Abstract
Zebrafish ( Danio rerio) teeth are increasingly used as a model to study odontogenesis in non-mammalians. Using serial semi-thin section histology and immunohistochemistry, the nerves innervating the pharyngeal jaws and teeth have been identified. The last pair of branchial arches, which are non-gill bearing but which carry the teeth, are innervated by an internal branch of a post-trematic ramus of the vagal nerve. Another, external, branch is probably responsible for the motor innervation of the branchiomeric musculature. Nerve fibres appear in the pulp cavity of the teeth only late during cytodifferentiation, and are therefore likely not involved in early steps of tooth formation. The precise role of the nervous system during continuous tooth replacement remains to be determined. Nonetheless, this study provides the necessary morphological background information to address this question.
Keywords: Danio rerio, innervation, pharyngeal jaws, teeth, teleosts, vagal nerve
Introduction
Over the last decades, extensive knowledge has been built up regarding the molecular mechanisms that govern tooth development (Jernvall & Thesleff, 2000; Thesleff, 2003, 2006; Jackman et al. 2004; Bei, 2009). However, apart from the presence of the correct developmental cues, teeth also need the appropriate environment to support development and growth. In particular, the presence of sensory and motor innervation is of vital importance for both the function and the protection of the tooth. Nonetheless, functional studies focussing on the role of nerves during tooth development and replacement are scarce (Lumsden & Buchanan, 1986; Harputluoglu, 1990; Tuisku & Hildebrand, 1994). This is remarkable given that the idea of a neuronal influence on the initiation of tooth development and replacement is not a new concept and has already been raised in the past (Pearson, 1977; Kollar & Lumsden, 1979; Lumsden, 1982; Chiego, 1995). This hypothesis is based on two observations. First, prospective dental nerves enter the mammalian jaw long before the development of teeth (Fried & Hildebrand, 1982). Second, early axons have a close spatial relationship to future sites of tooth development (Mohamed & Atkinson, 1983). Nevertheless, Lumsden & Buchanan (1986) have challenged this hypothesis through studies involvingin vitro culture of mouse mandibular arch fragments, with or without trigeminal ganglion explants. Their results have led to the conclusion that tooth initiation does not involve a nervous component, at least during early steps of odontogenesis (Lumsden & Buchanan, 1986). However, a more recent study by Tuisku & Hildebrand (1994) in a polyphyodont teleost fish,Tilapia mariae, has demonstrated arrest of tooth replacement after denervation of the trigeminal nerve, hence providing evidence in favour of a neuronal influence on tooth development and replacement. In addition, a recent study in mice has also highlighted the possible importance of nerves during odontogenesis, by identifying peripheral nerve-associated glia as a possible source of multipotent mesenchymal stem cells that produce pulp cells and odontoblasts in the continuously growing incisor (Kaukua et al. 2014). These apparent conflicting results, and the wish of the authors to explore exactly how tooth replacement is initiated, have been the incentive for the current study. Using the zebrafish (Danio rerio), this study explores the role of nerves during tooth development and replacement. However, prior to studying a functional relationship between nerves and developing teeth, the morphological baseline information regarding the innervation of zebrafish teeth must first be established.
The zebrafish is a small teleost fish belonging to the cyprinids, and is widely used as a vertebrate model organism for genetic, molecular and developmental research (Lele & Krone, 1996; Roush, 1996). Similar to most other tooth-bearing non-mammalian vertebrates, zebrafish replaces its teeth throughout life. The zebrafish has no oral dentition; its teeth are restricted to the pharyngeal region and are implanted on the fifth, i.e. last, branchial arch (Huysseune et al. 1998; Van der Heyden et al. 2000; Stock, 2007). The complete pharyngeal dentition consists of three rows of teeth on each side: a ventral row (V) of five teeth (1V–5V); a mediodorsal row (MD) of four teeth (1MD–4MD); and a dorsal row (D) of two teeth (1D–2D; Van der Heyden & Huysseune, 2000). The first tooth bud starts to develop at around 48 hpf (hours post-fertilization) at position 4V (Van der Heyden & Huysseune, 2000). The replacement of first-generation teeth starts already at 80 hpf (position 4V). First-generation teeth develop directly from the pharyngeal epithelium, whereas replacement teeth develop from an epithelial outgrowth at the base of the epithelial crypt surrounding the tip of the erupted functional tooth. This outgrowth is called the successional lamina (Huysseune, 2006).
The innervation of the teleost branchial arches has been described in several seminal papers and books (Goodrich, 1930; Grassé & Tetry, 1963; Nilsson, 1983; Beaumont & Cassier, 1994), albeit with inconsistent terminology and interpretation. In general, out of the 11 pairs of cranial nerves present, only numbers VII (facial), IX (glossopharyngeal) and X (vagus) are of major importance for branchial innervation, and are therefore called the branchial nerves (Nilsson, 1983, 1984; Sundin & Nilsson, 2002). These three nerves enter the branchial (or gill) arches dorsally and form large nerve trunks (Jonz & Nurse, 2008). The facial nerve (N.VII) projects efferent (motor) fibres to the most anterior pharyngeal arch, and receives afferent (sensory) fibres from the taste buds in the mouth region, gills, and in some species from large areas of the body surface (Laurent & Dunel, 1966; Ezeasor, 1982; Morita & Finger, 1985). The glossopharyngeal nerve (N.IX) receives sensory information from the pseudobranch, and contains both sensory and motor fibres innervating the first gill arch (third pharyngeal arch). The vagal nerve (N.X) innervates the other gill arches with afferent and efferent fibres (Nilsson, 1984). Upon entering the branchial region, these branchial nerves subdivide into a pre-trematic and post-trematic ramus that enclose the gill slits, rostrally and caudally, respectively (Nilsson, 1983, 1984; Sundin & Nilsson, 2002). The pre-trematic ramus carries sensory information, whereas the post-trematic nerve branch contains both sensory and motor fibres (Nilsson, 1984).
