Abstract
Anatomical studies of the cranium of crocodilians motivated by an interest in its function in feeding largely focused on bite force, the jaw apparatus and associated muscles innervated by the trigeminal nerve. However, the ossified and cartilaginous elements of the hyoid and the associated hyolingual muscles, innervated by the facial, hypoglossal and glossopharyngeal nerves, received much less attention. Crocodilians are known to retain what are ancestrally the ‘Rhythmic Hyobranchial Behaviors’ such as buccal oscillation, but show diminished freedom and movement for the hyobranchial apparatus and the tongue in food transport and manipulation. Feeding among crocodilians, generally on larger prey items than other reptilian outgroups, involves passive transport of the food within the mouth. The tongue in extant crocodilians is firmly attached to the buccal floor and shows little movement during feeding. Here, we present a detailed anatomical description of the myology of the hyolingual apparatus of Alligator mississippiensis, utilizing contrast-enhanced micro-computed tomography and dissection. We construct the first three-dimensional (3D) description of hyolingual myology in Alligator mississippiensis and discuss the detailed implications of these data for our understanding of hyolingual muscle homology across Reptilia. These anatomical data and an evaluation of the fossil record of hyoid structures also shed light on the evolution of feeding in Reptilia. Simplification of the hyoid occurs early in the evolution of archosaurs. A hyoid with only one pair of ceratobranchials and a weakly ossified or cartilaginous midline basihyal is ancestral to Archosauriformes. The comparison with non-archosaurian reptilian outgroup demonstrates that loss of the second set of ceratobranchials as well as reduced ossification in basihyal occurred prior to the origin of crown-clade archosaurs, crocodilians and birds. Early modification in feeding ecology appears to characterize the early evolution of the clade. Hyoid simplification has been linked to ingestion of large prey items, and this shift in hyoid-related feeding ecology may occur in early archosauriform evolution. A second transformation in hyoid morphology occurs within the crocodilian stem lineage after the split from birds. In Crocodyliformes, deflections in the ceratobrachials become more pronounced. The morphology of the hyoid in Archosauriformes indicates that aspects of the hyolingual apparatus in extant crocodilians are derived, including a strong deflection near the midpoint of the ceratobranchials, and their condition should not be treated as ancestral for Archosauria.
Keywords: Alligator, computed tomography, feeding, fossil, hyolingual apparatus, muscles, tongue
Introduction
In comparison with other non-avian reptiles, the hyoid apparatus and tongue in crocodilians is significantly simplified morphologically (Sewertzoff, 1929; Schumacher, 1973; Schwenk, 1986, 2000). Relatively few authors specifically focused on the hyoid apparatus or the associated hyobranchial muscles, compared with the prolific work on the jaw apparatus and associated trigeminal muscles in crocodilians (Sewertzoff, 1929; Gnanamuthu, 1937; Sondhi, 1958; Schumacher, 1973; Cong et al. 1998; Holliday et al. 2013). However, the hyolingual system for reptiles in general, which supports the posterior portion of the buccal floor with the larynx embedded, is not only importantly involved in drinking, food handling and transport, but also in respiration (e.g. gular pumping and panting) and in vocalization (Gnanamuthu, 1937; Britton, 2001; Holliday, 2006; Riede et al. 2011, 2015).
Although the cartilaginous and bony hyoid elements are simplified in crocodilians compared with their relatively complex counterparts of other reptiles (e.g. lepidosaurs, turtles; Schumacher, 1973; Schwenk, 2000; Figs1 and 2), the hyobranchial muscles show a similar pattern across all non-avian reptiles in general (Sewertzoff, 1929; Edgeworth, 1935; Gnanamuthu, 1937; Diogo & Abdala, 2010). Those muscles associated with the hyoid usually cross the ventral side of the head and neck, and are complex in both arrangement and function (Tanner & Avery, 1982; Cong et al. 1998). In Alligator, some of these hyoid muscles (e.g. M. branchiomandibularis visceralis or M. branchiohyoideus) have remarkably deep insertions in the gular region, and many of those attachments are covered by other cranial muscles and soft tissues. It is a challenge to reveal the anatomical detail of these relatively fine muscles associated with the hyolingual apparatus through either dissection photographs or reconstructions through illustrations. Previous authors who addressed cranial morphology in Alligator only depicted the hyoid muscles in ventral view (Sondhi, 1958; Schumacher, 1973; Cong et al. 1998; Bona & Desojo, 2011). This approach limits adequate and accurate data on either the insertion or the origin of these complex muscles. In addition, drawings can be difficult to interpret and inhibit comparisons between homologous structures even among species of extant crocodilians (Lubosch, 1933; Sondhi, 1958; Bona & Desojo, 2011).
Figure 1.
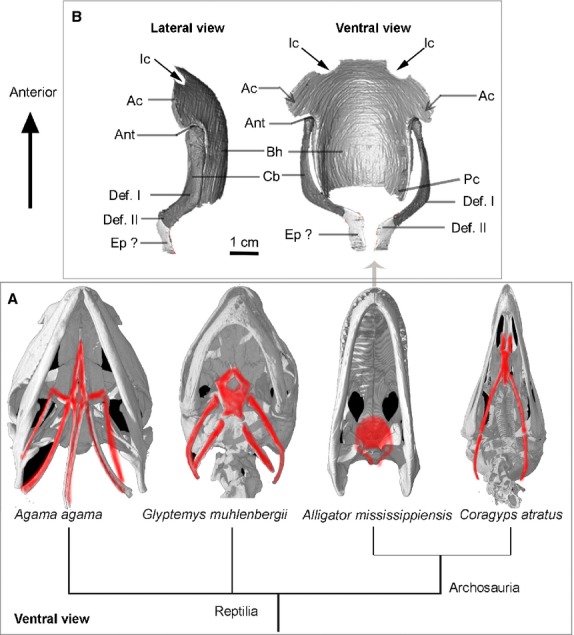
The bony hyoids in reptilians (A) with a detailed reconstruction of the hyoid in Alligator mississippiensis (B) based on X-ray CT data from this project. Anatomical abbreviations: Ac, anterior corner; Ant, anterolateral notch; Bh, basihyal; Cb, ceratobranchial; Def. I, deflection I; Def. II, deflection II; Ep?, epibranchial; Ic, incisure; pc, posterior corner (see main text); ventral views of the reptilian skulls are all adopted from ‘http://digimorph.org.’ Agama agama (FMNH 47531), Glyptemys muhlenbergii (UF 85274), Alligator mississippiensis (TMM M-983), Coragyps atratus (TMM Collections uncataloged).
Figure 2.
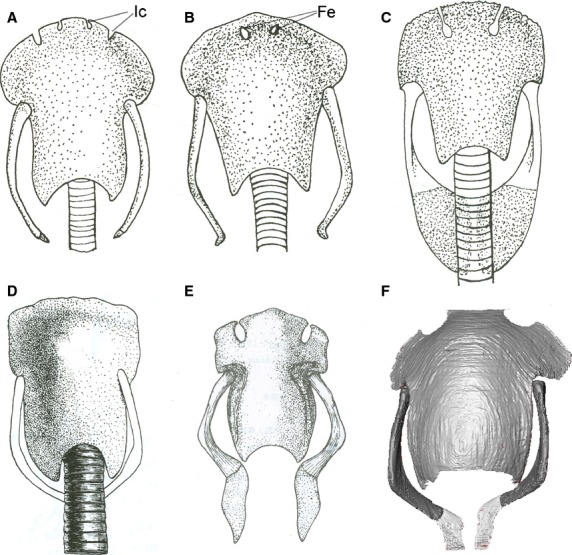
Ventral view of the hyoid apparatus in crocodilians. (A–E) After Corsy (1933); Schumacher (1973) and Cong et al. (1998); (F) from this project (specimen no. 3). (A and B) Crocodylus porosus (juvenile); (C) Caiman crocodilus; (D) Crocodylus cataphractus; (E) Alligator sinensis; (F) Alligator mississippiensis. Anatomical abbreviations: Fe, fenestra; Ic, incisure.
Previous work by Edgeworth (1935) provided a comprehensive review of the vertebrate cranial muscles and established proposed transformational homologies from fishes to mammals. The large scope of that classic work limited his detailed coverage of any one group. Most other early work on hyolingual structures included notes on the crocodilian hyoid and associated muscles with descriptions of other non-avian reptilians (e.g. lizards and snakes) without comparing birds or addressing homology issues between lepidosaurs and archosaurs (Gnanamuthu, 1937; Sondhi, 1958; Tanner & Avery, 1982). Detailed descriptions of jaw and hyolingual muscles from a variety of reptilians, including Alligator mississippiensis and turtles, was provided by Schumacher (1973). However, several labels for illustrations of the hyolingual muscles in Alligator mississippiensis are identified as incorrect here (e.g. M. coracohyoideus; fig. 56; Schumacher, 1973). Two studies treated the hyolingual apparatus in other crocodilians (Gavialis gangeticus and Caiman latirostris; Sondhi, 1958; Bona & Desojo, 2011). Their work is reviewed in a comparative framework. Laryngeal myology in Alligator has recently been described (Riede et al. 2015) and is not treated here.
All previous descriptions of hyolingual anatomy relied on traditional dissection and explanatory two-dimensional (2D) illustrations. However, the advent of X-ray computed tomography (CT) scans allows for the acquisition of detailed and accurate presentation of three-dimensional (3D) anatomical data that can be used in concert with data from dissection (Endo & Frey, 2008). Recently, the introduction of the use of contrasting agents in high-resolution micro-CT scans allowed for significant soft tissue definition (muscles, cartilages and major nerves) largely unavailable in CT scans of untreated tissues (Metscher, 2009; Holliday et al. 2013). In this study, we utilized both dissection and contrast-enhanced high-resolution X-ray CT to reveal the soft- and hard-tissue anatomy around the hyoid apparatus of Alligator mississippiensis in a few specimens ranging from post-hatching to juvenile to adult. The first 3D reconstruction of the hyoid apparatus and associated muscles was made possible by these new CT datasets (Figs3 and 4), and the detailed morphology of hyolingual elements and muscle attachments are described and illustrated (Figs8). These new data also allow for a comparative assessment of the anatomy of this region in extant crocodilians and identification of shared features within this clade.
