skeletal growth period of puberty in rats (from 22 to 55 days old; Hansson et al. 1972). Animals were examined for clinical signs and weighed on a daily basis, and the amount of food intake was also monitored. Body mass was measured to the nearest 0.1 g with an electronic scale (Dretec, Seoul, Korea), and recorded on the first day before caffeine feeding (initial) until the day of death (final). Treatments on caffeine and control animals were run in parallel. All the animals were killed 24 h after the last treatment following protocol and ethical procedures. Animals were anesthetized by isoflurane inhalation (Forane solution; Choongwae Pharma, Seoul, Korea), and killed by cervical dislocation under deep anesthesia. Blood samples were collected at 2 and 4 weeks into the experiment from tail vein and heart puncture, respectively, and obtained serum samples were stored at −70 °C until further analysed.
18F-NaF bone PET-computed tomography (CT)
Uptake of 18F-NaF using PET-CT in the tibiae was assessed on the day before death. Na18F was generated from a reaction of 18O (p, n) 18F using an in-house cyclotron (HM-12; Sumitomo Heavy Industries, Tokyo, Japan). Animals were anesthetized by 2% isoflurane inhalation, while being PET scanned using a Triumph LabPET 4 pre-clinical PET/CT scanner (Gamma Medica, Salem, NH, USA), with an image resolution of 1.0 mm. Each subject was positioned in the PET scanner, and a transmission scan was acquired to correct the measured emission data for attenuation. Then, 400 μCi per 100 g body mass of Na18F was injected into a tail vein as a bolus, and list mode data were acquired for 35 min. Following data acquisition, processing of PET list mode data, reconstruction and corrections were performed using a VIVID image analysis platform to form a high-quality image volume for defined regions of interest (ROIs). As shown in Fig. 2d, ROIs were manually defined on the PET images in the epiphyseal GP and diaphysis of the tibia. The measured radioactivity within the ROI was normalized to the average radioactivity concentration in the body, which is approximated as the injected dose divided by body mass (Adams et al. 2010). Resultant normalized values were reported as the standardized uptake value (SUV) in each region. An average value of both tibiae was calculated from the data for each animal and then pooled, and the mean value was calculated for each group.
Figure 2.
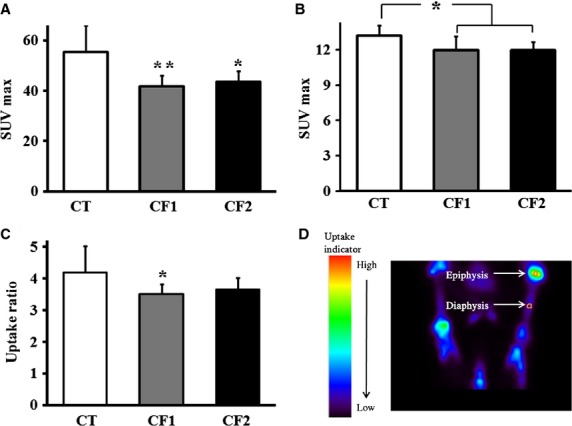
Comparison of maximum standardized 18F-NaF uptake value (SUVmax) in both tibiae in the control and caffeine-fed groups. SUVmax was measured (a) from epiphyseal GP, and (b) from the diaphysis. (c) Comparison of the ratios of 18F-NaF SUVs in the epiphyseal GP relative to the diaphysis. (d) Representative PET image showing how the ROIs in the epiphysis and diaphysis were defined. CT, control; CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day−1.Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 vs. CT.
Dual-energy X-ray absorptiometry (DXA)
All animals were evaluated for body composition at the end of the experiment with a DXA scanner (Discovery W QDR series; Hologic, Bedford, MA, USA), using software for small animals. Bone mineral content (BMC, g) and bone mineral density (BMD, g cm−2) of the whole body, lumbar vertebrae, both femurs and tibiae were analyzed, along with lean body mass (LBM) and body fat. Animals were anesthetized by isoflurane inhalation during examination.
Tibia and femur preparation
Both femurs and tibiae were dissected from each rat, and cleaned of fat, muscle and connective tissue at necropsy, they were then weighed and their lengths measured from the top of the plateau to the bottom of the lateral malleolar process with a precision digital caliper (± 0.03 mm; NA500-150S, Bluebird, China), as described by Weinreb et al. (1991).
Histomorphometric analysis
Both tibiae were fixed in 10% buffered formalin for 48 h and decalcified in a solution containing 10% ethylene-diamine tetraacetic acid in 0.1 m Tris at 4 °C. After decalcification, tibiae were cut in half longitudinally with a razor blade and embedded in paraffin. Serial longitudinal sections (5 μm thickness) were obtained from the proximal part of the tibiae, and stained with alcian blue to stain the cartilage matrix (Lison, 1954). All histomorphometric evaluations were performed by the same trained, calibrated and blinded examiner, using an image analysis system (leica las software) coupled to a light microscope (DM4000B, Leica) with final magnifications of 50 ×, 100 × or 200 ×. Quantitative assessment of longitudinal bone growth is frequently based upon measurement of the overall height of the growth cartilage (Hunziker & Schenk, 1989; Roach et al. 2003). Based on the zone definitions of the GP (Roach et al. 2003), it was divided into three: the resting zone; proliferative zone (PZ); and hypertrophic zone (HZ; Fig. S1A). PZ and HZ were analyzed in this study, because both zones are actively involved in synchronized processes of chondrogenesis resulting in longitudinal bone growth. The height of each zone and total GP were measured as indicated in Fig.4 using image analysis software (leica las software).
Figure 4.