Because branchial arches one–four bear the gills, considerable information is available on their innervation (Goodrich, 1930; Grassé & Tetry, 1963; Nilsson, 1983, 1984; Dunelerb et al. 1993; Beaumont & Cassier, 1994; Chandrasekhar et al. 1997; Sundin & Nilsson, 2002; Jonz & Zaccone, 2009; Young et al. 2011). Data regarding the innervation of the fifth arch are lacking, yet this is the arch that carries the pharyngeal teeth in zebrafish and in many other teleosts (Huysseune & Sire, 1997; Huysseune et al. 1998; Sire et al. 2002). Thus, there is also a complete lack of knowledge regarding the nervous supply of the teeth in zebrafish. In order to study the function of nerves during tooth development and replacement, first the nerves responsible for innervating the fifth ceratobranchials must be identified, whether or not the teeth themselves become innervated must be established, and the precise timing of these events must be determined. It is hypothesized that branches of the vagal nerve innervate the pharyngeal jaws and teeth; however, this needs to be confirmed.
Materials and methods
Animal husbandry
Wild-type zebrafish (Danio rerio) were bred and raised according to the methods described in Westerfield (1993). Briefly, fish were raised in a 14 h/10 h light/dark cycle at 28.5 °C. Embryos were obtained via natural mating and raised in egg water. Embryos, larvae, juvenile and adult fish were killed according to the Belgian law on the protection of laboratory animals (KB d.d. 13 September 2004) by an overdose of MS222 (3-aminobenzoic acid ethyl ester). Embryos/larvae aged 2, 3, 4, 5, and 6 days post-fertilization, juvenile zebrafish with standard length 6.0, 7.3, 8.0, 8.3, 9.5 and 11.0 mm, and five adult fish were used.
Histology
Fish were fixed and embedded in epon following Huysseune & Sire (1992). Briefly, they were fixed in a mixture of 1.5% glutaraldehyde and 1.5% paraformaldehyde buffered with 0.2 m cacodylate (pH 7.4) for 2 h at room temperature. Juvenile zebrafish were decalcified by adding 0.1 m EDTA to the fixative solution for one to several weeks at 4 °C. The decalcifying solution was refreshed every 2 days. After fixation, animals were rinsed in 0.2 m cacodylate buffer containing 10% sucrose, and post-fixed for 2 h at room temperature with 1% OsO4 in 0.2 m cacodylate buffer containing 8% sucrose. After rinsing in the same buffer, specimens were dehydrated through a graded series of ethanol and embedded in epon. Serial semi-thin (1 μm) sections were made using a Reichert-Jung Ultra-cut ultramicrotome (Leica, Vienna, Austria). These sections were stained with toluidine blue, and mounted in DePeX (Gurr, BDH Laboratory, UK). All sections were examined using a Zeiss Axio Imager Microscope and photographed using an Axiocam MRC video camera. Reconstructions were made using the software program amira® (v5.3.3.). Finally, 3D models were photographed from different perspectives using rhinoceros® (v5.0).
Tissue processing for immunohistochemistry
Adult fish were killed using a lethal dose of MS222, and the heads were fixed in 4% paraformaldehyde for 48 h. The heads were decalcified in Morse's solution for 2–4 weeks, and rinsed for 12 h in running tap water. Subsequently, they were dehydrated in an ascending series of ethanol (30%, 50%, 70% ethanol, 12 h each), and three times for 2 h in 96% ethanol. Next, the heads were immersed in three baths of Ultraclear (J.T. Baker, Deventer, the Netherlands) for 12 h and subsequently in three paraffin baths for 8 h at 60–70 °C. Heads were embedded in peel-away moulds [Ted Pella, Redding (CA), USA]. Transverse sections (5 μm) were made on a MicromHM360 microtome (Prosan, Merelbeke, Belgium).
Immunohistochemistry and visualization
The slides with paraffin sections were placed on a hot plate (70 °C) for 5 min, cooled down for 5 min, and heated again for 5 min. The paraffin was removed in two Ultraclear baths for 15 and 10 min, respectively, and the sections rehydrated in a descending ethanol series. The slides were next rinsed in 1 × phosphate-buffered saline (PBS)/1% dimethylsulphoxide (DMSO) three times for 5 min. Blocking in 3% bovine serum albumin/1% milk/1 × PBS/1% DMSO for 2 h at room temperature reduced the background signal. After blocking, the sections were demarcated with a hydrophobic pen (Dakopen, Heverlee, Belgium) to limit the amount of antibody needed. Per group of serial sections, one section was marked out and was incubated with pure block-solution to serve as a negative control. The monoclonal anti-acetylated alpha tubulin antibody produced in mouse (T5168; Sigma, Diegem, Belgium) was dissolved in block-solution at a 1/300 concentration. The antibody-covered slides were placed overnight in a wet chamber at 4 °C. The slides were next rinsed in 1 × PBS/1% DMSO three times for 5 min. The polyclonal anti-mouse antibody produced in goat (IgGDyLight 594- ab96881, Abcam, Cambridge, UK) was dissolved in blocking solution at a 1/300 concentration and was placed on the tissue for 2 h at room temperature, sheltered from light. The tissue was rinsed with 1 × PBS/1% DMSO and DAPI (1 mL/mL distilled water) was added for 5 min. Finally, the slides were rinsed with 1 × PBS/ 1% DMSO three times for 5 min, and mounted with Vectashield (Vector Laboratories, Peterborough, UK). Immunofluorescence was visualized using a NIKON eclipse TE2000-S confocal laser-scanning microscope [Nikon, Melville (NY), USA].