Figure 3.
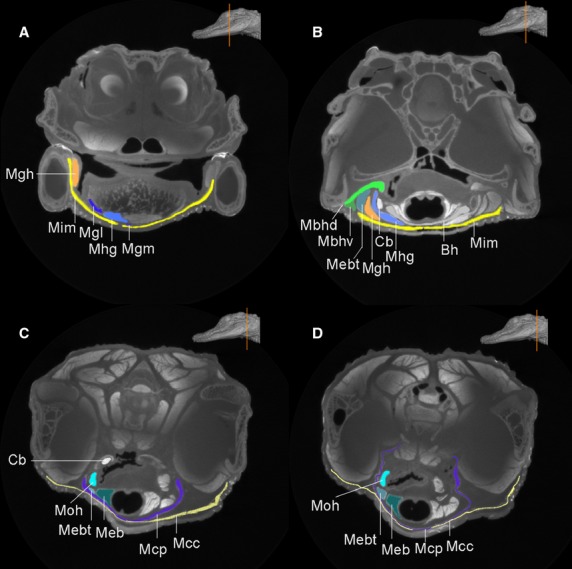
Select CT coronal sections from cranial to caudal (A–D) region in the head of Alligator mississippiensis. The inset (orange line) shows the locations of these coronal sections. The hyoid and the associated muscles are highlighted in different colors. Anatomical abbreviations: Bh, basihyal; Cb, ceratobranchial; Mbhd and Mbhv, M. branchiohyoideus (dorsalis and ventralis); Mcc, M. constrictor colli (pars anterior); Mcp, M. constrictor colli profundus; Meb, M. episternobranchialis; Mebt, M. episternobranchiotendineus; Mgh, M. geniohyoideus; Mgl and Mgm, M. genioglossus lateralis and medialis; Mhg, M. hyoglossus; Mim, M. intermanibularis; Moh, M. omohyoideus.
Figure 4.
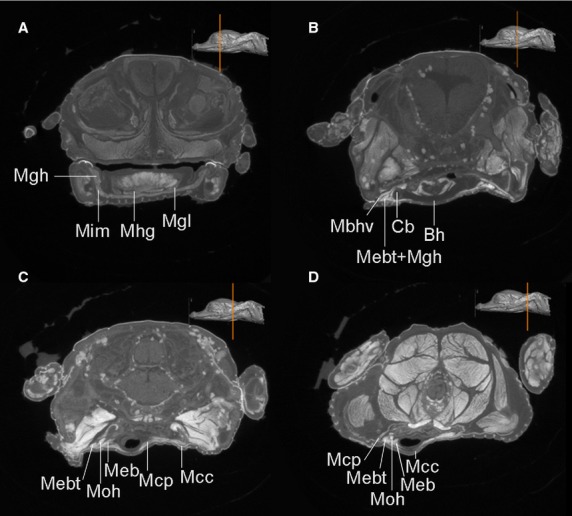
Select CT coronal sections from cranial to caudal (A–D) region of heads of Alligator mississippiensis. Similar muscles are discerned from this post-hatching specimen (no.1) as well. The hyoid and the associated muscles are labeled with the same anatomical abbreviations as in Fig.3.
Figure 8.
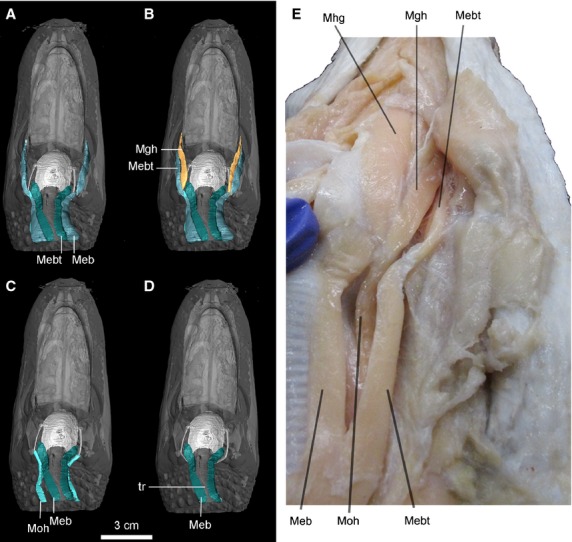
Ventral view of the highlighted hypobranchial longitudinal muscles viewed in CT reconstruction (A–D, specimen no. 2) and dissection (specimen, no. 6) for Alligator mississippiensis. Anatomical abbreviations: Meb, M. episternobranchialis; Mebt, M. episternobranchiotendineus; Mgh, M. geniohyoideus; Mhg, M. hyoglossus; Moh, M. omohyoideus; tr, trachea.
Materials and methods
Six specimens of Alligator mississippiensis were obtained from the Rockefeller Wildlife Refuge (Grand Chenier, Louisiana, USA), University of California, Irvine, USA, and University of Utah, Salt Lake City, USA (see Table1 for specimens list). These specimens were formalin-fixed prior to the study, and all specimens were prepared at the Texas Natural Historical Collection (TNHC). One adult and two juvenile specimens were dissected and photographed (Table1: no. 4, no. 5 and no. 6). Three specimens were prepared for contrast-enhanced CT scans, including the two heads from juveniles and the tongue from the adult. The two smaller juvenile heads (Table1: specimen no. 1 and no. 2; Figs3 and 4) were stained using I2KI solution (iodine and potassium iodine dissolved in aqueous formalin) using a similar approach to Jeffery et al. (2011). The isolated adult tongue (Table1: specimen no. 3) was dehydrated using ethanol (> 99.8%) for 8 days, and thereafter was stained used an elemental iodine solution (I2E, metal iodine dissolved in pure ethanol; Metscher, 2009). Both of these contrast-enhancing approaches provided adequate distinction between different tissues, such as bone, muscular fibers, connective tissues and even major nerve tissues (e.g. trigeminal ganglia) in the CT images (Figs3 and 4). The adoption of a low iodine concentration (via I2E) in the staining of that large, adult specimen was found to be superior to the I2KI staining approach in the rendering of bone during post-processing of the CT dataset. Even after staining, bony and cartilaginous parts still show distinctly higher grayscale values than the soft tissues in the CT images if the low concentration of I2E staining approach is used.
Table 1.
Information on processed Alligator specimens for staining and dissection
| Alligator mississippiensis (no.) | Specimen origin | Approximate head length | Age | Staining solution (W/V) | Incubation time | |
|---|---|---|---|---|---|---|
| Scanned specimen | No. 1 | TNHC uncataloged | 2 cm | Post hatching | 7% I2KI | over 3 weeks |
| No. 2 | TNHC uncataloged | 8 cm | Juvenile | 11% I2KI | 3 days | |
| No. 3 | TNHC uncataloged | 40 cm | Adult | 1% I2E | 10 days | |
| Dissected specimen | No. 4 | TNHC uncataloged | 20 cm | Adult | ||
| No. 5 | TNHC uncataloged | 10 cm | Juvenile | |||
| No. 6 | TNHC uncataloged | 10 cm | Juvenile | |||
All the CT scans were acquired at the University of Texas at Austin High-Resolution X-ray Computed Tomography Facility (UTCT). The head from the recently hatched juvenile specimen was able to be scanned on an Xradia MicroXCT scanner because of its small size (Table1: specimen no. 1). The other two specimens (no. 2 and no. 3) were scanned in the high-resolution subsystem. Scanning parameters are provided in Table2. Selected coronal sections (cranial to caudal) of the CT images are shown in Figs3 and 4 (specimens no. 1 and no. 2) with the target hyoid structures colored and labeled in Fig.3. Two CT datasets (for specimens no. 2 and no. 3) were used in the digital reconstruction of the major hyoid muscles, hyoid skeletal and cartilages. These were imported as TIFF files into Avizo 6.1 (FEI FEI Visualization Sciences Group) for segmentation. One of these specimens was used to generate a complete visualization of the hyoid apparatus and muscular tissues (specimen no. 2). The scan of the lingual specimen (no. 3; Figs1 and 2) was primarily used to illustrate the detailed anatomy of the bony hyoid, which is poorly ossified in smaller, juvenile specimens. Due to weak ossification of the hyoid elements in crocodilians generally, use of museum specimens to assess interspecific or intraspecific variation in hyoid skeletal materials was not possible. But, our new CT data for both juvenile and adult specimens do show slight differences in hyoid morphology.
Table 2.
Scanning parameters used for contrast-enhanced CT
| Specimen scanned | Images (tiff) | Voltage (kV) | Current (mA) | Slice thickness (mm) | Inter-slice spacing (mm) | Field of reconstruction (mm) | No. of slices |
|---|---|---|---|---|---|---|---|
| No. 2 (head) | 1024 × 1024 16-bit |
150 | 0.4 | 0.08638 | 0.08638 | 82 | 1538 |
| No. 1 (head-shoulder) | 1024 × 1024 16-bit |
90 | 0.11 | 0.03597 (voxel size) | 1390 | ||
| No. 3 (tongue) | 1024 × 1024 16-bit |
150 | 0.13 | 0.126 (voxel size) | 1732 |
Terminology and novelty
Previous anatomical descriptions of the hyolingual anatomy in Alligator mississippiensis are limited, and homology has been poorly understood because different terminology was used to describe these muscles in Alligator and other crocodilians, obscuring their homology across Reptilia (Edgeworth, 1935; Schumacher, 1973). While the general morphology of the hyoid and the associated muscles are known in Alligator mississippiensis, detailed attachments of these muscles on the hyoid elements have not been described or clearly visualized. By contrast, we describe and precisely visualize these attachments in 3D reconstructions from the CT data (Figs3 and 4), addressing outstanding homology issues. These new data clarify muscle homology among Alligator, lepidosaurs and birds, and provide a comparative basis and context to investigate hyoid variation and evolution in Reptilia.