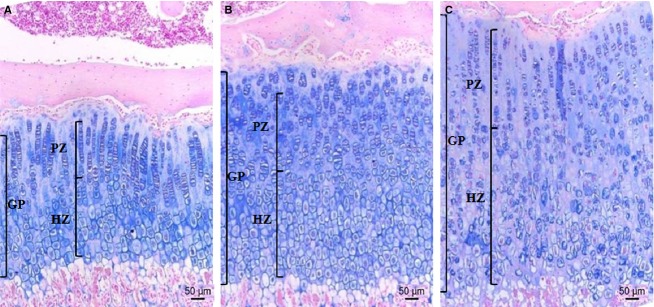
Representative proximal tibia sections from the control and caffeine-fed groups. The tissues were stained with alcian blue; collagen is blue. The GP of (A) the control, (B) CF1 and (C) CF2. The heights of the total GP, PZ and HZ are indicated. GP, growth plate; HZ, hypertrophic zone; PZ, proliferative zone. CT, control; CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day−1. Scale bar: 50 μm.
Four serial sections per animal were traced for each tibia, and five measurements per section were made for both height and cell number per column. The average of 20 GP measurements was calculated for each tibia at 100-fold magnification and combined to obtain a mean value per animal. The number of chondrocytes and columns of PZ and HZ were counted within the same defined region (0.307277 mm2) at 200-fold magnification, and the mean value of eight measurements was calculated from both tibiae.
For the metaphysis, 16 measurements per tibia were made for height of primary and secondary spongiosa (Sn) at 50-fold magnification. Histomorphometric analysis was performed on the whole secondary Sn, which is located under the primary Sn and characterized by a network of larger trabeculae (extending 1–1.9 mm distal to the epiphyseal GP; Fig. S1B). Whole fields of vision on four consecutive alcian blue-stained sections per animal for each tibia were evaluated for the analysis of secondary Sn at 100-fold magnification. All the standardized terms used in cancellous bone analysis are based on standard histomorphometry (Dempster et al. 2013). In this zone, the areas of trabecular bone within a reference area (1.229 mm2) of the proximal tibia were measured on printed copies by point counting using a square lattice (0.3 mm; Fig. S1C). Data are presented as the ratio of trabecular bone area to tissue area. Trabecular width (μm), number (mm−1) and separation (μm) were calculated according to stereological formulae described by a previous report (Dempster et al. 2013). An average was calculated from the data for each animal and then pooled, and the mean value was calculated for each group.
Hormone measurements
Serum levels of IGF-1, E2 and T4 were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Biotech, China) following the manufacturer protocol. Intra- and inter-assay coefficients of variance for IGF-1 (8%, 10%), E2 and T4 (< 15%) were < 15%. Under the test conditions, the limits of detection were 0.156 ng mL−1 for IGF-1, 15 pg mL−1 for E2 and 0.06 ng mL−1 for T4. The absorbance at 450 nm within 15 min was read in the ELISA reader blanking against a well (BioRad, Hercules, CA, USA). All samples were run in duplicate in the same assay, and each ELISA was repeated twice.
Statistical analysis
Data for each group are expressed as means with standard deviations. All data were analyzed using spss ver. 10.0 for Windows (SPSS, Chicago, IL, USA). The Kruskal–Wallis one-way analysis of variance on ranks was used for multiple group comparisons. The Mann–Whitney U-test was used for two-group comparisons.
Results
Body mass gain and food consumption
The effects of caffeine on body mass gain were observed, which can serve as an indicator of general growth in rats. The body mass of the rats was controlled between groups, and no difference was observed at the beginning of the experiment (52–54 g). Throughout the experimental period, there were four accidental deaths due to asphyxia caused by erroneous insertion of the feeding tube (N = 3) or anesthetic problem during blood sampling (N = 1) within the first 2 weeks, but no other treatment-related clinical signs were observed. General observations regarding the body mass, as well as food consumption, are summarized in Fig.1.
Figure 1.
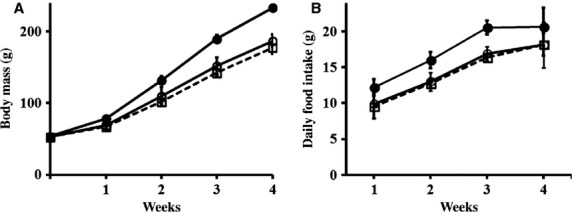
Effect of caffeine exposure on body mass and food intake in immature male rats. (a) The average body mass measured in the rats fed with vehicle or caffeine is depicted each week during the study period. (b) Average amounts of daily food intake calculated by dividing the amount of food consumed in a week by 7 and showing each week. Values are expressed as mean ± SD. Filled circle, CT (control); open circle, CF1 (caffeine 120 mg kg−1 day−1); open square, CF2 (caffeine 180 mg kg−1 day−1).
The body mass of all rats increased progressively throughout the study period as the rats were in a period of rapid growth. However, the caffeine-fed groups had a significantly reduced body mass gain in a time-dependent manner compared with the control group (Fig.1a). This reduction in mass gain was observed on and after 1 week of exposure (P < 0.001 vs. CT). On the other hand, a caffeine–dose effect was not observed. Overall, body mass gain throughout the study period was attenuated in the caffeine-fed groups compared with the controls. Likewise, food consumption was decreased in the caffeine-fed groups compared with the controls from the first week of caffeine exposure (P < 0.001 vs. CT), and this persisted until the end of the experiment (Fig.1b). However, no other difference was observed between the caffeine-fed groups.
Interpretation of bone PET
Among the parameters used to measure tracer accumulation in PET studies, SUV measurements are currently known as the most convenient method for quantitatively evaluating changes in metabolic activity (Adams et al. 2010). Here, maximum SUV (SUVmax) was used, because it is less observer-dependent and more reproducible than mean SUV (Adams et al. 2010). The data are depicted in Fig.2. SUVmax measured from epiphyseal GP (CT vs. CF1, P = 0.005; CT vs. CF2, P = 0.026) and diaphysis (CT vs. CF1, P = 0.027; CT vs. CF2, P = 0.011) in both tibiae was significantly reduced in caffeine-fed animals compared with the controls (Fig.2a,b). To evaluate the activity of the GP relative to the diaphysis, uptake ratio was calculated by dividing SUVmax for the GP to that of the diaphysis, which was chosen as the region with the lowest uptake value. The uptake ratio in caffeine-fed groups was reduced compared with the controls, although only statistically significant in CF1 (CT vs. CF1, P = 0.045; CT vs. CF2, P = 0.166; Fig.2c). On the other hand, there was no difference in uptake activity according to caffeine dose.