Results
The innervation pattern of the pharyngeal jaws and teeth was studied using both semi-thin sections and immunohistological staining of nerve fibres.
Vagal sensory ganglion and its branches
Study of toluidine blue-stained sections of 8.3 mm SL zebrafish revealed the presence of a large ganglion on each body side. Based on earlier publications of the zebrafish nervous system (Higashijima et al. 2000; Holzschuh et al. 2005), and the position of the ganglion on the postero-dorsal side of the dentition, just lateral of the inner ear, and dorsal of the posterior cardinal vein (Fig.1A,B), this ganglion could be identified as the large sensory ganglion of the vagal nerve. For complete visualization of the ganglion and its different branches, a 3D reconstruction of 111 consecutive semi-thin (1 μm) sections was made. A total of six different branches could be identified (Fig.1C,D). Two branches emerge from a common root and course ventrally towards the dentigerous region. The more anterior of these two branches was termed internal (branch 2 on Fig.1C,D), and the more posterior one external (branch 1 on Fig.1C,D). Three separate branches run medially towards the pharyngeal epithelium, and a final sixth branch extends caudally. The ganglion connects to the rhombencephalon more antero-dorsally (data not shown). These findings were confirmed in three different specimens.
Figure 1.
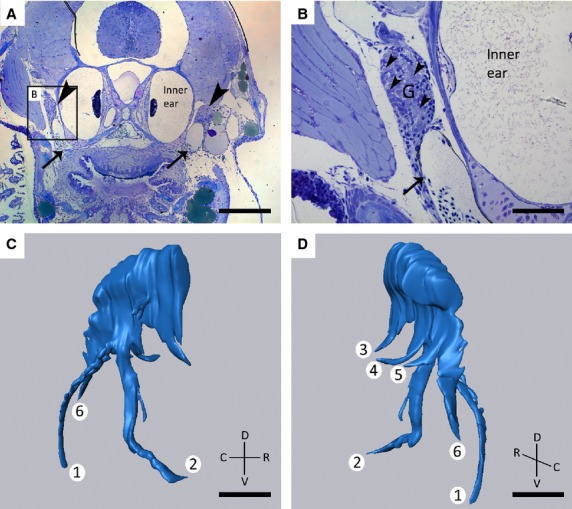
(A, B) Toluidine blue-stained transverse sections of 8.3 mm SL zebrafish showing the presence of two large ganglia (G) (A, arrowheads) dorsal to the developing dentition, lateral to the inner ear, and apposed to the posterior cardinal vein (arrow, A, B). Larger magnification of the area indicated in (A) clearly shows the cluster of neuronal cell bodies (B, arrowheads). (C, D) 3D reconstruction of the ganglion shown in (A) on the right body side. Note the six different branches, two of which (1, 2) have a common origin and course ventrally, three branches run medially (3, 4, 5), and a final branch runs caudally (6). C, caudal; D, dorsal; R, rostral; V, ventral. Scale bars: 200 μm (A); 50 μm (B–D).
Branches of the vagal nerve innervate the pharyngeal jaws
To study the innervation of the dentition, an in-depth light microscopical study of both transverse and sagittal toluidine blue-stained sections of 9.5 mm SL zebrafish was performed. Individual nerve bundles could be clearly traced and identified. A 3D reconstruction was made of 188 consecutive semi-thin (1 μm) sections for visualization and interpretation. Both on the anterior and posterior side of the dentigerous area, the nerve branches issuing from the vagal sensory ganglion could be clearly observed and visualized (Fig.2A).
Figure 2.
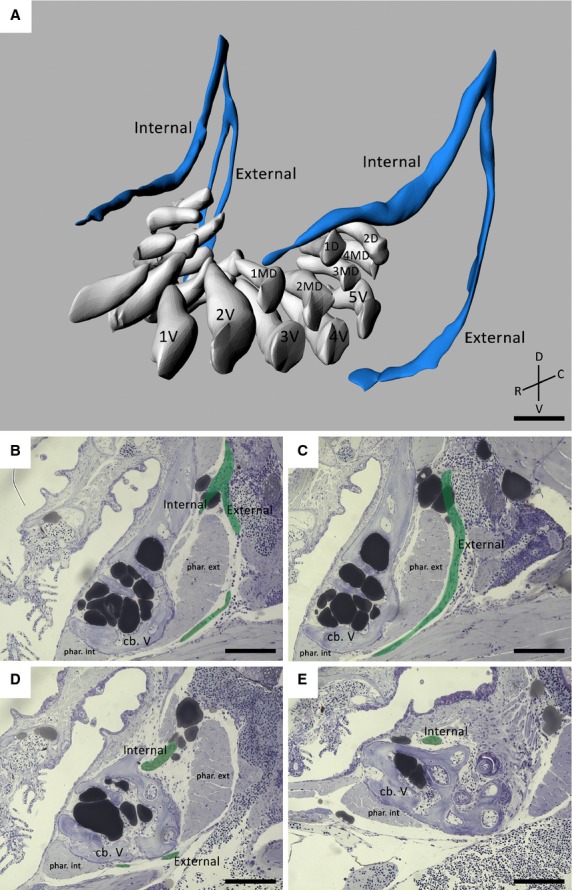
(A) 3D visualization of a 9.5 mm SL zebrafish dentition demonstrating the main nerve branches (blue) in the vicinity of the developing teeth (white). The internal and external branches emerge from a common stem on the postero-dorsal side of the dentition. Note that the internal branch is split up in two bundles on one body side. The teeth are organized in a ventral (1V–5V), mediodorsal (1MD–4MD) and dorsal (1D–2D) row. (B–E) Consecutive sagittal toluidine blue-stained sections of a wild-type zebrafish (SL = 8.3 mm) showing how the internal and external branch (pseudocoloured green) course in relation to both internal and external pharyngoclavicularis muscles (phar. int./ phar. ext.), and the fifth ceratobranchials (cb. V). The external branch extends ventrally and bends in a medial direction along the ventral side of the dentition. The internal branch on the other hand, bends medially almost instantly thus running along the dorsal side of the dorsal tooth row. C, caudal; D, dorsal; R, rostral; V, ventral. Scale bars: 50 μm (A); 100 μm (B–E).