Different myological nomenclature and grouping systems (e.g. based on origin, position or innervation) have been used in the description of hyoid muscles by previous authors (Gnanamuthu, 1937; Sondhi, 1958; Schumacher, 1973; Cong et al. 1998; Cleuren & De Vree, 2000). Because of a lack of a standardized terminology across crocodilians, birds and other reptiles (Diogo & Abdala, 2010), assignment of different names to the homologous muscles, and assignment of the same name for non-homologous muscles, has occurred. The hyolingual muscle terminology adopted here primarily follows Diogo & Abdala (2010) to facilitate a larger scope of comparison across tetrapods and to indicate proposed homology. However, we do also provide the names that have been used only within crocodilians (Schumacher, 1973; Cong et al. 1998) at first use, and treat the three groups of hyoid muscles identified by Sondhi (1958) and Schumacher (1973) in order to facilitate access to this literature (Table3).
Table 3.
Described hyoid muscles with their synonyms in the previous studies and proposed function
| Group | Muscle | Origin | Insertion | Proposed function | Estimated Directions of lingual motion involved |
|---|---|---|---|---|---|
| A | M. intermandibularis | Medial mandible | Middle raphe | Raising basihyal | Dorsal |
| M. constrictor colli | Medial mandible | Middle raphe | Raising pharynx, throat region | Dorsal | |
| M. constrictor profundus | Second cervical rib | Middle raphe | Throat contraction and raising hyoid | Dorsal | |
| B | M. genioglossus | Mandibular symphysis | Tongue | Protract the tongue cranial-dorsally | Dorsorostral |
| M. hyoglossus | Ceratobranchial shaft | Middle of the tongue | Lingual motion | Caudolateral | |
| M. branchiohyoideus | Later edge of ceratobranchial | Mandible | Hyoid suspension | Dorsoventral | |
| C | M. episternobranchiotendineus | Episternum | Mandible | Protract and retract hyoid | Rostra-caudal |
| M. episternobranchialis | Episternum | Basihyal | Retract hyoid | Caudal | |
| M. geniohyoideus | Ceratobranchial | Splenial bone | Cranial dorsal move hyoid | Rostrodorsal | |
| M. omohyoideus | Coracoid | Ceratobranchial | Retract hyoid | Caudal |
Simplified insertion and origin are indicated, and see details in the main text.Synonyms: M. intermandibularis (Edgeworth, 1935; Schumacher, 1973; Bona & Desojo, 2011)–M. mylohyoideus anterior principalis (Sondhi, 1958); M. constrictor colli (Edgeworth, 1935; Schumacher, 1973; Bona & Desojo, 2011)–M. constrictor pharyngis (Sondhi, 1958); M. constrictor profundus (Edgeworth, 1935; Schumacher, 1973)–M. constrictor pharyngis (Sondhi, 1958); M. branchiohyoideus (Diogo & Abdala, 2010)–M. branchiomandibularis viseralis (Schumacher, 1973; Bona & Desojo, 2011)–M. mandibulohyoideus (Sondhi, 1958)–M. branchiomandibularis (Edgeworth, 1935); M. episternobranchiotendineus (Schumacher, 1973; Bona & Desojo, 2011) and M. episternobranchialis (Schumacher, 1973)–M. sternohyoideus (Edgeworth, 1935; Sondhi, 1958); M. geniohyoideus (Edgeworth, 1935; Sondhi, 1958)–M. branchiomandibularis spinalis (Schumacher, 1973; Bona & Desojo, 2011); M. omohyoideus (Edgeworth, 1935; Sondhi, 1958)–M. coracohyoideus (Schumacher, 1973; Bona & Desojo, 2011).
Results
Bone and cartilage hyoid elements in Alligator mississippiensis
The hyoid apparatus (Figs1 and 2) of Alligator mississippiensis is composed of a midline basihyal (Bh, Corpus hyoidei, sensu Schumacher, 1973) and paired ceratobranchials (CB I, Cornu branchiale I, sensu Schumacher, 1973). Collectively, the hyoid elements (basihyal and ceratobranchials) were also called the hyobranchial apparatus, referencing their derivation from the hyoid and the branchial arches (McClearn & Noden, 1988). Although both basihyal and paired ceratobranchials are mostly cartilaginous, their outlines are discernible in CT images (Figs3 and 4: Bh and Cb) after the staining; the exterior surfaces of these cartilages and bones were brighter in grayscales than the interior portion, and were also distinct from the surrounding muscles. This is because the contrast agent (i.e. iodine molecules) tends to accumulate on the boundary surface, and accumulate within the muscular tissues. As shown in the reconstruction (Fig.1), the basihyal is a shovel-shaped cartilage, rounded in the front edge and convex ventrally. The incisures (or notch-like structure) are present in the anterior edge of the basihyal in adult specimens (Fig.1: Ic), but not apparent in juveniles. The notches were also reported to be enclosed as a fenestra and covered (Fig.2: Fe) by membranes in some crocodilians (Fig.2; Schumacher, 1973). No function for these notches has been identified experimentally, and they appear to be filled with thinner connective tissue. No nerves or vascular structures lie in them. They may be related to the mode of attachment of the fleshy tongue. The ceratobranchials are a pair of rod-like bones that are comparatively robust and commonly ossified in adults (Figs1 and 2: Cb). The proximal head of the ceratobranchial contacts the anterolateral notch (Fig.1: Ant) of the basihyal, and distal terminus of ceratobranchials is slightly expanded. The free end of the ceratobranchial, which is especially weakly ossified, was previously interpreted as possibly representing a cartilaginous epibranchial (Schumacher, 1973; Fig.1: Ep?), an osseous element in birds that has otherwise been considered an avian neomorph. However, the structure in Alligator mississippiensis and other crocodilians differs from those in birds in that it is not an independent element from the ceratobranchial. This element in crocodilians is absent in early ontogeny, and no separate ossification center was found for this element (Ep?) during later development (Schumacher, 1973). Ossification of these hyoid elements was reported to be generally greater in older individuals (Cleuren & De Vree, 2000), which was confirmed here through the comparison of the adult and juvenile specimens. In comparison with the juvenile, the hyoid skeleton of the adult has greater ossification overall. For example, ossification occurring at the anterolateral corner (Fig.1: Ac) of the basihyal in the adults is not present in juvenile (Schumacher, 1973; Cong et al. 1998), and no ossification of the epibranchial was observed at any stage. Ceratobranchials are better ossified than the basihyal in both juveniles and adults. Within an individual, ossification varied along the ceratobranchials, with the distal ends much less ossified than the proximal and medial portions in juveniles.
There is a notable proximal dorsomedial deflection in the anterior two-thirds of the ceratobranchial (Fig.1: here abbreviated ‘Def. I’). A second distal deflection (Fig.1: here abbreviated ‘Def. II’) is ventromedial and conspicuous in the adult but not well developed in the juvenile Alligator mississippiensis. Both of these deflections are linked to muscular attachments on the ceratobranchial described in the following section on myology.
The midline element (basihyal), the first ceratobranchial (CB I), and cartilaginous end (epibranchial?) have been homologized across reptilians based on their similar topographic position and generally similar morphology. The second paired ceratobranchials in Lepidosauria (CB II, or Cornu branchiale II, sensu Schumacher, 1973) is absent as a separate element in both crocodilians and birds, so homology (for CB II) across crocodilians, birds and other reptiles is unknown. The posterior corner (Fig.1: Pc) of the basihyal in crocodilians might represent the homologous portion of the CB II in other reptiles (Fürbringer, 1922; Schumacher, 1973).
Myology
Group A. Muscles of the buccal floor and neck region not connected with the hyoid. Muscles in ‘Group A’ have also been called ‘muscles of the mandibular segment’ (Kesteven, 1944) based on their derivations, or the ‘transverse muscles’ based on their general similar orientation of fibers (Gnanamuthu, 1937)
M. intermandibularis – M. intermandibularis is the most superficial muscle layer in the ventral head region of Alligator mississippiensis. This thin sheet-like muscle is broad and flat. It attaches to the mandibular rami and is oriented perpendicular to the cranial midline (Fig.5: Mim). It does not extend forward to cover the most anterior portion of the buccal floor. The muscle arises from the medial surface of the mandible, mostly on the dorsal splenial (Fig.3). Although this muscle is usually described along with the jaw musculature, functionally it acts on the hyoid apparatus when contracted by elevating the buccal floor at the base of the tongue (Holliday, 2006). This muscle also was called M. mylohyoideus anterior (Edgeworth, 1935; Gnanamuthu, 1937; Sondhi, 1958) or M. constrictor I ventralis (M.C1v; Kesteven, 1944), and was consistently described in other crocodilian species with similar attachment and morphology (Sondhi, 1958; Cong et al. 1998; Bona & Desojo, 2011). Because there is no attachment of this muscle with the hyoid apparatus, we prefer the name M. intermandibularis rather than M. mylohyoideus anterior. In addition to indicating the attachment, the name M. intermandibularis also better reflects the position of the muscle. The mandibular ramus of the trigeminal nerve (CN V) supplies this muscle (Sondhi, 1958; Cong et al. 1998).
Figure 5.
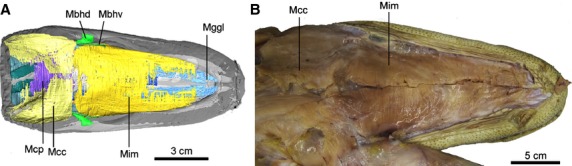
Ventral view showing the superficial layered muscles of head region in Alligator mississippiensis. (A) CT reconstruction of the specimen (no. 2); (B) dissection (no. 4). Anatomical abbreviations: Mbhd and Mbhv, M. branchiohyoideus (dorsalis and ventralis); Mcc, M. constrictor colli (pars anterior); Mcp, M. constrictor colli profundus; Mggl, M. genioglossus; Mim, M. intermanibularis.