Body composition
To determine whether caffeine exposure was associated with changes in body composition, LBM, body fat and bone mineral status were analyzed using DXA (Table1). In parallel with total body mass, body fat and LBM also significantly decreased in caffeine-fed groups compared with the controls (P < 0.001 vs. CT). However, body fat percentage was not significantly different between the control and caffeine-fed groups (CT = 17.9 ± 2.2% vs. CF1 = 16.2 ± 2.6%, P = 0.121; CT vs. CF2 = 16.1 ± 2.5%, P = 0.066), although a relative decrease in caffeine-fed groups was observed. Likewise, the percentage of LBM did not differ between the control and caffeine-fed groups, indicating a proportional reduction in body fat and LBM in caffeine-fed groups. Whole-body BMC was significantly reduced in the caffeine-fed groups (CF1 = 5.72 ± 0.47 g, CF2 = 5.44 ± 0.30 g), compared with the controls (7.31 ± 0.51 g; P < 0.001 vs. CT). Regional BMC measured from lumbar vertebrae and both femurs and tibiae was also significantly reduced in the caffeine-fed groups compared with the controls (P < 0.01 vs. CT). However, there was no difference between the caffeine-fed groups. On the other hand, whole-body BMD was significantly reduced in caffeine-fed groups compared with the controls (CT = 0.144 ± 0.01 g cm−2 vs. CF1 = 0.127 ± 0.01 g cm−2, P < 0.001; CT vs. CF3 = 0.121 ± 0.01 g cm−2, P < 0.001). Likewise, lumbar and tibial BMD was also significantly decreased in the caffeine-fed groups (CT vs. CF1, P < 0.05; CT vs. CF2, P < 0.001), but there was no difference between caffeine-fed groups. Femoral BMD was also decreased in the caffeine-fed groups compared with the controls, but the decrease was statistically significant only for the 180-mg dose (P = 0.003 vs. CT). In addition, the mean BMD of both femurs in the 180-mg dose group was lower than in the 120-mg dose group (P = 0.038).
Table 1.
Effects of caffeine on body composition as determined by DXA
| CT (N = 17) | CF1 (N = 16) | CF2 (N = 14) | |
|---|---|---|---|
| Fat | |||
| Mass (g) | 44.0 ± 6.0 | 31.3 ± 7.1*** | 29.1 ± 4.2*** |
| % of TBM | 17.9 ± 2.2 | 16.2 ± 2.6 | 16.1 ± 2.5 |
| LBM | |||
| Mass (g) | 194.6 ± 13.2 | 153.9 ± 10.2*** | 146.6 ± 13.9*** |
| % of TBM | 79.2 ± 0.51 | 80.6 ± 1.41 | 80.9 ± 1.56 |
| BMC (g) | |||
| Whole body | 7.31 ± 0.51 | 5.72 ± 0.47*** | 5.44 ± 0.30*** |
| Lumbar | 0.37 ± 0.03 | 0.30 ± 0.03*** | 0.28 ± 0.03*** |
| Femur | |||
| Mean | 0.24 ± 0.03 | 0.21 ± 0.01*** | 0.20 ± 0.02*** |
| Right | 0.24 ± 0.02 | 0.20 ± 0.02*** | 0.20 ± 0.02*** |
| Left | 0.25 ± 0.03 | 0.21 ± 0.01*** | 0.20 ± 0.02*** |
| Tibia | |||
| Mean | 0.17 ± 0.02 | 0.14 ± 0.02*** | 0.14 ± 0.02** |
| Right | 0.17 ± 0.02 | 0.14 ± 0.02*** | 0.14 ± 0.02** |
| Left | 0.17 ± 0.02 | 0.14 ± 0.02** | 0.14 ± 0.02** |
| BMD (g cm−2) | |||
| Whole body | 0.144 ± 0.01 | 0.127 ± 0.01*** | 0.121 ± 0.01*** |
| Lumbar | 0.249 ± 0.02 | 0.231 ± 0.01** | 0.224 ± 0.01*** |
| Femur | |||
| Mean | 0.273 ± 0.01 | 0.269 ± 0.01 | 0.260 ± 0.01**† |
| Right | 0.274 ± 0.01 | 0.269 ± 0.01 | 0.263 ± 0.01* |
| Left | 0.272 ± 0.01 | 0.270 ± 0.01 | 0.257 ± 0.01**,† |
| Tibia | |||
| Mean | 0.195 ± 0.01 | 0.182 ± 0.01* | 0.179 ± 0.01** |
| Right | 0.195 ± 0.01 | 0.182 ± 0.02* | 0.179 ± 0.01** |
| Left | 0.195 ± 0.02 | 0.182 ± 0.01* | 0.179 ± 0.01* |
BMC, bone mineral content; BMD, bone mineral density; CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day−1; CT, control; fat %, total body fat divided by TBM; LBM, lean body mass; LBM %, LBM divided by TBM; TBM, total body mass.
Values are expressed as mean ± SD.
P < 0.05,
P < 0.01,
P < 0.001 vs. CT.
P < 0.05, CF1 vs. CF2.
Bone length and weight
The postnatal growth spurt occurs between postnatal day (PD) 20 and 40 in male rats, and the accumulated growth reflected in length from the proximal tibia decreases after 60 days old (Hansson et al. 1972). Therefore, the treatment period covered approximately both the male rat's juvenile (PD21–35) and peripubertal (PD35–55) periods (Marty et al. 2003) in order to ensure a sensitive assessment of effects on skeletal development. Growth determination was made for the long bones such as femur, tibia and vertebral column (Fig.3). The length of vertebral column obtained with DXA was used as a measure of axial growth, because vertical growth of the vertebral body is known to follow the same process as long bone (Doskocil et al. 1993). The length of vertebral column (cm) was measured from the upper border of the first cervical vertebra to the lower border of the fourth sacral vertebra (Fig.3a, right panel), and it was significantly reduced in caffeine-fed groups compared with the controls in a dose-dependent manner (P < 0.001 vs. CT; CF1 vs. CF2, P = 0.027). Likewise, the length and weight of femur and tibia were significantly decreased in caffeine-fed groups in a dose-dependent manner (P < 0.001 vs. CT; Fig.3b,c). As clearly shown (Fig.3d), caffeine feeding caused a significant shortening and lightening of leg bones compared with the controls.