The internal and external nerve branch issue from a common stem connected to the vagal ganglion, as described earlier (Fig.2B). The external branch runs ventrally alongside the branchial musculature positioned posteriorly from the fifth ceratobranchials (Fig.2C). Eventually it bends medially and runs along the ventral side of the ventral tooth row and ceratobranchials (Fig.2D). The second, internal branch courses ventrally over a short distance and soon bends medially towards the teeth. Contrary to the external branch, the internal branch runs along the dorsal side of the ceratobranchials, and passes dorsally to the dorsal tooth row (Fig.2B,E). Surprisingly, in the specimen used for 3D reconstruction, the external branch bifurcated on one side, as opposed to the other side. This could not be observed in other specimens.
Next it was attempted to identify the targets that are innervated by both the external and internal branch (Fig.3). On sagittal semi-thin sections, the external branch can be seen running in very close proximity to the pharyngoclavicularis externus muscle, positioned just posteriorly of the fifth ceratobranchials (Fig.3A), and terminates at the pharyngoclavicularis internus muscle on the cranio-ventral side of the dentition (Fig.3B). The internal branch on the other hand, continues running anteriorly and, at tooth position 1D, penetrates the bone. The internal branch now runs at the base of the teeth in the cranio-ventral direction. Due to a decrease in diameter of the nerve bundle, distinguishing it from the surrounding tissue proved difficult on semi-thin sections. Hence, immunohistological staining was performed to visualize the nerve bundles. This allowed us to identify the internal branch to be responsible for the pulpal innervation of the teeth. As it passes at the base of the functional teeth, it gives off branches running towards the dental pulp (Fig.3C). Smaller branches can also be seen to ascend towards the pharyngeal epithelium (Fig.3D,E). These smaller branches likely innervate the taste buds located at the crests between adjacent epithelial crypts, and the oral mucosa that surrounds the teeth (Fig.3F).
Figure 3.
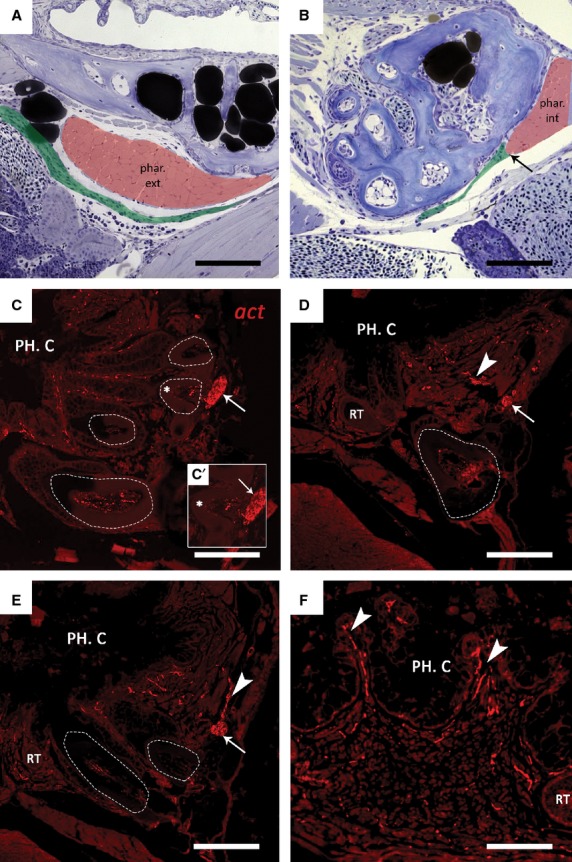
(A, B) Sagittal toluidine blue-stained sections of a wild-type 9.5 mm SL zebrafish. In both sections anterior is to the right. The external branch (pseudocoloured green) passes close to the external pharyngoclavicularis muscle (phar. ext, pseudocoloured red) at the posterior side of the dentition (A). The external branch appears to terminate (arrow) at the internal pharyngoclavicularis (phar. int) muscle on the cranioventral side of the dentition (B).(C–F) Immunohistochemical detection of nerve fibres in the dentition of zebrafish. Transverse paraffin sections of adult fish were stained with a primary antibody against acetylated tubulin (act). Functional teeth are indicated using dashed lines. (C) The internal branch (arrow) passes at the very base of the functional teeth (dashed line) after having penetrated the ceratobranchial bone at tooth position 1d (not shown). (C’) Enlargement of the functional tooth indicated in (c) (*); smaller branches appear to enter the pulp cavity of the tooth. (D, E) Furthermore, smaller axons (arrowheads) extend from the internal branch (arrow) towards the pharyngeal epithelium, where they probably innervate the oral mucosa and taste buds located between the epithelial crypts (F). PH. C., pharyngeal cavity; RT, replacement tooth. Scale bars: 100 μm (A–E); 50 μm (F).