M. constrictor colli (pars anterior) – This muscle is the superficial layer underlying the neck region, posterior to the M. intermandibularis (Figs6: Mcc). The muscle arises from a superficial tendinous aponeurosis of the M. pterygoideus posterior (Pterygoideus-tendon aponeuroses I; Schumacher, 1973; Cong et al. 1998). Near the posterior M. intermandibularis, the boundary between these two muscles can only be approximately detected by the small triangular-shaped gap (Trigonum intermandibulare posterius; Schumacher, 1973; Fig.6: Tr-g) on the medial surface of the mandible. Fibers of the M. constrictor colli are rather loosely attached to the ventral cervical fascia for insertions (Fig.3C,D; Schumacher, 1973). A ventral branch of the facial nerve (CN VII) supplies the muscle (Sondhi, 1958; Cong et al. 1998).
Figure 6.
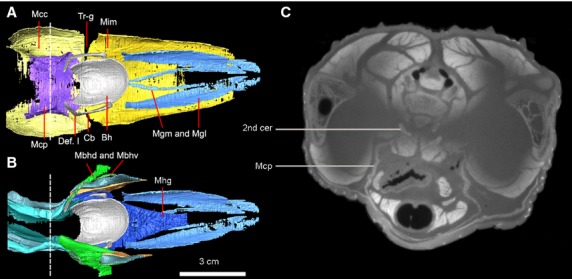
Dorsal view of the 3D reconstruction of the hyoid apparatus with the superficial layered (A) and deep-layered muscles (B) in Alligator mississippiensis (specimen no. 2). To better distinguish the two portions of M. branchiohyoideus, only partial of the M. branchiohyoideus (dorsalis) is visualized in the left side, in comparison with the whole set of the muscle in the right side of the head in (B). (C) The coronal section from CT image to indicate the attachment of M. constrictor colli profundus associated with the second cervical rib. The position of this slice is indicated as a dashed line in (A) and (B). Anatomical abbreviations: 2nd cer, second cervical rib; Bh, basihyal; Cb, ceratobranchial; Def. I, deflection I; Mbhd and Mbhv, M. branchiohyoideus (dorsalis and ventralis); Mcc, M. constrictor colli (pars anterior); Mcp, M. constrictor colli profundus; Mgl and Mgm, M. genioglossus lateralis and medialis; Mhg, M. hyoglossus; Mim, M. intermanibularis; Tr-g, triangular gap.
M. constrictor colli profundus – This thin muscle is deep to the M. constrictor colli and restricted to the gular region (Figs7: Mcp). The muscle arises from a thin, tendinous aponeurosis on the lateral surface of the second cervical rib (Figs3D and 6C). Its two lateral heads pass medially to insert on each other without forming a midline raphe. Medially, the M. constrictor colli and M. constrictor colli profundus are extremely thin and difficult to separate from each other in the gular region. The coalescence of these two muscles on the midline was noticed by Schumacher (1973), and is particularly notable as absent in juveniles (Fig.3C,D, Mcp), in which the two heads do not contact on the midline. We further confirm the status of the M. constrictor colli profundus as an independent muscular layer from the M. constrictor colli. However, mixing of fibers occurs more or less at the gular midline with those of the M. constrictor colli (Figs3C,D and 4C,D). The twisted shape of the dorsolateral edge of the muscular sling (Fig.3C,D) indicates a loose connection with the cervicals. The esophagus, trachea, hyoid plate (basihyal) and the hypobranchial longitudinal muscles all are wrapped inside the muscular sling formed by the M. constrictor colli profundus within the gular region (Figs3C,D and 7). Potential function of this muscle involves the constriction of the pharyngeal region and, therefore, it has also been called M. constrictor pharyngis (Gnanamuthu, 1937). A dorsal branch of the facial nerve (N VII) innervates this muscle (Sondhi, 1958; Cong et al. 1998).
Figure 7.
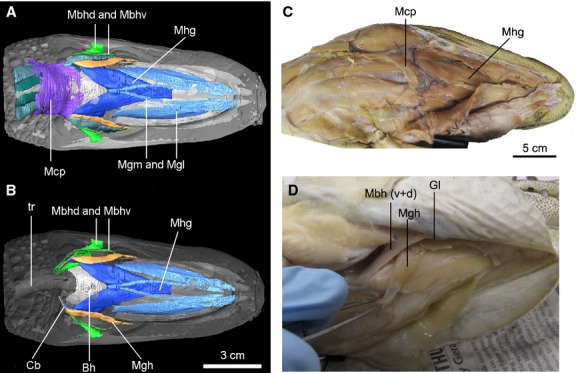
Ventral view of the deep layered muscles with CT reconstruction (A, B: specimen no. 2) and viewed in dissection (C, D; specimen no. 4 and no. 5), with emphasis on the glossopharyngeal muscles and tongue muscles in Alligator mississippiensis. Anatomical abbreviations: Bh, basihyal; Cb, ceratobranchial; Gl, mandibular gland; Mbh (v+d), M. branchiohyoideus (dorsalis and ventralis); Mcp, M. constrictor colli profundus; Mgh, M. geniohyoideus; Mgl and Mgm, M. genioglossus lateralis and medialis; Mhg, M. hyoglossus; tr, trachea.
Group B. Muscles of the deep layers of the buccal floor that are connected with the hyoid
Glossopharyngeal muscles
M. branchiohyoideus (M. branchiomandibularis visceralis, sensu Schumacher, 1973) – This muscle was called M. mandibulo-hyoideus in Gaviails (Sondhi, 1958). The same muscle had also been named as the M. branchiomandibularis visceralis in Alligator (Nishi, 1967), a name frequently used by subsequent authors (Schumacher, 1973; Cong et al. 1998). Here, we suggest using M. branchiohyoideus instead of M. branchiomandibularis visceralis to indicate potential homology across reptiles and correctly reference its branchial origins (Diogo & Abdala, 2010).
This muscle was identified as having only one part (Schumacher, 1973), but we observed two distinct parts, named here the pars dorsalis (deep) and ventralis (shallow) in Alligator mississippiensis. This muscle also was described as having two parts in Alligator sinensis (Cong et al. 1998), as again with deeper and shallower parts. Only small portions of each (Figs7: Mbhd and Mbhv) are visible ventrally; they are exposed between the M. intermandibularis and the M. constrictor colli. Tracing the extension of the muscle through a series of coronal sections in the CT images (Fig.3B), the muscle clearly extends dorsally and posteriorly along the primary axis of the ceratobranchial. The two parts both have an origin on the ceratobranchial and an insertion on the mandible.
The pars dorsalis of the M. branchiohyoideus is broad and extensive compared with the pars ventralis (Fig.6). The pars dorsalis originates widely on the distal ceratobranchial, and the connective tissue between the ceratobranchial and the dorsolateral wall of the esophagus (Fig.3B); this configuration makes it an important muscle for hyoid suspension. Anteriorly, its fibers run lateral to the pars ventralis and insert on the ventral surface of the mandible (Fig.7). The pars ventralis is rather slim and thin (Fig.5: Mbhv). Posteriorly, the muscular fibers originate from the distolateral ceratobranchial, running dorsally to the M. omohyoideus. Anteriorly, the muscle extends laterally to the M. episternobranchiotendineus and penetrates between the fibers of M. intermandibularis and M. constrictor colli, ultimately inserting on the mandible medially, close to the mandibular (or gular) gland (Figs6 and 7).
The dorsal attachment of the M. branchiohyoideus (both portions) on the hyoid is associated with the distal deflection (Def. II) of the ceratobranchial. The attachment site is distal to the deflection point. The muscle fibers have extensive contact with this distal area of the ceratobranchial, forming a half-sheath on the shaft (Fig.6). In addition to linking the hyoid with the mandible, this muscle also suspends the hyoid by connecting the ceratobranchial ramus to the lateral surface of the esophagus. This observation also is supported by the attachment of this muscle to the esophageal wall (Lubosch, 1933). Interestingly, it is the only muscle that is embryonically derived from branchial arches but innervated by the glossopharyngeal nerve (IX; Cong et al. 1998).
Tongue muscles
M. hyoglossus – This muscle comprises the major muscular portion of the tongue (Figs6 and 7: Mhg). It originates close to the midsection of the ceratobranchial ramus. The muscular bundles wrap around the dorsolateral and ventral sides of the ceratobranchial (Fig.3B: Mhg). The lateral portion of the muscle also arises from the lateral edge of the ventral basihyal. Anteriorly, muscular fibers from the left and right sides interweave with each other (Figs3A and 6). Dorsally, the muscle inserts over a broad area of the fatty pad of the tongue. The basal portion of this muscle, close to the junction of basihyal and ceratobranchial, was given a distinct name in Gavialis, M. ceratohyoideus (Gnanamuthu, 1937; Sondhi, 1958). The muscle was considered to be innervated by the hypoglossal nerve (N XII; Sondhi, 1958; Tanner & Avery, 1982). Given that this portion is not visibly separate from the main part of the muscle in Alligator mississippiensis, we do not adopt the name M. ceratohyoideus.
M. genioglossus (pars medialis and lateralis) – This muscle is comprised of two bundles, connecting the tongue to the mandibular symphysis. Anteriorly, the muscle arises from the mandibular symphysis via connective tissue. The pars lateralis and medialis (Figs6 and 7: Mgl and Mgm) are united at their origins on the mandible and then increasingly diverge toward their insertions. M. genioglossus pars lateralis inserts on the basal portion of the tongue, in contact with the lateral side of the M. hyoglossus. Pars medialis of the M. genioglossus is quite slim and is significantly narrower than the pars lateralis (Fig.6). Both the hyoglossus and genioglossus muscles are innervated by the lingual braches of the hypoglossal nerve (N XII; Cong et al. 1998).