Figure 3.
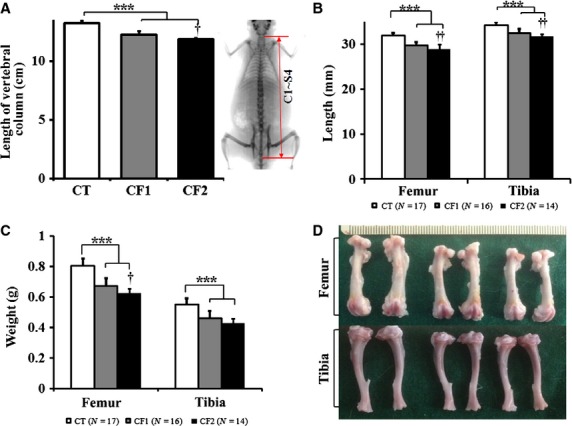
Effect of caffeine exposure on the length and weight of long bones. (a) The length of the vertebral column was measured from the DXA image as indicated in the right panel. (b) Mean lengths and (c) weights of femurs and tibiae measured from the control and caffeine-fed groups. (d) Representative pictures of leg bones dissected from the control and caffeine-fed groups. Femurs and tibiae are placed in the upper and lower panel, respectively. From the left in order, leg bones representing each group are designated as CT, CF1 and CF2. CT, control; CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day−1.Values are expressed as mean ± SD. ***P < 0.001 vs. CT; †P < 0.05, ††P < 0.01, CF1 vs. CF2.
Bone histomorphometry
Histomorphometric parameters analyzed from histological sections of proximal tibiae are summarized (Table2). Representative sections of GP are shown in Fig.4. GP in the caffeine-fed groups showed a significantly slower rate at which it closes in a dose-dependent manner compared with the controls (P < 0.001 vs. CT), and a similar change was also noted in PZ and HZ height. Although both PZ and HZ closing rate were significantly slower in the caffeine-fed groups, the proportion of PZ and HZ showed different directions of change in the GP. Caffeine-fed rats had a decreased proportion of PZ compared with the controls (P < 0.05 vs. CT), whereas in the proportion of HZ, caffeine-fed groups dose-dependently showed a reduced closing rate compared with the controls (P < 0.01 vs. CT; CF1 vs. CF2, P = 0.022). The PZ is formed of columns of rapidly dividing chondrocytes resembling stacks of coins (Fig.4). When a number of cells and columns in PZ were compared, there was no significant difference between the control and caffeine-fed groups (Table2). Considering the slower rate of closing in PZ in caffeine-fed groups, these discrepancies may be related to relatively smaller size of cells and loose cellularity (Fig.4). These abnormalities were more obvious as the caffeine dose increased. On the other hand, a number of cells and columns in HZ were increased in the caffeine-fed groups compared with the controls, apart from CF2 (P < 0.001 vs. CT; CF1 vs. CF2, P = 0.014, P = 0.026 for numbers of cell and column, respectively). In addition, relatively sparse and misaligned columns of HZ with irregular cell size and shape were noted in the caffeine-fed groups (Fig.4).
Table 2.
Effects of caffeine on GP parameters based on measurements of the proximal tibia
| CT (N = 17) | CF1 (N = 16) | CF2 (N = 14) | |
|---|---|---|---|
| GP height (μm) | |||
| Total | 525.4 ± 54.9 | 618.5 ± 75.2** | 853.0 ± 131.8***††† |
| PZ | 172.1 ± 15.2 | 190.1 ± 17.8** | 238.7 ± 41.9***††† |
| HZ | 314.8 ± 38.7 | 398.2 ± 60.7*** | 565.3 ± 119.1***††† |
| % to the GP | |||
| PZ | 33.1 ± 2.2 | 31.1 ± 2.9* | 29.1 ± 3.1** |
| HZ | 60.2 ± 2.6 | 64.2 ± 3.4** | 67.2 ± 3.7***† |
| PZ (no.) | |||
| Cell | 182.1 ± 29.9 | 191.9 ± 40.0 | 188.8 ± 33.5 |
| Column | 23.4 ± 3.2 | 21.6 ± 3.3 | 23.6 ± 5.1 |
| Cell/column | 8.1 ± 1.0 | 9.2 ± 1.7* | 8.5 ± 1.3 |
| HZ (no.) | |||
| Cell | 135.5 ± 16.0 | 142.0 ± 17.1 | 166.7 ± 25.5***† |
| Column | 21.7 ± 2.9 | 22.8 ± 2.9 | 26.4 ± 4.2**† |
| Cell/column | 6.4 ± 0.7 | 6.4 ± 0.6 | 6.5 ± 1.0 |
CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day−1; CT, control; GP, growth plate; HZ, hypertrophic zone; PZ, proliferative zone.
Values are expressed as mean ± SD. Measurements given in μm represent the longitudinal dimension of the structures parallel to the long axis of the bone. The data for the number of cells or columns represent the mean value of eight measurements counted within the same defined region (0.307277 mm2) at a 200-fold magnification from both tibiae; the cell number per column data for the PZ and HZ represent the mean number of cells in a single column spanning the longitudinal diameter of the zone.
P < 0.05,
P < 0.01,
P < 0.001 vs. CT.
P < 0.05,
P < 0.001, CF1 vs. CF2.