Innervation of individual teeth
Immunohistological staining revealed nerve fibres in the dental pulp but never in the dentine (Fig.4A). This was confirmed in two different individuals. All functional teeth studied in these two specimens (n = 44) showed specific staining in the pulp. However, nerve fibres in replacement teeth could not be detected, regardless of the stage of differentiation (initiation, morphogenesis, early cytodifferentiation; Fig.4B). Only at stages of very late cytodifferentiation, i.e. in teeth nearing attachment, could nerve fibres be observed penetrating the pulp cavity at the tooth base (Fig.4C).
Figure 4.
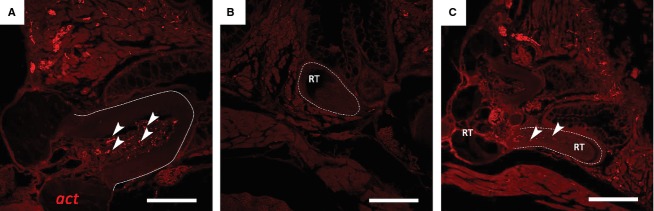
Immunohistochemical detection of nerve fibres in both functional teeth (full line) and replacement teeth (dashed line), on transverse paraffin sections of adult zebrafish stained with a primary antibody against acetylated tubulin (act). (A) Functional teeth clearly contain many small nerve fibres (arrowheads) in the dental pulp. (B) Replacement teeth (RT), however, are completely devoid of axons at all stages of differentiation. (C) Only at stages of very late cytodifferentiation, i.e. nearing attachment, could nerves (arrowheads) be seen entering the tooth at the base. Scale bars: 50 μm (A); 150 μm (B, C).
Discussion
This study aimed to improve our knowledge on the nervous supply of the fifth branchial, tooth-bearing, arch (pharyngeal jaw) in zebrafish, and the spatial relationship between nerve bundles and developing teeth. Using both serial semi-thin sections and immunohistochemistry, the main nerve branches coursing along the tooth-bearing ceratobranchials and the point from where they originate were able to be identified. In addition, evidence was found of nerve fibres penetrating the pulp.
Vagal sensory ganglion and its branches
A large vagal sensory ganglion on each body side gives off branches running towards the dentigerous region. This is in line with our hypothesis that the vagal nerve not only innervates the other, gill-bearing, branchial arches 1–4 (also termed pharyngeal arches 3–6), but also the fifth, tooth-bearing, branchial arch. According to Beaumont & Cassier (1994), a branchial nerve usually consists of a pharyngeal ramus, pre- and post-trematic ramus, and an intestinal ramus. Because the teeth develop on the arch behind the last gill slit (‘trema’), the ramus concerned is necessarily a post-trematic ramus. Therefore, the two branches running towards the teeth were termed the internal and external branch of the post-trematic ramus of the vagal nerve. The three nerve bundles running medially are potentially the pharyngeal branches, contradicting Beaumont & Cassier (1994) who only describes one pharyngeal branch. Finally, the bundle running caudally from the ganglion is probably the intestinal ramus responsible for the innervation of the gut, as described by several authors (Higashijima et al. 2000; Holzschuh et al. 2005; Jonz & Zaccone, 2009).
Branches of the vagal nerve innervate the pharyngeal jaws
Based on our light microscopical study and 3D reconstructions, and on published data (Higashijima et al. 2000; Holzschuh et al. 2005; Jonz & Zaccone, 2009), the vagal nerve was identified as responsible for the innervation of both the pharyngeal jaws and teeth (summarized in Fig.5). The fifth ceratobranchials in zebrafish appear to be innervated solely by a post-trematic ramus of the vagal nerve, issuing from a large sensory vagal ganglion. Several authors report the presence of not just the large sensory ganglion but also of several smaller sensory ganglia at the more anteriorly positioned gill arches giving off pre-trematic and post-trematic rami as well (Higashijima et al. 2000; Holzschuh et al. 2005; Jonz & Zaccone, 2009). However, contrary to what was expected, the post-trematic ramus on either body side is divided in both an internal and external branch. The bifurcation in the external branch on one side, as opposed to the other body side, was not observed in other specimens and could therefore be an artefact of the 3D rendering.
Figure 5.

Schematic representation of the main nerve branches in the vicinity of the dentition. The internal branch of the post-trematic ramus (int. pst. br.) of the vagal nerve is responsible for the innervation of the teeth along with the taste buds. The external branch of the post-trematic ramus (ext. pst. br.) on the other hand innervates the branchial musculature, i.e. the internal and external pharyngoclavicularis (phar. int./ phar. ext.). Both internal and external branches emerge from a large vagal ganglion (gX), which also gives rise to the intestinal ramus (ins. rm.). Also note the smaller vagal sensory ganglia (vs) positioned dorsally of gill slits two–four (G2–G4), which give rise to both pre-trematic (prt. rm.) and post-trematic (pst. rm.) rami. The first gill slit (G1) is innervated by branches of the glossopharyngeal nerve (nIX) (not shown).
The internal branch of the post-trematic ramus probably transmits sensory innervation from the teeth, taste buds and oral mucosa. A similar innervation pattern has already been observed in two other teleosts, the perch (Perca fluviatilis) and the rainbow trout (Oncorhynchus mykiss; Dunelerb et al. 1993), where taste buds are said to be innervated by both post- and pre-trematic rami of the vagal nerve. The external branch of the post-trematic ramus on the other hand likely transmits motor information to the branchiomeric musculature. This is also the case for several species of Tetraodontiformes, where post-trematic rami of the vagal nerve are involved in the innervation of the internal and external pharyngoclavicularis muscles (Nakae & Sasaki, 2008).