Group C. Longitudinal hypobranchial muscles
As the name indicates, these muscles all run longitudinally and parallel to the trachea (Fig.8). These muscles establish the link of the hyoid apparatus with the shoulder and pectoral girdle. Because these muscles are so closely situated to each other, individual muscles are visualized separately in Fig.8A–D. All these longitudinal hypobranchial muscles are innervated by the hypoglossal nerve (N XII; Cong et al. 1998).
M. geniohyoideus (M. branchiomandibularis spinalis, sensu Schumacher, 1973) – This is a wide ribbon-like muscle (Fig.7: Mgh). The muscle originates from the ventrolateral edge of the ceratobrachials, close to the dorsal deflection (Def. I). The M. geniohyoideus differs from the M. branchiohyoideus, which originates from the posterior half of the hyobranchial shaft, by arising from the anterior half of the shaft close to M. hyoglossus (Fig.7). The muscle extends anteriorly and laterally, inserting on the mandible dorsal to the splenial and medial to the attachment of the M. intermandibularis. Its fibers are so close to the anterior portion of M. episternobranchiotendineus that it was mislabeled by Schumacher (1973; figs 56 and 57). Here, these muscles are clearly shown to be independent of one another and are depicted separately (Fig.8A,B). M. geniohyoideus is conspicuously positioned ventromedially relative to the attachment of M. episternobranchiotendineus on the mandible (Fig.8). M. geniohyoideus was commonly called M. branchiomandibularis spinalis (Sewertzoff, 1929; Gnanamuthu, 1937; Sondhi, 1958) and was grouped with glossopharyngeal muscles with the potential confusion of its innervations of the glossopharyngeal nerve; it is actually innervated by the hypoglossal nerve (Schumacher, 1973; Rieppel, 1978).
M. omohyoideus and M. sternohyoideus (M. coracohyoideus, M. episternobranchiotendineus and M. episternobranchialis; sensu Schumacher, 1973) – M. omohyoideus and M. sternohyoideus generally reference muscles that connect the pectoral girdle elements to the hyoid in tetrapods (Diogo & Abdala, 2010). These muscles are diversified into three independent stripes in Alligator mississippiensis, which originated from coracoid (M. omohyoideus) and episternum, respectively (M. episternobranchialis and M. episternobranchiotendineus).
M. omohyoideus – This is the dorsal-most hypobranchial longitudinal muscle (Fig.3C,D: Moh). The muscle originates from the omal end of the coracoid with a ligamentous attachment. The muscular fibers run anteriorly and dorsally, inserting on the lateral ceratobranchial, close but slightly medial to the attachment of M. geniohyoideus (Fig.8C).
M. episternobranchiotendineus – This muscle is the lateral-most representative of the hypobranchial longitudinal muscles and the longest one as well. Its fibers originate from the ventral part of the episternum, close to the origination of M. episternobranchialis, but slightly lateral to the attachment site of M. episternobranchialis (Fig.8A,B). Anteriorly, its fibers become narrowed and constricted, with maximal constriction occurring close to its midpoint (Fig.8). Wrapping around the ceratobranchial at its proximal deflection point (Def. I), the muscle extends farther anteriorly and attaches to the mandible medial to the M. intermandibularis attachment and dorsal to that of the M. geniohyoideus. It was also called M. sternomandibularis or M. tendineomandibularis (Lubosch, 1933; Cleuren & De Vree, 2000).
M. episternobranchialis – This muscle is the medial-most representative of the hypobranchial longitudinal muscles, directly contacting the tracheal wall along the lateral surface. Its fibers also originate from the episternum, and insert anteriorly on the posterolateral corners of the basihyal (Fig.8D).
Discussion
Hyolingual apparatus variation within crocodilians
Previous authors have described aspects of the hyoid apparatus and associated muscles in Gavialis, Crocodylus, Caiman and Alligator (Gnanamuthu, 1937; Sondhi, 1958; Schumacher, 1973; Cong et al. 1998; Bona & Desojo, 2011). A comprehensive review reveals an overall consistency in the major morphologies of bony and muscular anatomy across different taxa. For the bony elements, the only variations noticed were in the shape of the basihyal: it is approximately a rectangular shape in Alligator and Crocodylus, but has a much narrower posterior margin in other crocodilians (Fig.2; Crocodylus and Caiman).
With respect to the hyoid and gular muscles of crocodilians, the most detailed work was done on Alligator sinensis (Cong et al. 1998), with less comprehensive studies of several other species (e.g. Gavialis and Caiman; Sondhi, 1958; Diogo & Abdala, 2010; Bona & Desojo, 2011). Review of this literature and comparison with the new data on Alligator mississippiensis indicates many similarities shared by Caiman and Alligator; while Gavialis has a few unique features. For instance, M. geniohyoideus has an insertion close to the middle of the ceratobranchial in Gavialis (Sondhi, 1958), rather than close to the proximal portion of the ceratobranchial in Caiman and Alligator. The M. omohyoideus was found to have both dorsal and ventral parts in Gavialis, but only one head is identified in Alligator and Caiman. In addition, one of the hypobrachial longitudinal muscles (M. episternobranchiotendineus) appears to be absent in Gavialis (Sondhi, 1958).
Within Alligator, the only difference noted between A. sinensis and A. mississippiensis was in one longitudinal hypobranchial muscle. A separate M. episternohyoideus muscle was reported in A. sinensis, very close to the M. episternobranchialis. The two muscles are similar in origin, but differ in their insertions in that taxon (Cong et al. 1998). By contrast, because in A. mississippiensis, two insertions are not discernible, we and other authors (Schumacher, 1973; Cleuren & De Vree, 2000) do not recognize the presence of a distinct M. episternohyoideus.
Despite minor variation in the hyoid skeletal and muscular anatomy among crocodilians noted above, most features described in Alligator are known from Caiman and Crocodylus (Gnanamuthu, 1937; Schumacher, 1973; Cleuren & De Vree, 2000; Bona & Desojo, 2011). A few unique features in Gavialis are inferred to represent autapomorphies, such as the lack of M. episternobranchialis and two parts of the M. omohyoideus discussed above. Data on the morphology of the hyolingual apparatus of Tomistoma and other rare crocodilian species is lacking. We believe the hyolingual features of Alligator we treat in our discussion here are a reasonable approximation of the ancestral condition of the crown-clade given the relatively basal phylogenetic position of Alligator (Oaks, 2011) and limited variability among studied extant crocodilians. The limited variation in hyolingual morphology among living is also consistent with their recently-proposed low rates of genome evolution compared with their extant sister taxa, birds (Green et al. 2014).
Hyoid muscle homology within Reptilia
A framework for investigating cranial muscle homology among the major clades of vertebrates was previously proposed from developmental data by Edgeworth (1935). More recently, Diogo et al. (2008) and Diogo & Abdala (2010) generated and synthesized new data from detailed dissection and developmental and molecular data, establishing in more detail the homologous muscles in Reptilia (Diogo & Abdala, 2010). The data from the present study served to evaluate some of these previous hypotheses of homology for crocodilians. Both morphologies (origin and insertion) and innervation were utilized. Although our assessments largely agree with previous hypotheses (Rieppel, 1978; Diogo & Abdala, 2010), here we particularly assess problematic homology issues (Fig.9) regarding the M. geniohyoideus and M. branchiohyoideus (both previously called the M. branchiomandibularis) in Lepidosauria and Archosauria.
Figure 9.
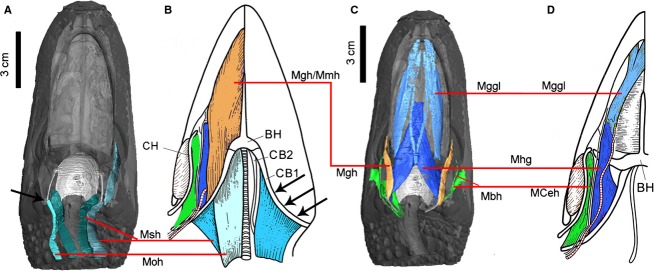
Proposed homologous hyoid muscles (linked by red line) mapped in Alligator mississippiensis (A, C) and Sphenodon punctatus (B, D) of the same color. The muscles of Sphenodon are modified from Jones et al. (2009). Anatomical abbreviations: BH, basihyal; CB 1, ceratobranchial I; CB 2, ceratobranchial II; CH, ceratohyal; Mbh, M. branchiohyoideus; Mceh, M. ceratohyoideus; Mggl, M. genioglossus; Mgh, M. geniohyoideus; Mhg, M. hyoglossus; Mmh: M. mandibulohyoideus; Moh, M. omohyoideus; Msh, M. sternohyoideus. Black arrows indicate the major attachments of the hyoid muscles on the ceratobranchial I.
The proposed homolog of the M. geniohyoideus in lepidosaurs is the M. mandibulohyoideus (Fig.9; Diogo & Abdala, 2010). The M. geniohyoideus and M. mandibulohyoideus are both innervated by the hypoglossal nerve (N XII). Because of significant differences in attachment and morphology, their potential homology has only been identified and proposed recently (Diogo & Abdala, 2010). As a second set of ceratobranchials (CB II) and the ceratohyal (CH) are absent in archosaurs (Fig.1), M. geniohyoideus only retains the attachment on CB I. This is true in A. mississippiensis, A. sinensis as well as other crocodilian species and birds (Sondhi, 1958; Vanden Berge & Zweers, 1993). The muscle was largely reduced along the development of neognathous birds and is reported to be absent from adult Gallus (Vanden Berge & Zweers, 1993; Müller & Weber, 1998).