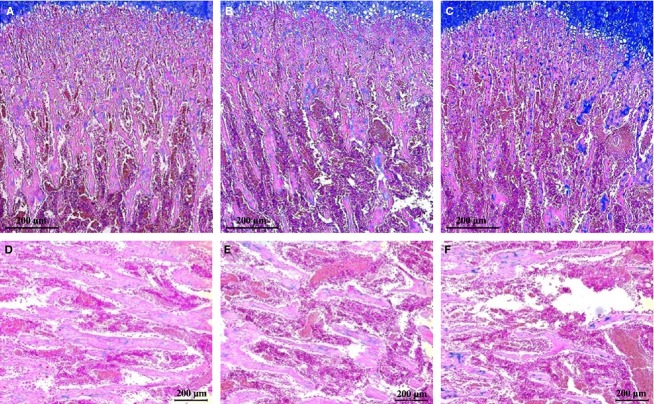
Indices of trabecular bone formation analyzed from mainly secondary Sn are summarized (Table3). In contrast to GP, total Sn height in the caffeine-fed groups was significantly reduced compared with the controls (P < 0.01 vs. CT). Parallel to this, primary and secondary Sn height were also proportionally reduced in the caffeine-fed groups (for primary Sn: P < 0.001 vs. CT; for secondary Sn: CT vs. CF1, P = 0.040; CT vs. CF2, P = 0.006). Along with a reduced height of Sn in caffeine-fed groups, all parameters related to bone formation were significantly decreased compared with the controls. As shown in Fig.5, the micro-architecture of secondary Sn was markedly modified in the caffeine-fed groups, with a decrease in the bone area ratio, trabecular number and width, thus resulting in increased trabecular separation. In caffeine-fed groups, a significant decrease in bone area ratio associated with a significant thinning of the newly formed trabeculae was apparent. Overall, negative influences of caffeine on cancellous bone formation are most likely dependent on dose level.
Table 3.
Effects of caffeine on histomorphometric parameters of the cancellous bone analyzed using the proximal tibia
| CT (N = 17) | CF1 (N = 16) | CF2 (N = 14) | |
|---|---|---|---|
| Height (mm) | |||
| Whole Sn | 3.30 ± 0.57 | 2.77 ± 0.40** | 2.50 ± 0.51** |
| 1° Sn | 0.65 ± 0.07 | 0.52 ± 0.07*** | 0.46 ± 0.08*** |
| 2° Sn | 2.65 ± 0.56 | 2.25 ± 0.40* | 2.03 ± 0.54** |
| % to the whole | |||
| 1° Sn | 20.87 ± 3.88 | 19.61 ± 4.10 | 20.05 ± 6.34 |
| 2° Sn | 79.13 ± 3.88 | 80.39 ± 4.10 | 79.95 ± 6.34 |
| 2° Sn | |||
| B.Ar (mm2) | 0.24 ± 0.05 | 0.18 ± 0.02** | 0.19 ± 0.03** |
| Bone perimeter (mm) | 13.52 ± 2.35 | 11.85 ± 1.60 | 12.53 ± 2.48 |
| B.Ar/T.Ar (%) | 19.66 ± 4.04 | 14.60 ± 1.59*** | 15.16 ± 2.70** |
| Tb.Wi (μm) | 35.96 ± 4.74 | 30.55 ± 2.43** | 30.11 ± 3.10** |
| Tb.N (mm−1) | 5.53 ± 1.01 | 4.85 ± 0.69 | 5.08 ± 1.01 |
| Tb.Sp (μm) | 154.30 ± 30.57 | 183.02 ± 31.98* | 178.99 ± 43.33 |
B.Ar./T.Ar., trabecular bone volume; CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day; CT, control; Sn, spongiosa; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Wi, trabecular width.
Values are expressed as mean ± SD. The height measurements represent the longitudinal dimension of the structure parallel to the long axis of the bone. Whole fields of vision on four consecutive alcian blue-stained sections of each tibia per animal were evaluated for the analysis of the secondary Sn at 100-fold magnification.
P < 0.05,
P < 0.01,
P < 0.001 vs. CT.
Figure 5.
Representative sections of the proximal tibia cancellous bone from the control and caffeine-fed groups. The tissues were stained with alcian blue; collagen is blue. (A–C) Sections including primary and secondary Sn at 50-fold magnification, and (D–F) sections showing a part of the secondary Sn at 100-fold magnification. (A, D) Control, (B, E) CF1, and (C, F) CF2. CT, control; CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day−1. Scale bar: 200 μm.
Hormones
The effects of caffeine exposure on serum levels of IGF-1, E2 and T4 were analyzed (Fig.6). Serum level of IGF-1 was 1.22 ± 0.38 μg mL−1 at the beginning of the experiment (at 21 days old; data not shown), and profoundly increased to 1.5- and 2.2-fold of its initial level at 2 and 4 weeks into the experiment in the controls (1.84 ± 0.42, 2.69 ± 0.48, respectively; μg mL−1; Fig.6a). However, increases in IGF-1 levels by age were substantially attenuated in the caffeine-fed groups. Although there was no significant difference in IGF-1 level after 2 weeks of the study between the control and caffeine-fed groups, IGF-1 levels were relatively lower in the caffeine-fed groups in a dose-dependent manner compared with the controls (P = 0.08 vs. CT). Likewise, after 4 weeks of caffeine exposure, IGF-1 level was decreased compared with the controls, and was statistically significant for the 180-mg dose group (P = 0.032 vs. CT).
Figure 6.
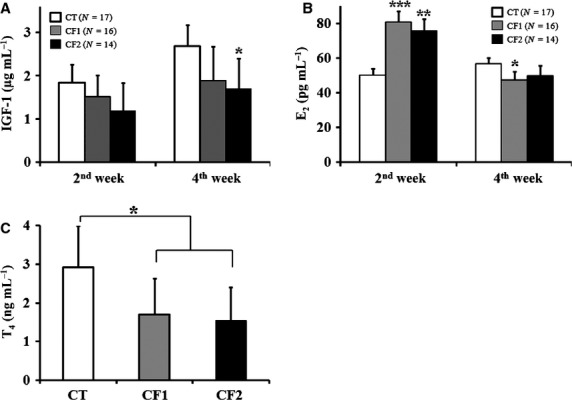
Effect of caffeine on serum levels of (a) IGF-1, (b) E2, and (c) T4 after 2 or 4 weeks of study. Data are presented as mean ± SD at each designated time point, based on two independent measurements. CT, control; CF1, caffeine 120 mg kg−1 day−1; CF2, caffeine 180 mg kg−1 day−1; E2, estradiol; IGF-1, insulin-like growth factor-1; T4, testosterone. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CT.