Both internal and external branches of the post-trematic ramus emerge directly from their dorsal ganglion. Therefore, one could assume these nerves to contain solely viscero-afferent fibres, as somato-efferent fibres have been described in zebrafish to emerge directly from nuclei in the central nervous system and to course directly towards their targets, i.e. branchiomeric musculature (Beaumont & Cassier, 1994; Chandrasekhar et al. 1997). However, given the possible involvement of the external branch of the post-trematic ramus in delivering motor information to both the internal and external pharyngoclavicularis, it was assumed these post-trematic branches not only transmited sensory information, but also motor signals. In humans, motor fibres of the trigeminal nerve likewise pass through the trigeminal ganglion without having their cell bodies in the ganglion; rather they are located in nuclei in the central nervous system (Nilsson, 1983).
The combination of motor and sensory nerve fibres has also been described in the light of vasomotoric control of the vasculature in the gills (Nilsson, 1983; Beaumont & Cassier, 1994; Jonz & Zaccone, 2009). In mammals, axons present in the pulp take part in the regulation of pulpal blood flow (Kim, 1990; Kerezoudis et al. 1992; Olgart, 1996; Pagella et al. 2014). Given a possible role of motor fibres in regulating blood flow, it is important to note that the dentigerous area in zebrafish is richly supplied with blood (Crucke & Huysseune, 2013). The hypothesis has recently been raised that blood vessels act instructively to initiate tooth development in zebrafish (Crucke & Huysseune, 2013). However, in a follow-up study, it was demonstrated that blood vessels are not required for tooth initiation, but rather allow the teeth to further grow and develop (Crucke & Huysseune, 2015). Nerves in the dentigerous region could be involved in influencing the vascular elements present through secretion of so-called angioneurins, i.e. signalling molecules affecting both neural and vascular functions (Zacchigna et al. 2008a). Moreover, it has been shown in the skin of mice that peripheral nerves provide a template for developing arteries to guide their growth and development toward their target (Mukouyama et al. 2002). This cross-talk between vascular and neural networks is termed ‘the neurovascular link’ (Carmeliet, 2003; Zacchigna et al. 2008b; Ulrich et al. 2011). To what degree the nervous system influences the vascular network and vice versa during the process of tooth development, still needs to be determined.
Innervation of individual teeth
Apart from studies performed on the cichlidTilapia mariae (Holje et al. 1986; Tuisku & Hildebrand, 1994, 1996), little information is available on innervation of teleost teeth. Here, for the first time, the presence of nerve fibres in the pulp cavity of functional teeth in zebrafish has been clearly demonstrated. While this may be expected based on a comparative basis (e.g. data from mouse, rat, human; Zmijewska et al. 2003; Luukko et al. 2005, 2008), it may be somewhat surprising considering the continuous turnover of the teeth and their short functional lifetime. Therefore, nerves are probably more involved in concepts such as vasomotoric control rather than pain sensation (teeth are replaced anyway). The nerve fibres in the pulp of zebrafish issue from an internal branch of the post-trematic ramus of the vagal nerve. InT. mariae and mammals, on the other hand, dental nerves originate from the trigeminal nerve (Holje et al. 1986) and the inferior/superior alveolar nerve (Hildebrand et al. 1995), respectively. Given the location of zebrafish teeth deep within the oral cavity, a difference in innervation when compared with oral teeth is to be expected. Furthermore, different from what is known for mice and humans, the dental nerves in zebrafish appear to be limited to the pulp cavity, whereas in mammals, nerve fibres penetrate into the dentine as well (Fearnhead, 1957; Byers, 1980).
Interestingly, no evidence was found for nerve fibres in developing replacement teeth. All stages of differentiation were devoid of nerve fibres. Only at very late stages of cytodifferentiation, i.e. nearing attachment, were nerves seen entering at the base of the replacement tooth. At this stage, teeth become functional allowing nerves to serve their function for the tooth. Likewise, blood vessels could only be seen penetrating at the base of replacement teeth at very late stages of differentiation (Crucke & Huysseune, 2013), further suggesting a developmental and/or functional link between both. Moreover, the initiation of a replacement tooth (i.e. upon attachment of the predecessor) might then be potentially achieved through the secretion of certain growth factors by sensory neurons that in turn could activate mesenchymal stem cells, for example, periarterial cells as was shown for the neurovascular bundle present in the mouse incisor (Zhao et al. 2014). In mice as well, nerves enter the dental pulp only at late bell stage, i.e. after the onset of enamel formation (Moe et al. 2008).
The late penetration of nerves into the dental pulp, both in mice and zebrafish, suggests a relative independence between the establishment of the innervation on the one hand, and the process of tooth development on the other hand. Yet, prior to the penetration of nerves in the dental pulp, growth of the dental trigeminal axons in mice occurs concomitantly with advancing tooth formation (Hildebrand et al. 1995). This coordinated development between nerves and teeth hints towards a possible neuronal influence on tooth development as was already suggested by Pearson (1977). Nonetheless, only two studies have yet been able to show a direct influence of nerves on the processes of tooth development and replacement. InT. mariae, unilateral denervation of the trigeminal nerve ceased tooth turnover on the operated side (Tuisku & Hildebrand, 1994). Removing inferior alveolar neurovascular structures in puppies with erupted deciduous teeth stopped the eruption of permanent teeth (Harputluoglu, 1990; Tuisku & Hildebrand, 1994). These two studies suggest an instructive role of dental nerves in tooth development and replacement. The arrest in tooth development in these studies might have succeeded through removing the potential source of mesenchymal stem cells (e.g. nerve-associated glia) or in removing the inductive signal produced by sensory nerves to induce the differentiation of periarterial cells as was demonstrated in the mouse incisor (Zhao et al. 2014).