Another muscle, which was easily confused with M. geniohyoideus in Alligator, is the M. branchiohyoideus because of the misleading name previously used for both muscles (i.e. M. branchiomandibularis; Schumacher, 1973). Although the two muscles share a few general similarities in their position and shape (Fig.7), detailed examination suggested they are quite distinctive from each other in both innervation and development. M. geniohyoideus is a hypobranchial muscle, which is derived from anterior somites in development (Oisi et al. 2015); while M. branchiohyoideus is a branchial muscle, which derived from more posterior somites (Noden, 1984). Here, we agree that the muscle homologous with the M. branchiohyoideus in archosaurs is the M. branchiohyoideus or the M. ceratohyoideus in lepidosaurs (Fig.9; Jones et al. 2009; Diogo & Abdala, 2010). In lepidosaurs, those muscles link the hyoid elements (CH, CB I; Herrel et al. 2005), but in Alligator, and very likely in other members of Archosauria, because the ceratohyal is absent, the homologous glossopharyngeal muscle (M. branchiohyoideus) alters its anterior insertion to the mandible rather than on the ceratobranchial. This muscle is further derived in birds (M. branchiomandibularis; Vanden Berge & Zweers, 1993) where it is significantly more elongate (associated with elongation of the epibranchials), but its origin and insertion are considered homologous as those observed in Alligator. The extension of the M. branchiohyoideus to the mandible in archosaurs represents a derived condition in comparison with that of basal lepidosaurs in reptilian evolution; that is why the name M. branchiomandibularis was commonly used in both Alligator and birds. This usage has confused its homology in other reptiles. The extension of this muscle on to the mandible for the insertion has also been noticed in turtles, which potentially support their close relationship with other extant archosaurs (Chiari et al. 2012; Field et al. 2014). Functionally, this muscle plays a critical role in the hyoid suspension as well as hyobranchial kinetics within archosaurs, especially for birds (Homberger & Meyers, 1989). Both M. branchiohyoideus (or M. ceratohyoideus) and/or the M. branchiomandibularis (for birds) are innervated by the glossopharyngeal nerve (N IX) in lepidosaurs, turtles, Alligator and birds (Edgeworth, 1935; Meyers et al. 2002).
Implications for inferring hyolingual function during inertial feeding
Employed by many reptiles, inertial feeding is a passive way for transporting food to the back of the oral cavity by mainly utilizing the posterior inertial motion of the food object as it is released from the jaw (Gans, 1969). The role the hyolingual apparatus plays in the inertial feeding of crocodilians is not clearly understood (Cleuren & De Vree, 2000). Previously interpretations restricted the hyolingual apparatus to a role in the passive dorsoventral movement associated with the raising and lowering of the buccal floor in crocodilians (Sondhi, 1958; Busbey, 1989). However, data from cineradiography revealed an unexpected role for the hyoid and lingual apparatus during feeding in both Alligator mississippiensis and Caiman crocodilus, in which both a slight dorsoventral and anteroposterior movement of the hyoid and tongue during the feeding cycles were found (Busbey, 1989; Cleuren & De Vree, 1992). Although a potentially active role of the hyolingual apparatus was tentatively proposed from these primary observations, no explicit anatomical structures or muscles have been identified as playing a role in these movements. Meantime, no electromyography studies have tested the role of specific hyoid muscles during feeding or other related activities in crocodilians. Here we propose muscles that may be involved to inform, and subject to testing by, electromyographic work on hyolingual muscle activation during feeding.
The hyoid role in intra-oral transport was considered limited because of the reduction of tongue in crocodilians. Cleuren & De Vree (1992), however, revealed the tongue in helping to push the food item dorsally before releasing in the intra-oral transport process. In addition, in the middle of the swallowing phase, the hyoid has an important role in pushing food back into the esophagus by moving circularly. This observed motion by Busbey (1989) involves the depression of the ceratobranchial against the throat skin during swallowing. As seen from the new CT data, the distal deflection of the ceratobranchial (Fig.1: Def. II) hangs over the dorsolateral wall of the esophagus when relaxed. Because it is intimately attached with both the lateral and medial portions of the distal ceratobranchial, contraction of the M. branchiohyoideus (dorsal portion) would pull the shaft ventrolaterolly, further leading to the ventral-most portion of the ceratobranchial pressing against the skin around the throat. This particular skeletal-muscular arrangement, first noticed here in Alligator mississippiensis, explains well unexpected hyoid function observed by Busbey (1989). The deflected distal portion of the ceratobranchial (Def. II) serves not only for the suspension of hyoid with the esophagus, but also serves as a stable anchor for the dorsal portion of the M. branchiohyoideus, enforcing the muscular control of the hyoid. For the suspension role of hyolingual muscles, M. constrictor colli profundus might also be important due to the special muscular attachment with the cervical ribs. This bony attachment is helpful in stabilizing the tissues encased by the muscular sling. In addition, contraction of this muscle can also raise the gular region to facilitate food transportation during swallowing.
Another series of motions that have been confirmed are the protraction and retraction of the hyolingual apparatus during intra-oral transport of feeding in Alligator mississippiensis, which are closely related to arrangements of the hypobranchial longitudinal muscles. With the direct or indirect attachment on the hyoid, muscles affecting the protraction–retraction of the tongue include M. geniohyoideus, M. omohyoideus and M. sternohyoideus (i.e. M. episternobrachialis and M. episternobranchiotendineus). The contraction of the M. omohyoideus and the M. episternobrachialis would retract the hyoid backward and downward; the M. geniohyoideus can pull the hyoid slightly anteriorly. The well-developed nature of these muscles even in juvenile specimens of Alligator mississippiensis supports their potential role in relation to hyolingual movement during transport of food from the buccal floor to the esophagus.
The hyoid apparatus also plays an active role in food acquisition and movement within the buccal cavity in birds, the small circular movements in crocodilians could imply at least some role for the tongue in feeding as a homologous condition of Archosauria. However, evaluation of the outgroup condition in basal lepidosaurs such as Sphenodon and in turtles as well as the morphology of fossil taxa is key to assessing this hypothesis. Below we discuss evidence bearing on the evolution of hyolingual function.
Hyolingual structures in Archosauriformes and crocodilian-line archosaurs
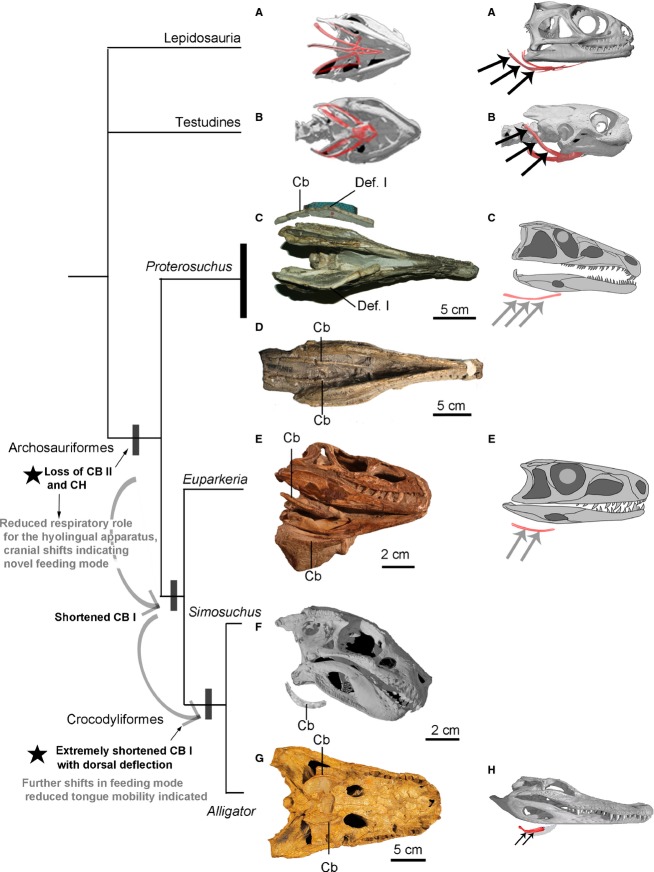
Comparison of the bony hyoids in extant crocodilians, birds, turtles and lepidosaurs indicates that the shared homologous elements comprise the basihyal and the first ceratobranchial (CB I), although those elements are variable in their degree of ossification. One remarkable feature that characterizes the crown-clade archosaur (crocodilians and birds) hyoid is the absence of Ceratobranchial II and ceratohyal. The largely cartilaginous basihyal and better ossified single pair of ceratobranchials are inferred to represent the ancestral condition for this clade (Archosauria). An ossified basihyal has not been reported from extinct archosaurs even in Archosauriformes, with the exception of several derived dinosaurian species (e.g. Hongshanornis; Zhou & Zhang, 2005). Ossified single sets of ceratobranchials are quite frequently found in extinct Archosauriformes and dinosaurian species (Ewer, 1965; Weishampel et al. 1990; Zhou & Zhang, 2002; Li et al. 2006; Delfino et al. 2008; Lü et al. 2008; Kley et al. 2010; De França et al. 2013). Assessment of available ceratobranchial morphology in Archosauriformes indicates their length relative to total skull length is significantly reduced to less than a third of the skull close to crown-clade Crocodylia in Simosuchs (Crocodyliformes; Kley et al. 2010; Nesbitt, 2011; Fig.10). The general appearance of the hyoid of Simosuchus is quite similar to that of extant alligators (Fig.10), with a markedly dorsomedial deflection (Def. I) in the short ceratobranchials. By contrast, they are relatively thin, elongate elements in basal archosaurs including basal dinosaurs (e.g. Qianosuchus, Jeholosaurus and Syntarsus; Rowe, 1989; Li et al. 2006; Han et al. 2012). In Archosauriformes, outside crown-clade Archosauria, they are relatively more elongate with only a slight medial curvature in the ceratobranchials (Fig.10; Ewer, 1965; Cruickshank, 1972). A relatively long CB I with the markedly dorsal deflection was found in both the lepidosaurs and turtles (Lemell et al. 2000; Jones et al. 2009); therefore, the lack of these features in basal archosaurs indicates the hyoid reduction might occur during their early evolution, possibly associated with a shift in feeding and respiratory behavior (Fig.10). The acquisition of other novel cranial features also occurs early in Archosauriformes, for example, ‘socketed’ teeth (‘thecodont tooth implantation’) and novel dental morphologies, as well as antorbital and lateral mandibular fenestrae and increased cranial kinesis (Nesbitt, 2011; Holliday & Nesbitt, 2013). Reduction of the bony hyoid apparatus and major changes in hyolingual muscles may be related to consumption of larger prey items or acquisition of a hypercarnivorous behavior within the clade.