Estradiol levels in the controls were relatively consistent, without age variation (Fig.6b). However, caffeine-fed rats exhibited a significantly higher E2 level after 2 weeks of caffeine exposure compared with the controls (P < 0.01 vs. CT). After 4 weeks of caffeine exposure, E2 levels in the caffeine-fed groups were profoundly decreased compared with those measured at 2 weeks, and even lower than for the controls. In addition, total T4 levels of serum samples obtained after 4 weeks of caffeine exposure only were measured, due to a shortage of 2-week serum samples (Fig.6c). Caffeine-fed groups showed significantly reduced levels of T4 compared with the controls (P < 0.05 vs. CT), no difference was observed between the caffeine-fed groups.
Discussion
This is the first study to clearly show the negative influence of caffeine on the in vivo osteogenic activity of growing bones and important endocrine factors such as IGF-1, E2 and T4, leading to retarded longitudinal bone growth during the pubertal growth spurt. Based on the current results, shortening of long bones and decreased BMD or BMC by caffeine exposure appears to be a result of decreased osteogenic activity, which may indicate decreased chondrocytic or osteoblastic activity and metabolism rather than mineral deficiency.
The available animal and human experimental data support a possible influence of caffeine on body size. The current results clearly demonstrated that caffeine reduced body mass gain, with its inhibitory effects noted even after the first week of caffeine exposure, and the difference augmented as the exposure time increased (Fig.1a). Like the negative influence of prenatal caffeine exposure on fetal growth observed in human and rodent models (Huang et al. 2012), prepubertal exposure also had an effect on body mass gain. In addition, caffeine is known to enhance the catabolism of fatty acids, speeding up the rate of resting metabolism (Dulloo & Miller, 1987). Therefore, the reduced mass gain may be attributed to both poor feeding and increased catabolism. Given that body mass and food intake influence the initiation of puberty (Kennedy & Mitra, 1963), the reduced body mass after caffeine exposure could contribute to the delayed pubertal growth.
Increased muscle mass and body fat are some of the major physical changes characterizing normal pubertal development. Because pubertal skeletal growth is also regulated by the mechanical loading imposed on the skeleton (Pietrobelli et al. 2002), the reduced body mass and LBM due to caffeine exposure may negatively affect calcium deposition in bone during pubertal growth spurts. Considering that puberty is the critical period for maximal calcium deposition in bone, caffeine may cause more adverse effects in this period than in adulthood when development has finished. In fact, caffeine consumption has been found to reduce BMD and to increase mineral loss in young or adult rats (Tsuang et al. 2006). Consistent with this, a negative influence of caffeine on BMC and BMD was also shown, and further that this is more detrimental at a younger age.
A major advantage of PET is its ability to quantify radiotracer accumulation. Na18F PET is often used to localize and characterize bone lesions (Grant et al. 2008) because of its high bone-to-background ratios within a short time frame. Until now, most human or animal studies of caffeine exposure have mainly compared bone mineral status with DXA, which reflects final bone status. Here, high-resolution Na18F PET was used for in vivo assessment of the osteogenic activity of rapidly growing bones, as increased 18F-fluoride ion deposition in bone can occur in areas of increased osteogenic activity during growth (Grant et al. 2008). As shown in Fig.2, caffeine exposure decreased Na18F uptake, particularly at the epiphyseal GP. Considering that Na18F is delivered to bone via the blood, and is preferentially retained at the site of newly mineralizing bone, caffeine may interfere with the blood flow and metabolic activity relevant to osteogenic activity. In addition, changes in metabolite availability due to the reduced blood supply to the epiphysis may disturb chondrogenesis during tissue maturation. Therefore, the decreased bone minerals and reduced bone formation in caffeine-fed animals is most likely attributable to reduced in vivo osteogenic activity.
Consistent with these observations, significant changes in histomorphometric parameters of the GP and cancellous bone were noted in the caffeine-fed groups. The change in GP closing rate was highly correlated with the size and volume of the HZ chondrocytes (Hunziker & Schenk, 1989; Roach et al. 2003). Likewise, significantly reduced GP closing rate was accompanied by a higher proportion of HZ in the caffeine-fed groups (Table2). As the postnatal growth spurt occurs between PD20 and 40 in male rats (Hansson et al. 1972), peak GP height, which represents the slowest rate at which GP closes, is attained at that time. In this study, histomorphometric analysis was performed after PD50, which may be a time of rapid closing rate of GP. Therefore, the slower GP closing rate in the caffeine-fed groups points to delayed bone growth rather than increased chondrocyte activity. Although the numbers of cells and columns in the HZ increased in the caffeine-fed groups, small and irregularly aligned chondrocytes were observed in specimens from the caffeine-fed groups, and were especially prominent in CF2 (Fig.4; Table2). The degree of impairment in chondrocyte hypertrophy and organization into columns could be correlated with the retarded bone elongation observed, because chondrocytes that are organized into columns are generally believed to represent the functional units for longitudinal bone growth (Hansson et al. 1972). In addition, relatively sparse columns in the PZ were noted in the caffeine-fed groups, in particular in CF2. Thus, caffeine may also inhibit chondrocyte proliferation and impair column formation in the PZ. Given that chondrocyte enlargement has been shown to be a much faster and more efficient means for rapidly extending the columnar units (Hunziker & Schenk, 1989; Roach et al. 2003), impaired chondrocyte hypertrophy seems to be the major cause of the shortening of long bones in caffeine-fed animals.
In contrast to the change pattern of the GP, the heights of primary and secondary Sn were decreased in the caffeine-fed groups (Table3). Because successful bone elongation was preceded by formation of primary and secondary Sn (Hunziker & Schenk, 1989), this may be a reflection of impaired osteoblast activity or mineralization. As expected from the few fairly thin spicules in the caffeine-fed groups, as shown in Fig.5, all parameters related to bone formation were profoundly decreased. In addition, rats continuously exposed to caffeine from in utero to young adulthood exhibited impaired osteoblast activity and reduced thickness of their femoral bones, which was not changed by a caffeine-free diet in young adulthood (Sasahara et al. 1994). This suggests that caffeine exposure during the prenatal or postnatal growth spurt could result in irreversible impairment of long bone growth. However, further study is needed to examine whether the negative impact of peripubertal caffeine on the long bones persists later in life.