In conclusion, having established the essential morphological background information on innervation of zebrafish teeth, it can now be investigated whether or not dental nerves in zebrafish fulfil an instructive or rather permissive role during odontogenesis. It is clear that apart from investigating direct effects on tooth development, future research should also focus on mutual interactions of the nerves with the vascular system (i.e. the neurovascular link).
Acknowledgments
The authors thank Mieke Soenens and Dennis Vlaeminck for expert technical assistance, and the lab of Evolutionary Developmental Biology for support and discussions. J.C. acknowledges a grant from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
Author contributions
J. Crucke, contributed to conception, design, data acquisition, analysis and interpretation, drafted the manuscript; A. Van de Kelft contributed to data acquisition, analysis and interpretation; A. Huysseune, contributed to conception, design and data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
References
- Beaumont A, Cassier P. Biologie animale. Paris: Dunod; 1994. [Google Scholar]
- Bei M. Molecular genetics of tooth development. Curr Opin Genet Dev. 2009;19:504–510. doi: 10.1016/j.gde.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers MR. Development of sensory innervation in dentin. J Comp Neurol. 1980;191:413–427. doi: 10.1002/cne.901910307. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A, Moens CB, Warren JT. Development of branchiomotor neurons in zebrafish. Development. 1997;124:2633–2644. doi: 10.1242/dev.124.13.2633. [DOI] [PubMed] [Google Scholar]
- Chiego DJ. The early distribution and possible role of nerves during odontogenesis. Int J Dev Biol. 1995;39:191–194. [PubMed] [Google Scholar]
- Crucke J, Huysseune A. Unravelling the blood supply to the zebrafish pharyngeal jaws and teeth. J Anat. 2013;223:399–409. doi: 10.1111/joa.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crucke J, Huysseune A. Blocking VEGF signaling delays development of replacement teeth in zebrafish. J Dent Res. 2015;94:157–165. doi: 10.1177/0022034514557156. [DOI] [PubMed] [Google Scholar]
- Dunelerb S, Bailly Y, Laurent P. Pattern of gill innervation in 2 teleosts, the perch and the trout. Can J Zool. 1993;71:18–25. [Google Scholar]
- Ezeasor DN. Distribution and ultrastructure of taste-buds in the oropharyngeal cavity of the rainbow-trout, Salmo-Gairdneri Richardson. J Fish Biol. 1982;20:53–68. [Google Scholar]
- Fearnhead RW. Histological evidence for the innervation of human dentine. J Anat. 1957;91:267–277. [PMC free article] [PubMed] [Google Scholar]
- Fried K, Hildebrand C. Qualitative structural development of the feline inferior alveolar nerve. J Anat. 1982;134:517–531. [PMC free article] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the Structure and Development of Vertebrates. London: Macmillan; 1930. [Google Scholar]
- Grassé PP, Tetry A. Zoologie. Paris: Gallimard; 1963. [Google Scholar]
- Harputluoglu S. Effects of removing inferior alveolar neurovascular structures on mandibular growth and the eruption of permanent dentition in puppies. Oral Surg Oral Med Oral Pathol. 1990;70:147–149. doi: 10.1016/0030-4220(90)90107-4. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the Islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C, Fried K, Tuisku F. Teeth and tooth nerves. Prog Neurobiol. 1995;45:165–222. doi: 10.1016/0301-0082(94)00045-j. [DOI] [PubMed] [Google Scholar]
- Holje L, Hildebrand C, Fried K. On nerves and teeth in the lower jaw of the cichlidTilapia-mariae. Anat Rec. 1986;214:304–311. doi: 10.1002/ar.1092140310. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Huysseune A. Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation. Int J Dev Biol. 2006;50:637–643. doi: 10.1387/ijdb.052098ah. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire J-Y. Development of cartilage and bone tissues of the anterior part of the mandible in cichlid fish: a light and TEM study. Anat Rec. 1992;233:357–375. doi: 10.1002/ar.1092330304. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire JY. Structure and development of first-generation teeth in the cichlidHemichromis bimaculatus (Teleostei, Cichlidae) Tissue Cell. 1997;29:679–697. doi: 10.1016/s0040-8166(97)80044-4. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Van der Heyden C, Sire JY. Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae) Anat Embryol. 1998;198:289–305. doi: 10.1007/s004290050185. [DOI] [PubMed] [Google Scholar]
- Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274:139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Jonz MG, Nurse CA. New developments on gill innervation: insights from a model vertebrate. J Exp Biol. 2008;211:2371–2378. doi: 10.1242/jeb.010587. [DOI] [PubMed] [Google Scholar]
- Jonz MG, Zaccone G. Nervous control of the gills. Acta Histochem. 2009;111:207–216. doi: 10.1016/j.acthis.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–554. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- Kerezoudis NP, Olgart L, Edwall L. Activation of sympathetic fibres in the pulp by electrical stimulation of rat incisor teeth. Arch Oral Biol. 1992;37:1013–1019. doi: 10.1016/0003-9969(92)90033-5. [DOI] [PubMed] [Google Scholar]
- Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endod. 1990;16:48–53. doi: 10.1016/S0099-2399(06)81563-3. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Lumsden AGS. Tooth morphogenesis – role of the innervation during induction and pattern formation. J Biol Buccale. 1979;7:49–60. [PubMed] [Google Scholar]
- Laurent P, Dunel S. [Studies on the innervation of the pseudobranchia of teleostean fish] Arch Anat Microsc Morphol Exp. 1966;55:633–656. [PubMed] [Google Scholar]