Figure 10.
Hyoid remains preserved in close-to-life position in Archosuriformes and crown-clade crocodilians compared with selected extant outgroups. (A) Lepidosauria: Sphenodon punctatus (YPM 9194); (B) Testudines: Glyptemys muhlenbergii (UF 85274); (C, D) Proterosuchus fergusi (C, NMC. 3016 and D, BP/1/4016); (E) Euparkeria capensis (SAM 5867); (F) Simosuchus clarki (UA 8679; adopted from Kley et al. 2010); (G) Alligator prenasalis (SDSM 243) and (H) Alligator mississippiensis. The major features and related functional shifts inferred along crocodilian-line archosaurs are labeled (see text also). Phylogenetic relationships within Archosauriformes are based on Nesbitt (2011). Curved arrows and black stars indicate major shifts in hyoid morphology and inferred feeding ecology in Archosauriformes; the parallel black and gray arrows indicate the attachment site of the major hypobranchial muscles on ceratobranchial I in lepidosaurs, turtles, Alligator and the position of these attachments inferred in extinct Archosauriformes. Anatomical abbreviations: Cb, ceratobranchial; Def. I, deflection I.
Associated with skeletal reduction, the hyoid muscles that are attached to these bony components show a major shift or rearrangement in archosaurs. The attachments of the hypobranchial muscles shift (Fig.9: parallel arrows), and the muscles in the buccal floor of crocodilians and birds are significantly reduced or simplified. In addition to the M. intermandibularis shared by all reptilians, the massive and multilayered muscles of the M. mandibulohyoideus (also called M. geniohyoideus; Schwenk, 2000), which are present in lepidosaurs and turtles, are reduced or absent in archosaurs (Müller & Weber, 1998). They are the muscular sheets that connect the mandibular ramus to the hyobranchials (CB I, CB II and CH). These muscles are variably developed in turtles and lepidosaurs; as many as three parts are identified, namely M. mandibulohyoideus I, II and III (Schwenk, 2000; Herrel et al. 2005). In addition, the intrinsic tongue muscles are absent in crown archosaurs with the exception of a few birds (e.g. parrots) in which they are considered to be secondarily derived features (Tomlinson, 2000). Therefore, less ossification of the bony hyoid, loss of the hyobranchial elements, and the rearrangement of various hyoid muscles and the reduction of intrinsic tongue muscles are inferred to occur prior to the origin of the clade. However, due to limited fossil hyoid evidence and the lack of solid evidence to assess these soft tissues associated with the hyoid, the inferences for timing of these changes are only a minimum estimation. The longitudinal hypobranchial muscles are largely retained in archosaurs (Edgeworth, 1935) even though they have different attachments. These muscles have been observed to be extensively active during gular or buccal pumping and oscillation, a respiratory behavior intensified during locomotion in lizards (Brainerd, 1999; Owerkowicz et al. 1999, 2001). In Alligator, although a similar gular oscillation is present, differing from lizards, this behavior does not increase their lung ventilation (Brainerd & Owerkowicz, 2006). This reduction in hyoid complexity thus may be linked to the loss of a role in respiration (Brainerd, 1999).
Conclusions
Assessment of the hyolingual morphology across crocodilian-line archosaurs and early Archosauriformes indicates, compared with outgroups, both the hyobranchials and the anterior portions of the hyolingual muscles are largely rearranged or reduced as characterizing the clade Archosauria or even the Archosauriformes. By contrast, the longitudinal hypobranchial muscles, involved mostly in swallowing, food handling, as well as buccal pumping and panting (the ‘rhythmic hyobranchial behaviors’; Brainerd, 1999), are retained in extant archosaurs and inferred to be present in basal archosaurs but with a different attachment (Figs9 and 10). These findings imply that beyond described locomotion novelties, shifts in feeding ecology in Archosauriformes could be another key factor in the rapid diversification of Archosauria in the Triassic (Nesbitt, 2011). The evolutionary changes in the hyolingual apparatus that a shift in feeding ecology occur prior to the origin of Archosauria, perhaps linked to hypercarnivory and/or feeding on larger prey. This hypothesis is consistent with a more inclusive morphological analysis of basal stem archosaurs, in which the synapomorphies identified for Archosauriformes are exclusively cranial features (Nesbitt, 2011) that may be related to this shift in feeding ecology. Combined assessment of hyoid structure with other cranial and postcranial features is needed to provide further insights into potential links between the evolution of novel feeding, respiratory and locomotor modes in Archosauriformes.
Acknowledgments
The authors are grateful to T. Owerkowicz (California State Univ., San Bernardino, USA), R. M. Elsey (Rockefeller Wildlife Refuge, Louisiana, USA) and T. Riede (Midwestern University, USA) for providing the specimens used in the study. The authors are grateful to S. Nesbitt, M. Stocker, and for providing photos of extinct archosaurs, and Palaeontological Association for use, with permission, of the illustration of Sphenodon musculature. The authors are further grateful to T. Riede, C. Bell and two reviewers for providing insightful comments on the draft. M. Colbert and J. Maisano (UTCT) are thanked for performing the scans. This study was supported by the Jackson School of Geosciences, the University of Texas at Austin to J.A.C. and Z.L.
Author contributions
Conceived project: J.A.C., Z.L.; collected data: Z.L.; analyzed and interpreted data: Z.L, J.A.C.; wrote paper: J.A.C, Z.L; created figures: Z.L.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
An interactive 3D PDF showing the myology of the hyolingual apparatus in Alligator mississippiensis.
References
- Bona P, Desojo JB. Osteology and cranial musculature of Caiman latirostris (Crocodylia: Alligatoridae) J Morphol. 2011;272:780–795. doi: 10.1002/jmor.10894. [DOI] [PubMed] [Google Scholar]
- Brainerd EL. New perspectives on the evolution of lung ventilation mechanisms in vertebrates. Exp Biol Online. 1999;4:1–28. [Google Scholar]
- Brainerd EL, Owerkowicz T. Functional morphology and evolution of aspiration breathing in tetrapods. Respir Physiol Neurobiol. 2006;154:73–88. doi: 10.1016/j.resp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Britton A. Review and classification of call types of juvenile crocodilians and factors affecting distress calls. In: Grigg GC, Seebacher F, Franklin F, editors. Crocodilian Biology and Evolution. Chipping Norton: Surrey Beatty; 2001. pp. 364–377. ) In: [Google Scholar]
- Busbey AB. Form and function of the feeding apparatus of Alligator mississippiensis. J Morphol. 1989;202:99–127. doi: 10.1002/jmor.1052020108. [DOI] [PubMed] [Google Scholar]
- Chiari Y, Cahais V, Galtier N. Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria) BMC Biol. 2012;10:65. doi: 10.1186/1741-7007-10-65. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleuren J, De Vree F. Kinematics of the jaw and hyolingual apparatus during feeding in Caiman crocodilus. J Morphol. 1992;212:141–154. doi: 10.1002/jmor.1052120205. [DOI] [PubMed] [Google Scholar]
- Cleuren J, De Vree F. Feeding in crocodilians. In: Schwenk K, editor. Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. San Diego, CA: Academic Press; 2000. pp. 337–358. ) In: [Google Scholar]
- Cong L, Hou L, Wu X. The Gross Anatomy of Alligator Sinensis Fauvel (Yang-Zi-E Da Ti Jie Pou) Beijing: Scientific Publisher; 1998. , et al. ( In Chinese (with English summary) [Google Scholar]
- Corsy F. Evolution de L'appareil hyo-Branchial. 1933. . Thèse de doctorat es sciences.Université de Paris. [Google Scholar]
- Cruickshank ARI. The proterosuchian thecodonts. In: Joysey KA, Kemp TS, editors. Studies in Vertebrate Evolution. Edinburgh: Oliver and Boyd; 1972. pp. 89–119. ) In: [Google Scholar]
- De França MAG, Langer MC, Ferigolo J. The skull anatomy of Decuriasuchus quartacolonia (Pseudosuchia: Suchia: Loricata) from the middle Triassic of Brazil. Geol Soc London Spec Publ. 2013;379:469–501. [Google Scholar]
- Delfino M, Martin JE, Buffetaut E. A new species of Acynodon (Crocodylia) from the upper Cretaceous (Santonian–Campanian) of Villaggio del Pescatore, Italy. Palaeontology. 2008;51:1091–1106. [Google Scholar]
- Diogo R, Abdala V. Muscles of Vertebrates. Comparative Anatomy, Evolution, Homologies and Development. New Hampshire: Science Publishers Enfield; 2010. [Google Scholar]
- Diogo R, Yaniv H, Simon H. Development of mandibular, hyoid and hypobranchial muscles in the zebrafish: homologies and evolution of these muscles within bony fishes and tetrapods. BMC Dev Biol. 2008;8:24. doi: 10.1186/1471-213X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgeworth FH. The Cranial Muscles of Vertebrates. London: Cambridge University Press; 1935. [Google Scholar]