Experimental in vivo studies have not found consistent effects on bone elongation in caffeine-treated males. Some studies have suggested that caffeine has a negative influence (Sasahara et al. 1994), but others have suggested no effect or even a positive influence on longitudinal growth (Burdan et al. 2000; Huang et al. 2002) depending on the stage when treatment was initiated, along with its duration and the dose. Longitudinal growth, as assessed by femoral, tibial and vertebral length, was dose-dependently reduced in caffeine-fed animals (Fig.3). This effect is analogous to the decreased body and femur lengths observed in fetal animals after prenatal exposure to caffeine (Huang et al. 2012; Tan et al. 2012). The current results clearly demonstrated that caffeine inhibits longitudinal bone growth in a dose-dependent manner in pubertal male rats.
Insulin-like growth factor-1 is one of the key endocrine factors regulating longitudinal bone growth during the fetal and pubertal growth spurt in male rats (Venken et al. 2007; Tan et al. 2012). As shown in Fig.6a, an increase in IGF-1 levels between 2 and 4 weeks into the experimental period (corresponding to PD35 and PD50, respectively) in the controls may have contributed to simultaneous longitudinal bone growth, whereas reduced levels of IGF-1 were observed at each time point in caffeine-fed groups as compared with the controls. In fact, prenatal caffeine exposure has been shown to decrease hepatic IGF-1 production in fetal rats (Tan et al. 2012). Although the appropriate level of IGF-1 for the growth spurt is not known, maintenance of certain levels of IGF-1 might be important for regulating the growth spurt. Therefore, caffeine exposure may impair long bone growth by interfering with the physiological changes associated with IGF-1 levels during the growth spurt.
In addition, maternal caffeine consumption has been shown to significantly decrease T4 levels in the male offspring of humans (Ramlau-Hansen et al. 2008) and rats (Dorostghoal et al. 2012). Likewise, pubertal caffeine exposure was shown to have similar effects on T4 levels (Fig.6c). In contrast, several studies with adult humans or animals showed increased serum T4 levels due to chronic caffeine consumption (Ramlau-Hansen et al. 2008; Sarobo et al. 2012), suggesting that different responses exist depending on age. It is obvious that caffeine consumption can interfere with serum T4 levels in immature male rats, contributing to impaired bone growth. In addition, decreases in IGF-1 and T4 may also contribute to changes in body composition in caffeine-fed animals (Table1) because both are important stimulators of skeletal muscle development in men (Venken et al. 2007).
Estrogen is another regulating factor for long bone growth during the pubertal growth spurt in humans and animals (Cutler, 1997), although appropriate E2 levels are not as clearly defined as levels of IGF-1 and T4. As shown in Fig.6b, the caffeine-fed groups showed decreased E2 levels compared with the controls after 4 weeks of exposure, whereas profoundly increased E2 levels were noted after 2 weeks of exposure. The influence of caffeine on serum levels of E2 may differ by age, and younger rats may be more sensitive to caffeine exposure. Because estrogen has a biphasic dose–response relationship for epiphyseal growth with maximal stimulation at low levels and a reduced effect at high levels (Cutler, 1997), the increased E2 levels after 2 weeks of exposure (corresponding to PD35) may interfere with long bone growth. These observations support the hypothesis that certain physiological levels of estrogen are needed to maintain a stimulatory effect on longitudinal growth in male rodents. So far, there have been no data on the interaction between key hormones regulating long bone growth and caffeine exposure during the pubertal growth spurt.
In conclusion, caffeine altered in vivo osteogenic activity, and delays peripubertal longitudinal bone growth and maturation. Given that osteogenic cells undergo dynamic changes in metabolic activity, and pubertal growth spurt is mainly stimulated by growth hormone/IGF-1 and sex steroids during pubertal development, caffeine may suppress ossification by interfering with both physiological changes in hormonal secretion and osteogenic activity during this critical period. Further study is needed to investigate the cellular/molecular mechanisms by which caffeine affects osteogenesis.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education and Science (NRF-2013R1A1A3006054, 2014R1A1A2053601). All authors state that they have no conflicts of interest.
Author contributions
Study design: J.S., Y.C., Y.-Y.C. and J.R. Study conduct: J.S., Y.C., J.K., A.-R.Y., J.-S.S., Y.-Y.C. and J.R. Data collection: J.S., Y.C., J.K., A.-R.Y. and J.-S.S. Data interpretation: J.S., Y.C. and J.R. Drafting manuscript: J.S., Y.C. and J.R. J.R. takes responsibility for the integrity of the data analysis.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
(A) Histological section of GP showing three zones and column. GP, growth plate; RZ, reserve zone; PZ, proliferative zone; HZ, hypertrophic zone. Yellow circle indicates column. (B) Representative section of cancellous bone showing primary and secondary Sn. Sn, spongiosa. (C) Measurement of trabecular bone formation parameters from secondary Sn. B.Ar, bone area; Tb.Wi, trabecular width; Tb.Sp, trabecular separation.