- Lele Z, Krone PH. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol Adv. 1996;14:57–72. doi: 10.1016/0734-9750(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Lumsden AGS. The developing innervation of the lower jaw and its relation to the formation of tooth germs in mouse embryos. In: Björn Kurtén., editor. Teeth: Form, Function, and Evolution. New York: Colombia University Press; 1982. pp. 32–43. [Google Scholar]
- Lumsden AG, Buchanan JA. An experimental study of timing and topography of early tooth development in the mouse embryo with an analysis of the role of innervation. Arch Oral Biol. 1986;31:301–311. doi: 10.1016/0003-9969(86)90044-0. [DOI] [PubMed] [Google Scholar]
- Luukko K, Kvinnsland IH, Kettunen P. Tissue interactions in the regulation of axon pathfinding during tooth morphogenesis. Dev Dyn. 2005;234:482–488. doi: 10.1002/dvdy.20586. [DOI] [PubMed] [Google Scholar]
- Luukko K, Moe K, Sijaona A. Secondary induction and the development of tooth nerve supply. Ann Anat. 2008;190:178–187. doi: 10.1016/j.aanat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Moe K, Kettunen P, Kvinnsland IH. Development of the pioneer sympathetic innervation into the dental pulp of the mouse mandibular first molar. Arch Oral Biol. 2008;53:865–873. doi: 10.1016/j.archoralbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mohamed SS, Atkinson ME. A histological study of the innervation of developing mouse teeth. J Anat. 1983;136:735–749. [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Finger TE. Topographic and laminar organization of the vagal gustatory system in the goldfish, Carassius-Auratus. J Comp Neurol. 1985;238:187–201. doi: 10.1002/cne.902380206. [DOI] [PubMed] [Google Scholar]
- Mukouyama Y, Shin D, Britsch S. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Nakae M, Sasaki K. Branchial arch muscle innervation by the glossopharyngeal (IX) and vagal (X) nerves in Tetraodontiformes, with special reference to muscle homologies. J Morphol. 2008;269:674–690. doi: 10.1002/jmor.10611. [DOI] [PubMed] [Google Scholar]
- Nilsson S. Autonomic Nerve Function in the Vertebrates. Berlin: Springer; 1983. [Google Scholar]
- Nilsson S. Innervation and pharmacology of the gills. In: Hoar WS, Randall DJ, editors. Fish Physiology. San Diego: Academic Press; 1984. pp. 137–147. [Google Scholar]
- Olgart L. Neural control of pulpal blood flow. Crit Rev Oral Biol Med. 1996;7:159–171. doi: 10.1177/10454411960070020401. [DOI] [PubMed] [Google Scholar]
- Pagella P, Jimenez-Rojo L, Mitsiadis TA. Roles of innervation in developing and regenerating orofacial tissues. Cell Mol Life Sci. 2014;71:2241–2251. doi: 10.1007/s00018-013-1549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson AA. The early innervation of the developing deciduous teeth. J Anat. 1977;123:563–577. [PMC free article] [PubMed] [Google Scholar]
- Roush W. Zebrafish embryology builds better model vertebrate. Science. 1996;272:1103–1103. doi: 10.1126/science.272.5265.1103. [DOI] [PubMed] [Google Scholar]
- Sire JY, Davit-Beal T, Delgado S. First-generation teeth in nonmammalian lineages: evidence for a conserved ancestral character? Microsc Res Tech. 2002;59:408–434. doi: 10.1002/jemt.10220. [DOI] [PubMed] [Google Scholar]
- Stock DW. Zebrafish dentition in comparative context. J Exp Zool B Mol Dev Evol. 2007;308B:523–549. doi: 10.1002/jez.b.21187. [DOI] [PubMed] [Google Scholar]
- Sundin L, Nilsson S. Branchial innervation. J Exp Zool. 2002;293:232–248. doi: 10.1002/jez.10130. [DOI] [PubMed] [Google Scholar]
- Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140A:2530–2535. doi: 10.1002/ajmg.a.31360. [DOI] [PubMed] [Google Scholar]
- Tuisku F, Hildebrand C. Evidence for a neural influence on tooth germ generation in a Polyphyodont species. Dev Biol. 1994;165:1–9. doi: 10.1006/dbio.1994.1228. [DOI] [PubMed] [Google Scholar]
- Tuisku F, Hildebrand C. Occurrence of axons with certain immunohistochemical markers in teleost gingiva and teeth. Brain Res. 1996;729:137–141. [PubMed] [Google Scholar]
- Ulrich F, Ma LH, Baker RG. Neurovascular development in the embryonic zebrafish hindbrain. Dev Biol. 2011;357:134–151. doi: 10.1016/j.ydbio.2011.06.037. [DOI] [PubMed] [Google Scholar]
- Van der Heyden C, Huysseune A. Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae) Dev Dyn. 2000;219:486–496. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1069>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Van der Heyden C, Huysseune A, Sire JY. Development and fine structure of pharyngeal replacement teeth in juvenile zebrafish (Danio rerio) (Teleostei, Cyprinidae) Cell Tissue Res. 2000;302:205–219. doi: 10.1007/s004410000180. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene: University of Oregon Press; 1993. The zebrafish book. 385 pp. [Google Scholar]
- Young HM, Cane KN, Anderson CR. Development of the autonomic nervous system: a comparative view. Auton Neurosci. 2011;165:10–27. doi: 10.1016/j.autneu.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Zacchigna S, De Almodovar CR, Carmeliet P. Similarities between angiogenesis and neural development: what small animal models can tell us. Curr Top Dev Biol. 2008a;80:1–55. doi: 10.1016/S0070-2153(07)80001-9. [DOI] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008b;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- Zhao H, Feng J, Seidel K. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–173. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewska C, Surdyk-Zasada J, Zabel M. Development of innervation in primary incisors in the foetal period. Arch Oral Biol. 2003;48:745–752. doi: 10.1016/s0003-9969(03)00155-9. [DOI] [PubMed] [Google Scholar]