- Endo H, Frey R. Anatomical Imaging: Towards a new Morphology. Tokyo: Springer; 2008. [Google Scholar]
- Ewer RF. The anatomy of the thecodont reptile Euparkeria capensis Broom. Philos Trans R Soc Lond B Biol Sci. 1965;248:379–435. [Google Scholar]
- Field DJ, Gauthier JA, King BL. Toward consilience in reptile phylogeny: miRNAs support an archosaur, not lepidosaur, affinity for turtles. Evol Dev. 2014;16:189–196. doi: 10.1111/ede.12081. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürbringer M. Das Zungenbein der Wirbeltiere insbesondere der Reptilien und Vögel. Abh Heidelb. 1922;11:1–164. [Google Scholar]
- Gans C. Comments on inertial feeding. Copeia. 1969;4:855–857. [Google Scholar]
- Gnanamuthu CP. Comparative study of the hyoid and tongue of some typical genera of reptiles. Proc Zool Soc London. 1937;107:1–63. [Google Scholar]
- Green RE, Braun EL, Armstrong J. Three crocodilian genomes reveal ancestral patterns of evolution among archosaurs. Science. 2014;346:12 54 449. doi: 10.1126/science.1254449. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F, Barrett PM, Butler RJ. Postcranial anatomy of Jeholosaurus shangyuanensis (Dinosauria, Ornithischia) from the Lower Cretaceous Yixian Formation of China. J Vert Paleontol. 2012;32:1370–1395. , et al. ( [Google Scholar]
- Herrel A, Canbek M, Özelmas Ü. Comparative functional analysis of the hyolingual anatomy in lacertid lizards. Anat Rec. 2005;284:561–573. doi: 10.1002/ar.a.20195. , et al. ( [DOI] [PubMed] [Google Scholar]
- Holliday CM. Evolution and Function of the Jaw Musculature and Adductor Chamber of Archosaurs (Crocodilians, Dinosaurs, and Birds) Ohio: Ohio University; 2006. . PhD thesis, [Google Scholar]
- Holliday CM, Nesbitt SJ. Morphology and diversity of the mandibular symphysis of archosauriforms. Geol Soc London Spec Publ. 2013;379:555–571. [Google Scholar]
- Holliday CM, Tsai HP, Skiljan RJ. A 3D interactive model and atlas of the jaw musculature of Alligator mississippiensis. PLoS One. 2013;8:e62806. doi: 10.1371/journal.pone.0062806. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberger DG, Meyers RA. Morphology of the lingual apparatus of the domestic chicken, Gallus gallus, with special attention to the structure of the fasciae. Am J Anat. 1989;186:217–257. doi: 10.1002/aja.1001860302. [DOI] [PubMed] [Google Scholar]
- Jeffery NS, Stephenson RS, Gallagher JA. Micro-computed tomography with iodine staining resolves the arrangement of muscle fibres. J Biomech. 2011;44:189–192. doi: 10.1016/j.jbiomech.2010.08.027. , et al. ( [DOI] [PubMed] [Google Scholar]
- Jones ME, Tennyson AJ, Worthy JP. The head and neck muscles associated with feeding in Sphenodon (Reptilia: Lepidosauria: Rhynchocephalia) Palaeontol Electronica. 2009;12:56p. , et al. ( [Google Scholar]
- Kesteven HL. The evolution of the skull and the cephalic muscles. Part III. The Sauria. The Reptilia. Aust Mus Mem. 1944;8:237–269. [Google Scholar]
- Kley NJ, Sertich JJ, Turner AH. Craniofacial morphology of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. J Vert Paleontol. 2010;30:13–98. , et al. ( [Google Scholar]
- Lemell P, Beisser CJ, Weisgram J. Morphology and function of the feeding apparatus of Pelusios castaneus (Chelonia: Pleurodira) J Morphol. 2000;244:127–136. doi: 10.1002/(SICI)1097-4687(200005)244:2<127::AID-JMOR3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Li C, Wu X, Sato T. An unusual archosaurian from the marine Triassic of China. Naturwissenschaften. 2006;93:200–206. doi: 10.1007/s00114-006-0097-y. , et al. ( [DOI] [PubMed] [Google Scholar]
- Lü J, Xu L, Ji Q. Restudy of Liaoxipterus (Istiodactylidae: Pterosauria), with comments on the Chinese istiodactylid pterosaurs. In: Hone DWE, Buffetaut E, editors. Flugsaurier: Pterosaur Papers in Honour of Peter Wellnhofer. Bayerische Staatssammlung für Paläontologie und Geologie: München; 2008. pp. 229–241. ) In: Zitteliana. [Google Scholar]
- Lubosch W. Untersuchungen über die Visceralmuskulatur der Sauropsiden. Gegenbaurs Morph Jb. 1933;72:584–666. [Google Scholar]
- McClearn D, Noden DM. Ontogeny of architectural complexity in embryonic quail visceral arch muscles. Am J Anat. 1988;183:277–293. doi: 10.1002/aja.1001830402. [DOI] [PubMed] [Google Scholar]
- Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009;9:11. doi: 10.1186/1472-6793-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JJ, Herrel A, Nishikawa KC. Comparative study of the innervation patterns of the hyobranchial musculature in three iguanian lizards: Sceloporus undulatus Pseudotrapelus sinaitus, and Chamaeleo jacksonii. Anat Rec. 2002;267:177–189. doi: 10.1002/ar.10096. [DOI] [PubMed] [Google Scholar]
- Müller W, Weber E. Re-discovery of a supposedly lost muscle in palaeognathous birds and its phylogenetic implications. Zoosyst Evol. 1998;74:11–18. [Google Scholar]
- Nesbitt SJ. The early evolution of archosaurs: relationships and the origin of major clades. Bull Am Mus Nat Hist. 2011;352:1–292. [Google Scholar]
- Nishi S. Muskeln des Rumpfes. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere. Vol. 5. Amsterdam: A Asher; 1967. pp. 341–446. ) In:, Vol. [Google Scholar]
- Noden DM. Craniofacial development: new views on old problems. Anat Rec. 1984;208:1–13. doi: 10.1002/ar.1092080103. [DOI] [PubMed] [Google Scholar]
- Oaks JR. A time-calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution. 2011;65:3285–3297. doi: 10.1111/j.1558-5646.2011.01373.x. [DOI] [PubMed] [Google Scholar]
- Oisi Y, Fujimoto S, Ota KG. On the peculiar morphology and development of the hypoglossal, glossopharyngeal and vagus nerves and hypobranchial muscles in the hagfish. Zool Lett. 2015;1:6. doi: 10.1186/s40851-014-0005-9. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owerkowicz T, Farmer CG, Hicks JW. Contribution of gular pumping to lung ventilation in monitor lizards. Science. 1999;284:1661–1663. doi: 10.1126/science.284.5420.1661. , et al. ( [DOI] [PubMed] [Google Scholar]
- Owerkowicz T, Brainerd EL, Carrier DR. Electromyographic pattern of the gular pump in monitor lizards. Bull Mus Comp Zool. 2001;156:237–248. [Google Scholar]
- Riede T, Tokuda IT, Farmer CG. Subglottal pressure and fundamental frequency control in contact calls of juvenile Alligator mississippiensis. J Exp Biol. 2011;214:3082–3095. doi: 10.1242/jeb.051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Li Z, Tokuda IT. Functional morphology of the Alligator mississippiensis larynx and implications for vocal production. J Exp Biol. 2015;218:991–998. doi: 10.1242/jeb.117101. , et al. ( jeb-117101. [DOI] [PubMed] [Google Scholar]
- Rieppel O. The throat musculature of Sphenodon, with comments on the primitive character states of the throat muscles in lizards. Anat Anz. 1978;144:429–440. [PubMed] [Google Scholar]
- Rowe T. A new species of the theropod dinosaur Syntarsus from the Early Jurassic Kayenta Formation of Arizona. J Vert Paleontol. 1989;9:125–136. [Google Scholar]
- Schumacher GH. The head muscles and hyolaryngeal skeleton of turtles and Crocodilians. In: Gans C, Parsons TS, editors. Biology of the Reptilia. Vol. 4. London: Academic Press; 1973. pp. 101–199. ) In:, Vol. [Google Scholar]
- Schwenk K. Morphology of the tongue in the tuatara, Sphenodon punctatus (Reptilia: Lepidosauria), with comments on function and phylogeny. J Morph. 1986;188:129–156. doi: 10.1002/jmor.1051880202. [DOI] [PubMed] [Google Scholar]
- Schwenk K. Feeding in lepidosaurs. In: Schwenk K, editor. Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. San Diego, CA: Academic Press; 2000. pp. 175–291. ) In: [Google Scholar]
- Sewertzoff SA. Zur Entwicklungsgeschichte der Zunge bei den Reptilien. Acta Zool. 1929;10:231–341. [Google Scholar]
- Sondhi KC. The hyoid and associated structures in some Indian reptiles. Ann Zool (Agra) 1958;2:155–240. [Google Scholar]
- Tanner WW, Avery DF. Buccal floor of reptiles, a summary. Great Basin Nat. 1982;42:273–349. [Google Scholar]
- Tomlinson CA. Feeding in Paleognathous Birds. In: Schwenk K, editor. Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. San Diego, CA: Academic Press; 2000. pp. 359–394. ) In: [Google Scholar]
- Vanden Berge J, Zweers GA. Myologia. In: Baumel JJ, King AS, Breazile JE, editors. Handbook of Avian Anatomy: Nomina Anatomica Avium. 2nd edn. Cambridge, MA: Publications of the Nuttall Ornithological Club; 1993. pp. 189–247. , et al. ) In: [Google Scholar]
- Weishampel DB, Dodson P, Osmólska H. The Dinosauria. Berkeley, CA: University of California Press; 1990. [Google Scholar]
- Zhou Z, Zhang F. A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature. 2002;418:405–409. doi: 10.1038/nature00930. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhang F. Discovery of an ornithurine bird and its implication for Early Cretaceous avian radiation. Proc Natl Acad Sci USA. 2005;102:18 998–19 002. doi: 10.1073/pnas.0507106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An interactive 3D PDF showing the myology of the hyolingual apparatus in Alligator mississippiensis.