References
- Adams MC, Turkington TG, Wilson JM. A systematic review of the factors affecting accuracy of SUV measurements. Am J Roentgenol. 2010;195:310–320. doi: 10.2214/AJR.10.4923. , et al. ( [DOI] [PubMed] [Google Scholar]
- Brighton CT, Hunt RM. Histochemical localization of calcium in the fracture callus with potassium pyroantimonate. Possible role of chondrocyte mitochondrial calcium in callus calcification. J Bone Joint Surg Am. 1986;68:703–715. [PubMed] [Google Scholar]
- Burdan F, Madej B, Wojtowicz Z. The effects of short-time caffeine administration on skeleton development in Wistar rats. Folia Morphol (Warsz) 2000;59:91–95. , et al. ( [PubMed] [Google Scholar]
- Cutler GB., Jr The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61:141–144. [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorostghoal M, Erfani Majd N, Nooraei P. Maternal caffeine consumption has irreversible effects on reproductive parameters and fertility in male offspring rats. Clin Exp Reprod Med. 2012;39:144–152. doi: 10.5653/cerm.2012.39.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskocil M, Valouch P, Pazderka V. On vertebral body growth. Funct Dev Morphol. 1993;3:149–155. [PubMed] [Google Scholar]
- Dulloo AG, Miller DS. Reversal of obesity in the genetically obese fa/fa Zucker rat with an ephedrine/methylxanthines thermogenic mixture. J Nutr. 1987;117:383–389. doi: 10.1093/jn/117.2.383. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Evolution, development and timing of puberty. Trends Endocrinol Metab. 2006;17:7–12. doi: 10.1016/j.tem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grant FD, Fahey FH, Packard AB. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. , et al. ( [DOI] [PubMed] [Google Scholar]
- Hansson LI, Menander-Sellman K, Stenstrom A. Rate of normal longitudinal bone growth in the rat. Calcif Tissue Res. 1972;10:238–251. doi: 10.1007/BF02012553. , et al. ( [DOI] [PubMed] [Google Scholar]
- Heaney RP. Effects of caffeine on bone and the calcium economy. Food Chem Toxicol. 2002;40:1263–1270. doi: 10.1016/s0278-6915(02)00094-7. [DOI] [PubMed] [Google Scholar]
- Huang TH, Yang RS, Hsieh SS. Effects of caffeine and exercise on the development of bone: a densitometric and histomorphometric study in young Wistar rats. Bone. 2002;30:293–299. doi: 10.1016/s8756-3282(01)00659-7. , et al. ( [DOI] [PubMed] [Google Scholar]
- Huang J, Zhou S, Ping J. Role of p53-dependent placental apoptosis in the reproductive and developmental toxicities of caffeine in rodents. Clin Exp Pharmacol Physiol. 2012;39:357–363. doi: 10.1111/j.1440-1681.2012.05676.x. , et al. ( [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lison L. Alcian blue 8 G with chlorantine fast red 5 B: a technique for selective staining of mycopolysaccharides. Stain Technol. 1954;29:131–138. doi: 10.3109/10520295409115457. [DOI] [PubMed] [Google Scholar]
- Marty MS, Chapin RE, Parks LG. Development and maturation of the male reproductive system. Birth Defects Res B Dev Reprod Toxicol. 2003;68:125–136. doi: 10.1002/bdrb.10015. , et al. ( [DOI] [PubMed] [Google Scholar]
- Oddy WH, O'Sullivan TA. Energy drinks for children and adolescents. BMJ. 2009;339:b5268. doi: 10.1136/bmj.b5268. [DOI] [PubMed] [Google Scholar]
- Olchowik G, Chadaj-Polberg E, Tomaszewski M. The influence of caffeine on the biomechanical properties of bone tissue during pregnancy in a population of rats. Folia Histochem Cytobiol. 2011;49:504–511. doi: 10.5603/fhc.2011.0071. , et al. ( [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Faith MS, Wang J. Association of lean tissue and fat mass with bone mineral content in children and adolescents. Obes Res. 2002;10:56–60. doi: 10.1038/oby.2002.8. , et al. ( [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Bonde JP. Semen quality according to prenatal coffee and present caffeine exposure: two decades of follow-up of a pregnancy cohort. Hum Reprod. 2008;23:2799–2805. doi: 10.1093/humrep/den331. , et al. ( [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks–a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach HI, Mehta G, Oreffo RO. Temporal analysis of rat growth plates: cessation of growth with age despite presence of a physis. J Histochem Cytochem. 2003;51:373–383. doi: 10.1177/002215540305100312. , et al. ( [DOI] [PubMed] [Google Scholar]
- Sarobo C, Lacorte LM, Martins M. Chronic caffeine intake increases androgenic stimuli, epithelial cell proliferation and hyperplasia in rat ventral prostate. Int J Exp Pathol. 2012;93:429–437. doi: 10.1111/j.1365-2613.2012.00843.x. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahara H, Cheuk SL, Wink CS. Alteration of femoral structure in later life by chronically feeding caffeine during rapid growing period in newborn female rats. Toxicol Lett. 1994;73:55–64. doi: 10.1016/0378-4274(94)90188-0. , et al. ( [DOI] [PubMed] [Google Scholar]
- Schiepers C, Hoh CK. Positron emission tomography as a diagnostic tool in oncology. Eur Radiol. 1998;8:1481–1494. doi: 10.1007/s003300050579. [DOI] [PubMed] [Google Scholar]
- Seifert SM, Schaechter JL, Hershorin ER. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011;127:511–528. doi: 10.1542/peds.2009-3592. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Liu J, Deng Y. Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicol Lett. 2012;214:279–287. doi: 10.1016/j.toxlet.2012.09.007. , et al. ( [DOI] [PubMed] [Google Scholar]
- Tsuang YH, Sun JS, Chen LT. Direct effects of caffeine on osteoblastic cells metabolism: the possible causal effect of caffeine on the formation of osteoporosis. J Orthop Surg Res. 2006;1:7. doi: 10.1186/1749-799X-1-7. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K, Moverare-Skrtic S, Kopchick JJ. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J Bone Miner Res. 2007;22:72–82. doi: 10.1359/jbmr.060911. , et al. ( [DOI] [PubMed] [Google Scholar]
- Weinreb M, Rodan GA, Thompson DD. Depression of osteoblastic activity in immobilized limbs of suckling rats. J Bone Miner Res. 1991;6:725–731. doi: 10.1002/jbmr.5650060710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Histological section of GP showing three zones and column. GP, growth plate; RZ, reserve zone; PZ, proliferative zone; HZ, hypertrophic zone. Yellow circle indicates column. (B) Representative section of cancellous bone showing primary and secondary Sn. Sn, spongiosa. (C) Measurement of trabecular bone formation parameters from secondary Sn. B.Ar, bone area; Tb.Wi, trabecular width; Tb.Sp, trabecular separation.